Abstract
Detailed characterization of neural circuitries furthers our understanding of how nervous systems perform specific functions and enables the use of those systems to test hypotheses. We have characterized the sensory input to the cutaneous trunk muscle (CTM; also cutaneus trunci (rat) or cutaneus maximus (mouse)) reflex (CTMR), which manifests as a puckering of the dorsal thoracolumbar skin and is selectively driven by noxious stimuli. CTM electromyography (EMG) and neurogram recordings in naïve rats revealed that CTMR responses were elicited by natural stimuli and electrical stimulation of all segments from C4 to L6, a much greater extent of segmental drive to the CTMR than previously described. Stimulation of some subcutaneous paraspinal tissue can also elicit this reflex. Using a selective neurotoxin, we also demonstrate differential drive of the CTMR by trkA-expressing and non-expressing small diameter afferents. These observations highlight aspects of the organization of the CTMR system which make it attractive for studies of nociception and anesthesiology and plasticity of primary afferents, motoneurons, and the propriospinal system. We use the CTMR system to qualitatively and quantitatively demonstrate that experimental pharmacological treatments can be compared to controls applied either to the contralateral side or to another segment, with the remaining segments providing controls for systemic or other treatment effects. These data indicate the potential for using the CTMR system as both an invasive and non-invasive quantitative assessment tool providing improved statistical power and reduced animal use.
Keywords: sensory neurons, pain, plasticity, animal models, spinal cord, anesthesiology, pharmacology
Introduction
Nociception, the sensing of conditions that are actually or potentially tissue-damaging, is one of the most important sensory functions performed by any nervous system. Nociception is the first stage not only of generating self-protective behaviors, but also of generating the perception of evoked pain. Determining the neural circuitry involved in nociception and pain can facilitate our understanding of the mechanisms underlying different behaviors. The different neural circuitry underlying different behaviors, both across species and within different regions of the same animal, can provide not only insight into mechanism, but also opportunity to test hypotheses regarding those mechanisms. The circuitry underlying activation of the cutaneous trunk muscle (CTM; also called cutaneus trunci, cutaneus maximus, or panniculus carnosus) may represent this kind of system. Here we characterize the organization of the spinal sensory input that leads to reflexive activation of the CTM and demonstrate the utility of this system for examining nociception-related hypotheses.
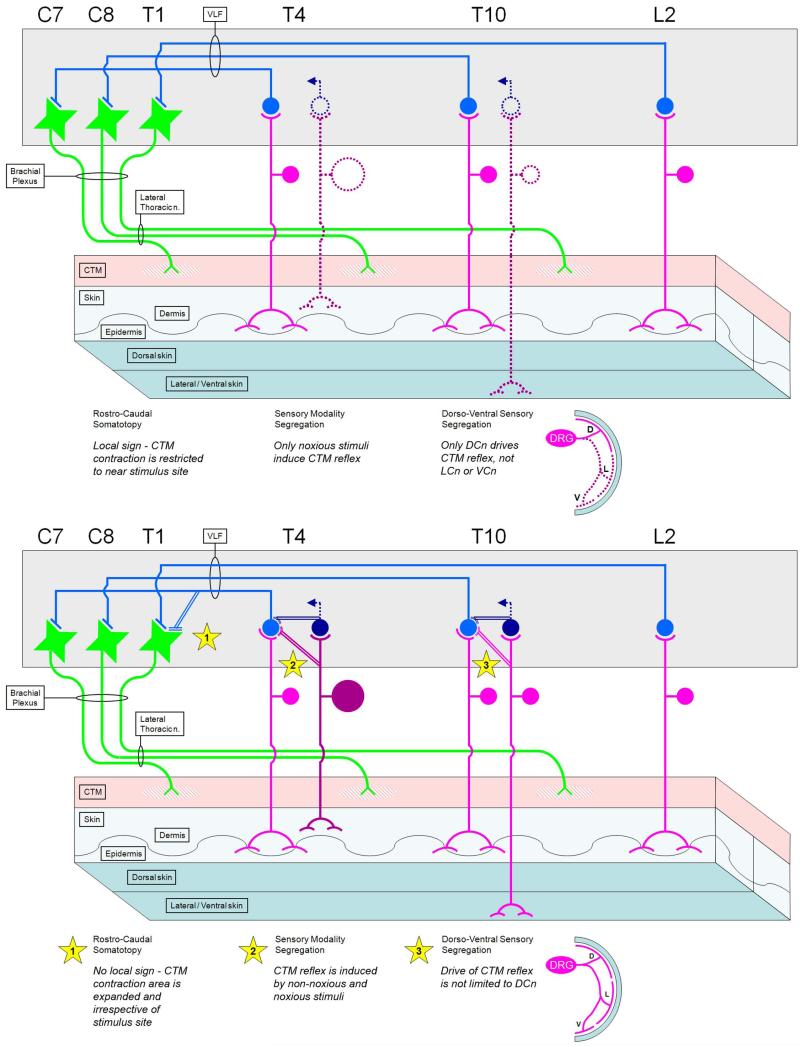
The CTM is a thin sheet of striated muscle originating on the upper humerus and inserting into the dorsolateral thoracolumbar dermis. The CTM motoneuron pool extends from C7-T1 in rat (Baulac and Meininger, 1981; Theriault and Diamond, 1988a), and innervation of the muscle is from the brachial plexus-derived lateral thoracic nerve (LTn)(Krogh and Towns, 1984). It is considered an appendicular muscle in spite of its axial location owing to its innervation and embryological origins (Krogh and Towns, 1984). The LTn branches extend in parallel along the CTM in a rostro-caudal manner, giving off small branches along their course (Griffin et al., 2010; Theriault and Diamond, 1988a). The motoneuron pool is somatotopically organized in both a rostro-caudal and dorso-ventral manner (Theriault and Diamond, 1988a).
Reflexive activation of the CTM (CTM reflex; CTMR) is readily visible as a flicking or puckering of the skin. In rats and mice the CTMR is nociceptive-specific, i.e., activated only in response to noxious stimulation of the skin (e.g., heat, pinch)(Krogh and Denslow, 1979; Krogh and Towns, 1984; Theriault and Diamond, 1988b). In many other species including horses, cats, and guinea pigs, reflex activation can also be induced in response to light touch of the fur/hair (e.g., Blight et al., 1990; Krogh and Denslow, 1979; Krogh and Towns, 1984). The CTMR is polysynaptic, involving at least 3 neurons (Blight et al., 1990; Theriault and Diamond, 1988b). Additional details of comparative anatomy and physiology are available elsewhere (e.g., Krogh and Denslow, 1979; Krogh and Towns, 1984).
Aspects of the CTM and CTMR organization make this system enticing for use as a model to study 1) nociception and anesthesiology, 2) plasticity of primary afferents, motoneurons, and the propriospinal system, and 3) reactions to spinal cord injury/disease and treatment. The CTMR has already proven to be highly useful as a monitor for the progress and extent of sensory neuron axonal collateral sprouting and motoneuron axonal regeneration (e.g., Diamond et al., 1992; Griffin et al., 2010), for the recovery of function after dorsal root injuries (Jiang et al., 2003) and spinal cord injuries (SCI) and treatments (Blight, 1991; Blight et al., 1991; Borgens et al., 1990; Borgens and Bohnert, 2001; Borgens et al., 2002; Tansey et al., 2007) and a readout for anesthesiological experimentation (Binshtok et al., 2009; Colvin et al., 2011; Duarte et al., 2005; Gerner et al., 2006; Khan et al., 2002a; Khan et al., 2002b; Lim et al., 2007; Mujenda et al., 2007; Shieh et al., 2009; Wang et al., 2008; Yoshitomi et al., 2008).
A more detailed description of the organization and characteristics of the CTM system could further our understanding of how different species process the different types of stimuli that are capable of driving the CTMR and other similar systems. Although humans do not possess a CTM, they do possess numerous cutaneous muscles, and reflexes induced by cutaneous stimulation are common neurologic diagnostic tools used to assess the functional status of the underlying neural circuitry, particularly spinal circuitry (Bates, 2007). This knowledge will also advance efforts to develop quantitative readouts of the CTM system which could then be used in multiple species to test hypotheses. Although it has been suggested from electromyography and behavior that the sensory supply to the CTMR in the rat is via dorsal cutaneous nerves (DCnn) from the T4 to L2 segments (Theriault and Diamond, 1988b), the full extent of the segmental sensory contribution to the CTMR has not been described. We therefore used behavioral observations, EMG of the CTM, and neurogram recordings from branches of the LTn to determine which segments, nerves, and tissues contribute to the CTMR.
In addition to determining the segmental organization, we have addressed some aspects of the cellular specificity of afferent drive of the CTMR. While the CTM reflex appears to be driven selectively by noxious stimuli, nociceptive sensory neurons are a heterogeneous population. They are distinguished by numerous specific classifications, but are often classified according to their expression of receptors for trophic factors. In the adult, the two major subpopulations are those that express the trkA receptor for nerve growth factor (NGF), and those that lack trkA but express the GFR-alpha/ret receptor complex for glial cell line-derived neurotrophic factor-family ligands (e.g., Bennett et al., 1998; Molliver et al., 1995; Molliver et al., 1997). These populations are largely separate but are both predominantly nociceptive (e.g., Fang et al., 2005a; Fang et al., 2006; Fang et al., 2005b), and display different peripheral and central termination patterns (Bennett et al., 1998; Braz et al., 2005; Molliver and Snider, 1997; Molliver et al., 1997; Zylka et al., 2005). We therefore addressed whether the populations were equally able to drive the CTMR by destroying DRG neurons expressing the trkA receptor, but sparing those not expressing the neurotrophin receptors, and assessing the CTMR.
We also demonstrate how the CTMR, used as a model system, can improve statistical power and animal use efficiency by providing internal controls. Using the peripheral nerve components of the CTMR system, we examined whether the CTMR system, monitored either invasively or non-invasively, could detect the known extension of the local anesthetic actions of bupivacaine by the alpha-2 adrenergic agonist dexmedetomidine (Brummett et al., 2008; Petruska et al., 2008; Soto et al., 2007; Yoshitomi et al., 2008). Using the spinal cord component of the CTMR system, we demonstrate how the CTMR can be used to detect the effects of the neurotrophin BDNF on spinal nociceptive processing (Garraway et al., 2003; Thompson et al., 1999). Some of this work has been presented in abstract form (Petruska et al., 2005; Petruska et al., 2008; Soto et al., 2007).
Methods
All procedures were approved by the Institutional Animal Care and Use Committees of the University of Louisville, SUNY Stony Brook, and the University of Florida and were in accord with the guidelines set forth by the NIH (Guide for the Care and Use of Laboratory Animals) and Society for Neuroscience (Handbook for the Use of Animals in Neuroscience Research). All procedures were carried out with the animal under pentobarbital anesthesia (30-40mg/kg for sedation, 40-60mg/kg for surgery), and euthanasia was induced by overdose of pentobarbital given either iv or ip. The CTMR is resistant to pentobarbital anesthesia (Theriault and Diamond, 1988b), is highly variable with isoflorane, and is eliminated by ketamine (unpublished observations). All experiments used Sprague-Dawley female rats (Taconic, Hudson, NY)(Table 1). Animals were cannulated via the jugular vein for supplemental anesthesia and intubated via the trachea to monitor end-tidal pCO2. Body temperature was monitored with a rectal thermistor and was maintained at 35-37 degrees C with a heated water pad. EKG leads were also placed to monitor heart rate. For all preparations the entire dorsal skin was shaved. A summary of the animals used for the various preparations is provided in Table 1.
Table 1.
Summary of animals and experiments.
| Experiment | n | Experiment | n |
|---|---|---|---|
| Characterization | [31] | Anesthetic | [18] |
| EMG (natural) | 3 | Bupiv+Dex − Behavior | 9 |
| EMG (electrical) |
5 | Bupiv+Dex − EMG | 3 |
| neurogram | 10 | Saline+Dex | 4 |
| LCnn test | 3 | Nerve conduction | 2 |
| subcutaneous | 2 | ||
| 192-saporin | 8 | BDNF | 1 |
Mapping of the CTM reflex cutaneous receptive field (n=8) did not use dedicated animals, but was performed before some of the invasive experimental procedures.
CTM reflex organization experiments
Behavioral observation of the reflex was performed by applying noxious mechanical stimuli (lightly pricking or pinching small, discrete areas of skin with sharp forceps) in a matrix fashion (n=8). Ink marks were placed on responsive sites to create a visual map of the responsive skin. In some cases mechanical pressure or pinch was applied to subcutaneous tissue to determine if these tissues could drive the reflex.
For EMG recordings with natural stimulation (n=3), a longitudinal incision was made in the skin lateral to the CTM, leaving the cutaneous nerves and the muscle intact. Silver EMG electrodes were inserted into numerous sites, including the belly of the CTM muscle between its origin and the rostral-most insertion in the dermis. For neurogram and EMG recordings with electrical stimulation of the sensory nerves (n=15), an incision was made in the midline skin extending from the occipital protuberance to the base of the tail. The skin was then reflected and cutaneous nerves cut close to the skin and prepared for recording. Branches of the lateral thoracic nerve innervating the CTM (Figure 1) were isolated and transected to be placed on bipolar electrodes. In many cases the transected nerve was also stripped of its epineurial sheath to enhance the signal:noise ratio. In most cases, all branches of the LTn were transected even if not used for recordings, but in other cases some branches were left intact to allow comparison of the neurogram and behavioral responses. Neurogram output recorded from the different LTn branches varied depending on the DCn stimulated - i.e., there was a somatotopic arrangement such that the same branch of the LTn could not be used for recording the responses following stimulation of all DCnn. Therefore, we did not perform a quantitative comparison of responses following stimulation of different DCnn. Segmental identity of DCnn not previously reported to drive the CTMR was confirmed in some animals post-mortem by gross anatomical tracing of the nerve back to the spinal nerve and dorsal root ganglion. A schematic of the DCnn with identifying landmarks is provided in Figure 2.
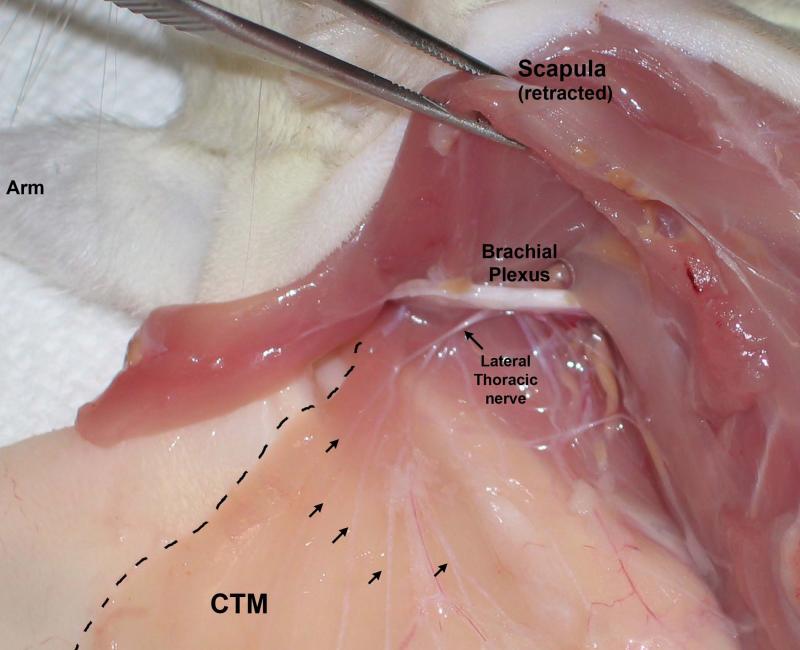
Figure 1.
Gross dissection of the CTM neural supply (Lateral Thoracic nerve; LTn) emerging from the brachial plexus and branching to run through the muscle (arrows). View is of the left side of the rat, head at top left, with skin incised at midline and reflected laterally (left). The dashed line demarcates the edge of the CTM.
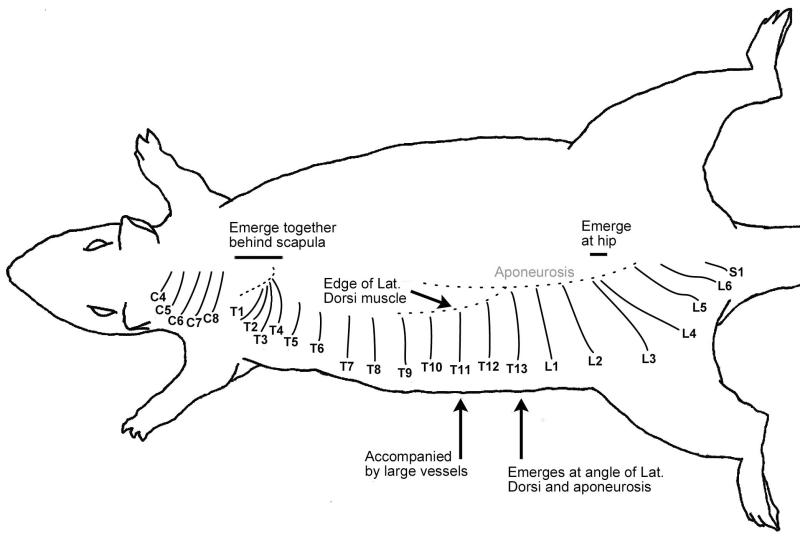
Figure 2.
Schematic drawing of dorsal cutaneous nerves with some distinguishing landmarks.
Electrical stimulation of the cutaneous nerves sufficient to recruit A-delta fibers (1-3 mA, 0.05msec) or both A-delta and C-fibers (3-5mA, 0.5 msec) was delivered with bipolar Ag/AgCl electrodes. Neurogram and EMG responses were recorded through a Grass P511 amplifier and digitized with a Digidata 1322A A-D converter (Axon Instruments). In most cases the cumulative responses from 20 trials were used for analysis.
In some cases, the subcutaneous tissue was stimulated with pressure or pinch to determine if a CTMR could be evoked. Two rats were dedicated to making these observations, but this was tested in numerous animals during the course of other preparations. Subcutaneous tissue that elicited a CTMR following stimulation was excised and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 2-4 hours, then placed in 30% sucrose in PBS until it was sectioned on a cryostat.
In order to directly test the potential contribution of the lateral cutaneous nerves to the CTMR, we mapped the CTMR receptive field and then, after a dorsal midline skin incision, unilaterally transected all DCnn from T11-S1 (n=3). The skin shown prior to surgery to be part of the CTMR receptive field, particularly the lateral aspect of the field, was then tested with noxious pinch. The contralateral skin and rostral ipsilateral skin provided controls for the status of the CTMR generally.
In order to distinguish between two possible routes of innervation to subcutaneous structures that could drive a CTMR in response to natural stimulation, a different surgical approach was used (n=2 rats). Parallel longitudinal incisions were made well off midline in an effort to spare the DCnn bilaterally. As the reflexive contraction of the CTM induced by stimulation of subcutaneous structures was focused in the medial-most region of the CTM, we do not expect that any damage of the lateral CTM associated with this lateral incision would have introduced any confound to our interpretation. The region of skin between the lateral longitudinal incisions was then tested to determine if pinch could elicit a CTMR. This procedure was repeated after transecting multiple DCnn serving the caudal skin, first on one side, then the other. Stimulation of the rostral skin, whose DCnn had likewise been exposed but not transected, served as an internal control for the status of the CTMR generally.
Population-specific neurotoxin experiments
To determine if induction of the CTMR required input from those neurons expressing neurotrophin receptors (trkA, trkB, trkC, p75), or if input from non-trk-expressing neurons alone was sufficient, the selective neurotoxin 192-saporin (Advanced Targeting Systems, Inc.) was injected into the T13 DRG in 8 animals and the CTMR integrity tested by behavioral and electrophysiological assessments. The left T13 DRG was exposed with care not to damage the T12-L1 dorsal primary branches or DCnn. The capsule of the DRG was pierced with a 28g needle, and 75-90ng (in 1.0-1.2μl) of the 192-saporin conjugate was injected into the DRG with a 10μl Hamilton syringe coupled to a glass micropipette with a tip outer diameter of 30-50μm. The injection was done in 0.2μl increments with a few minutes between each. The tissue was closed in layers and secured with wound clips. Animals (n=8) were housed individually and allowed to survive for 7-23 days.
At the end of the survival period they were prepared for a terminal physiological examination. They were anesthetized with sodium pentobarbital (40-60mg/kg, ip) and placed on a circulating water heating pad to maintain their core temperature (monitored with an esophageal thermistor). A jugular catheter was placed for infusion of supplemental pentobarbital anesthesia. The trachea was intubated to monitor end-expired pCO2. They were given 0.1cc of atropine (0.1mg/ml, s.c.) every 2 hours. The T11, T12, T13, L1 and L2 dorsal cutaneous nerves (DCnn) were isolated bilaterally. The CTMR in response to pinch of the skin was tested to confirm its presence, and then all but the T13 DCnn (bilateral) were transected close to the skin. In naïve animals this pattern of transection results in a strip of skin with spared innervation bordered rostrally and caudally by denervated skin. The skin was again pinched to determine if the CTMR could be induced. After the assessment using pinch and behavioral observation, the T13 DCnn were transected, and the central portion placed on bipolar Ag/AgCl electrodes. In some cases, a branch of the lateral thoracic nerve (LTn) was also dissected free from where it entered the CTM and was placed on recording electrodes to assess the CTMR neurogram. The electrical stimulation threshold to elicit a visible contraction of the CTM, or a recordable LTn neurogram response, was then established for each of the DCnn, with the corresponding contralateral nerve serving as a control. For electrical stimuli, direct current stimuli of 1.5mA, 0.05ms (supramaximal to recruit A-delta fibers, but subthreshold for C-fibers) and 3-5mA, 0.5ms (to recruit A-delta and C-fibers) were delivered to the DCn. After the CTMR assessments, a laminectomy was performed in order to expose the T11-L1 dorsal roots. The dorsal roots were transected and placed on bipolar recording electrodes and the compound action potential (CAP) elicited by stimulation of the corresponding DCn recorded by averaging 25 traces. After the electrophysiological preparation the injected and contralateral DRG were removed and immersion-fixed in 4% paraformaldehyde for 24 hours. They were then cryoprotected in 30% sucrose and cryostat sectioned for histological assessment of the effectiveness of the neurotoxin.
Antibody characterization
Table 2 describes the primary antibodies used in these studies.
Table 2.
Summary of antibodies used.
| Target | Immunogen | Species, clonality, manufacturer, catalogue#, lot# |
Dilution | Specificity Controls |
|---|---|---|---|---|
| PGP9.5 | Entire bovine protein |
Rabbit, polyclonal, Biogenesis/AbD Serotech, Cat#7863-0504 |
1:1000 | Antibody purified from rabbit serum by adsorption against purified PGP9.5 protein, and determined that it labelled a single band of appropriate size( 27kDa) on Western blots of rat brain cell lysates (Wilkinson et al., 1989; Wilson et al., 1988). |
| trkA | Extracellular domain of rat trkA (aa 1-416) |
Chemicon, rabbit polyclonal, #06-574 | 1:1000 | Protein localization matches with mRNA (Manca et al., 2012); NGF-induced responses observed only in neurons labelled as trkA- expressing (Michael et al., 1997); Manufacturer shows single band of correct size on Western blot of human A431 cells with no cross-reaction with trkB or trkC. |
| SOM | Somatostatin- 28 |
Peninsula Laboratories, rabbit polyclonal, #IHC-8004 |
1:1000 | Manufacturer shows reactivity for somatostatin-25 and -28, but not CCK, SP, or VIP. Staining in tissue is identical to that for SOMA-8 monoclonal antibody, which is characterized to recognize somatostatin-28, as well as both cyclic and linear forms of somatostatin-14, and has its staining abolished by preadsorbtion against somatostatin-14 (Buchan et al., 1985; Muller et al., 2007). |
| P2X3 | C-terminus of rat P2X3, aa 383-397 (VEKQSTDSGA YSIGH) |
Guinea pig, polyclonal, Neuromics, Cat#GP10108 |
1:1000 | Antibody recognizes appropriate size band on WB from P2X3-transfected, but not P2x2-transfected or non- transfected HEK293 cells. Staining on tissue is absent when preadsorbed against antigen peptide (Vulchanova et al., 1997). |
The polyclonal antibody against PGP9.5 recognizes a single band of approximately 27 kDa in Western blot of rat brain. This size is consistent with its recognition of the 27-kDa native enzyme against which it was raised (manufacturer’s datasheet). In our work it robustly labels axons in cutaneous structures (Petruska et al., 1997) in agreement with numerous other reports (e.g., Fundin et al., 1997a; Rice et al., 1998).
The polyclonal P2X3 antibody was previously described as recognizing a Western blot band of appropriate size (about 57kDa) from homogenate of HEK293 cells transfected with plasmid for P2X3, but not from non-transfected cells or those transfected with P2X2 (Vulchanova et al., 1997). Immunocytochemical staining of cells and tissues was blocked by preadsorbtion of the antibody against the antigen peptide. Our previous work using this antibody yielded results consistent with prior reports of labeling principally of many non-trkA-expressing sensory neurons in DRG, and concluded that staining was present only on dissociated neurons which also had an electrophysiological response consistent with the presence of functional P2X3 channels (Petruska et al., 2000a; Petruska et al., 2000b; Petruska et al., 2000c).
The polyclonal trkA antibody was shown by the manufacturer to recognize a single band of appropriate size (140 kDa) on Western blot of human A431 cell homogenates and does not cross-react with family members trkB or trkC. The staining pattern in DRG sections is consistent with previously described expression of trkA mRNA and protein and high-affinity NGF-binding (Michael et al., 1997; Verge et al., 1992; Verge et al., 1989).
The polyclonal somatostatin antibody was shown in manufacturer quality-control studies to have immunoreactivity to somatostatin-28 and somatostatin-25, but no cross-reactivity for other neuropeptides (CCK, substance P, or VIP). In our work it labels a population consistent with previous descriptions of SOM expression in a small group of small-diameter DRG neurons lacking trkA receptors (Kashiba et al., 1996; Verge et al., 1989). Studies by Muller and colleagues demonstrated that this antibody showed identical staining in the amygdala to that shown by the SOMA-8 monoclonal antibody (Muller et al., 2007). The SOMA-8 antibody has been well-characterized and recognizes somatostatin-28, as well as both cyclic and linear forms of somatostatin-14 (Buchan et al., 1985). Preadsorption of the SOMA-8 antibody with somatostatin-14 abolished staining in tissue sections (Muller et al., 2007).
Histology
Tissue was sectioned and mounted on slides coated with gelatin and poly-L-lysine. It was then stained to visualize axons in peripheral tissue samples or neurons in DRG samples. Methods are similar to those previously published (Petruska et al., 2000a). Briefly, sections were incubated for 1 hour in phosphate-buffered saline (PBS; pH 7.2-7.4) with 10% normal goat serum (NGS) and 0.4% Triton X-100 to block non-specific reagent binding. Sections were then incubated overnight at room temperature in rabbit primary antibody solution (diluted 1:1000 in PBS with 1%NGS and 0.4% Triton X-100) - aponeurosis sections in anti-PGP 9.5, and DRG sections in either anti-trkA or anti-Somatostatin. The following day sections were rinsed and incubated for 3 hours in a solution of goat anti-rabbit antibody (diluted 1:100). For aponeurosis sections for which the secondary antibody was conjugated to peroxidase (Jackson ImmunoResearch), sections were rinsed and then incubated with diaminobenzidine/H2O2 to visualize the peroxidase. For DRG sections the anti-rabbit secondary antibody was conjugated to AlexaFluor-594 (Invitrogen). After the 3 hour incubation, sections were rinsed and incubated overnight in either 10ug/ml IB4-lectin (Sigma Chemicals, St. Louis, MO, USA) conjugated to peroxidase (applied to sections with anti-trkA) or guinea pig anti-P2X3 (applied to sections with anti-SOM). The following day sections with anti-P2X3 were rinsed and incubated for 3 hours either in a 1:100 solution of goat anti-guinea pig-AlexaFluor-488 antibody (Invitrogen) and then rinsed. Those sections with IB4-HRP were rinsed and incubated for 6 minutes with TSA-AlexaFluor-488 diluted in amplification buffer containing H2O2 (Invitrogen) and then rinsed to quench the reaction. Sections incubated without primary antiserum or lectin showed no staining signal. Sections were viewed with a Zeiss Axiophot microscope. For the DRG sections from the neurotoxin experiments, samples from the injected DRG from all eight animals were viewed to determine a set of image capture parameters which would be uniformly applicable, for each individual label, across the dynamic range of the staining intensities. These parameters were applied when capturing images from all samples.
Digital image manipulation
Manipulation of any digital image demonstrating raw data was restricted to rotation, cropping, full-image brightness and contrast adjustments, and application of labels, all performed with Adobe Photoshop software. Other figures were generated using Photoshop, SigmaPlot (Jandel Scientific), and/or Microsoft PowerPoint.
Dexmedetomidine experiments
We used the CTMR system to perform an internally-controlled assessment of the duration of action of bupivacaine with saline versus bupivacaine with dexmedetomidine (Dex) using the “latency-to-first-return” of the CTMR – how long after treatment of the nerve did the stimulation-mediated CTMR first return. The characterization of the sensory input to the CTMR presented herein makes it clear that there is a large extent of CTMR-involved skin and many nerves that can be used for testing, providing the opportunity for targeted treatments. That is, one can treat some nerves (or skin areas) and spare others to act as internal controls. The motor output is via the brachial plexus (not the dorsal cutaneous nerves), providing a system in which effects of treatments on the sensory and motor sides of the reflex may be discriminated.
We quantified the effects of Dex alone and in conjunction with bupivacaine using the CTMR system in a manner that modeled a clinical peripheral nerve block. Female Sprague-Dawley rats (weight range 230-280g) were anesthetized with pentobarbital i.p. (20-30 mg/kg) and the dorsal thoracolumbar skin shaved. Rats (n=9) were treated on opposite sides with both 1) 0.15 ml bupivacaine 0.125% (Marcaine™, Hospira, Chicago IL) with saline and 2) 0.15ml bupivacaine 0.125% + 7.5 ug dexmedetomidine (Precedex™, Hospira, Chicago IL) administered subcutaneously/perineurally. The high-threshold (but not low-threshold) dermatomes of single nerves are known to completely overlap each other (Bajrovic and Sketelj, 1998; Diamond et al., 1992; Takahashi et al., 2003; Takahashi and Nakajima, 1996). Thus, elimination of a single cutaneous nerve leads to a region of skin that lacks low-threshold reception in its autonomous (non-overlapped) zone (Bailey et al., 1984), but retains high-threshold reception from adjacent nerves. In order to properly assess the effects of agents in vivo, it is necessary to account for this and to treat more than a single cutaneous nerve (or to treat a region of skin itself). If treatments are applied directly to surgically-exposed nerves, any set of nerves may be used. For treatments applied without surgical exposure, regions where two or more nerves are in close proximity may be preferable. This exists where the T1-4 dorsal cutaneous nerves emerge together from behind the scapula, and also where the L3 and L4 dorsal cutaneous nerves emerge close together at the angle of the hip flexors and abdominal muscles (Figure 2). We targeted this latter region, which generated an anesthetic area of skin from which the CTMR could not be evoked. The anesthetic mixtures were applied, one to each side of the rat, alternating which side received which treatment. Additional rats (n=4) received 15 ug dexmedetomidine (left side) or an equal volume of saline (right side) to the same locations to test for effects of Dex alone.
The CTMR was evoked by lightly pinching the skin with #5 Dumont forceps. In non-anesthetic skin this reliably elicited a CTMR. The CTMR was tested at 10 minute intervals following the subcutaneous injections. For each test session, induction of the CTMR from non-treated / non-anesthetized region of skin was assessed (positive control), and then the anesthetized skin was stimulated. This was repeated for both sides, alternating which side was tested first. The CTMR “latency-to-first-return” was considered as the session during which a CTMR could be observed in 2 consecutive trials, even if the CTMR was weak or brief compared to normal.
An additional set of adult female rats (n=3) were prepared for CTMR electrophysiological assessment as described above. Platinum EMG electrodes were placed into the CTM such that they recorded strong CTMR in response to stimulation of the isolated nerves. Stimulation sessions were 20 pulses at 1 Hz. Records were taken through a Grass P511 amplifier and an Axon Instruments Digidata 1322A digitizer. Baseline records were taken from both sides prior to drug injection. The drug combinations (as above) were injected, with surgical microscope guidance, perineurally into the superficial fascia, creating an enclosed fluid space that clearly contacted the nerve (we used T12 or T13), similar to successful ultrasound-guided injections (Gray, 2006). The investigator performing the injections and the electrophysiological recordings was blinded to the identity of the treatment solutions. The CTMR was tested on both sides sequentially. This process was repeated at 10 minute intervals, alternating which side was tested first. The CTMR “latency-to-first-return” was considered as the session during which a CTMR EMG could be observed in a trace averaged from the 20 trials, even if the CTMR was weak or brief compared to normal.
Because the effects of Dex alone on axonal conduction had not been determined, an additional 2 rats were prepared for examination of the effects of agents applied directly to the sciatic nerve on sensory conduction. Briefly, a laminectomy was performed to expose the L4/5 spinal roots and recording electrodes were placed on the cut end of the sectioned L4 and L5 dorsal roots to record compound action potentials (CAP). The common peroneal nerve was isolated in the popliteal fossa, sectioned, and placed on bipolar stimulating electrodes in mineral oil. The sciatic nerve was exposed through a separate incision at the hip for application of agents. The exposure was held open with silk ties and the cavity filled with agar, in which an opening was made for delivering agents directly to the nerve. Dex 0.1 cc (10 ug) was applied to the sciatic nerve. Following these trials the specific reversal agent atipamezole (Antisedan™, Pfizer, Groton, CT, USA) was administered i.v. (0.1 cc, 0.5mg).
BDNF demonstration
To demonstrate the utility of the system for using 2 segments for different purposes, one animal was prepared as described above to expose the dorsal cutaneous nerves. Branches of the lateral thoracic nerve (supplying the CTM) were isolated, transected, and placed on bipolar platinum electrodes in a pool of mineral oil for neurogram recordings. The animal also had small laminectomies performed to expose the spinal cord at 2 different segments. The dura was opened and pools were made with agar and filled with mineral oil. Bipolar recording electrodes were rested gently on the exposed dorsal root and different DCnn stimulated to identify which nerve contributed to the exposed root. The contributing nerves were identified as T7 and T12. Baseline CTMR recordings were made for each segment. 500ul of BDNF (500ng/ml)(generous gift of Regeneron Pharmaceuticals, Tarrytown, NY, USA) in artificial cerebrospinal fluid (aCSF)(Arvanian and Mendell, 2001) was then added to the T12 pool. The T7 pool had 500ul of aCSF added. Fast Green dye was included with both solutions to allow visualization of leaks, etc. The solutions were left in place for the duration of the experiment. Recordings of the CTMR neurogram in response to C-fiber strength stimulation of the T7 and T12 DCnn were made every 8 minutes (20 pulses per trial, 0.9Hz; pulse parameters were 5ms duration and 5mA amplitude). Which nerve was stimulated first was alternated every trial.
Captured data were averaged and rectified. The latency to early and late responses and area under the curve for the early and the late responses at various post-treatment times were measured and compared between the control and BDNF-treated segments. To ensure that responses were not artificially cropped, the duration of the temporal window for calculating area was based on the profile of the broadest response.
Statistical analyses
Since individual animals received both control and experimental treatments (i.e., internal controls) in the Dexmedtomidine experiments, analysis of the anesthetic duration data from the CTMR assays (behavioral and electrophysiological) was performed with a paired t-test. This was not necessary for the assays in which Dex alone was compared with saline, as the time-to-return of the CTMR occurred during the same trial for both sides. MANOVA was performed using SigmaStat (Jandel Scientific) to examine whether a difference existed between the regressions of the data generated by the two forms of CTMR assessment (behavioral and electrophysiological). The degree of variability is indicated throughout by standard deviation (SD). Statistical analyses were not performed on the data from the in vivo conduction assessment or the BDNF demonstration.
Results
Assessing the CTMR segmental organization by motor neurogram
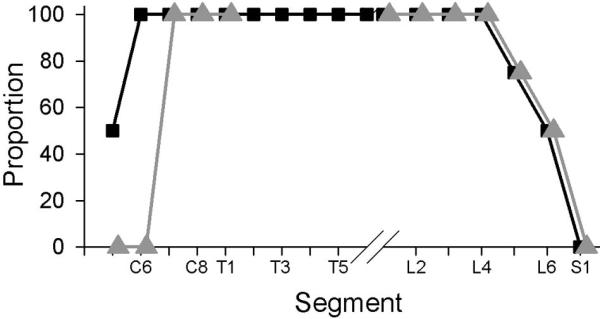
We examined which segments were capable of eliciting a neurogram response in one or more branches of the LTn in response to A-delta and C-fiber strength electrical stimulation of the ipsilateral dorsal cutaneous nerves (DCnn). Responses from stimulation of each of the segments displayed the same characteristic double-burst which others have reported is representative of input from A-delta (earlier burst) and C-fibers (later burst) (Theriault and Diamond, 1988b). As expected from prior reports, stimulation of lower thoracic and upper lumbar DCnn gave robust responses in multiple LTn branches. The CTMR was not observed with stimulation of the DCnn at A-beta strength, which is important because A-beta axons are necessarily recruited by electrical stimuli of sufficient strength to recruit the smaller A-delta and C-fibers. The CTMR was also not observed with stimulation of various lateral cutaneous nerves (LCnn) at any strength or frequency tested (data not shown). We found that moderate to relatively strong reflex neurogram activity could be recorded in response to stimulation of DCnn of all lumbar and thoracic segments. The CTMR induced by electrical stimulation of the L5 DCn, when present, generally retained a similar profile though it was much weaker than from segments T1-L4. Responses following stimulation of the L6 segment were detectible in about half of the experiments, and no CTMR responses were recorded in any LTn branch with stimulation of the S1 DCn, despite the presence of local reflexive responses to the high threshold stimulation (Figure 3). Increasing the intensity of electrical stimuli to additionally recruit C-fibers did not induce a CTMR in a case where one was not detected in response to stimulation of A-delta fibers alone.
Figure 3.
The proportion of animals in which the CTMR can be observed either by LTn neurogram (black squares) or CTM EMG (gray triangles) in response to electrical stimulation of segmental dorsal cutaneous nerves is highest from C7-L4. Stimuli were single pulses at C-fiber strength (3-5mA amplitude, 0.5ms duration).
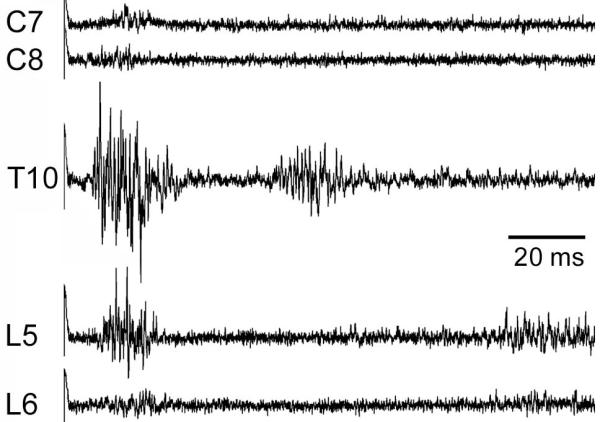
The relative strength of the CTMR varied according to which segment was stimulated. Qualitatively, the most robust (amplitude, duration) responses were associated with stimulation at A-delta or C-fiber strength of segments T4 through L2, with the CTMR decreasing beyond those segments. A LTn neurogram response to stimulation of lower cervical segments was consistently present, but very weak (Figure 4).
Figure 4.
Neurogram recordings reveal that the CTMR is weaker following stimulation of segments at the rostral and caudal borders of sensory input (C7-8 and L5-6, respectively) than from main sensory input segments (T1-L4), represented by the response from stimulating the T10 DCn. Traces are averages of 20 responses.
Assessing the CTM reflex segmental organization by EMG and behavior
We also examined the CTMR using EMG recordings and behavioral observation in response to electrical and natural stimulation of various segments and structures. EMG electrodes were inserted into various sites along the CTM. As expected from prior work describing the “local sign” of the CTMR (Theriault and Diamond, 1988b), the strongest EMG recordings were found where the EMG site was close to the stimulated segment. All segments that demonstrated strong responses by neurogram also showed responses by EMG (Figure 3). Interestingly, stimulation of cervical DCnn gave a stronger EMG response than would have been predicted from the neurogram recordings, but only if the EMG electrodes were placed in the belly of the CTM, proximal to its insertion in the dermis. Even so, the EMG response of the CTM to stimulation of the cervical DCnn was very weak, and detectable only for C7/8 (Figure 3).
A map of the area of skin from which visibly detectable CTMR can be elicited by pinch was made (Figure 5). Little or no behavioral CTMR was visible following stimulation of the cervical skin unless the arm was pulled forward, stretching the CTM. Even visualized in this manner the reflex was inconsistent and weak. Because the innervation territories of cutaneous high-threshold, small-diameter sensory axons from the same and adjacent segments are known to overlap each other and that the lumbar LCnn extend dorsally into the territory of the DCnn of some species (Bailey et al., 1984), we further examined the possibility that axons of the lumbar LCnn might be capable of driving the reflex. We determined that noxious pinch of skin innervated by only LCnn, and shown prior to transection of the corresponding DCnn to be part of the CTMR receptive field, did not induce a CTMR. This agreed with the results obtained using electrical stimulation of individual LCnn.
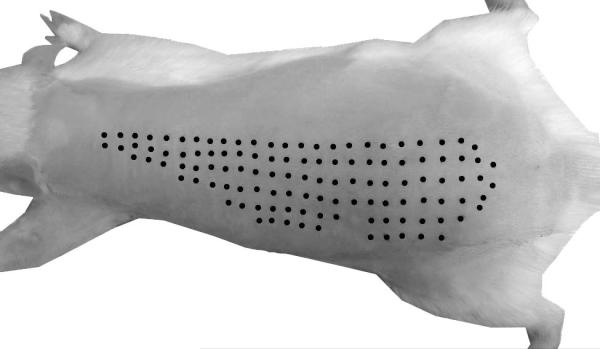
Figure 5.
The CTMR is visible in response to pinch stimulation over a large area of dorsal skin. Black dots indicate sites of light pinch that induced a CTMR. Some dots were digitally enhanced.
Interestingly, it could be readily observed during the early stages of the surgical preparation that mechanical contact with certain subcutaneous and deep tissues in the paraspinal region induced a robust CTMR. Pinching or application of pressure to subcutaneous fascia and aponeuroses, and particularly the tissue overlying and between the vertebral spinous processes, resulted in very strong responses (Figure 6). While a CTMR could reliably be induced by stimulation of the tissue associated with the vertebrae, induction via stimulation of the paraspinal tissues was sporadic and we could identify no discernible pattern in terms of tissue type or rostro-caudal gradient of frequency, though it was clear that a CTMR could most frequently be induced with more medially-placed stimuli, with stimuli delivered to tissue more than 1cm lateral to the vertebral articulating processes rarely inducing a CTMR. There are two possible sources of afferents that mediate this response. The first is that putative small nerve bundles may be traveling dorsally after having branched off from the DCnn before it emerges from the musculature to innervate the overlying dorsomedial skin near the midline were simply activated by the mechanical stimuli “en passant”. That is, stimulation of the subcutaneous structures could have been activating axons that were ultimately destined for the skin, but were getting there through an undescribed route through the muscle and fascia. The second is that sensory axons innervating the deep subcutaneous tissue are actually capable of driving the reflex. Using a surgical approach that provided lateral access to the DCnn, but did not interrupt the interface between the skin and the dorsal-medial subcutaneous structures (those most frequently found to give rise to a CTMR), we mapped the CTMR field bilaterally (between the lateral longitudinal incisions). Pinch of the interposed skin revealed that the CTMR was present. This was expected because although the skin had been incised laterally, the DCnn serving the interposed medial skin as well as the majority of the CTM remained intact. Numerous adjacent DCnn serving the caudal region of the interposed medial skin were then transected from the lateral approach and the CTMR tested again by pinching the same areas of skin. High intensity pinch throughout the area of skin served by the transected DCnn did not elicit any CTMR, though stimulation of skin innervated by intact DCnn did elicit a response (i.e., the reflex had not habituated). This implies that the reflex generated in response to stimulation of the subcutaneous tissue was not due to activation of en passant axon bundles serving the overlying skin. The areas of subcutaneous tissue where stimuli could generate a response did not contain a grossly-identifiable nerve, but were often not contiguous with each other, and some included a small artery. It is possible that the natural stimuli applied to them may be activating very small bundles of axons, but it is unlikely they are supplying skin as part of the CTMR circuitry.
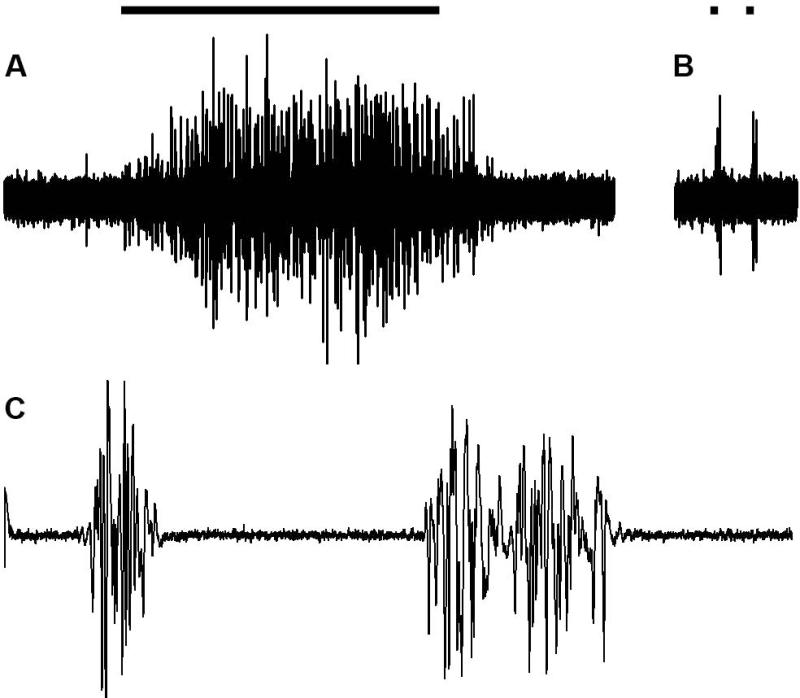
Figure 6.
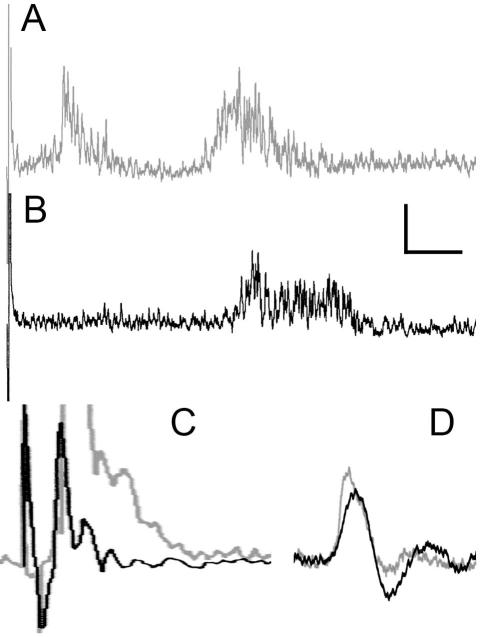
A CTMR EMG can be recorded in response to different types of stimuli including skin pinch (A), tap of subcutaneous superficial fascia over vertebral spinous process (B), or electrical stimulation of L3 DCn (C). Application of pinch (A) and tap (B) are indicated as bars above the EMG traces. (A) and (B) are single sweeps and (C) is the average of 20 sweeps (each sweep was from a single stimulus at C-fiber strength). Duration of (A) is 2.5s, (B) is 500ms, and (C) is 250ms.
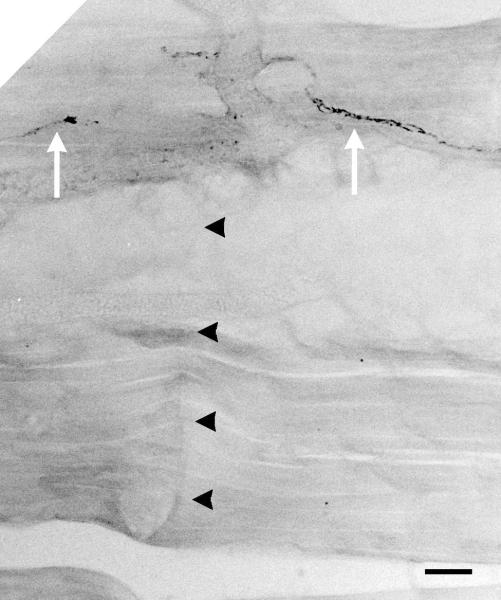
It was often observed that forceps pinch to a circumscribed area of aponeurosis was able to induce a CTMR. In one case, a small slit was made in the aponeurosis adjacent to the sensitive area and it was gently freed from the underlying tissue and a thin metal platform, supported by an arm attached to the surgical table, was inserted underneath to prevent stimulation of other tissues. Tapping certain areas of the aponeurosis induced a CTMR. Forceps pinch across its thickness generated a robust CTMR. The induction of the reflex could be induced again by pinching to one side of the original pinch site while pinching the other side resulted in no response. Immunohistochemical analysis of this area of tissue revealed few axons expressing the pan-neuronal marker PGP9.5 (Figure 7). Further, marks from the forceps pinch intersected with a single axon. Thus, the stimuli appear to have activated axons and/or sense organs that were the native sensory innervation of the tissue and are likely the distal arborizations of the medial branch of the dorsal primary ramus which innervate the deep tissue near the midline including the paraspinal muscles, tendons, and ligaments.
Figure 7.
Pinch of this subcutaneous aponeurosis tissue induced a CTMR. Pinch-marks (black arrowheads) cross axon(s) (white arrows) in a section of. Scale bar indicates 10 microns.
Assessing the afferent drive of the CTM reflex by different sensory populations
Previous studies have demonstrated that the CTMR in rodents is driven selectively by noxious natural stimuli, or high-amplitude electrical stimuli (Doucette et al., 1987; Theriault and Diamond, 1988b). While these studies strongly indicate that low-threshold afferents are not generally capable of driving the CTMR, they did not directly correlate the afferents activated with the characteristics of the CTMR at given intensities of electrical stimuli. Therefore, in some experiments, after CTMR recordings, the stimulated DCn was dissected proximally and prepared for recording. Bipolar electrodes placed proximal on the DCn recorded the compound action potential in response to the same stimulus intensities as were used for the CTMR recordings. This allowed the CTMR characteristics and the sensory population being stimulated to be directly correlated. We determined that the stimulus threshold for generating a readily detectible CTMR neurogram was at the lowest A-delta level. That is, the CTMR was not elicited by single electrical stimuli that activated only A-beta axons, but could be elicited with single electrical stimuli which recruited only a small portion of the A-delta population as reflected in the emergence of a small A-delta CAP deflection on the falling phase of the A-beta CAP. The earlier CTMR burst increased in amplitude as the stimulus strength was increased and recruited additional A-delta fibers, as reflected in a continued increase in the amplitude and duration of the A-delta deflection on the falling phase of the A-beta CAP. The later/second component of the CTMR emerged only as the stimulus recruited C-fibers, reflected in the emergence of a C-fiber CAP at relatively longer latencies consistent with C-fiber conduction velocity. Additional data regarding these relationships are presented below (see Case 8 in neurotoxin experiments).
Although the evidence is strong that low-threshold afferents do not provide drive to the CTMR in rats, at least as assessed here and elsewhere, it is not clear whether different populations of high-threshold afferents provide similar or different drive to the CTMR. In order to determine whether or not the small-diameter afferents expressing trk receptors and those lacking trk receptors contributed similarly to the CTMR system, we endeavored to eliminate the trk-expressing population and then to test the ability of the remaining afferents to drive the CTMR. We employed the selective neurotoxin 192-sap to destroy the neurons bearing p75 receptors, which includes all small- and medium-diameter sensory neurons expressing trk receptors, and nearly all large-diameter neurons expressing trk receptors (Wright and Snider, 1995), while sparing those that lacked p75 (and trk receptors).
Of the eight animals receiving intraganglionic injections of 192-sap, the CTMR was appreciably affected in five (Table 3, cases 2, 4, 6, 7, 8). In two of those five (cases 2 and 8), the CTMR was detectible but had a higher threshold for activation when elicited by electrical stimulation of the DCn of the treated DRG (ipsilateral T13) than when elicited by electrical stimulation of the contralateral T13 DCn, and appeared weaker. In three of those five (cases 4, 6, and 7), the CTMR could not be elicited by any natural stimuli applied to the skin innervated by the T13 DCn or by any electrical stimuli we tested when applied to the ipsilateral T13 DCn. This was in spite of a normal-appearing CTM being evoked by electrical or natural stimuli applied to the nerves and dermatomes, respectively, of non-treated DRG (Figure 8A).
Table 3.
Summary of assessments in the 192-saporin-treated animals. Cases are presented in order of progressively-increasing effect on the CTM reflex, represented by the T-ratio measure. Assessment of the dorsal root A-fiber CAP is irrespective of the small A-wave that is present in all cases. In trkA histochemistry, +/− denotes the finding that trkA was depleted in some regions of the DRG, but expressed strongly in others.
| Case | Dose (ng) |
Volume (uL) |
Time (days) |
CTM Reflex |
T-Ratio (L:R) |
Dorsal Root | Histochemistry | ||
|---|---|---|---|---|---|---|---|---|---|
| A-fiber | C-fiber | trkA | Non-trkA | ||||||
| 1 | 75 | 1 | 7 | +++ | 1 | +++ | +++ | +++ | +++ |
| 5 | 90 | 1.2 | 21 | +++ | 1 | +++ | +++ | +++ | +++ |
| 3 | 75 | 1 | 17 | +++ | 1.3 | +++ | +++ | +++ | +++ |
| 8 | 90 | 1.2 | 21 | ++ | 10.5 | − | ++ | +/− | +++ |
| 2 | 75 | 1 | 13 | + | 16.2 | ++ | ++ | +/− | +++ |
| 4 | 75 | 1 | 23 | − | ∞ | − | + | − | +++ |
| 6 | 90 | 1.2 | 22 | − | ∞ | − | + | − | +++ |
| 7 | 90 | 1.2 | 16 | − | ∞ | − | + | − | +++ |
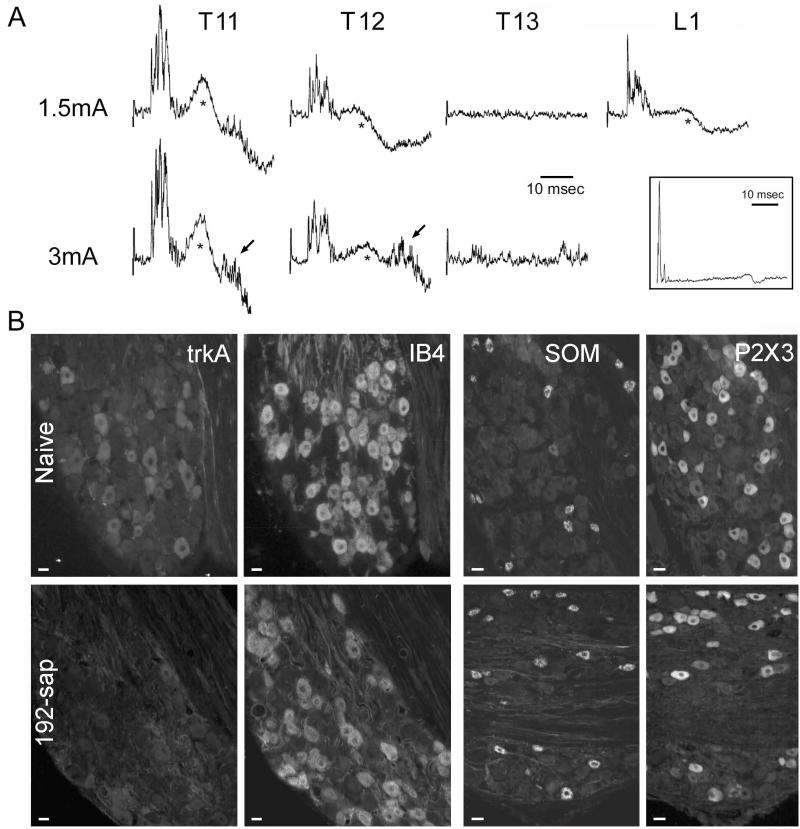
Figure 8.
Elimination of p75-bearing neurons affects the ability of cutaneous sensory nerves to drive a CTMR. A) Electrophysiological measurement of the CTMR from an animal that received an injection of 192-saporin into the left T13 DRG. Traces are averaged neurogram recordings (20 sweeps) from the lateral thoracic nerve (LTn) in response to electrical stimulation (current intensity indicated to left) of various DCnn (indicated below traces). The neurograms also reveal motion artifact (*) due to CTMR-induced contraction of the muscle which occurred because the non-recorded branches of the LTn were left intact to enable comparison of LTn motor neurogram and behavioral CTMR. The presence of a CTMR in response to stimulation of the L1 DCn indicates that the surgical procedures used to prepare the dorsal roots for recording did not damage the spinal cord. A-inset) Electrophysiological recording of the dorsal root compound action potential (average of 25 traces) in response to stimulation of the T13 DCn to confirm conduction in spared C-fiber primary afferents (arrow). B) Histochemical assessment of naive and 192-saporin-injected DRG for IB4-binding, and immunoreactivity for trkA, somatostatin (SOM), and P2X3. Scale bars indicate 25μm.
In all eight animals, recordings from the dorsal roots demonstrated the presence of a clear C-fiber CAP in response to the same electrical stimuli that were used to test the CTMR when applied to both treated and non-treated nerves. In the three animals in which the CTMR could not be elicited by stimulation of the ipsilateral T13 DCn (cases 4, 6, and 7; Figure 8A), the C-fiber CAP was clearly present, but appeared reduced in amplitude (Figure 8A-inset). The two animals in which a CTMR, albeit an altered one, could be elicited by stimulation of the ipsilateral T13 DCn (cases 2 and 8) displayed a clear C-fiber CAP which may have been reduced from normal. In the three animals in which the CTMR appeared unaffected by toxin injection (cases 1, 3, 5), both the A-fiber and C-fiber waves elicited by stimulation of the ipsilateral T13 DCn appeared normal. In the five animals in which the CTMR was appreciably affected (cases 2, 4, 6, 7, 8), the A-fiber CAP wave recorded from the dorsal root in response to stimulation of the ipsilateral T13 DCn was of variable amplitude. All five had some A-fiber response which, by latency, was likely A-beta-mediated. A small proportion of large-diameter DRG neurons lacks the p75 receptor (Wright and Snider, 1995), and would thus be expected to not be directly affected by the 192-sap toxin. Of those five with an affected CTMR (sases 2, 4, 6, 7, 8), the three animals in which no CTMR could be elicited from the ipsilateral T13 dermatome or DCn (sases 4, 6, and 7) displayed no A-delta CAP.
Case 8 was unique in that it displayed no A-delta CAP to electrical stimulation of the ipsilateral T13 DCn, but displayed a C-fiber CAP that appeared unaffected, or possibly slightly weakened. In this case, the CTMR output had only one burst, as opposed to the usual two, in response to C-fiber strength stimulation. Specifically, it appeared to lack the usual “earlier” burst which is elicited by A-delta strength electrical stimulation. However, the “later” burst that emerges with C-fiber strength electrical stimulation was present (Figure 9).
Figure 9.
In Case 8 where the 192-sap toxin was only partially effective, killing nearly all A-fibers, but sparing C-fibers, the “earlier” CTMR component evoked in response to C-fiber-strength electrical stimulation of the DCn was selectively lost, while the “later” CTMR component remained. CTMR neurograms are shown in A (to stimulation of the control segment) and B ((to stimulation of the 192-sap-injected segment). Dorsal root recordings to the same stimulus parameters that evoked the CTMR responses in A and B are shown in C (A-fiber response), and D (C-fiber response; traces are overlaid to enable comparison). Traces from control segments are in gray, and from 192-sap-injected segments are in black. The scale bar indicates 10ms for A, B, and D, and 2ms for C, and 100mV for A and B, and 500mV for C and D.
In the five cases where the CTMR was detectible (cases 1, 2, 3, 5, and 8), all displayed trkA immunoreactivity. In three of those cases it appeared strong/normal throughout the DRG (cases 1, 3, and 5). These were the same cases in which the CTMR appeared near normal. In two cases the trkA immunoreactivity was variable in that it appeared strong/normal in some regions of the DRG, but essentially absent in other regions (Cases 2 and 8). These were the same cases in which the CTMR had an increased threshold and appeared weaker than normal. In the three cases where the CTMR was not detectible in response to stimulation of the ipsilateral T13 DCn or dermatome (cases 4, 6, and 7), all displayed severely depleted, potentially absent, trkA immunoreactivity. In all eight cases the markers of the non-trkA-expressing small diameter neurons (SOM, P2X3, IB4) appeared strong, indicating that the 192-sap toxin did not kill these neurons (Figure 8B). Thus, in all cases where a CTMR was inducible in response to stimulation of the ipsilateral T13 DCn, trkA immunoreactivity was clearly present in the DRG, even if sporadically, while in all cases where the CTMR was not inducible from the injected segment, trkA was severely depleted, possibly absent, from the entire DRG. Irrespective of either CTMR inducibility or expression of trkA, both a C-fiber CAP and histological markers of the non-trkA small diameter neurons were clearly present in all cases.
Assessing the effect of dexmedetomidine on the duration of bupivacaine-mediated conduction block using contralateral controls
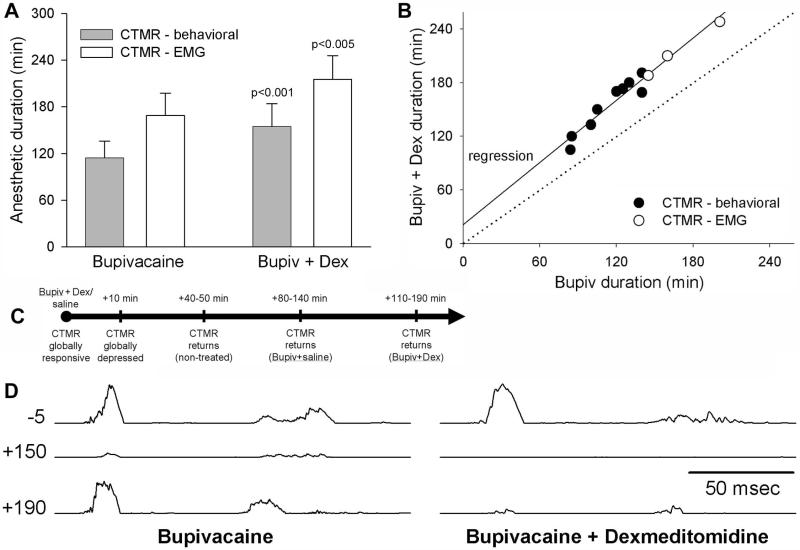
The anesthetic duration of Bupivacaine (Bup) + saline and Bup + Dex was tested (n=9), assessed by observing the CTMR in response to pinch of the skin innervated by the treated nerves. In all cases, the CTMR on the side treated with Bup + Dex returned later than on the side treated with Bup + saline (Figure 10). The mean duration for the Bup + saline side was 114.3±21.7 min, while that for the Bup + Dex side was 154.6±29.4 min (paired t-test, p>0.001), an average increase of 26%.
Figure 10.
Dexmedetomidine extends the anesthetic actions of bupivacaine assessed with CTMR induced by skin pinch (for behavioral assay) or electrical stimulation of the treated nerve (for EMG assay). (A) Dex-mediated extension of anesthetic duration over Bupivacaine alone is significant (p-values shown in bars); 26% when assessed behaviorally and 28.1% when assessed electrophysiologically. Bars represent mean ± standard deviation. (B) Scatterplot and regression analysis showing results from individual animals. Points of “no effect” of Dexmedetomidine are indicated by the dotted line. (C) Schematic timeline of the effects of Dexmedetomidine on the CTMR. (D) EMG recordings of the CTMR induced by electrical stimulation of the treated nerves. Time (minutes) relative to treatment is indicated to the left of the traces. The traces represent the baseline (-5 minutes) and the trial during which the reflex began to return on the Bupivacaine side (left panel; +150 min) and bupivacaine + dexmedetomidine side (right panel; +190 min).
The anesthetic duration of Bup + saline and Bup + Dex was also assessed electrophysiologically (n=3) in a fully-exposed surgical preparation by recording the CTMR - EMG in response to electrical stimulation of the treated nerves. In all cases, the CTMR on the side treated with Bup + Dex returned significantly later than that treated with Bup + saline (Figure 10). The mean duration for the Bup + saline side was 168.7±29.0 min, while that for the Bup + Dex side was 215.3±30.4 min (paired t-test, p<0.01), an average increase of 28.1%, matching very well with the effect observed with behavioral assessments. In order to determine the similarity of the effect of the dexmedetomidine as revealed by the behavioral and by the EMG assessments, we ran a MANOVA comparing the slopes of the regressions. The resulting p-value was not significant (p=0.599), indicating the behavioral and the EMG assessment methods yielded essentially the same relationship.
It was noted that perineural administration of Bup + Dex (or Dex alone) eliminated the CTMR (behaviorally and electrophysiologically) for up to 30-40 minutes. This was true for all areas of skin that would normally drive the CTMR, regardless of treatment status of the nerves. However, the CTMR always returned in response to pinch of non-experimental regions of skin (positive control territory) well before it returned in response to pinch of the treated regions (Figure 10C); i.e., this brief global loss of the CTMR did not interfere with the ability to determine the duration of local anesthetic actions. We assume that this effect was caused by a systemic sedative action of dexmedetomidine (Doze et al., 1989; Xu et al., 2000), since a unilateral injection eliminated the CTMR bilaterally and throughout the entire rostro-caudal extent of its normal receptive field, and this effect had an onset latency of many minutes. We directly tested this possibility by perineural administration of Dex alone versus saline on opposite sides (n=4). In all cases, the CTMR (assessed by EMG) was eliminated bilaterally (as occurred with Bup + Dex) in response either to electrical stimulation of both of the treated nerves or to pinch of skin innervated by the (intact) treated nerves. Also in all cases, the CTMR returned on both sides during the same stimulation/recording trial (i.e., for both dexmedetomidine alone and saline treated nerves), and with a time-course of recovery similar to that observed for activation of the CTMR from non-treated nerves in Bup + Dex experiments. We also noted that C-fiber strength stimulation of the DCnn during the “CTMR global depression” period induced localized segmental nociceptive responses, indicating that conduction in nociceptive sensory axons was not blocked. Also, electrical stimulation of the LTn during this period induced CTM contraction, indicating that conduction in the motor axons was not blocked. Thus, the brief global loss of CTMR responsiveness associated with perineural Dex administration was most likely due to central actions of dexmedetomidine.
Further, since the brief global CTMR depression was the same in time-course and character for both Dex-alone and Bup + Dex experiments, the effect is most likely due to dexmedetomidine, and not bupivacaine. This conclusion is further buoyed by the demonstration that a perineural bupivacaine dose 3-fold greater than that used in the anesthetic duration assays did not induce global depression of the CTMR (n=1; rat was also used for above Dex-vs-saline experiment), in spite of producing the expected local anesthetic effect.
Although both treated nerves received the same volume of fluid in the peri-nerve injections, we used the same preparation to determine if the injection itself (fluid pressure) might have some effect on the CTMR-EMG. Using different nerves from those that were treated with anesthetic, we recorded the CTMR-EMG before, during, and after performing peri-nerve injections. In all cases, injection of lactated Ringer’s in the same manner as the anesthetic mixtures did not alter the CTMR-EMG.
Nerve conduction assays
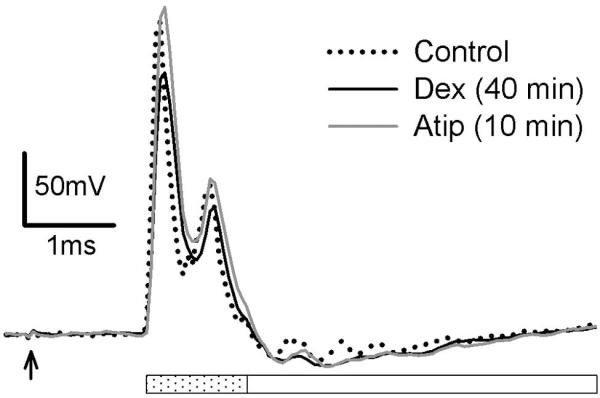
As noted above, perineural injection of Dex led to a global depression in CTMR responsiveness. The local segmental nociceptive reflexes in response to C-fiber strength stimulation of the DCn during this period indicated that conduction in nociceptive axons was not blocked, but it did not address the possibility that Dex may have affected action potential propagation in fast-conducting axons with low-threshold receptors. It should be noted, however, that even if conduction in these axons were blocked it should not affect the CTMR, since the CTMR is not driven by low-threshold axons. In order to assess whether Dex alone had any effect on conduction in large-diameter low-threshold afferent axons, we performed an in vivo assessment of the effect of Dex on the amplitude and area of the dorsal root A-fiber compound action potential (CAP). Dex, applied directly to the sciatic nerve in vivo (n=2), had a small effect on the earliest component of the A-fiber CAP, but a greater effect on the later component. We therefore measured these components separately. Dex-induced reduction of the peak of the early CAP component (11% mean decrease) was completely reversed 10 minutes after i.v. application of the alpha-2-specific antagonist atipamezole (Figure 11). Dex-induced reduction of the area of the early CAP component (9% mean decrease) was also completely reversed 10 minutes after application of atipamezole. Dex-induced reduction of the peak of the late CAP component (45% of control) was only weakly restored after application of atipamezole (59% of control). Dex-induced reduction of the area of the late CAP component (64% of control) was essentially unaffected by application of atipamezole. These data suggest that the effects of Dex on the fastest-conducting A-fibers was rapidly reversible, but that the effects on the slower-conducting A-fibers was not. We interpret the finding that Dex affected both peak and area to indicate that Dex likely induced conduction block in a small proportion of the largest/fastest A-fibers, as opposed to inducing conduction velocity (CV) decreases, which would likely have manifest as reduction of peak without reduction of area. The same could be true for the smaller/slower A-fibers, though decreased CV alone without conduction block could result in a loss of both peak and area in this population as their extracellular voltage-profile can be lost in the noise.
Figure 11.
The effect of dexmedetomidine, applied directly to the sciatic nerve in vivo, on nerve conduction was assessed by dorsal root recordings in response to stimulation of the peroneal nerve. Effect on the early component of the compound action potential (indicated by dotted bar) was rapidly reversed by the competitive alpha-2 antagonist atipamezole (Atip), administered iv. Effect on the later component of the CAP (indicated by open bar) was minimally reversed by Atip in this timeframe. Each sweep represents the average of 30 traces (delivered at 1Hz); arrow indicates stimulus artifact.
Demonstrating the effect of BDNF on spinal nociception using different segments as controls
Testing hypotheses regarding sensory processing often requires examining the spinal cord in vivo. Though necessary, these types of experiments are often complicated by 1) possible/actual systemic effects of the treatment and/or anesthetic, and 2) the concurrent treatment of sensory and motor components of reflexes whose circuitry resides in the same or adjacent segments. Anesthesia and the systemic effects of the treatment make it difficult to compare readouts across animals, necessitating the use of a large number of animals to achieve statistical significance. The sharing of anatomical space by sensory and motor components makes interpreting the mechanism of treatment effects very difficult, often necessitating the use of additional levels of investigation such as intracellular electrophysiology. The CTMR has a large spatial extent which allows numerous assessments to be simultaneously made of the same readout in the same animal, thus controlling for anesthetic and systemic effects of the treatment by assessing untreated and/or control-treated segments. Statistical assessment of the data across animals can then be performed more easily by using the control segments/nerves from each animal to normalize the systemic effects. Further, because the sensory and motor components of the CTMR system reside in largely separate segments, each can be tested independently by treating the proper segment and/or peripheral nerves. We demonstrate one way in which this type of experiment can be designed, and the type of data it can produce, by examining the effect of the neurotrophin brain-derived neurotrophic factor (BDNF) on nociceptive processing using the CTMR as a readout.
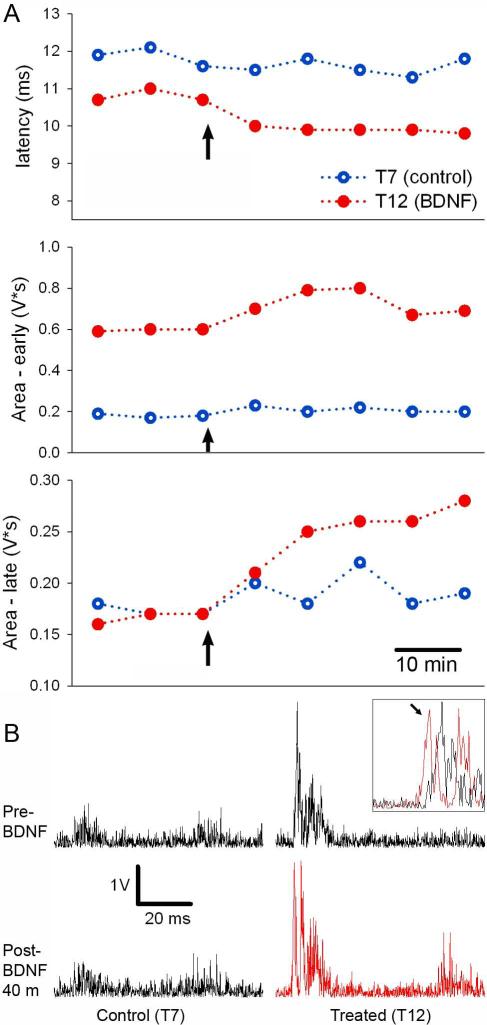
BDNF was applied to a single spinal segment (T12), while in the same animal the vehicle solution alone was applied to a separate spinal segment (T7). The latency and area of the CTMR driven by stimulation of the T12 segment (BDNF-treated) was compared to the CTMR driven by stimulation of the T7 segment (vehicle) over time. These parameters changed little over time for the control segment (T7). However, it was clear that they changed in the BDNF-treated segment in the same rat (Figure 12). Notably, the late response, driven by C-fiber afferent input, appeared to be preferentially increased. This agrees with the specific enhancement by BDNF of C-fiber synapses onto spinal cord dorsal horn neurons observed with neonatal spinal cord slice preparations (Kerr et al., 1999).
Figure 12.
The effects of BDNF on spinal nociceptive processing were assessed with the CTMR. (A) The values for each parameter are plotted over time. Each point corresponds to a single trial (20 single pulses delivered at 0.9Hz, 0.5ms duration, 5mA amplitude). Arrow indicates the time at which aliquots (BDNF or aCSF) were added to the pools. (B) The neurogram traces (20 traces added and rectified) from the baseline trial and the final trial are presented. The inset shows overlaid traces magnified at the CTMR onset from the T12 segment before and after (arrowed) BDNF treatment to demonstrate the latency shift.
Thus, in this design the CTMR induced by stimulation of the T7 DCn acted as a control for any effects of the anesthetic level of the animal, and also for any systemic effects of the BDNF. In this way, statistical analyses can use the ratio of the outcome measures between the experimental (T12) and control (T7) segments. Analyses would compare the ratio determined in animals in which the segments were treated differently (experimental and control) with the ratio from separate animals in which the segments were treated identically. This experimental design could replace the design which compares the CTMR value of single treated segments in separate animals (from separate groups). The number of animals required could be notably reduced using the design we demonstrate here.
Discussion
Anatomical organization of CTM reflex drive
We have described the organization of the sensory input to the CTMR in rat. Using motor neurogram recordings we have shown that the CTMR circuit can be driven from nearly every segment rostral to the sacrum. In terms of gross anatomy, the primary input is from the dorsal cutaneous nerves, exclusive of the lateral and ventral cutaneous nerves, whose contribution to the CTMR differs according to species (Krogh and Towns, 1984). However, stimuli delivered to medial subcutaneous tissue, particularly paraspinal and intervertebral tissue, also elicited a behaviorally-observable CTMR, an observation perhaps akin to the viscero-pannicular response described in the cat (Ashkenaz and Spiegel, 1935). This likely indicates that the ability to drive the CTMR lies not with the DCn per se, but with the dorsal ramus of the spinal nerve, of which the DCn is the major branch, with the subcutaneous CTMR-inducing activity likely traveling via the medial muscular branch of the dorsal primary ramus of the spinal nerve, which innervates the paraspinal tissues and has no cutaneous component (Bailey et al., 1984).
Histological examination of the deep tissue from which natural stimulation elicited a CTMR indicated that very few axons were present. We and others have observed that pinprick to the cutaneous CTMR receptive field, a stimulus that presumably activates few axons, is certainly capable of activating the CTMR (e.g., Diamond et al., 1992; Duarte et al., 2005), indicating that activation of very few axons is required for eliciting a behaviorally-observable CTMR.
Though the system can be detectably driven by a large number of segments, not all of these inputs lead to a behaviorally detectable reflex contraction. The different results obtained with EMG and neurogram methods following stimulation of cervical DCnn may be explained by the CTM motoneuron termination pattern. If the pattern defined by Theriault and Diamond (Theriault and Diamond, 1988a) holds for innervation of the muscle belly, then it is likely that the rostal-most CTM motoneurons – those expected to receive afferent drive from the most rostral segments (i.e., cervical) – would likely terminate in the muscle belly rostral to the point where the neurogram recording electrodes were placed on the nerve. The CTM output (neurogram and/or EMG) in response to stimulation of the cervical sensory nerves was very weak. This may correspond to findings in the cat which indicate poor input to the CTM motoneurons from cervical propriospinal axons, unlike the propriospinal input from other segments (Holstege and Blok, 1989). Activity of CTM motoneurons could be detected only with EMG electrodes placed in the belly of the muscle between the origin on the humerus and the insertion on the dermis, a site where contraction is unlikely to elicit a meaningful movement of the trunk skin. This could be viewed as being in accord with the somatotopic patterning of other CTMR afferents and motoneurons. However, it is possible that the LTn neurogram and CTM EMG observed in response to stimulation of cervical DCnn do not actually constitute reflexive output. The activity recorded as neurogram and EMG does not appear to be of sufficient magnitude to generate a behaviorally-detectible and relevant CTMR, i.e., generating movement of the skin. Thus, although the cervical cutaneous afferents appear capable of providing drive to CTM motoneurons, the output should perhaps be referred to as a CTM response.
Cellular-level organization of CTM reflex drive
Duarte et al. (2005) mention a “low-threshold” CTM response that disappears when the animals have been handled regularly. The loss of this response with reduction of animal stress, along with the data indicating that there is no low threshold-drive of CTMR under even light pentobarbital anesthesia, indicates that the response they observe is likely not a spinal reflex, but is part of the broader startle response. This would be expected if the descending neural circuitry of the rat is organized in a manner similar to the cat where brainstem neurons projecting to the abdominal motoneuron pools also project to the CTM motoneuron pool where they appear to recruit CTM participation in the startle and abdominal guarding responses, and likely also in mating (Gerrits et al., 2000; Holstege and Blok, 1989).
The CTMR appears to be driven differentially by two separate populations of small-diameter sensory neurons – those afferents expressing trk receptors and those not. These two populations display predominantly nociceptive characteristics, many of which are shared (Dirajlal et al., 2003; Elitt et al., 2006; Fang et al., 2005a; Fang et al., 2006; Malin et al., 2006; Petruska et al., 2000a; Petruska et al., 2002; Petruska et al., 2000c; Stucky and Lewin, 1999; Stucky et al., 2002). However, they also display analogous but different peripheral termination patterns in skin (Davis et al., 1997; Fundin et al., 1997a; Fundin et al., 1997b; Rice et al., 1998; Zylka et al., 2005), different central termination patterns in spinal cord (Molliver et al., 1995; Molliver et al., 1997), and appear to participate in separate ascending pathways (Braz et al., 2005). It has been suggested that each may separately represent the peripheral extension of the neural circuitry underlying two components of pain, with the trkA-expressing population driving the sensory/discriminative component and the non-trkA-expressing population driving the affective/motivational component (Braz et al., 2005; Zylka et al., 2005), including a possible contribution to interoception (Craig, 2002; 2003a; b). Although our functional data seem to be in accord with the anatomical data of those other groups, the greater meaning of these anatomical and functional distinctions is yet to be determined.
While we interpret the results of the 192-sap toxin experiments to indicate that there is a difference in the central processing of information from small diameter sensory neurons expressing or lacking trkA, other possible explanations must be considered. It is possible that the “spared sensory neurons” were not entirely spared and were somehow injured, and/or the intra-DRG inflammatory/cell-death response affected the surviving neurons such that the action potentials clearly being conducted along dorsal root axons were not effective in the spinal dorsal horn, whereas they would have been in the naïve animal. While this is certainly possible, data we present and those present in the literature argue against this. First, in our own data there were three animals in which the toxin failed to kill p75/trk-expressing neurons, thus acting as an excellent control group for the injection procedure itself. There were also two animals in which the toxin was only partially-effective, making these animals controls for both the injection procedure and also the effect of toxin-induced cell death. Second, regarding the possibility of the procedures injuring the neurons that were spared by the toxin, it is important to note that all of the histological markers used to report on the status of the non-trkA-expressing small neurons (IB4, SOM, P2X3) are downregulated by injury (Bennett et al., 1998; Bradbury et al., 1998; Hammond et al., 2004). Their clear presence in all of the injected DRG argues against massive injury. This does not indicate that there were no neurons that were injured, but does indicate that many were not injured. Third, injury and other processes, such as compression or inflammation, which might affect nociceptive DRG neuronal sensitivity/activity and central synaptic transmission generally act to enhance nociceptive transmission, not depress it (Cervero, 1995; Coull et al., 2005; D’Mello and Dickenson, 2008; Hylden et al., 1989; Lu et al., 2009; Ma et al., 2006; Simone, 1992; Trang et al., 2011; Zhang et al., 2000). These considerations suggest that neither the ganglion injection procedure itself, nor the intraganglionic cell death, are likely to account sufficiently for the data regarding the lack of a CTMR in response to stimuli applied to a nerve depleted of trk-expressing sensory neurons. This in turn supports the conclusion that trkA-expressing and non-trkA-expressing small-diameter neurons at very least have differential drive to the CTMR, and at most the non-trkA-expressing are not involved in driving the CTMR.
Doucette and colleagues (1987) demonstrated that while the A-delta-mediated CTMR was intact after capsaicin treatment of the rats as neonates, the CTMR could not be evoked by electrical stimulation of C-fibers. The literature indicates a highly-variable effect of neonatal-capsaicin treatment on non-trkA-expressing small diameter neurons, but generally agrees that this treatment does not kill all of the C-fiber axons/neurons, and often spares many of them (Doucette et al., 1987; Jancso and Kiraly, 1981; Lawson, 1987; Nagy et al., 1981; Nagy et al., 1983; Priestley et al., 1982), a point considered in the Doucette manuscript (Discussion, page 590). Direct comparison of SOM-expression with capsaicin-sensitivity of acutely dissociated DRG neurons indicates that SOM-expressing neurons have a low sensitivity to capsaicin (Petruska et al., 2000c), suggesting they may be preferentially-spared. Further, the Doucette study also showed that no Evan’s Blue dye extravasation was achieved with C-fiber strength electrical stimulation of peripheral nerve. This indicates that in their model the sensory neurons were depleted of the vasoactive peptides necessary for dye extravasation, peptides not found in the majority of the non-trkA-expressing IB4-binding DRG neurons. These findings support our contention that the CTMR is selectively driven by the trkA-expressing small-diameter afferents.
Many forms of natural stimuli recruit multiple types of afferents, and C-fiber strength electrical stimuli necessarily recruit all fiber types. All of the stimuli used thus far to examine the CTMR, in our study and others, have not distinguished between different populations of high-threshold neurons, nor between A-delta and C-fiber inputs.. Therefore, it is possible that the C-fiber-mediated CTMR may be affected in some way by the A-fiber input. Although we are not aware of any systemic examination of this possibility for the CTM, we can draw some inferences from one of our cases in which the 192-sap toxin was partially effective. By chance, case 8 produced what appears to be effectively an A-delta knockout as indicated by the dorsal root recording in which the A-delta portion of the A-fiber CAP is reduced or absent (Figure 9). In this animal, no CTMR was recorded in response to A-delta strength stimulation, but was induced in response to C-fiber stimulation of the DCn of the injected DRG. The C-fiber evoked CTMR had a latency that was in accord with what would be expected for the later of the two CTMR waves, but the earlier wave was not observed. This suggests that A-delta fiber input is not necessary for the C-fiber input to manifest in a CTMR. When considered in conjunction with the fact that A-delta electrical stimulation evokes a CTMR independent of C-fiber input, this suggests an independent ability of A-delta and C-fibers to drive a CTMR.
Demonstrations of quantitative assessments using the CTM reflex system
One of the major advantages of the CTMR system is that the sensory and motor components reside in separate locations, allowing one to discriminate effects on either population, or to treat one population independently of the other (barring systemic effects). Few other reflex models have this capability (Vadakkan et al., 2005). We have demonstrated the utility of this organization for testing treatments of peripheral nerves in our experiments examining the effects of dexmedetomidine on nerve conduction and the action of bupivicaine, where another control-treated nerve acted as a control for the treatment process itself, and the other non-treated nerves acted as controls for systemic effects and the status of the readout and the animal. In vivo studies often suffer from an inability to separate systemic effects from those related to a localized treatment. The CTMR provides a system that may alleviate this issue for certain applications, particularly for studies of spinal nociception. In addition, the ability to use a quantitative internal control (in this case the contralateral nerve) enables statistical assessment using a paired t-test, which reduces the number of animals required and/or raises the power. For example, our assessment of the effects of Dex using the behavioral CTMR used 9 rats and a paired t-test. However, only 5 were necessary to maintain power at >0.8. Alternatively, if the experimental design did not have an internal control and therefore used a non-paired t-test, 12 animals would have been necessary. For a mean-difference half as great (with same SD) to maintain power at >0.8 would have required only 11 animals using the CTMR system, but 37 animals if the design used separate groups. The same holds for the electrophysiological assessment, where our data using the internal control had a power of 1 with only 3 animals, but this same mean-difference and SD for separate animals would have required 15 rats to reach suitable power. This example assumes the same SD for the design with an internal control and the design using separate groups. However, the reality of inter-animal variability and effects of anesthetics and the resulting increase in SD would only act to enhance the differences described above regarding the required number of animals and statistical power.
We also demonstrated how one spinal segment could act as a control for another (BDNF demonstration). Using the CTMR system in this manner could avoid effects of treatments on the motoneurons directly, which could occur with functional assessments where the sensory and motor components are both exposed to treatments because they are located in the same or adjacent segments. For example, intrathecal sequestration of BDNF prevents sensitization of the hindlimb withdrawal reflex, induced by peripheral inflammatory mediators which increase the BDNF content of DRG neurons and the spinal cord (Kerr et al., 1999). However, the sensory, spinal, and motor components of that reflex system are spatially concentrated in a few adjacent segments, making it difficult to determine the cellular and synaptic site(s) of BDNF action. This is important given that BDNF has been shown to affect sensory neurons (e.g., Shu et al., 1999), sensory synapses onto spinal dorsal horn neurons (e.g., Bardoni et al., 2007; Garraway et al., 2003; Kerr et al., 1999; Merighi et al., 2008), synapses onto motoneurons (e.g., Arvanian and Mendell, 2001; Seebach et al., 1999), motoneurons (e.g., Boyce et al., 2012; Gonzalez and Collins, 1997), and non-neuronal cells that in turn influence neural function (e.g., Coull et al., 2005; Ferrini et al., 2013; Lu et al., 2009; Trang et al., 2011).
In general, the CTMR system displays a number of characteristics that may be useful. The sensory axons/segments are separate from the motor axons/segments, enabling separate testing of the involved units. The CTM itself is a large muscle readily accessed for either acute transcutaneous or chronic indwelling EMG instrumentation. The dorsal cutaneous nerves are also easily accessible. The system displays intra-segmental somatotopy (driven by the dorsal ramus but not ventral ramus of the spinal nerves), inter-segmental somatotopy (zone of shortest latency response and local-sign), and differential drive based on sensory modality (nociceptive-specific in rodents). We have demonstrated how some of these characteristics can be used (i.e., effects of Dex on bupivacaine and of BDNF). In addition, one can see how both the intersegmental (rostrocaudal) and intrasegmental (dorsoventral) somatotopy could be used to detect plasticity of the afferents, propriospinal neurons, and/or motoneurons (Figure 13). Further, in the rodent the CTMR is nociceptive specific. If low-threshold afferents gain access to the nociceptive pathways at the segmental second-order neurons, or ascending first- or second-order neurons of the low-threshold channels gain access to the CTM motor neurons, this plasticity may be readily detected with characteristics that could indicate the level(s) where the plasticity occurred (Figure 13).
Figure 13.
This schematic represents the basic proposed spinal circuitry of the CTMR, not including other sources of input to the CTM motoneurons such as those from brainstem, and some characteristics that may provide indications of different forms of plasticity. It is arranged as if the animal were prone with its head to the left and midline at the top of the image, with the skin reflected laterally (toward bottom of image), causing the CTM underlying the skin to be at the “top” of the skin and the epidermis to be at the “bottom”. Dotted lines represent neural elements or stimulus modalities that do not drive the reflex normally (dark large-diameter DRG neuron, LCn, DCn). T4, T10, and L2 segments were chosen to graphically indicate rostrocaudal progression and do not indicate that the schematized characteristics are present only at those segments. The lower panel represents some of the possible sites of plasticity and how the characteristics of the CTMR may change accordingly (numbered stars). Plasticity is noted by double lines not present in the top panel, and the conversion of dotted lines to solid lines.
As with any model system, caution must be exercised when designing experiments and interpreting data. It is clear that the CTM reflex in the naïve rat cannot be used to assess input from low-threshold sensory neurons and it appears that the two major populations of high-threshold sensory neurons have different involvement in driving the CTM reflex. These characteristics must be properly considered for experimental designs using the CTM reflex system.
Acknowledgements
The authors thank the KSCIRC Statistical Core for consultation, Drs. Keith Tansey, Kris Rau, and Ben Harrison for critical review of an earlier version of the manuscript, Drs. Wolfgang J. Streit, Louis A. Ritz, Edwin M. Meyer, and John B. Munson for their role in discussing and shaping this work, Drs. Ron Wiley and Doug Lappi with Advanced Targeting Systems for valuable discussions and generous assistance with reagents, and Alyssa Tuthill, Vicki Dugan, Dana Kubinski, and Scott Ferriera for excellent technical assistance throughout the course of this work. We thank Regeneron Pharmaceuticals for the kind gift of BDNF.
This work was supported in part by the National Institutes of Health (NS080091 – JCP; NS016996 – LMM; NS027511 – RDJ; P30GM103507 (PI – S.R. Whittemore) supporting the KSCIRC Core facilities), the Florida Brain and Spinal Cord Injury Rehabilitation Trust Fund (RDJ), The Christopher and Dana Reeve Foundation (LMM), the UofL Dept. of Neurological Surgery (JCP), the Kentucky Spinal Cord and Head Injury Research Trust (09-12A and 10-10 to JCP), the SUNY Stony Brook Department of Anesthesiology (Chair Peter Glass supporting RT, RGS, PAS), and the University of Florida Medical Guild (JCP).
This work was supported in part by the National Institutes of Health (NS080091 – JCP; NS016996 – LMM; NS027511 – RDJ; P30GM103507 (PI – S.R. Whittemore) supporting the KSCIRC Core facilities), the Brain and Spinal Cord Injury Trust Fund of Florida (RDJ), the Kentucky Spinal Cord and Head Injury Research Trust (09-12A, 10-10; JCP), the SUNY-SB Department of Anesthesiology (PAS, RGS, RT), and the University of Florida Medical Guild (JCP).
Footnotes
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
REFERENCES
- Institute of Laboratory Animal Resources Commission on Life Sciences . Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- Society for Neuroscience . Handbook for the Use of Animals in Neuroscience Research. Society for Neuroscience; Washington, D.C.: 1991. [Google Scholar]
- Arvanian VL, Mendell LM. Acute modulation of synaptic transmission to motoneurons by BDNF in the neonatal rat spinal cord. Eur J Neurosci. 2001;14(11):1800–1808. doi: 10.1046/j.0953-816x.2001.01811.x. [DOI] [PubMed] [Google Scholar]
- Ashkenaz DM, Spiegel EA. The viscero-pannicular reflex. Am J Physiol. 1935;112(4):573–576. [Google Scholar]
- Bailey CS, Kitchell RL, Haghighi SS, Johnson RD. Cutaneous innervation of the thorax and abdomen of the dog. Am J Vet Res. 1984;45(9):1689–1698. [PubMed] [Google Scholar]
- Bajrovic F, Sketelj J. Extent of nociceptive dermatomes in adult rats is not primarily maintained by axonal competition. Exp Neurol. 1998;150(1):115–121. doi: 10.1006/exnr.1997.6734. [DOI] [PubMed] [Google Scholar]
- Bardoni R, Ghirri A, Salio C, Prandini M, Merighi A. BDNF-mediated modulation of GABA and glycine release in dorsal horn lamina II from postnatal rats. Developmental neurobiology. 2007;67(7):960–975. doi: 10.1002/dneu.20401. [DOI] [PubMed] [Google Scholar]
- Bates B. The Nervous System: Cranial Nerves, Motor System, Sensory System, and Reflexes. In: Bickley LS, Szilagyi PG, editors. Bates’ Guide to Physical Examination and History Taking. 9th ed Williams, and Wilkins; Philadelphia: Lippincott: 2007. pp. 595–667. [Google Scholar]
- Baulac M, Meininger V. Organization of pectoral muscle motor neurons in the rat. Contribution to the study of the axillary arch (Achselbogen) Acta Anat (Basel) 1981;109(3):209–217. [PubMed] [Google Scholar]
- Bennett DL, Michael GJ, Ramachandran N, Munson JB, Averill S, Yan Q, McMahon SB, Priestley JV. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci. 1998;18(8):3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binshtok AM, Gerner P, Oh SB, Puopolo M, Suzuki S, Roberson DP, Herbert T, Wang CF, Kim D, Chung G, Mitani AA, Wang GK, Bean BP, Woolf CJ. Coapplication of lidocaine and the permanently charged sodium channel blocker QX-314 produces a long-lasting nociceptive blockade in rodents. Anesthesiology. 2009;111(1):127–137. doi: 10.1097/ALN.0b013e3181a915e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blight AR. Morphometric analysis of a model of spinal cord injury in guinea pigs, with behavioral evidence of delayed secondary pathology. J Neurol Sci. 1991;103(2):156–171. doi: 10.1016/0022-510x(91)90159-5. [DOI] [PubMed] [Google Scholar]
- Blight AR, McGinnis ME, Borgens RB. Cutaneus trunci muscle reflex of the guinea pig. J Comp Neurol. 1990;296(4):614–633. doi: 10.1002/cne.902960408. [DOI] [PubMed] [Google Scholar]
- Blight AR, Toombs JP, Bauer MS, Widmer WR. The effects of 4-aminopyridine on neurological deficits in chronic cases of traumatic spinal cord injury in dogs: a phase I clinical trial. J Neurotrauma. 1991;8(2):103–119. doi: 10.1089/neu.1991.8.103. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Blight AR, McGinnis ME. Functional recovery after spinal cord hemisection in guinea pigs: the effects of applied electric fields. J Comp Neurol. 1990;296(4):634–653. doi: 10.1002/cne.902960409. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Bohnert D. Rapid recovery from spinal cord injury after subcutaneously administered polyethylene glycol. Journal of neuroscience research. 2001;66(6):1179–1186. doi: 10.1002/jnr.1254. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Shi R, Bohnert D. Behavioral recovery from spinal cord injury following delayed application of polyethylene glycol. J Exp Biol. 2002;205(Pt 1):1–12. doi: 10.1242/jeb.205.1.1. [DOI] [PubMed] [Google Scholar]
- Boyce VS, Park J, Gage FH, Mendell LM. Differential effects of brain-derived neurotrophic factor and neurotrophin-3 on hindlimb function in paraplegic rats. Eur J Neurosci. 2012;35(2):221–232. doi: 10.1111/j.1460-9568.2011.07950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci. 1998;12(4-5):256–268. doi: 10.1006/mcne.1998.0719. [DOI] [PubMed] [Google Scholar]
- Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47(6):787–793. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Brummett CM, Norat MA, Palmisano JM, Lydic R. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology. 2008;109(3):502–511. doi: 10.1097/ALN.0b013e318182c26b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan AM, Sikora LK, Levy JG, McIntosh CH, Dyck I, Brown JC. An immunocytochemical investigation with monoclonal antibodies to somatostatin. Histochemistry. 1985;83(2):175–180. doi: 10.1007/BF00495150. [DOI] [PubMed] [Google Scholar]
- Cervero F. Visceral pain: mechanisms of peripheral and central sensitization. Annals of medicine. 1995;27(2):235–239. doi: 10.3109/07853899509031965. [DOI] [PubMed] [Google Scholar]
- Colvin AC, Wang CF, Soens MA, Mitani AA, Strichartz G, Gerner P. Prolonged cutaneous analgesia with transdermal application of amitriptyline and capsaicin. Reg Anesth Pain Med. 2011;36(3):236–240. doi: 10.1097/AAP.0b013e31820c2c30. [DOI] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438(7070):1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature reviews Neuroscience. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Current opinion in neurobiology. 2003a;13(4):500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. Annual review of neuroscience. 2003b;26:1–30. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- D’Mello R, Dickenson AH. Spinal cord mechanisms of pain. Br J Anaesth. 2008;101(1):8–16. doi: 10.1093/bja/aen088. [DOI] [PubMed] [Google Scholar]
- Davis BM, Fundin BT, Albers KM, Goodness TP, Cronk KM, Rice FL. Overexpression of nerve growth factor in skin causes preferential increases among innervation to specific sensory targets. J Comp Neurol. 1997;387(4):489–506. doi: 10.1002/(sici)1096-9861(19971103)387:4<489::aid-cne2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Diamond J, Holmes M, Coughlin M. Endogenous NGF and nerve impulses regulate the collateral sprouting of sensory axons in the skin of the adult rat. J Neurosci. 1992;12(4):1454–1466. doi: 10.1523/JNEUROSCI.12-04-01454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirajlal S, Pauers LE, Stucky CL. Differential response properties of IB(4)-positive and - negative unmyelinated sensory neurons to protons and capsaicin. J Neurophysiol. 2003;89(1):513–524. doi: 10.1152/jn.00371.2002. [DOI] [PubMed] [Google Scholar]
- Doucette R, Theriault E, Diamond J. Regionally selective elimination of cutaneous thermal nociception in rats by neonatal capsaicin. J Comp Neurol. 1987;261(4):583–591. doi: 10.1002/cne.902610409. [DOI] [PubMed] [Google Scholar]
- Doze VA, Chen BX, Maze M. Dexmedetomidine produces a hypnotic-anesthetic action in rats via activation of central alpha-2 adrenoceptors. Anesthesiology. 1989;71(1):75–79. doi: 10.1097/00000542-198907000-00014. [DOI] [PubMed] [Google Scholar]
- Duarte AM, Pospisilova E, Reilly E, Mujenda F, Hamaya Y, Strichartz GR. Reduction of postincisional allodynia by subcutaneous bupivacaine: findings with a new model in the hairy skin of the rat. Anesthesiology. 2005;103(1):113–125. doi: 10.1097/00000542-200507000-00018. [DOI] [PubMed] [Google Scholar]
- Elitt CM, McIlwrath SL, Lawson JJ, Malin SA, Molliver DC, Cornuet PK, Koerber HR, Davis BM, Albers KM. Artemin overexpression in skin enhances expression of TRPV1 and TRPA1 in cutaneous sensory neurons and leads to behavioral sensitivity to heat and cold. J Neurosci. 2006;26(33):8578–8587. doi: 10.1523/JNEUROSCI.2185-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Djouhri L, McMullan S, Berry C, Okuse K, Waxman SG, Lawson SN. trkA is expressed in nociceptive neurons and influences electrophysiological properties via Nav1.8 expression in rapidly conducting nociceptors. J Neurosci. 2005a;25(19):4868–4878. doi: 10.1523/JNEUROSCI.0249-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Djouhri L, McMullan S, Berry C, Waxman SG, Okuse K, Lawson SN. Intense isolectin-B4 binding in rat dorsal root ganglion neurons distinguishes C-fiber nociceptors with broad action potentials and high Nav1.9 expression. J Neurosci. 2006;26(27):7281–7292. doi: 10.1523/JNEUROSCI.1072-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, McMullan S, Lawson SN, Djouhri L. Electrophysiological differences between nociceptive and non-nociceptive dorsal root ganglion neurones in the rat in vivo. J Physiol. 2005b;565(Pt 3):927–943. doi: 10.1113/jphysiol.2005.086199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrini F, Trang T, Mattioli TA, Laffray S, Del’Guidice T, Lorenzo LE, Castonguay A, Doyon N, Zhang W, Godin AG, Mohr D, Beggs S, Vandal K, Beaulieu JM, Cahill CM, Salter MW, De Koninck Y. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl(−) homeostasis. Nature neuroscience. 2013;16(2):183–192. doi: 10.1038/nn.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fundin BT, Pfaller K, Rice FL. Different distributions of the sensory and autonomic innervation among the microvasculature of the rat mystacial pad. J Comp Neurol. 1997a;389(4):545–568. [PubMed] [Google Scholar]
- Fundin BT, Silos-Santiago I, Ernfors P, Fagan AM, Aldskogius H, DeChiara TM, Phillips HS, Barbacid M, Yancopoulos GD, Rice FL. Differential dependency of cutaneous mechanoreceptors on neurotrophins, trk receptors, and P75 LNGFR. Developmental biology. 1997b;190(1):94–116. doi: 10.1006/dbio.1997.8658. [DOI] [PubMed] [Google Scholar]
- Garraway SM, Petruska JC, Mendell LM. BDNF sensitizes the response of lamina II neurons to high threshold primary afferent inputs. Eur J Neurosci. 2003;18(9):2467–2476. doi: 10.1046/j.1460-9568.2003.02982.x. [DOI] [PubMed] [Google Scholar]
- Gerner P, Srinivasa V, Zizza AM, Zhuang ZY, Luo S, Zurakowski D, Eappen S, Wang G. Doxepin by topical application and intrathecal route in rats. Anesth Analg. 2006;102(1):283–287. doi: 10.1213/01.ane.0000183639.37428.4d. [DOI] [PubMed] [Google Scholar]
- Gerrits PO, Vodde C, Holstege G. Retroambiguus projections to the cutaneus trunci motoneurons may form a pathway in the central control of mating. J Neurophysiol. 2000;83(5):3076–3083. doi: 10.1152/jn.2000.83.5.3076. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Collins WF., 3rd. Modulation of motoneuron excitability by brain-derived neurotrophic factor. J Neurophysiol. 1997;77(1):502–506. doi: 10.1152/jn.1997.77.1.502. [DOI] [PubMed] [Google Scholar]
- Gray AT. Ultrasound-guided regional anesthesia: current state of the art. Anesthesiology. 2006;104(2):368–373. doi: 10.1097/00000542-200602000-00024. discussion 365A. [DOI] [PubMed] [Google Scholar]
- Griffin JW, Pan B, Polley MA, Hoffman PN, Farah MH. Measuring nerve regeneration in the mouse. Exp Neurol. 2010 doi: 10.1016/j.expneurol.2009.12.033. [DOI] [PubMed] [Google Scholar]
- Hammond DL, Ackerman L, Holdsworth R, Elzey B. Effects of spinal nerve ligation on immunohistochemically identified neurons in the L4 and L5 dorsal root ganglia of the rat. J Comp Neurol. 2004;475(4):575–589. doi: 10.1002/cne.20209. [DOI] [PubMed] [Google Scholar]
- Holstege G, Blok BF. Descending pathways to the cutaneous trunci muscle motoneuronal cell group in the cat. J Neurophysiol. 1989;62(6):1260–1269. doi: 10.1152/jn.1989.62.6.1260. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Nahin RL, Traub RJ, Dubner R. Expansion of receptive fields of spinal lamina I projection neurons in rats with unilateral adjuvant-induced inflammation: the contribution of dorsal horn mechanisms. Pain. 1989;37(2):229–243. doi: 10.1016/0304-3959(89)90135-8. [DOI] [PubMed] [Google Scholar]
- Jancso G, Kiraly E. Sensory neurotoxins: chemically induced selective destruction of primary sensory neurons. Brain Res. 1981;210(1-2):83–89. doi: 10.1016/0006-8993(81)90886-6. [DOI] [PubMed] [Google Scholar]
- Jiang S, Khan MI, Wang J, Middlemiss PJ, Werstiuk ES, Wickson R, Rathbone MP. Enteric glia promote functional recovery of CTM reflex after dorsal root transection. Neuroreport. 2003;14(10):1301–1304. doi: 10.1097/01.wnr.0000077547.91466.e1. [DOI] [PubMed] [Google Scholar]
- Kashiba H, Ueda Y, Senba E. Coexpression of preprotachykinin-A, alpha-calcitonin gene-related peptide, somatostatin, and neurotrophin receptor family messenger RNAs in rat dorsal root ganglion neurons. Neuroscience. 1996;70(1):179–189. doi: 10.1016/0306-4522(95)00334-f. [DOI] [PubMed] [Google Scholar]
- Kerr BJ, Bradbury EJ, Bennett DL, Trivedi PM, Dassan P, French J, Shelton DB, McMahon SB, Thompson SW. Brain-derived neurotrophic factor modulates nociceptive sensory inputs and NMDA-evoked responses in the rat spinal cord. J Neurosci. 1999;19(12):5138–5148. doi: 10.1523/JNEUROSCI.19-12-05138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MA, Gerner P, Kuo Wang G. Amitriptyline for prolonged cutaneous analgesia in the rat. Anesthesiology. 2002a;96(1):109–116. doi: 10.1097/00000542-200201000-00023. [DOI] [PubMed] [Google Scholar]
- Khan MA, Gerner P, Sudoh Y, Wang GK. Use of a charged lidocaine derivative, tonicaine, for prolonged infiltration anesthesia. Reg Anesth Pain Med. 2002b;27(2):173–179. doi: 10.1053/rapm.2002.28710. [DOI] [PubMed] [Google Scholar]
- Krogh JE, Denslow JS. The cutaneus trunci muscle in spinal reflexes. Electromyogr Clin Neurophysiol. 1979;19(1-2):157–164. [PubMed] [Google Scholar]
- Krogh JE, Towns LC. Location of the cutaneous trunci motor nucleus in the dog. Brain Res. 1984;295(2):217–225. doi: 10.1016/0006-8993(84)90970-3. [DOI] [PubMed] [Google Scholar]
- Lawson SN. The morphological consequences of neonatal treatment with capsaicin on primary afferent neurones in adult rats. Acta physiologica Hungarica. 1987;69(3-4):315–321. [PubMed] [Google Scholar]
- Lim TK, Macleod BA, Ries CR, Schwarz SK. The quaternary lidocaine derivative, QX-314, produces long-lasting local anesthesia in animal models in vivo. Anesthesiology. 2007;107(2):305–311. doi: 10.1097/01.anes.0000270758.77314.b4. [DOI] [PubMed] [Google Scholar]
- Lu VB, Biggs JE, Stebbing MJ, Balasubramanyan S, Todd KG, Lai AY, Colmers WF, Dawbarn D, Ballanyi K, Smith PA. Brain-derived neurotrophic factor drives the changes in excitatory synaptic transmission in the rat superficial dorsal horn that follow sciatic nerve injury. J Physiol. 2009;587(Pt 5):1013–1032. doi: 10.1113/jphysiol.2008.166306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Greenquist KW, Lamotte RH. Inflammatory mediators enhance the excitability of chronically compressed dorsal root ganglion neurons. J Neurophysiol. 2006;95(4):2098–2107. doi: 10.1152/jn.00748.2005. [DOI] [PubMed] [Google Scholar]
- Malin SA, Molliver DC, Koerber HR, Cornuet P, Frye R, Albers KM, Davis BM. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci. 2006;26(33):8588–8599. doi: 10.1523/JNEUROSCI.1726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merighi A, Bardoni R, Salio C, Lossi L, Ferrini F, Prandini M, Zonta M, Gustincich S, Carmignoto G. Presynaptic functional trkB receptors mediate the release of excitatory neurotransmitters from primary afferent terminals in lamina II (substantia gelatinosa) of postnatal rat spinal cord. Developmental neurobiology. 2008;68(4):457–475. doi: 10.1002/dneu.20605. [DOI] [PubMed] [Google Scholar]
- Michael GJ, Averill S, Nitkunan A, Rattray M, Bennett DL, Yan Q, Priestley JV. Nerve growth factor treatment increases brain-derived neurotrophic factor selectively in TrkA-expressing dorsal root ganglion cells and in their central terminations within the spinal cord. J Neurosci. 1997;17(21):8476–8490. doi: 10.1523/JNEUROSCI.17-21-08476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliver DC, Radeke MJ, Feinstein SC, Snider WD. Presence or absence of TrkA protein distinguishes subsets of small sensory neurons with unique cytochemical characteristics and dorsal horn projections. J Comp Neurol. 1995;361(3):404–416. doi: 10.1002/cne.903610305. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Snider WD. Nerve growth factor receptor TrkA is down-regulated during postnatal development by a subset of dorsal root ganglion neurons. J Comp Neurol. 1997;381(4):428–438. doi: 10.1002/(sici)1096-9861(19970519)381:4<428::aid-cne3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19(4):849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- Mujenda FH, Duarte AM, Reilly EK, Strichartz GR. Cutaneous endothelin-A receptors elevate post-incisional pain. Pain. 2007;133(1-3):161–173. doi: 10.1016/j.pain.2007.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Postsynaptic targets of somatostatin-containing interneurons in the rat basolateral amygdala. J Comp Neurol. 2007;500(3):513–529. doi: 10.1002/cne.21185. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Hunt SP, Iversen LL, Emson PC. Biochemical and anatomical observations on the degeneration of peptide-containing primary afferent neurons after neonatal capsaicin. Neuroscience. 1981;6(10):1923–1934. doi: 10.1016/0306-4522(81)90032-4. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Iversen LL, Goedert M, Chapman D, Hunt SP. Dose-dependent effects of capsaicin on primary sensory neurons in the neonatal rat. J Neurosci. 1983;3(2):399–406. doi: 10.1523/JNEUROSCI.03-02-00399.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertens E, Urschel-Gysbers BA, Holmes M, Pal R, Foerster A, Kril Y, Diamond J. Intraspinal and behavioral consequences of nerve growth factor-induced nociceptive sprouting and nerve growth factor-induced hyperalgesia compared in adult rats. J Comp Neurol. 1999;410(1):73–89. [PubMed] [Google Scholar]
- Petruska JC, Barker DB, Garraway SM, Mendell LM. BDNF sensitizes the nociceptive-specific CTM reflex. Abstract Viewer/Itinerary Planner. 2005:982–983. [Google Scholar]
- Petruska JC, Cooper BY, Gu JG, Rau KK, Johnson RD. Distribution of P2X1, P2X2, and P2X3 receptor subunits in rat primary afferents: relation to population markers and specific cell types. J Chem Neuroanat. 2000a;20(2):141–162. doi: 10.1016/s0891-0618(00)00080-6. [DOI] [PubMed] [Google Scholar]
- Petruska JC, Mena N, Nakatsuka T, Cooper BY, Johnson RD, Gu JG. P2X1 receptor subunit immunoreactivity and ATP-evoked fast currents in adult rat dorsal root ganglion neurons. Neuroreport. 2000b;11(16):3589–3592. doi: 10.1097/00001756-200011090-00037. [DOI] [PubMed] [Google Scholar]
- Petruska JC, Napaporn J, Johnson RD, Cooper BY. Chemical responsiveness and histochemical phenotype of electrophysiologically classified cells of the adult rat dorsal root ganglion. Neuroscience. 2002;115(1):15–30. doi: 10.1016/s0306-4522(02)00409-8. [DOI] [PubMed] [Google Scholar]
- Petruska JC, Napaporn J, Johnson RD, Gu JG, Cooper BY. Subclassified acutely dissociated cells of rat DRG: histochemistry and patterns of capsaicin-, proton-, and ATP-activated currents. J Neurophysiol. 2000c;84(5):2365–2379. doi: 10.1152/jn.2000.84.5.2365. [DOI] [PubMed] [Google Scholar]
- Petruska JC, Streit WJ, Johnson RD. Localization of unmyelinated axons in rat skin and mucocutaneous tissue utilizing the isolectin GS-I-B4. Somatosens Mot Res. 1997;14(1):17–26. doi: 10.1080/08990229771187. [DOI] [PubMed] [Google Scholar]
- Petruska JC, Trainer R, Seidman P, Glass P, Soto R. Dexmedetomidine extension of local anesthetic effect of bupivacaine in a peripheral nerve block in rats; Anesth Analg IARS Meeting; 2008. Abstracts:S:203. [Google Scholar]
- Priestley JV, Bramwell S, Butcher LL, Cuello AC. Effect of capsaicin on neuropeptides in areas of termination of primary sensory neurones. Neurochemistry international. 1982;4(1):57–65. doi: 10.1016/0197-0186(82)90027-4. [DOI] [PubMed] [Google Scholar]
- Rice FL, Albers KM, Davis BM, Silos-Santiago I, Wilkinson GA, LeMaster AM, Ernfors P, Smeyne RJ, Aldskogius H, Phillips HS, Barbacid M, DeChiara TM, Yancopoulos GD, Dunne CE, Fundin BT. Differential dependency of unmyelinated and A delta epidermal and upper dermal innervation on neurotrophins, trk receptors, and p75LNGFR. Developmental biology. 1998;198(1):57–81. [PubMed] [Google Scholar]
- Seebach BS, Arvanov V, Mendell LM. Effects of BDNF and NT-3 on development of Ia/motoneuron functional connectivity in neonatal rats. J Neurophysiol. 1999;81(5):2398–2405. doi: 10.1152/jn.1999.81.5.2398. [DOI] [PubMed] [Google Scholar]
- Shieh JP, Chu CC, Wang JJ, Lin MT. Epinephrine, phenylephrine, and methoxamine induce infiltrative anesthesia via alpha1-adrenoceptors in rats. Acta pharmacologica Sinica. 2009;30(9):1227–1236. doi: 10.1038/aps.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu XQ, Llinas A, Mendell LM. Effects of trkB and trkC neurotrophin receptor agonists on thermal nociception: a behavioral and electrophysiological study. Pain. 1999;80(3):463–470. doi: 10.1016/S0304-3959(99)00042-1. [DOI] [PubMed] [Google Scholar]
- Simone DA. Neural mechanisms of hyperalgesia. Current opinion in neurobiology. 1992;2(4):479–483. doi: 10.1016/0959-4388(92)90183-l. [DOI] [PubMed] [Google Scholar]
- Soto R, Trainer R, Petruska JC, Glass P, Seidman P. The in vitro effect of dexmedetomidine on rat sciatic nerve transmission; Anesthesiology ASA Meeting; 2007. Abstracts:A:976. [Google Scholar]
- Stucky CL, Lewin GR. Isolectin B(4)-positive and -negative nociceptors are functionally distinct. J Neurosci. 1999;19(15):6497–6505. doi: 10.1523/JNEUROSCI.19-15-06497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucky CL, Rossi J, Airaksinen MS, Lewin GR. GFR alpha2/neurturin signalling regulates noxious heat transduction in isolectin B4-binding mouse sensory neurons. J Physiol. 2002;545(Pt 1):43–50. doi: 10.1113/jphysiol.2002.027656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Chiba T, Kurokawa M, Aoki Y. Dermatomes and the central organization of dermatomes and body surface regions in the spinal cord dorsal horn in rats. J Comp Neurol. 2003;462(1):29–41. doi: 10.1002/cne.10669. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Nakajima Y. Dermatomes in the rat limbs as determined by antidromic stimulation of sensory C-fibers in spinal nerves. Pain. 1996;67(1):197–202. doi: 10.1016/0304-3959(96)03116-8. [DOI] [PubMed] [Google Scholar]
- Tansey KE, Ortiz N, Zinkhan G, Huntington P, Botterman BR. Neurorehab Neural Repair. Asilomar, CA: 2007. Plasticity in an intersegmental pain reflex following spinal cord lateral hemisection in the rat; p. 606. [Google Scholar]
- Theriault E, Diamond J. Intrinsic organization of the rat cutaneus trunci motor nucleus. J Neurophysiol. 1988a;60(2):463–477. doi: 10.1152/jn.1988.60.2.463. [DOI] [PubMed] [Google Scholar]
- Theriault E, Diamond J. Nociceptive cutaneous stimuli evoke localized contractions in a skeletal muscle. J Neurophysiol. 1988b;60(2):446–462. doi: 10.1152/jn.1988.60.2.446. [DOI] [PubMed] [Google Scholar]
- Thompson SW, Bennett DL, Kerr BJ, Bradbury EJ, McMahon SB. Brain-derived neurotrophic factor is an endogenous modulator of nociceptive responses in the spinal cord. Proc Natl Acad Sci U S A. 1999;96(14):7714–7718. doi: 10.1073/pnas.96.14.7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang T, Beggs S, Salter MW. Brain-derived neurotrophic factor from microglia: a molecular substrate for neuropathic pain. Neuron glia biology. 2011;7(1):99–108. doi: 10.1017/S1740925X12000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzabazis A, Klyukinov M, Manering N, Nemenov MI, Shafer SL, Yeomans DC. Differential activation of trigeminal C or Adelta nociceptors by infrared diode laser in rats: behavioral evidence. Brain Res. 2005;1037(1-2):148–156. doi: 10.1016/j.brainres.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Vadakkan KI, Jia YH, Zhuo M. A behavioral model of neuropathic pain induced by ligation of the common peroneal nerve in mice. J Pain. 2005;6(11):747–756. doi: 10.1016/j.jpain.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Verge VM, Merlio JP, Grondin J, Ernfors P, Persson H, Riopelle RJ, Hokfelt T, Richardson PM. Colocalization of NGF binding sites, trk mRNA, and low-affinity NGF receptor mRNA in primary sensory neurons: responses to injury and infusion of NGF. J Neurosci. 1992;12(10):4011–4022. doi: 10.1523/JNEUROSCI.12-10-04011.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verge VM, Richardson PM, Benoit R, Riopelle RJ. Histochemical characterization of sensory neurons with high-affinity receptors for nerve growth factor. Journal of neurocytology. 1989;18(5):583–591. doi: 10.1007/BF01187079. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Riedl MS, Shuster SJ, Buell G, Surprenant A, North RA, Elde R. Immunohistochemical study of the P2X2 and P2X3 receptor subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacology. 1997;36(9):1229–1242. doi: 10.1016/s0028-3908(97)00126-3. [DOI] [PubMed] [Google Scholar]
- Wang CF, Gerner P, Schmidt B, Xu ZZ, Nau C, Wang SY, Ji RR, Wang GK. Use of bulleyaconitine A as an adjuvant for prolonged cutaneous analgesia in the rat. Anesth Analg. 2008;107(4):1397–1405. doi: 10.1213/ane.0b013e318182401b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DE, Snider WD. Neurotrophin receptor mRNA expression defines distinct populations of neurons in rat dorsal root ganglia. J Comp Neurol. 1995;351(3):329–338. doi: 10.1002/cne.903510302. [DOI] [PubMed] [Google Scholar]
- Xu M, Wei H, Kontinen VK, Kalso E, Pertovaara A. The dissociation of sedative from spinal antinociceptive effects following administration of a novel alpha-2-adrenoceptor agonist, MPV-2426, in the locus coeruleus in the rat. Acta Anaesthesiol Scand. 2000;44(6):648–655. doi: 10.1034/j.1399-6576.2000.440604.x. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Pirec V, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: behavioral evidence. Pain. 1996;68(1):133–140. doi: 10.1016/S0304-3959(96)03176-4. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Proudfit HK. Characterization of the foot withdrawal response to noxious radiant heat in the rat. Pain. 1994;59(1):85–94. doi: 10.1016/0304-3959(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: electrophysiological evidence. Pain. 1996;68(1):141–150. doi: 10.1016/S0304-3959(96)03177-6. [DOI] [PubMed] [Google Scholar]
- Yoshitomi T, Kohjitani A, Maeda S, Higuchi H, Shimada M, Miyawaki T. Dexmedetomidine enhances the local anesthetic action of lidocaine via an alpha-2A adrenoceptor. Anesth Analg. 2008;107(1):96–101. doi: 10.1213/ane.0b013e318176be73. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Goldstein BD, Yeomans DC. Low but not high rate noxious radiant skin heating evokes a capsaicin-sensitive increase in spinal cord dorsal horn release of substance P. Brain Res. 1997;752(1-2):143–150. doi: 10.1016/s0006-8993(96)01466-7. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Li H, Brull SJ. Perfusion of the mechanically compressed lumbar ganglion with lidocaine reduces mechanical hyperalgesia and allodynia in the rat. J Neurophysiol. 2000;84(2):798–805. doi: 10.1152/jn.2000.84.2.798. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45(1):17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]
References
- Buchan AM, Sikora LK, Levy JG, McIntosh CH, Dyck I, Brown JC. An immunocytochemical investigation with monoclonal antibodies to somatostatin. Histochemistry. 1985;83(2):175–180. doi: 10.1007/BF00495150. [DOI] [PubMed] [Google Scholar]
- Manca A, Capsoni S, Di Luzio A, Vignone D, Malerba F, Paoletti F, Brandi R, Arisi I, Cattaneo A, Levi-Montalcini R. Nerve growth factor regulates axial rotation during early stages of chick embryo development. Proc Natl Acad Sci U S A. 2012;109(6):2009–2014. doi: 10.1073/pnas.1121138109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael GJ, Averill S, Nitkunan A, Rattray M, Bennett DL, Yan Q, Priestley JV. Nerve growth factor treatment increases brain-derived neurotrophic factor selectively in TrkA-expressing dorsal root ganglion cells and in their central terminations within the spinal cord. J Neurosci. 1997;17(21):8476–8490. doi: 10.1523/JNEUROSCI.17-21-08476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Postsynaptic targets of somatostatin-containing interneurons in the rat basolateral amygdala. J Comp Neurol. 2007;500(3):513–529. doi: 10.1002/cne.21185. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Riedl MS, Shuster SJ, Buell G, Surprenant A, North RA, Elde R. Immunohistochemical study of the P2X2 and P2X3 receptor subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacology. 1997;36(9):1229–1242. doi: 10.1016/s0028-3908(97)00126-3. [DOI] [PubMed] [Google Scholar]
- Wilkinson KD, Lee KM, Deshpande S, Duerksen-Hughes P, Boss JM, Pohl J. The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science. 1989;246(4930):670–673. doi: 10.1126/science.2530630. [DOI] [PubMed] [Google Scholar]
- Wilson PO, Barber PC, Hamid QA, Power BF, Dhillon AP, Rode J, Day IN, Thompson RJ, Polak JM. The immunolocalization of protein gene product 9.5 using rabbit polyclonal and mouse monoclonal antibodies. Br J Exp Pathol. 1988;69(1):91–104. [PMC free article] [PubMed] [Google Scholar]