Abstract
Purpose
Outcomes in men with NCCN high-risk prostate cancer (PCa) can vary substantially--some will have excellent cancer-specific survival, whereas others will experience early metastasis even after aggressive local treatments. Current nomograms, which yield continuous risk probabilities, do not separate high-risk PCa into distinct sub-strata. Here we derive a binary definition of very-high-risk (VHR) localized PCa to aid in risk stratification at diagnosis and selection of therapy.
Materials and Methods
We queried the Johns Hopkins radical prostatectomy database to identify 753 men with NCCN high-risk localized PCa (Gleason sum 8–10, PSA >20 ng/ml, or clinical stage ≥T3). 28 alternate permutations of adverse grade, stage, and cancer volume were compared by their hazard ratios for metastasis and cancer-specific mortality. VHR criteria with top-ranking hazard ratios were further evaluated by multivariable analyses and inclusion of a clinically meaningful proportion of the high-risk cohort.
Results
The VHR cohort was best defined by primary pattern 5 present on biopsy, or ≥5 cores with Gleason sum 8–10, or multiple NCCN high-risk features. These criteria encompassed 15.1% of the NCCN high-risk cohort. Compared to other high-risk men, VHR men were at significantly higher risk for metastasis (H.R. 2.75) and cancer-specific mortality (H.R. 3.44) (p <0.001 for both). Among high-risk men, VHR men also had significantly worse 10-year metastasis-free survival (37% vs 78%) and cancer-specific survival (62% vs 90%).
Conclusions
Men who meet VHR criteria form a subgroup within the current NCCN high-risk classification who have particularly poor oncologic outcomes. Use of these characteristics to distinguish VHR localized PCa may help in counseling and selection optimal candidates for multimodal treatments or clinical trials.
Keywords: Prostate cancer, risk stratification, metastasis, survival, high-risk prostate cancer
Introduction
Risk-stratification in newly diagnosed prostate cancer (PCa) aids physicians and patients to choose an optimal management approach. The most widely used risk-classification system (D’Amico) was developed in 1998 (1) and has been adapted by the National Comprehensive Cancer Network (NCCN). (2) Currently, the NCCN high-risk localized PCa classification (comprising up to 26% of newly diagnosed men (3)), defined as biopsy Gleason sum ≥8 or PSA >20 ng/ml or clinical stage ≥T3a, helps identify men with localized disease who are at high recurrence and progression risk after treatment.
However, cancer-specific outcomes within high-risk PCa vary dramatically. Ten-year metastasis free survival (MFS) can range from 70% to 95% depending on biopsy Gleason sum and clinical stage among high-risk men who undergo radical prostatectomy (RP). (4) Biochemical recurrence among high-risk men can vary by over 50% at 10 years. (5) Pre-operative factors that predict adverse outcomes in high-risk men have been subsequently identified, including multiple high-risk features, Gleason sum 9–10, PSA >10 ng/ml, clinical stage ≥T2b, and higher volume of high-grade cancer. (6,7)
This heterogeneity of clinical outcomes suggests the existence of high-risk sub-populations. We hypothesize that commonly used clinical variables can be used to distinguish subsets of men with high-risk disease, while preserving the ease-of-use and point-of-care clinical applicability of existing NCCN risk strata. Identification of men within the high-risk cohort who either experience relatively good oncologic outcomes (high-risk) or the worst outcomes (very-high-risk, or VHR) despite aggressive treatment can aid pre-treatment risk stratification and also assist in the selection of potential candidates for multimodal therapy or clinical trials. (8)
Here, by exploring alternate permutations of known pre-treatment prognostic variables, we identified a subset of men within the NCCN high-risk cohort who were at very-high risk (VHR) for adverse oncologic outcomes.
Materials and Methods
The IRB-approved institutional RP database containing 21039 men was queried to identify men with NCCN high-risk localized PCa (Gleason sum 8–10, PSA >20 ng/ml, or clinical stage ≥T3a) (2) who underwent RP. The following groups were excluded from analysis: men treated in the pre-PSA era (1571); those who received neoadjuvant hormonal treatments (876); men with no follow-up data (6499); and men with incomplete pre-treatment staging (307). Of 11786 evaluable patients, 7085 (60.1%) were NCCN very-low or low-risk, 3948 (33.5%) were intermediate-risk, and 753 (6.4%) were high-risk. In the high-risk cohort detailed biopsy information was evaluated, including number of positive/total cores, primary/secondary Gleason patterns in each core, and maximum percent cancer in each core for each Gleason pattern present. Multiple high-risk features was defined as having two or three of the individual NCCN high-risk criteria.
As a preliminary analysis, associations of previously described prognostic factors (number of NCCN high risk features, increasing biopsy Gleason sum, increasing PSA, PSA velocity, more advanced clinical stage, and increasing volume of high-grade cancer on biopsy) with metastasis and cancer-specific death were calculated in order to validate their further use in the present high-risk cohort. Pre-operative PSA velocity data was available for 234 men. Twenty-eight alternate permutations of adverse prognostic factors served as test definitions of VHR criteria. For each VHR test definition, Cox proportional hazards modeling was used to calculate unadjusted hazard ratios for metastasis, cancer-specific, and all-cause mortality. VHR test definitions that had hazard ratios in the top quartile for both metastasis and cancer-specific mortality were selected for further evaluation by multivariable analysis after adjusting for variables that were not already incorporated into the VHR test definitions: age, year of surgery, and perineural invasion. Only patients with NCCN high-risk cancer were included in all analyses.
The final criteria for VHR PCa were selected based on adjusted hazard ratios for metastasis and cancer-specific mortality and cohort size. The resulting VHR cohort was compared to the remainder of high-risk men, analyzing freedom from biochemical recurrence (BFS), metastasis free survival (MFS), cancer-specific survival (CSS), and overall survival (OS). Appropriate comparative tests were used. Survival estimates were derived from Kaplan-Meier life tables. Minimum follow-up for all patients included annual symptom assessments and prostate specific antigen (PSA) measurements. Biochemical recurrence was defined as a post-operative increase in PSA (≥0.2 ng/ml), metastasis was defined as radiographic evidence of extra-pelvic disease spread, and cancer-specific mortality was defined as death due to PCa. In order to reflect contemporary practice patterns, sub-analyses were also performed including only men with extended sampling (≥10 cores at biopsy)(9) and who were treated in the modern Gleason grading era (adopted at our center in 2004) (10).
The final VHR criteria performance characteristics were also compared with all possible binary cut-points of the Cancer of the Prostate Risk Assessment scoring system (CAPRA), a validated pre-operative oncologic risk classification that yields a distinct integer score between 0 and 10. (11–13). Because calculation of CAPRA depends on proportion of positive biopsy cores, only patients with known total number of biopsy cores were included in this analysis (n=566). The median number of biopsy cores was 12.0, and 62% had extended sampling with ≥10 cores.
The two-tailed level of statistical significance was pre-defined as p <0.05. Statistical analyses were performed with STATA 11.0 (StataCorp, College Station, Texas, USA).
Results
We first validated known risk factors for metastasis and cancer-specific mortality including number of NCCN high-risk features, biopsy Gleason sum, and volume of high-grade cancer (as assessed by proportion of positive cores on biopsy with Gleason pattern 4 or 5), all of which were significant predictors of metastasis and death in this cohort. In contrast, PSA, PSA velocity, and clinical stage were not prognostic for either outcome (data not shown). Alternate permutations of adverse risk factors (number of high-risk features, volume of high-grade cancer on biopsy, and pattern of high-grade cancer) comprised 28 alternate VHR criteria. Within these VHR test definitions, hazard ratios for metastasis ranged from 1.82–3.98 and those for cancer=specific mortality ranged from 1.86–4.82 (Table 1). VHR test definitions with hazard ratios in the highest quartile for both MFS and CSS were subject to multivariable analysis
Table 1.
Univariate hazard ratios for metastasis, cancer-specific mortality, and all-cause mortality among 28 tested very-high-risk definitions
| Metastasis | Cancer-specific mortality | All-cause mortality | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tested very-high-risk definitions | H.R. | p | 95% C.I. | H.R. | p | 95% C.I. | H.R. | p | 95% C.I. | JHU very high risk sample size | |
| 1 | Any pattern 5 or pattern 4 + PSA >20 or pattern 4 + ≥cT3a | 2.26 | <0.001 | 1.53, 3.32 | 2.54 | <0.001 | 1.52, 4.26 | 1.80 | 0.002 | 1.24, 2.61 | 328 (43.6%) |
| 2 | Any pattern 5 or Gleason 8 + PSA >20 or Gleason 8 + ≥cT3a | 2.38 | <0.001 | 1.60, 3.55 | 3.78 | <0.001 | 2.31, 6.18 | 2.55 | <0.001 | 1.74, 3.74 | 190 (25.2%) |
| 3 | Multiple high-risk features | 2.53 | <0.001 | 1.44, 4.43 | 2.91 | <0.001 | 1.55, 5.45 | 2.12 | 0.005 | 1.25, 3.59 | 45 (6.0%) |
| 4 | Any pattern 5 | 2.16 | <0.001 | 1.42, 3.27 | 3.44 | <0.001 | 2.08, 5.69 | 2.47 | <0.001 | 1.66, 3.68 | 171 (22.7%) |
| 5 | Primary pattern 5 | 3.98 | <0.001 | 2.28, 6.96 | 4.82 | <0.001 | 2.43, 9.58 | 3.20 | <0.001 | 1.78, 5.76 | 52 (6.9%) |
| 6 | Any pattern 5 or multiple high-risk features | 2.40 | <0.001 | 1.62, 3.55 | 3.90 | <0.001 | 2.39, 6.36 | 2.57 | <0.001 | 1.76, 3.75 | 197 (26.2%) |
| 7 | Any pattern 5 or ≥4 cores with pattern 4 | 2.31 | <0.001 | 1.57, 3.39 | 2.97 | <0.001 | 1.81, 4.89 | 2.05 | <0.001 | 1.39, 3.01 | 286 (38.0%) |
| 8 | Any pattern 5 or ≥4 cores with pattern 4 or multiple high-risk feat | 2.54 | <0.001 | 1.73, 3.73 | 3.28 | <0.001 | 2.00, 5.39 | 2.06 | <0.001 | 1.42, 3.00 | 304 (40.4%) |
| 9 | Primary pattern 5 or multiple high-risk features | 3.15 | <0.001 | 2.02, 4.91 | 3.73 | <0.001 | 2.20, 6.33 | 2.57 | <0.001 | 1.67, 3.97 | 90 (12.0%) |
| 10 | Primary pattern 5 or ≥4 cores with pattern 4 | 2.55 | <0.001 | 1.70, 3.83 | 2.52 | <0.001 | 1.44, 4.43 | 1.62 | 0.038 | 1.03, 2.66 | 201 (26.7%) |
| 11 | Primary pattern 5 or ≥4 cores with pattern 4 or multiple high-risk feat | 2.78 | <0.001 | 1.89, 4.10 | 2.76 | <0.001 | 1.66, 4.57 | 1.76 | 0.007 | 1.17, 2.66 | 227 (30.1%) |
| 12 | Any pattern 5 or max pattern 4 or 5 per core >50% | 2.31 | <0.001 | 1.56, 3.41 | 2.32 | <0.001 | 1.41, 3.81 | 1.82 | 0.002 | 0.25, 2.65 | 368 (48.9%) |
| 13 | Any pattern 5 or max pattern 4 or 5 per core >50% or mult hi risk feat | 2.63 | <0.001 | 1.76, 3.92 | 2.74 | <0.001 | 1.65, 4.55 | 1.92 | 0.002 | 1.32, 2.80 | 384 (51.0%) |
| 14 | Primary pattern 5 or ≥4 cores with pattern 4/5 | 2.51 | <0.001 | 1.67, 3.77 | 2.50 | <0.001 | 1.42, 4.40 | 1.63 | 0.042 | 1.02, 2.63 | 204 (27.1%) |
| 15 | Primary pattern 5 or ≥4 cores with pattern 4/5 or mult high-risk feat | 2.74 | <0.001 | 1.86, 4.05 | 2.74 | <0.001 | 1.65, 4.54 | 1.75 | 0.007 | 1.16, 2.64 | 230 (30.5%) |
| 16 | Primary pattern 5 or ≥4 cores with pattern 4/5 or PNI | 2.12 | <0.001 | 1.45, 3.10 | 1.86 | <0.001 | 1.14, 3.05 | 1.32 | 0.150 | 0.90, 1.94 | 308 (40.9%) |
| 17 | Primary pattern 5 or PNI | 2.21 | <0.001 | 1.51, 3.23 | 1.95 | <0.001 | 1.18, 3.20 | 1.42 | 0.076 | 0.96, 2.10 | 234 (31.1%) |
| 18 | Primary pattern 5 or ≥1 core with sum 8–10 or multiple high-risk features | 1.82 | <0.001 | 1.22, 2.70 | 2.93 | <0.001 | 1.71, 5.05 | 1.84 | 0.002 | 1.25, 2.69 | 455 (60.4%) |
| 19 | Primary pattern 5 or ≥2 cores with sum 8–10 or multiple high-risk features | 2.25 | <0.001 | 1.54, 3.30 | 2.79 | <0.001 | 1.71, 4.56 | 1.75 | 0.004 | 1.19, 2.56 | 267 (35.5%) |
| 20 | Primary pattern 5 or ≥3 cores with sum 8–10 or multiple high-risk features | 2.72 | <0.001 | 1.83, 4.04 | 3.17 | <0.001 | 1.92, 5.23 | 2.04 | 0.001 | 1.36, 3.07 | 176 (23.4%) |
| 21 | Primary pattern 5 or ≥4 cores with sum 8–10 or multiple high-risk features | 2.55 | <0.001 | 1.68, 3.87 | 3.06 | <0.001 | 1.82, 5.13 | 2.05 | 0.001 | 1.34, 3.13 | 138 (18.3%) |
| 22 | Primary pattern 5 or ≥5 cores with sum 8–10 or multiple high-risk features | 3.21 | <0.001 | 2.10, 4.89 | 3.54 | <0.001 | 2.10, 5.96 | 2.39 | <0.001 | 1.56, 3.67 | 114 (15.1%) |
| 23 | Primary pattern 5 or ≥6 cores with sum 8-10 or multiple high-risk features | 3.22 | <0.001 | 2.10, 4.96 | 3.74 | <0.001 | 2.22, 6.30 | 2.52 | <0.001 | 1.64, 3.87 | 102 (13.5%) |
| 24 | Primary pattern 5 or ≥7 cores with sum 8-10 or multiple high-risk features | 3.35 | <0.001 | 2.17, 5.17 | 3.90 | <0.001 | 2.31, 6.57 | 2.63 | <0.001 | 1.71, 4.03 | 94 (12.5%) |
| 25 | Primary pattern 5 or mult hi risk features or PNI | 2.41 | <0.001 | 1.65, 3.52 | 2.44 | <0.001 | 1.50, 3.97 | 1.70 | 0.005 | 1.17, 2.46 | 257 (34.1%) |
| 26 | Primary pattern 5 or mult hi risk features or PNI+ ≥1 cores with Gleason sum 8–10 | 2.83 | <0.001 | 1.90, 4.20 | 3.17 | <0.001 | 1.92, 5.23 | 2.22 | <0.001 | 1.49, 3.30 | 191 (25.4%) |
| 27 | Primary pattern 5 or mult hi risk features or PNI+ ≥2 cores with Gleason sum 8–10 | 3.04 | <0.001 | 2.04, 4.53 | 3.20 | <0.001 | 1.92, 5.31 | 2.15 | <0.001 | 1.43, 3.25 | 152 (20.2%) |
| 28 | Primary pattern 5 or mult hi risk features or PNI+ ≥3 cores with Gleason sum 8–10 | 3.20 | <0.001 | 2.12, 4.82 | 3.26 | <0.001 | 1.94, 5.46 | 2.18 | <0.001 | 1.43, 3.33 | 125 (16.6%) |
Among the five VHR test definitions analyzed by multivariable modeling, ‘primary pattern 5’ had the highest adjusted hazard ratios but it included only 6.9% of the high-risk cohort; therefore it was not considered any further. Of the four remaining VHR test definitions, adjusted hazard ratios for metastasis and cancer-specific mortatlity were similar (Table 2), indicating nearly equivalent abilities to discriminate outcomes within the high-risk cohort. Subsequently the ultimate VHR definition was selected according to inclusion of the highest proportion of the high-risk cohort (15.1%) to maximize clinical utility. This definition included men presenting with: primary Gleason pattern 5, or ≥5 cores with Gleason sum 8–10, or multiple NCCN high-risk features (i.e. Gleason sum 8–10 and PSA >20).
Table 2.
Hazard ratios for metastasis, cancer-specific mortality, and all-cause mortality among top-ranking definitions, adjusted for age, year of surgery, and perineural invasion
| Metastasis | Cancer-specific mortality | All-cause mortality | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tested very-high-risk definitions | H.R. | p | 95% C.I. | H.R. | p | 95% C.I. | H.R. | p | 95% C.I. | JHU very high risk sample size | |
| 5 | Primary pattern 5 | 4.11 | <0.001 | 2.35, 7.17 | 4.70 | <0.001 | 2.37, 9.33 | 3.14 | <0.001 | 1.75, 5.63 | 52 (6.9%) |
| 9 | Primary pattern 5 or multiple high-risk features | 2.86 | <0.001 | 1.81, 4.50 | 3.33 | <0.001 | 1.93, 5.76 | 2.56 | <0.001 | 1.65, 4.00 | 90 (12.0%) |
| 22 | Primary pattern 5 or ≥5 cores with sum 8–10 or multiple high-risk features | 2.93 | <0.001 | 1.90, 4.53 | 3.22 | <0.001 | 1.88, 5.53 | 2.44 | <0.001 | 1.57, 3.79 | 114 (15.1%) |
| 23 | Primary pattern 5 or ≥6 cores with sum 8–10 or multiple high-risk features | 2.92 | <0.001 | 1.88, 4.54 | 3.37 | <0.001 | 1.96, 5.77 | 2.53 | <0.001 | 1.63, 3.92 | 102 (13.5%) |
| 24 | Primary pattern 5 or ≥7 cores with sum 8–10 or multiple high-risk features | 3.05 | <0.001 | 1.96, 4.75 | 3.50 | <0.001 | 2.04, 6.00 | 2.62 | <0.001 | 1.96, 4.07 | 94 (12.5%) |
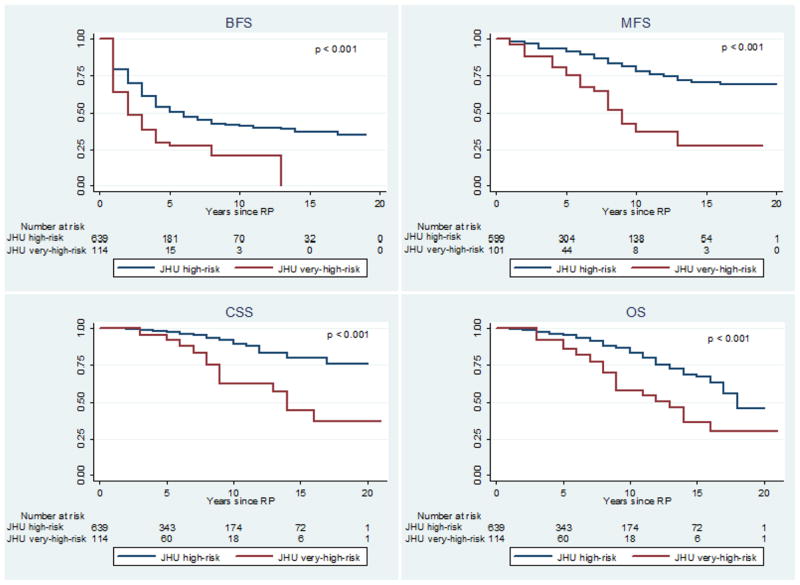
When compared to the remainder of the high-risk cohort, VHR men presented more commonly with clinical T3 disease (14.9% vs 5.8%, p<0.001) and perineural invasion on biopsy (36.8% vs 24.4%, p=0.005) but had equivalent positive surgical margin rates (26.3% vs 28.5%, p=0.609) (Table 3). Median follow-up was 5.0 years in both the high-risk and VHR cohorts. VHR criteria discriminated men with significantly divergent BFS, MFS, CSS, and OS Kaplan-Meier curves (log-rank p<0.001 for all measures) (Figure 2). At 10 years, BFS for VHR was 0.21 (95% C.I. 0.09, 0.36) compared to 0.41 (95% C.I. 0.36, 0.46) for the remainder of the high-risk cohort (Table 4). Ten-year MFS for VHR men was 0.37 (95% C.I. 0.20, 0.54) compared to 0.78 (95% C.I. 0.72, 0.83) for the remainder of the high-risk cohort (Table 4). Similarly, 10-year CSS for VHR men was 0.62 (95% C.I. 0.45, 0.76) compared to 0.90 (95% C.I. 0.85, 0.93) for high-risk men (Table 4). The independent contributions of each component of the VHR criteria were assessed in multivariable models (Table S3). All three components were significantly associated with risk of metastasis. In a sub-analysis of men diagnosed with extended biopsy sampling in the modern Gleason grading era (n=275), VHR criteria remained the strongest predictors of metastasis in univariate and multivariable analyses (Table S4).
Table 3.
Pre-operative & pathologic characteristics of very-high-risk men
| JHU high-risk | JHU very-high-risk | p | |
|---|---|---|---|
|
| |||
| N | 639 | 114 | |
|
| |||
| Median Age (IQR) | 60.0 (54.0, 64.0) | 59.0 (54.0, 63.0) | 0.482* |
|
| |||
| AA race | 74 (11.6%) | 10 (8.8%) | 0.380 |
|
| |||
| Positive family history | 128/356 (36.0%) | 23/64 ((35.9%) | 0.998 |
|
| |||
| Median PSA (ng/dl) (IQR) | 11.1 (5.5, 24.1) | 10.2 (6.2, 21.4) | 0.597* |
|
| |||
| Median PSA density (IQR) | 0.43 | 0.35 | 0.806* |
|
| |||
| PSA >20 ng/dl | 261 (40.8%) | 36 (31.6%) | 0.062 |
|
| |||
| Clinical stage | <0.001 | ||
| T1 | 351 (54.9%) | 45 (39.5%) | |
| T2 | 251 (39.3%) | 52 (45.6%) | |
| T3 | 37 (5.8%) | 17 (14.9%) | |
|
| |||
| Biopsy Gleason | <0.001^ | ||
| ≤6 | 164 (25.7%) | 3 (2.6%) | |
| 7 | 134 (30.0%) | 4 (3.5%) | |
| 8 | 278 (43.5%) | 50 (43.9%) | |
| 9 | 63 (9.9%) | 52 (45.6%) | |
| 10 | 0 (0%) | 5 (4.4%) | |
|
| |||
| Primary pattern 5 | 0 (0%) | 52 (45.6%) | <0.001^ |
|
| |||
| Multiple high-risk features | 0 (0%) | 45 (39.5%) | <0.001^ |
|
| |||
| ≥5 cores with Gleason sum 8 | 0 (0%) | 36 (31.6%) | <0.001^ |
|
| |||
| Perineural invasion present on biopsy | 156 (24.4%) | 42 (36.8%) | 0.005 |
|
| |||
| Laparoscopic or Robotic RP | 59 (9.2%) | 12 (10.5%) | 0.663 |
|
| |||
| Pathologic stage | <0.001 | ||
| pT2N0 | 206 (32.2%) | 21 (18.4%) | |
| pT3aN0 | 287 (44.9%) | 43 (37.7%) | |
| pT3bN0 | 78 (12.2%) | 22 (19.3%) | |
| pN1 | 68 (10.6%) | 28 (24.6%) | |
|
| |||
| Pathologic Gleason | <0.001^ | ||
| ≤6 | 93 (14.6%) | 1 (0.9%) | |
| 7 | 294 (46.0%) | 27 (23.7%) | |
| 8 | 126 (19.7%) | 36 (31.6%) | |
| 9 | 124 (19.4%) | 47 (41.2%) | |
| 10 | 0 (0%) | 3 (2.6%) | |
|
| |||
| Positive surgical margin | 182 (28.5%) | 30 (26.3%) | 0.609 |
|
| |||
| Received radiation, androgen deprivation, or chemotherapy prior to metastasis | 225 (35.2%) | 59 (51.8%) | 0.001 |
|
| |||
| Median follow-up (years) (IQR) | 5.0 (2.0, 10.0) | 5.0 (3.0, 8.0) | 0.430* |
p-value derived from Wilcoxon-Mann-Whitney test, IQR=interquartile range
p-value derived from Fisher’s exact test
Table 4.
Five- & 10-year survival probabilities* (BFS, MFS, CSS, & OS) stratified by very-high-risk classification
| JHU high-risk | JHU very-high-risk | ||||||
|---|---|---|---|---|---|---|---|
| Years after RP | Survival | 95% C.I. | Survival | 95% C.I. | |||
| BFS | 5 | 0.503 | 0.457 | 0.548 | 0.275 | 0.182 | 0.376 |
| 10 | 0.412 | 0.360 | 0.462 | 0.206 | 0.090 | 0.356 | |
| MFS | 5 | 0.914 | 0.884 | 0.937 | 0.749 | 0.630 | 0.835 |
| 10 | 0.779 | 0.722 | 0.826 | 0.369 | 0.198 | 0.542 | |
| CSS | 5 | 0.976 | 0.956 | 0.987 | 0.922 | 0.833 | 0.965 |
| 10 | 0.895 | 0.850 | 0.928 | 0.622 | 0.447 | 0.755 | |
| OS | 5 | 0.951 | 0.927 | 0.968 | 0.858 | 0.757 | 0.920 |
| 10 | 0.833 | 0.782 | 0.872 | 0.579 | 0.416 | 0.711 | |
Kaplan-Meier life table estimates
Rates of additional treatment post-RP (adjuvant or salvage radiation, androgen deprivation and/or chemotherapy) were compared between the VHR and high risk groups. Post-operative, pre-metastatic treatments occurred in 51.8% of VHR men but only 35.2% of the remainder of the high-risk cohort (p=0.001) (Table 3).
Further, we evaluated our high risk cohort in regards to their CAPRA score. Like our dichotomous classifier, CAPRA has the advantage over typical nomograms of being fairly easy to apply in the clinical setting with minimal calculation. (11–13)
Within the entire NCCN high risk cohort, the mean CAPRA score was 5.3 (median 5.0, range 2–9). No metastases or PCa-specific deaths were seen among men with CAPRA <3 or ≥9, both of which contained small numbers of patients (Table S1). In unadjusted (Table S2) and adjusted (Table 5) models, the final VHR criteria, compared with all CAPRA cut-points, was the only significant predictor of both MFS and CSS, though multiple CAPRA cut-points were significantly associated with MFS. The CAPRA cut-point that best discriminated MFS and CSS was 6. In MFS and CSS survival curves stratified by CAPRA ≥6 and the VHR criteria, MFS stratified similarly between the two different criteria, but the log-rank p-value for CSS by CAPRA ≥6 was not significant (p=0.140). Among 28 men with intermediate-risk CAPRA (3 to 5) who subsequently developed metastases, 4/28 (14.3%) met VHR criteria.
Table 5.
Hazard ratios for metastasis and cancer-death comparing all possible CAPRA cut-points and JHH Very High Risk criteria, among 566 NCCN high-risk men with evaluable CAPRA scores, adjusted for age, year of surgery, and perineural invasion
| Metastasis | Cancer-specific mortality | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tested very-high-risk definitions | H.R. | p | 95% C.I. | c-index | H.R. | p | 95% C.I. | c-index | Very high risk sample size |
| CAPRA ≥4 | 2.81 | 0.305 | 0.39, 20.3 | 0.6100 | 1.12 | 0.913 | 0.15, 8.23 | 0.6599 | 540 (95.4%) |
| CAPRA ≥5 | 3.08 | 0.009 | 1.32, 7.18 | 0.6531 | 1.01 | 0.980 | 0.41, 2.49 | 0.6592 | 405 (71.6%) |
| CAPRA ≥6 | 2.48 | 0.001 | 1.47, 4.20 | 0.6487 | 1.40 | 0.351 | 0.69, 2.82 | 0.6553 | 231 (40.8%) |
| CAPRA ≥7 | 2.27 | 0.003 | 1.33, 3.87 | 0.6538 | 1.77 | 0.135 | 0.84, 3.73 | 0.6676 | 99 (17.5%) |
| CAPRA ≥8 | 1.65 | 0.220 | 0.74, 3.67 | 0.6161 | 2.00 | 0.134 | 0.81, 4.94 | 0.6560 | 34 (6.0%) |
| Primary pattern 5 or ≥5 cores with sum 8–10 or multiple high-risk features | 2.26 | 0.005 | 1.28, 4.00 | 0.6413 | 2.35 | 0.031 | 1.08, 5.12 | 0.6874 | 93 (16.4%) |
Discussion
After systematic evaluation of adverse prognostic variables, we report criteria that dichotomize men with NCCN high-risk PCa groups with distinct clinical outcomes. The very-high-risk (VHR) group is defined by the following criteria at diagnosis: primary Gleason pattern 5, or ≥5 cores with Gleason sum 8–10, or multiple NCCN high-risk features. VHR PCa defined in this way has an estimated prevalence of 15.1% of high-risk PCa. Furthermore, VHR criteria identify a subgroup of men within high-risk disease who have significant oncologic disparities: a 41% worse MFS and a 27% worse CSS at 10 years.
By design, we studied only pre-treatment variables, because pathologic data (as reported in Table 3), are not pertinent to pre-treatment risk stratification or consideration of initial management options. D’Amico et al have previously shown that PSA velocity >2.0ng/ml/yr is an independent predictor of worse CSS after RP. (14) In our cohort higher PSA velocities were not associated with MFS or CSS, which may be related to the relatively small sample size of men with adequate preoperative PSA data to allow velocity calculations (n=234). When ranking hazard ratios, we focused on metastasis and cancer-specific mortality as endpoints most relevant to the biological behavior of PCa. Biochemical recurrence after RP in high-risk men is extremely common (over 60% at 10 years) (6) but does not have a direct impact on symptoms or further therapy. (8) Similarly, all-cause mortality is an endpoint confounded by co-morbidities: competing-risks mortality can exceed 40% at 10 years. (15) Thus we felt the most appropriate study design to define VHR criteria should evaluate the association of commonly available pre-treatment variables with MFS and CSS. (16)
When evaluating MFS and CSS between the VHR cohort and remaining high-risk men, it is important to consider 1) follow-up and 2) additional therapies that were administered after RP. Median follow-up was 5.0 years in both groups, thus strengthening the comparison of incident cancer-related events. As shown in table 3, rates of additional therapies (radiation, androgen deprivation, and/or chemotherapies) after RP (and prior to metastasis) were higher among VHR men (52% versus 35%). The reasons for this difference are unknown as triggers for secondary therapies were not available for analysis. Historically, we have we have favored salvage treatments reserved for evidence of clinical metastasis and/or rapid PSA doubling time. These therapies may have been too delayed to alter VHR patients’ clinical course, which suggests that the higher rate of pre-metastatic secondary treatments in VHR men reflected their more aggressive cancer rather than treating cancer progression, that VHR men may be better candidates therefore for aggressive adjuvant rather than salvage therapies, or that our best available systemic therapies are inadequate to treat VHR PCa, thus providing a greater sense of urgency for clinical trials to investigate neoadjuvant and adjuvant agents that can be truly therapeutic for aggressive PCa.
Other investigators have developed tools to predict MFS and CSS after PCa treatment. Several nomograms rely on post-operative data such as pathologic stage and PSA kinetics to predict metastasis (17–20) or cancer-specific death. (21–24)
Tools to predict MFS and CSS using only pre-treatment variables are less common. For example some investigators have examined prostate cancer-specific mortality after curative local therapy in men stratified by the original D’Amico classification, (25,26) though as previously discussed, the D’Amico and NCCN high-risk strata encompass a broad range of outcomes. One nomogram (Kattan et al) uses pre-treatment PSA, clinical stage, and biopsy Gleason sum to predict the 5-year probability of metastasis after external beam radiotherapy. (27) Nomograms, which are typically complex multivariable tools, provide accurate risk estimates for groups of patients, but they require paper- or software-based calculation, which is potentially cumbersome to apply in the live clinical setting. (16)
A system that improves on the difficulty-of-use of multivariable nomograms is CAPRA, originally developed to predict biochemical recurrence after RP using pre-operative patient and tumor characteristics. (11–13) CAPRA requires minimal calculation, and it correlates with the development of metastasis after RP. (28,29) Interestingly, in our cohort of NCCN high risk men, 59.2% were classified as CAPRA low (1.6) or intermediate (57.6) risk. When the high risk group was stratified by CAPRA, men meeting NCCN high risk criteria that additionally CAPRA 6 had MFS similar to VHR men.
In a study by Cooperberg et al (28) (similar findings were found by Budäus et al (29)), among high-risk men (defined as CAPRA ≥6), the range of the worst and best 10-year MFS was 79.2% to 84.7% (5.5 point difference), compared to 36.9% to 77.9% (41.0 point difference) within high-risk men stratified by VHR criteria. Among the NCCN high-risk cohort in the present study, though CAPRA ≥5 to ≥7 was significantly associated with MFS, no CAPRA score dichotomized the high-risk cohort in a way that was associated with CSS. These findings suggest that VHR criteria may better differentiate high-risk men who perform well compared to the group that suffers with disproportionately worse MFS and CSS. However, nearly one third of high-risk men who developed metastases did not meet either CAPRA ≥6 or VHR, suggesting a need for future classifiers (such as genomic predictors) to achieve superior risk-stratification. (30)
The objective of the VHR classification is to identify those who suffer the worst oncologic outcomes despite curative local treatments, thus informing additional treatment decisions. Among established therapies, VHR men may be suitable candidates for early radiation therapy and/or and androgen deprivation after RP, which is a clinical dilemma that has been raised by other investigators. (31,32) VHR criteria may also aid in patient selection for clinical trials. Selecting men with localized PCa who are most prone to develop subsequent adverse oncologic outcomes may help clinical investigations illustrate which novel therapeutics can meaningfully alter the course of aggressive PCa.
There are several limitations. First, it was retrospective and therefore vulnerable to unrecognized selection bias--there were no explicit criteria to select high-risk men for surgery. Second, this is a single-center study where the cohort is largely referral-based and the treating surgeons are high-volume. The outcomes reported may therefore not reflect community-based or international practice patterns, and external validation of the VHR criteria are required before routine use. Third, although all tissues were analyzed centrally, pathological specimens were not re-reviewed. Thus in order to correct for changes in Gleason grading over time, we performed additional analyses including men diagnosed in the modern grading era, which commenced at our center in 2004. Additionally, all patients in this study underwent RP, and though there has been increasing evidence to support the use of surgery in high-risk men (33,34), any pre-treatment risk stratification, including VHR, is relevant to men considering all management approaches.
Conclusions
Criteria for very-high-risk localized PCa as defined in this population are any primary Gleason pattern 5, or ≥5 cores with Gleason sum 8–10 at biopsy, or multiple NCCN high-risk features present at diagnosis. These criteria identify a group of men within the current NCCN high-risk category who are at greatest risk of developing metastasis or cancer-related death despite conventional treatment. Use of the VHR classification scheme can help improve pre-treatment risk stratification and help to select those patients who have the most to gain from multimodal treatments or novel clinical trial approaches.
Supplementary Material
Distribution of CAPRA scores stratified by very-high-risk criteria, metastasis, and prostate cancer specific mortality
Univariate hazard ratios for metastasis and cancer-death comparing all possible CAPRA cut-points and JHH Very High Risk criteria, among 566 NCCN high-risk men with evaluable CAPRA scores
Independent associations of individual very high risk criteria components in multivariable Cox proportional hazards models
Subgroup analysis of men treated in the modern Gleason grading era (2004=present) and diagnosed with extended sampling (≥10 biopsy cores), n=275: Univariate and multivariable hazard ratios for metastasis, cancer-specific mortality, and all-cause mortality among top-ranking definitions (multivariable adjusted for age, year of surgery, and perineural invasion)
Figure 1.
Kaplan-Meier freedom from biochemical recurrence (BFS), metastasis (MFS), cancer death (CSS), and all-cause mortality (OS) stratified by very-high-risk classification
Acknowledgments
NIH Training Grant T32DK007552 (DS), Howard Hughes Clinician-Scientist Early Careers Award (EMS), AUA/Astellas Rising Star Award (EMS), Johns Hopkins Clinician Scientist Award (AER)
Footnotes
Conflicts of interest
None
References
- 1.D’Amicoa V, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick Ga, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998 Sep 16;280(11):969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 2.NCCN. Clinical practice guidelines in oncology: prostate cancer. National comprehensive cancer network. 2012 doi: 10.6004/jnccn.2010.0012. Version 3. Available from: NCCN.org. [DOI] [PubMed]
- 3.Meng MV, Elkin EP, Latini DM, Duchane J, Carroll PR. Treatment of patients with high risk localized prostate cancer: results from cancer of the prostate strategic urological research endeavor (CaPSURE) The Journal of urology. 2005 May;173(5):1557–61. doi: 10.1097/01.ju.0000154610.81916.81. [DOI] [PubMed] [Google Scholar]
- 4.Loeb S, Schaeffer EM, Trock BJ, Epstein JI, Humphreys EB, Walsh PC. What are the outcomes of radical prostatectomy for high-risk prostate cancer? Urology. Elsevier Inc. 2010 Sep;76(3):710–4. doi: 10.1016/j.urology.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooperberg MR, Cowan J, Broering JM, Carroll PR. High-risk prostate cancer in the United States, 1990–2007. World journal of urology. 2008 Jun;26(3):211–8. doi: 10.1007/s00345-008-0250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierorazio PM, Ross AE, Han M, Epstein JI, Partin AW, Schaeffer EM. Evolution of the clinical presentation of men undergoing radical prostatectomy for high-risk prostate cancer. BJU international. 2012 Apr;109(7):988–93. doi: 10.1111/j.1464-410X.2011.10514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pierorazio PM, Ross AE, Lin BM, Epstein JI, Han M, Walsh PC, et al. Preoperative characteristics of high-Gleason disease predictive of favourable pathological and clinical outcomes at radical prostatectomy. BJU international. 2012 Feb;28:1–7. doi: 10.1111/j.1464-410X.2012.10986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yossepowitch O, Eggener SE, Serio AM, Carver BS, Bianco FJ, Scardino PT, et al. Secondary therapy, metastatic progression, and cancer-specific mortality in men with clinically high-risk prostate cancer treated with radical prostatectomy. European urology. 2008 May;53(5):950–9. doi: 10.1016/j.eururo.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sundi D, Ross A, Humphreys E, Han M, Partin AW, Carter HB, et al. African American men with very low risk prostate cancer exhibit adverse oncologic outcomes after radical prostatectomy. Should active surveillance still be an option for them? Journal of Clinical Oncology. 2013 Jun 17; doi: 10.1200/JCO.2012.47.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein JI, Allsbrook WC, Amin MB, Egevad LL. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. The American journal of surgical pathology. 2005 Oct;29(9):1228–42. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 11.Cooperberg MR, Freedland SJ, Pasta DJ, Elkin EP, Presti JC, Amling CL, et al. Multiinstitutional validation of the UCSF cancer of the prostate risk assessment for prediction of recurrence after radical prostatectomy. Cancer. 2006 Nov 15;107(10):2384–91. doi: 10.1002/cncr.22262. [DOI] [PubMed] [Google Scholar]
- 12.Loeb S, Carvalhal GF, Kan D, Desai A, Catalona WJ. External validation of the cancer of the prostate risk assessment (CAPRA) score in a single-surgeon radical prostatectomy series. Urologic oncology. Elsevier Inc. 2012 Sep;30(5):584–9. doi: 10.1016/j.urolonc.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooperberg MR, Pasta DJ, Elkin EP, Litwin MS, Latini DM, Du Chane J, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. The Journal of urology. 2005 Jun;173(6):1938–42. doi: 10.1097/01.ju.0000158155.33890.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Amico AV, Chen M-H, Roehl KA, Catalona WJ. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. The New England journal of medicine. 2004 Mar;351(2):125–35A. doi: 10.1056/NEJMoa032975. [DOI] [PubMed] [Google Scholar]
- 15.Kutikov A, Cooperberg MR, Paciorek AT, Uzzo RG, Carroll PR, Boorjian S. Prostate cancer and prostatic diseases. February. Nature Publishing Group; 2012. Jun 19, Evaluating prostate cancer mortality and competing risks of death in patients with localized prostate cancer using a comprehensive nomogram; pp. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephenson AJ. Re: Zhao et al. External Validation of University of California, San Francisco, Cancer of the Prostate Risk Assessment Score (Urology 2008; 72: 396–400) Urology. 2008;72(3):719–20. doi: 10.1016/j.urology.2007.11.165. [DOI] [PubMed] [Google Scholar]
- 17.Dotan Z, Bianco FJ, Rabbani F, Eastham J, Fearn P, Scher HI, et al. Pattern of prostate-specific antigen (PSA) failure dictates the probability of a positive bone scan in patients with an increasing PSA after radical prostatectomy. Journal of clinical oncology. 2005 Mar 20;23(9):1962–8. doi: 10.1200/JCO.2005.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slovin SF, Wilton AS, Heller G, Scher HI. Time to detectable metastatic disease in patients with rising prostate-specific antigen values following surgery or radiation therapy. Clinical cancer research. 2005 Dec 15;11(24 Pt 1):8669–73. doi: 10.1158/1078-0432.CCR-05-1668. [DOI] [PubMed] [Google Scholar]
- 19.Porter CR, Suardi N, Kodama K, Capitanio U, Gibbons RP, Correa R, et al. A nomogram predicting metastatic progression after radical prostatectomy. International journal of urology: official journal of the Japanese Urological Association. 2008 Oct;15(10):889–94. doi: 10.1111/j.1442-2042.2008.02105.x. [DOI] [PubMed] [Google Scholar]
- 20.Inman B, Frank I, Boorjian S, Akornor JW, Karnes RJ, Leibovich BC, et al. Dynamic prediction of metastases after radical prostatectomy for prostate cancer. BJU international. 2011 Dec;108(11):1762–8. doi: 10.1111/j.1464-410X.2011.10208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2013;294(4):433–9. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 22.Eggener SE, Scardino PT, Walsh PC, Han M, Partin AW, Trock BJ, et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. The Journal of urology. 2011 Mar;185(3):869–75. doi: 10.1016/j.juro.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Svatek R, Karakiewicz PI, Shulman M, Karam J, Perrotte P, Benaim E. Pre-treatment nomogram for disease-specific survival of patients with chemotherapy-naive androgen independent prostate cancer. European urology. 2006 Apr;49(4):666–74. doi: 10.1016/j.eururo.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 24.Porter CR, Gallina A, Kodama K, Gibbons RP, Correa R, Perrotte P, et al. Prostate cancer-specific survival in men treated with hormonal therapy after failure of radical prostatectomy. European urology. 2007 Aug;52(2):446–52. doi: 10.1016/j.eururo.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 25.D’Amico AV, Moul J, Carroll PR, Sun L, Lubeck D, Chen M-H. Cancer-specific mortality after surgery or radiation for patients with clinically localized prostate cancer managed during the prostate-specific antigen era. Journal of clinical oncology. 2003 Jun 1;21(11):2163–72. doi: 10.1200/JCO.2003.01.075. [DOI] [PubMed] [Google Scholar]
- 26.D’Amico AV, Kote K, Loffredo M, Renshaw AA, Schultz D. Determinants of Prostate Cancer-Specific Survival After Radiation Therapy for Patients With Clinically Localized Prostate Cancer. Journal of Clinical Oncology. 2002 Dec 1;20(23):4567–73. doi: 10.1200/JCO.2002.03.061. [DOI] [PubMed] [Google Scholar]
- 27.Kattan MW, Zelefsky MJ, Kupelian P, Cho D, Scardino PT, Fuks Z, et al. Pretreatment nomogram that predicts 5-year probability of metastasis following three-dimensional conformal radiation therapy for localized prostate cancer. Journal of clinical oncology. 2003 Dec 15;21(24):4568–71. doi: 10.1200/JCO.2003.05.046. [DOI] [PubMed] [Google Scholar]
- 28.Cooperberg MR, Broering JM, Carroll PR. Risk assessment for prostate cancer metastasis and mortality at the time of diagnosis. Journal of the National Cancer Institute. 2009 Jun 16;101(12):878–87. doi: 10.1093/jnci/djp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Budaus L, Isbarn H, Tennstedt P, Salomon G, Schlomm T, Steuber T, et al. Risk assessment of metastatic recurrence in Cancer of the Prostate Risk Assessment score: results from 2937 European patients. BJU international. 2012;110:1714–20. doi: 10.1111/j.1464-410X.2012.11147.x. [DOI] [PubMed] [Google Scholar]
- 30.Cuzick J, Swanson GP, Fisher G, Brothman AR, Berney DM, Reid JE, et al. The lancet oncology. 3. Vol. 12. Elsevier Ltd; 2011. Mar, Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: a retrospective study; pp. 245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moul JW, Wu H, Sun L, McLeod DG, Amling C, Donahue T, et al. Early versus delayed hormonal therapy for prostate specific antigen only recurrence of prostate cancer after radical prostatectomy. The Journal of urology. 2008;171(March 2004):1141–7. doi: 10.1097/01.ju.0000113794.34810.d0. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen ME, Trock BJ, Walsh PC. Salvage or adjuvant radiation therapy: counseling patients on the benefits. Journal of the National Comprehensive Cancer Network. 2010 Feb;8(2):228–37. doi: 10.6004/jnccn.2010.0015. [DOI] [PubMed] [Google Scholar]
- 33.Zelefsky MJ, Eastham JA, Cronin AM, Fuks Z, Zhang Z, Yamada Y, et al. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: a comparison of clinical cohorts adjusted for case mix. Journal of clinical oncology. 2010 Mar 20;28(9):1508–13. doi: 10.1200/JCO.2009.22.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffman RM, Koyama T, Fan K-H, Albertsen PC, Barry MJ, Goodman M, et al. Mortality after radical prostatectomy or external beam radiotherapy for localized prostate cancer. Journal of the National Cancer Institute. 2013 May 15;105(10):711–8. doi: 10.1093/jnci/djt059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of CAPRA scores stratified by very-high-risk criteria, metastasis, and prostate cancer specific mortality
Univariate hazard ratios for metastasis and cancer-death comparing all possible CAPRA cut-points and JHH Very High Risk criteria, among 566 NCCN high-risk men with evaluable CAPRA scores
Independent associations of individual very high risk criteria components in multivariable Cox proportional hazards models
Subgroup analysis of men treated in the modern Gleason grading era (2004=present) and diagnosed with extended sampling (≥10 biopsy cores), n=275: Univariate and multivariable hazard ratios for metastasis, cancer-specific mortality, and all-cause mortality among top-ranking definitions (multivariable adjusted for age, year of surgery, and perineural invasion)