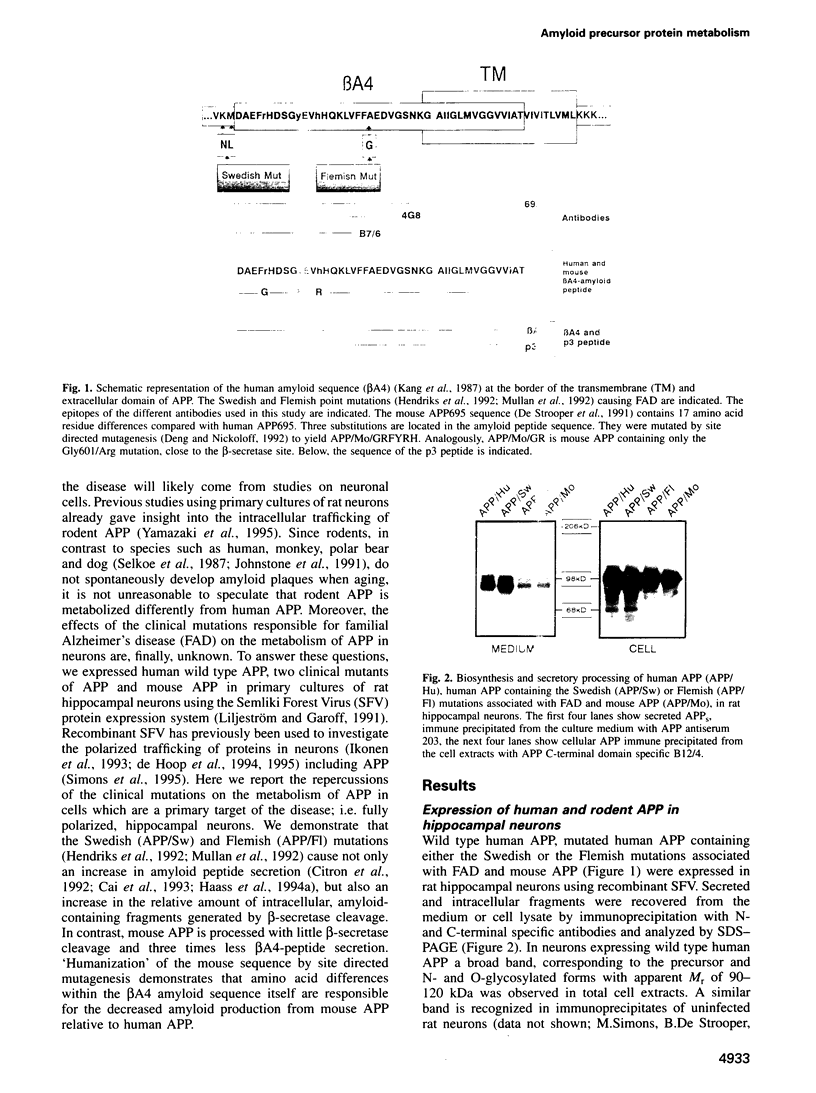
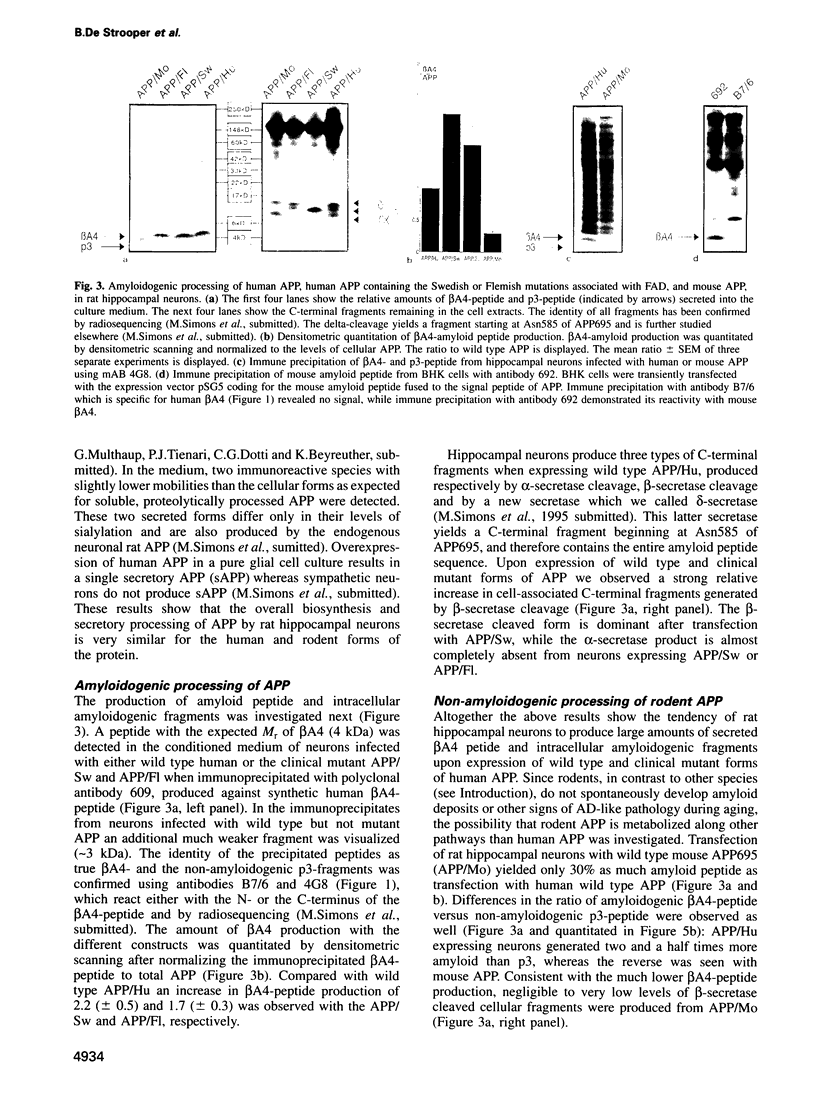
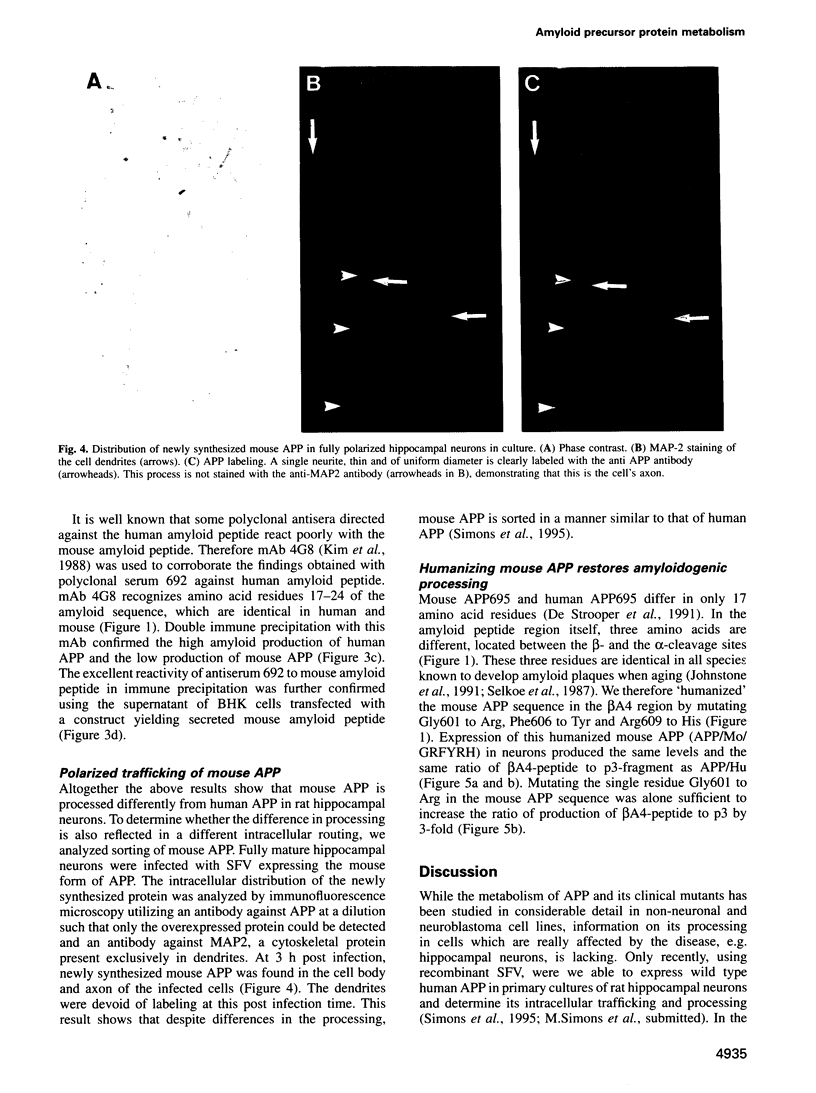
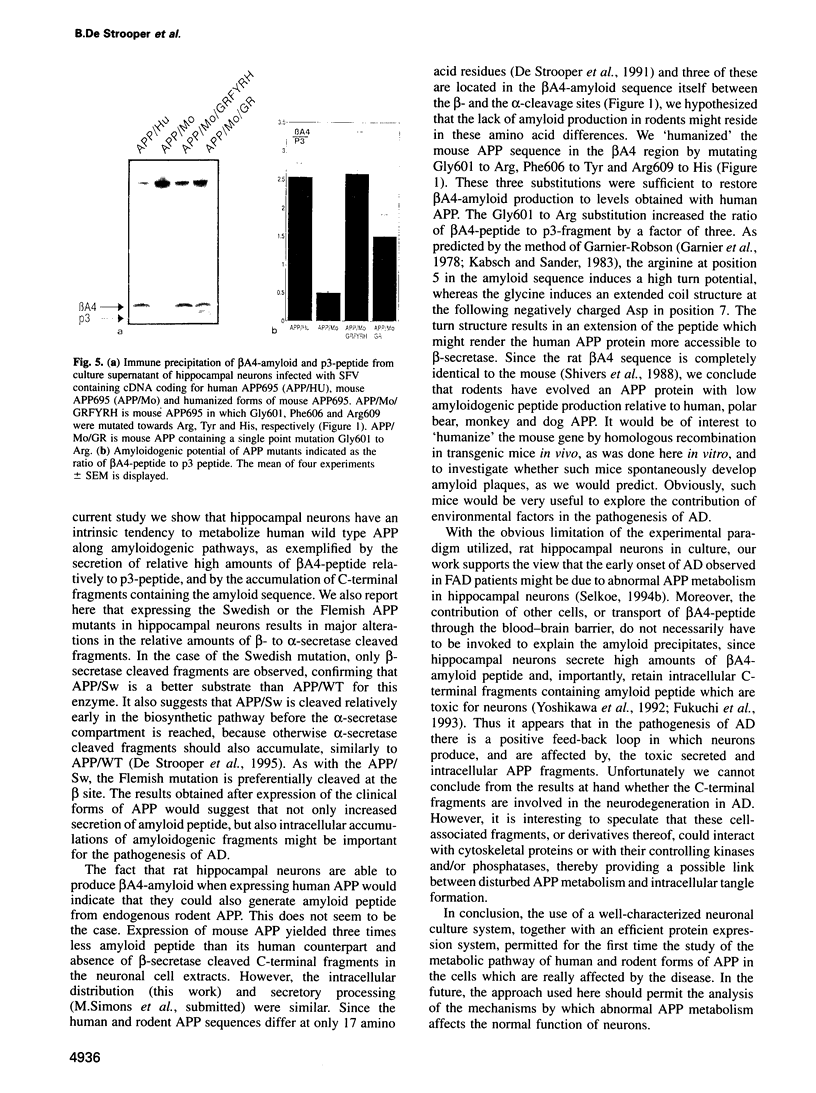
Abstract
A distinguishing feature of Alzheimer's disease (AD) is the deposition of amyloid plaques in brain parenchyma. These plaques arise by the abnormal accumulation of beta A4, a proteolytic fragment of amyloid precursor protein (APP). Despite the fact that neurons are dramatically affected in the course of the disease, little is known about the neuronal processing of APP. To address this question we have expressed in fully mature, synaptically active rat hippocampal neurons, the neuronal form of human APP (APP695), two mutant forms of human APP associated with AD, and the mouse form of APP (a species known not to develop amyloid plaques). Protein expression was achieved via the Semliki Forest Virus system. Expression of wild type human APP695 resulted in the secretion of beta A4-amyloid peptide and the intracellular accumulation of potential amyloidogenic and non-amyloidogenic fragments. The relative amount of amyloid-containing fragments increased dramatically during expression of the clinical mutants, while it decreased strongly when the mouse form of APP was expressed. 'Humanizing' the rodent APP sequence by introducing three mutations in the beta A4-region also led to increased production of amyloid peptide to levels similar to those obtained with human APP. The single Gly601 to Arg substitution alone was sufficient to triple the ratio of beta A4-peptide to non-amyloidogenic p3-peptide. Due to the capacity of these cells to secrete and accumulate intracellular amyloid fragments, we hypothesize that in the pathogenesis of AD there is a positive feed-back loop where neurons are both producers and victims of amyloid, leading to neuronal degeneration and dementia.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buxbaum J. D., Koo E. H., Greengard P. Protein phosphorylation inhibits production of Alzheimer amyloid beta/A4 peptide. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9195–9198. doi: 10.1073/pnas.90.19.9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X. D., Golde T. E., Younkin S. G. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science. 1993 Jan 22;259(5094):514–516. doi: 10.1126/science.8424174. [DOI] [PubMed] [Google Scholar]
- Citron M., Oltersdorf T., Haass C., McConlogue L., Hung A. Y., Seubert P., Vigo-Pelfrey C., Lieberburg I., Selkoe D. J. Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature. 1992 Dec 17;360(6405):672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- Citron M., Teplow D. B., Selkoe D. J. Generation of amyloid beta protein from its precursor is sequence specific. Neuron. 1995 Mar;14(3):661–670. doi: 10.1016/0896-6273(95)90323-2. [DOI] [PubMed] [Google Scholar]
- De Strooper B., Craessaerts K., Dewachter I., Moechars D., Greenberg B., Van Leuven F., Van den Berghe H. Basolateral secretion of amyloid precursor protein in Madin-Darby canine kidney cells is disturbed by alterations of intracellular pH and by introducing a mutation associated with familial Alzheimer's disease. J Biol Chem. 1995 Feb 24;270(8):4058–4065. doi: 10.1074/jbc.270.8.4058. [DOI] [PubMed] [Google Scholar]
- De Strooper B., Umans L., Van Leuven F., Van Den Berghe H. Study of the synthesis and secretion of normal and artificial mutants of murine amyloid precursor protein (APP): cleavage of APP occurs in a late compartment of the default secretion pathway. J Cell Biol. 1993 Apr;121(2):295–304. doi: 10.1083/jcb.121.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B., Van Leuven F., Van den Berghe H. The amyloid beta protein precursor or proteinase nexin II from mouse is closer related to its human homolog than previously reported. Biochim Biophys Acta. 1991 Dec 2;1129(1):141–143. doi: 10.1016/0167-4781(91)90231-a. [DOI] [PubMed] [Google Scholar]
- Deng W. P., Nickoloff J. A. Site-directed mutagenesis of virtually any plasmid by eliminating a unique site. Anal Biochem. 1992 Jan;200(1):81–88. doi: 10.1016/0003-2697(92)90280-k. [DOI] [PubMed] [Google Scholar]
- Esch F. S., Keim P. S., Beattie E. C., Blacher R. W., Culwell A. R., Oltersdorf T., McClure D., Ward P. J. Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science. 1990 Jun 1;248(4959):1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- Fukuchi K., Sopher B., Furlong C. E., Smith A. C., Dang N., Martin G. M. Selective neurotoxicity of COOH-terminal fragments of the beta-amyloid precursor protein. Neurosci Lett. 1993 May 14;154(1-2):145–148. doi: 10.1016/0304-3940(93)90192-n. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Golde T. E., Estus S., Younkin L. H., Selkoe D. J., Younkin S. G. Processing of the amyloid protein precursor to potentially amyloidogenic derivatives. Science. 1992 Feb 7;255(5045):728–730. doi: 10.1126/science.1738847. [DOI] [PubMed] [Google Scholar]
- Haass C., Hung A. Y., Selkoe D. J., Teplow D. B. Mutations associated with a locus for familial Alzheimer's disease result in alternative processing of amyloid beta-protein precursor. J Biol Chem. 1994 Jul 1;269(26):17741–17748. [PubMed] [Google Scholar]
- Haass C., Koo E. H., Capell A., Teplow D. B., Selkoe D. J. Polarized sorting of beta-amyloid precursor protein and its proteolytic products in MDCK cells is regulated by two independent signals. J Cell Biol. 1995 Feb;128(4):537–547. doi: 10.1083/jcb.128.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C., Koo E. H., Teplow D. B., Selkoe D. J. Polarized secretion of beta-amyloid precursor protein and amyloid beta-peptide in MDCK cells. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1564–1568. doi: 10.1073/pnas.91.4.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., Lieberburg I., Koo E. H., Schenk D., Teplow D. B. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992 Sep 24;359(6393):322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- Haass C., Selkoe D. J. Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell. 1993 Dec 17;75(6):1039–1042. doi: 10.1016/0092-8674(93)90312-e. [DOI] [PubMed] [Google Scholar]
- Hendriks L., van Duijn C. M., Cras P., Cruts M., Van Hul W., van Harskamp F., Warren A., McInnis M. G., Antonarakis S. E., Martin J. J. Presenile dementia and cerebral haemorrhage linked to a mutation at codon 692 of the beta-amyloid precursor protein gene. Nat Genet. 1992 Jun;1(3):218–221. doi: 10.1038/ng0692-218. [DOI] [PubMed] [Google Scholar]
- Hung A. Y., Haass C., Nitsch R. M., Qiu W. Q., Citron M., Wurtman R. J., Growdon J. H., Selkoe D. J. Activation of protein kinase C inhibits cellular production of the amyloid beta-protein. J Biol Chem. 1993 Nov 5;268(31):22959–22962. [PubMed] [Google Scholar]
- Ikonen E., Parton R. G., Hunziker W., Simons K., Dotti C. G. Transcytosis of the polymeric immunoglobulin receptor in cultured hippocampal neurons. Curr Biol. 1993 Oct 1;3(10):635–644. doi: 10.1016/0960-9822(93)90061-r. [DOI] [PubMed] [Google Scholar]
- Johnstone E. M., Chaney M. O., Norris F. H., Pascual R., Little S. P. Conservation of the sequence of the Alzheimer's disease amyloid peptide in dog, polar bear and five other mammals by cross-species polymerase chain reaction analysis. Brain Res Mol Brain Res. 1991 Jul;10(4):299–305. doi: 10.1016/0169-328x(91)90088-f. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Sander C. How good are predictions of protein secondary structure? FEBS Lett. 1983 May 8;155(2):179–182. doi: 10.1016/0014-5793(82)80597-8. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Koo E. H., Squazzo S. L. Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J Biol Chem. 1994 Jul 1;269(26):17386–17389. [PubMed] [Google Scholar]
- Kuentzel S. L., Ali S. M., Altman R. A., Greenberg B. D., Raub T. J. The Alzheimer beta-amyloid protein precursor/protease nexin-II is cleaved by secretase in a trans-Golgi secretory compartment in human neuroglioma cells. Biochem J. 1993 Oct 15;295(Pt 2):367–378. doi: 10.1042/bj2950367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljeström P., Garoff H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology (N Y) 1991 Dec;9(12):1356–1361. doi: 10.1038/nbt1291-1356. [DOI] [PubMed] [Google Scholar]
- Lo A. C., Haass C., Wagner S. L., Teplow D. B., Sisodia S. S. Metabolism of the "Swedish" amyloid precursor protein variant in Madin-Darby canine kidney cells. J Biol Chem. 1994 Dec 9;269(49):30966–30973. [PubMed] [Google Scholar]
- Lowery D. E., Pasternack J. M., Gonzalez-DeWhitt P. A., Zürcher-Neely H., Tomich C. C., Altman R. A., Fairbanks M. B., Heinrikson R. L., Younkin S. G., Greenberg B. D. Alzheimer's amyloid precursor protein produced by recombinant baculovirus expression. Proteolytic processing and protease inhibitory properties. J Biol Chem. 1991 Oct 15;266(29):19842–19850. [PubMed] [Google Scholar]
- Mullan M., Crawford F., Axelman K., Houlden H., Lilius L., Winblad B., Lannfelt L. A pathogenic mutation for probable Alzheimer's disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet. 1992 Aug;1(5):345–347. doi: 10.1038/ng0892-345. [DOI] [PubMed] [Google Scholar]
- Näslund J., Jensen M., Tjernberg L. O., Thyberg J., Terenius L., Nordstedt C. The metabolic pathway generating p3, an A beta-peptide fragment, is probably non-amyloidogenic. Biochem Biophys Res Commun. 1994 Oct 28;204(2):780–787. doi: 10.1006/bbrc.1994.2527. [DOI] [PubMed] [Google Scholar]
- Roses A. D. Apolipoprotein E affects the rate of Alzheimer disease expression: beta-amyloid burden is a secondary consequence dependent on APOE genotype and duration of disease. J Neuropathol Exp Neurol. 1994 Sep;53(5):429–437. doi: 10.1097/00005072-199409000-00002. [DOI] [PubMed] [Google Scholar]
- Sambamurti K., Shioi J., Anderson J. P., Pappolla M. A., Robakis N. K. Evidence for intracellular cleavage of the Alzheimer's amyloid precursor in PC12 cells. J Neurosci Res. 1992 Oct;33(2):319–329. doi: 10.1002/jnr.490330216. [DOI] [PubMed] [Google Scholar]
- Sandbrink R., Masters C. L., Beyreuther K. Beta A4-amyloid protein precursor mRNA isoforms without exon 15 are ubiquitously expressed in rat tissues including brain, but not in neurons. J Biol Chem. 1994 Jan 14;269(2):1510–1517. [PubMed] [Google Scholar]
- Selkoe D. J. Alzheimer's disease: a central role for amyloid. J Neuropathol Exp Neurol. 1994 Sep;53(5):438–447. doi: 10.1097/00005072-199409000-00003. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Bell D. S., Podlisny M. B., Price D. L., Cork L. C. Conservation of brain amyloid proteins in aged mammals and humans with Alzheimer's disease. Science. 1987 Feb 20;235(4791):873–877. doi: 10.1126/science.3544219. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Cell biology of the amyloid beta-protein precursor and the mechanism of Alzheimer's disease. Annu Rev Cell Biol. 1994;10:373–403. doi: 10.1146/annurev.cb.10.110194.002105. [DOI] [PubMed] [Google Scholar]
- Shivers B. D., Hilbich C., Multhaup G., Salbaum M., Beyreuther K., Seeburg P. H. Alzheimer's disease amyloidogenic glycoprotein: expression pattern in rat brain suggests a role in cell contact. EMBO J. 1988 May;7(5):1365–1370. doi: 10.1002/j.1460-2075.1988.tb02952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji M., Golde T. E., Ghiso J., Cheung T. T., Estus S., Shaffer L. M., Cai X. D., McKay D. M., Tintner R., Frangione B. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992 Oct 2;258(5079):126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- Simons M., Ikonen E., Tienari P. J., Cid-Arregui A., Mönning U., Beyreuther K., Dotti C. G. Intracellular routing of human amyloid protein precursor: axonal delivery followed by transport to the dendrites. J Neurosci Res. 1995 May 1;41(1):121–128. doi: 10.1002/jnr.490410114. [DOI] [PubMed] [Google Scholar]
- Sisodia S. S. Beta-amyloid precursor protein cleavage by a membrane-bound protease. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6075–6079. doi: 10.1073/pnas.89.13.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N., Cheung T. T., Cai X. D., Odaka A., Otvos L., Jr, Eckman C., Golde T. E., Younkin S. G. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994 May 27;264(5163):1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- Weidemann A., König G., Bunke D., Fischer P., Salbaum J. M., Masters C. L., Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell. 1989 Apr 7;57(1):115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Yamazaki T., Selkoe D. J., Koo E. H. Trafficking of cell surface beta-amyloid precursor protein: retrograde and transcytotic transport in cultured neurons. J Cell Biol. 1995 Apr;129(2):431–442. doi: 10.1083/jcb.129.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa K., Aizawa T., Hayashi Y. Degeneration in vitro of post-mitotic neurons overexpressing the Alzheimer amyloid protein precursor. Nature. 1992 Sep 3;359(6390):64–67. doi: 10.1038/359064a0. [DOI] [PubMed] [Google Scholar]
- de Hoop M. J., Huber L. A., Stenmark H., Williamson E., Zerial M., Parton R. G., Dotti C. G. The involvement of the small GTP-binding protein Rab5a in neuronal endocytosis. Neuron. 1994 Jul;13(1):11–22. doi: 10.1016/0896-6273(94)90456-1. [DOI] [PubMed] [Google Scholar]
- de Hoop M., von Poser C., Lange C., Ikonen E., Hunziker W., Dotti C. G. Intracellular routing of wild-type and mutated polymeric immunoglobulin receptor in hippocampal neurons in culture. J Cell Biol. 1995 Sep;130(6):1447–1459. doi: 10.1083/jcb.130.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]