Abstract
Objective
To describe speech, neurological and imaging characteristics of a series of patients presenting with progressive spastic dysarthria (PSD) as the first and predominant sign of a presumed neurodegenerative disease.
Methods
Participants were 25 patients with spastic dysarthria as the only or predominant speech disorder. Clinical features, pattern of MRI volume loss on voxel-based morphometry, and pattern of hypometabolism with F18-Fluorodeoxyglucose (FDG-PET) scan are described.
Results
All patients demonstrated speech characteristics consistent with spastic dysarthria, including strained voice quality, slow speaking rate, monopitch and monoloudness, and slow and regular speech alternating motion rates. Eight patients did not have additional neurological findings on examination. Pseudobulbar affect, upper motor neuron pattern limb weakness, spasticity, Hoffman sign and positive Babinski reflexes were noted in some of the remaining patients. Twenty-three patients had electromyographic assessment and none had diffuse motor neuron disease or met El Escorial criteria for ALS. Voxel-based morphometry revealed striking bilateral white matter volume loss, , affecting the motor cortex (BA 4), including the frontoparietal operculum (BA 43) with extension into the middle cerebral peduncle. FDG-PET showed subtle hypometabolism affecting the premotor and motor cortices in some patients, particularly in those who had a disease duration longer than two years.
Conclusions
We have characterized a neurodegenerative disorder that begins focally with spastic dysarthria due to involvement of the motor and premotor cortex and descending corticospinal and corticobulbar pathways. We propose the descriptive label “progressive spastic dysarthria” to best capture the dominant presenting feature of the syndrome.
Keywords: dysarthria, neuromuscular disease, MRI, PET
Introduction
Progressive speech impairment (dysarthria, apraxia of speech) is not uncommon as a presenting complaint heralding neurodegenerative disease(1). Unique patterns of speech impairment are associated with specific sites of lesion and may inform the neurologic differential diagnosis(1, 2). For example, ataxic dysarthria is typically associated with lesions in the cerebellum(3) or its efferent or afferent pathways hypokinetic dysarthria with basal ganglia control circuit dysfunction, and spastic dysarthria with bilateral upper motor neuron lesions (see Kent(4) for review).
Some patterns of progressive dysarthria strongly suggest particular underlying pathology. A prime example is mixed spastic-flaccid dysarthria commonly associated with amyotrophic lateral sclerosis (ALS)(2, 5); about 20% of ALS cases present with bulbar signs, among which dysarthria is usually the most prominent(6, 7). In some patients, speech symptoms at initial evaluation reflect an isolated spastic or flaccid dysarthria that typically evolves to include both upper and lower motor neuron (LMN) features(8) (i.e., mixed spastic-flaccid dysarthria). Other patients eventually exhibit speech and/or nonspeech signs that establish a diagnosis other than ALS (e.g., corticobasal syndrome, progressive supranuclear palsy syndrome). However, an apparently small number of patients presenting with isolated progressive spastic dysarthria (PSD) do not develop additional symptoms to warrant these diagnoses. In such cases, the spastic dysarthria may represent primary lateral sclerosis (PLS) or a neurodegenerative variant of a rare motor syndrome such as anterior opercular syndrome, also known as Foix-Chavany-Marie syndrome(9–11). One barrier to a fuller understanding of PSD and its possible explanation is the sparse description of the specific speech attributes that characterize it, its other clinical neurologic features, and its neuroimaging correlates. Thus, the purpose of the current study was to describe the specific speech features, non-speech neurologic findings, and imaging findings for a relatively large series of patients presenting with PSD as the first and predominant sign of a presumed neurodegenerative disease.
Methods
This retrospective study was conducted with the approval of the Mayo Clinic Institutional Review Board.
Participants
Participants were identified through a database search of patients seen at Mayo Clinic Rochester, in the Division of Speech Pathology, between 2006 and 2012, with a communication disorder diagnosis of spastic dysarthria as the only or the predominant speech disorder and a null history of acute medical diagnoses to explain the dysarthria (e.g., stroke, traumatic brain injury). Inclusion criteria were: (1) Speech difficulties as the presenting or primary neurologic complaint; (2) Duration of symptoms at final follow-up of at least 6 months; (3) Speech diagnosis of spastic dysarthria (defined under Results) as the primary communication deficit; and (4) No evidence of speech or bulbar symptoms indicative of LMN involvement (i.e., flaccid dysarthria, atrophy, fasciculations), or neurological findings indicative of lower motor neuron involvement elsewhere.
A total of 25 patients (18 females) met inclusion and exclusion criteria. The mean age of onset of spastic dysarthria was 63.1 years (range 46 to 87 years); mean age of presentation 66.1 years (range 47 to 89 years); and mean duration of symptoms at final evaluation 30 months (range 6 – 84 months). All patients reported normal speech prior to onset of the presenting dysarthria.
MRI processing
All PSD patients completed head imaging with an MRI scan. Seven had a volumetric MRI performed with a standardized protocol(12) suitable for volumetric analysis. The mean age at scan of these patients was 65.4 years (range 50-74 years) and 57% were female. These patients were matched 1:2 by age and gender to a group of 14 healthy controls (mean age 65.4 years, range 50-74, 43% male).
All images underwent pre-processing correction for gradient non linearity(13) and intensity non-uniformity(14). Voxel-based morphometry (VBM)(15) (http://www.fil.ion.ucl.ac.uk/spm) in SPM5 characterized patterns of grey and white matter atrophy in PSD patients compared to controls. Briefly, all images were normalized to a customized template and segmented into grey matter, white matter and CSF using customized tissue probability maps and the unified segmentation routine(16) followed by the HMRF cleanup step. Grey and white matter images were modulated and smoothed at 8 mm full width at half maximum. A two-sample t-test was used to assess grey and white matter loss in PSD subjects compared to controls. Results were assessed for multiple comparisons using the false discovery rate (FDR)(17) at a statistical threshold of p<0.05 and uncorrected at p<0.001.
FDG-PET processing
Nine PSD patients, seven of whom also had MRI imaging, had 18F Flurodeoxyglucose positron emission tomography (FDG-PET) performed according to a standardized protocol(18). Fully automated three-dimensional stereotactic surface projections (SSP) was used for the interpretation of the FDG-PET. Scans were realigned and spatially normalized and underwent nonlinear warping. The scans were sampled at 16,000 predefined cortical locations and projected on a 3-dimensional image. The activity was normalized to the pons and compared with an age-segmented normative database, yielding a 3-dimensional SSP z score image. The image produced by this analysis produces a metabolic map using the z scores as calculated for each surface pixel(19). The software package used to perform this analysis was CortexID (GE Healthcare, Waukesha, Wisconsin).
Results
Speech
All patients demonstrated speech characteristics consistent with spastic dysarthria(1, 2) (Table 1). Although many abnormal speech characteristics may be present, the key distinguishing and defining features of spastic dysarthria typically include strained voice quality, slow speaking rate, monopitch and monoloudness, and slow and regular speech alternating motion rates (AMRs)(1). Strained voice quality and slow, regular speech AMRs were present in all patients. All but two patients additionally exhibited slow rate, monopitch and monoloudness, and imprecise articulation. More than half exhibited hypernasality, and approximately one-third exhibited short phrases. All of these features are compatible with spastic dysarthria.
Table 1.
Demographic features, non-speech bulbar and non-bulbar neurologic findings
| Patient | Sex | Age at onset |
Duration of symptoms at final follow-up (years) |
Non-speech bulbar neurological findings |
Non-speech non- bulbar neurological findings |
EMG findings |
|---|---|---|---|---|---|---|
| 1 | F | 87 | 2 | lip and tongue weakness, pseudobulbar affect, mild periorbital weakness, occasional dysphagia | normal | |
| 2 | M | 69 | 5 | reduced range, strength, and speed of lip and tongue movements, mild right facial droop, minor dysphagia | right UMN pattern weakness, Hoffman sign on right | normal |
| 3 | F | 62 | 4 | mild tongue weakness and slowness, subtle nonverbal oral apraxia, pseudobulbar affect | bilateral (right > left) upper extremity spasticity with slow AMRs | normal |
| 4 | M | 57 | 1 | pseudobulbar affect, drooling, dysphagia | normal | |
| 5 | F | 79 | 2 | right lower face and tongue weakness, dysphagia | UMN limb weakness | normal |
| 6 | F | 46 | 3.5 | tongue weakness, brisk jaw jerk | normal | |
| 7 | F | 79 | 3 | drooling, dysphagia, mild lip weakness | mild left upper extremity spasticity with slow AMRs | normal |
| 8 | F | 63 | 1 | mild lower facial weakness | normal | |
| 9 | F | 74 | 3 | dysphagia, drooling, facial and tongue weakness, neck flexor weakness | spasticity left>right leg | normal |
| 10 | M | 58 | 7 | equivocal nonverbal oral apraxia | spastic paraparesis and left upper extremity spasticity, left Babinski; mild postural/kinetic tremor | evidence for longstanding motor neuronopathy or neuropathy without fasciculation or fibrillation potential |
| 11 | F | 60 | 3 | dysphagia, pseudobulbar affect, nonverbal oral apraxia | mildly brisk, symmetric reflexes and decreased limb AMRs | normal |
| 12 | M | 46 | 1 | dysphagia, tongue weakness and slowness, bifacial weakness | left side UMN type weakness, left Babinski | normal |
| 13 | F | 72 | 1 | dysphagia, pseudobulbar affect, mild tongue and lower facial weakness | brisk (non-pathologic) reflexes, mild slow limb AMRs | normal |
| 14 | F | 66 | 5 | dysphagia, drooling, minimal jaw opening, mild left facial weakness | left upper extremity spasticity with slow AMRs; 2–3 beat left ankle clonus; | normal |
| 15 | M | 63 | 1 | dysphagia, pseudobulbar affect, tongue and facial weakness | mild left upper extremity UMN weakness | normal |
| 16 | M | 48 | 2 | minor dysphagia, drooling, brisk jaw, jerk, jaw clonus, pseudobulbar affect, tongue weakness | normal | |
| 17 | F | 56 | 1 | minor dysphagia, tongue and lip weakness, positive suck and snout reflexes | normal | |
| 18 | M | 71 | 2 | pseudobulbar affect, nonverbal oral apraxia | normal | |
| 19 | F | 50 | 2 | dysphagia, pseudobulbar affect, tongue weakness, brisk jaw jerk reflex | mild hyperreflexia in upper and lower extremities (not pathologic) | normal |
| 20 | F | 63 | 2 | dysphagia, pseudobulbar affect, jaw clonus, reduced tongue strength and speed | left upper and lower extremity spasticity with slow AMRs; spastic/apraxic gait | normal |
| 21 | F | 66 | 1 | dysphagia, subtle jaw clonus, mild tongue weakness, brisk jaw jerk reflex | mild hyperreflexia in upper and lower extremities (not pathologic) | normal |
| 22 | M | 56 | 6 | drooling, minor dysphagia, pseudobulbar affect, jaw clonus | left UMN pattern weakness, bilateral limb spasticity (left > right), spastic gait circumducting left leg; subtle left Babinski | n/a |
| 23 | M | 62 | 1.5 | dysphagia, pseudobulbar affect, facial, tongue, and velar weakness | frontal release signs, hyper-reflexia, reduced right arm swing, unsustained clonus at both ankles | n/a |
| 24 | F | 55 | 1 | minor dysphagia, jaw and tongue weakness | normal | |
| 25 | F | 70 | 1 | dysphagia, pseudobulbar affect, tongue and palatal weakness | normal |
n/a: not administered
Four patients (16%) also had concomitant motor speech disorders. One patient had ataxic dysarthria characterized by irregular articulatory breakdowns. Three had apraxia of speech (AOS), defined as a speech planning/programming disorder characterized by distorted substitutions, vowel distortions, pronounced difficulty with multisyllabic words (e.g., increased errors with increasing length), segmentation of syllables within or between words, and/or intrusive neutral vowel in consonant clusters (e.g."buhlend”/blend). In all cases, spastic dysarthria was the predominant speech disorder.
Nonspeech Neurologic findings
Nonspeech neurologic signs considered compatible with spastic dysarthria were observed during speech examination (Table 2). These included orofacial weakness, slow oral movements, reduced velar movement during vowel prolongation, reduced sharpness of cough and/or glottal coup, and pathologic oral reflexes (i.e., suck, snout, palmomental, jaw jerk). Jaw clonus was occasionally evident. Drooling and/or dysphagia were common complaints. Approximately 40% of the patients had pseudobulbar affect.
Table 2.
Motor speech characteristics
| Patient | Intelligibility Impairment |
Strained Voice Quality |
Slow rate | Monopitch- Monoloudness |
Hypernasality | Articulatory Imprecision |
Slow Regular AMRS |
Other |
|---|---|---|---|---|---|---|---|---|
| 1 | moderate | marked | marked | marked-severe | marked | marked | short phrases | |
| 2 | marked | marked | marked | mild | mild | moderate | mild | reduced loudness |
| 3 | mild | moderate-marked | marked | marked | mild-moderate | marked | excess equal stress, reduced loudness | |
| 4 | normal | mild | mild | mild-moderate | minimal | minimal | moderate | |
| 5 | moderate | marked | marked | marked-severe | mild-moderate | marked-severe | moderate-marked | short phrases |
| 6 | mild | mild-moderate | moderate | mild | mild-moderate | mild | ||
| 7 | normal | moderate | mild-moderate | moderate | moderate | mild | reduced loudness | |
| 8 | mild | moderate | mild-moderate | mild-moderate | mild | mild | irregular articulatory breakdown | |
| 9 | mild-moderate | moderate-marked | moderate | moderate | mild | mild-moderate | moderate | apraxia of speech |
| 10 | normal | minimal | moderate | moderate | mild-moderate | mild | moderate | excess, equal stress, apraxia of speech |
| 11 | marked | marked | marked-severe | marked | marked | marked | apraxia of speech | |
| 12 | normal | mild | mild | mild | mild | mild-moderate | ||
| 13 | mild | marked-severe | marked | marked | minimal | marked | ||
| 14 | severe | marked-severe | severe | severe | severe | mild-moderate | ||
| 15 | mild | moderate | mild | moderate | moderate | mild-moderate | moderate | |
| 16 | normal | minimal | mild-moderate | moderate | mild-moderate | moderate | excess, equal stress | |
| 17 | moderate | marked | marked | marked | marked | marked | ||
| 18 | severe | marked | severe | severe | severe | moderate-marked | ||
| 19 | mild | mild | minimal | minimal | mild-moderate | mild | ||
| 20 | moderate | marked-severe | severe | marked-severe | marked-severe | marked | marked | |
| 21 | normal | moderate | mild-moderate | mild-moderate | mild | mild | moderate-marked | |
| 22 | normal | moderate | moderate | moderate | mild-moderate | minimal | mild-moderate | |
| 23 | severe | marked-severe | marked-severe | severe | marked-severe | severe | marked-severe | |
| 24 | mild | moderate | mild | mild | mild | reduced loudness | ||
| 25 | moderate | moderate | moderate-marked | marked | marked-severe | moderate-marked | moderate |
Upper extremity weakness and/or spasticity resulting in slow AMRs were observed in over half of the patients. Lower limb spasticity and hyper-reflexia were present in approximately one-third of the patients; frank lower limb weakness was not reported. Half of the patients had asymmetric motor findings predominantly affecting the left side. Observed less frequently were a unilateral Hoffman sign and unilateral Babinski reflex. No patients had striking eye movement abnormalities. Eight patients had no abnormal neurological findings.
Electromyographic (EMG) assessment was conducted on 23 patients. No patients demonstrated evidence of diffuse motor neuron disease or met El Escorial criteria for ALS(20). One patient exhibited evidence of long-standing motor neuronopathy without fasciculations or fibrillation potentials.
The neurologic diagnoses at the time of initial assessment were primary lateral sclerosis (PLS; n= 12), pseudobulbar palsy (n = 6; defined as a syndrome consisting of spastic dysarthria, dysphagia, pathologic oral reflexes and pseudobulbar affect), or progressive spastic dysarthria (n = 7).
Follow up evaluation
Ten patients were seen for one or more speech evaluations up to four years after the initial evaluation. Seven patients continued to display speech features consistent with those noted at the initial evaluation. One patient who had irregular articulatory breakdowns suggestive of ataxic dysarthria at the time of initial assessment was no longer exhibiting this feature at follow-up evaluation; the speech diagnosis changed from mixed spastic-ataxic to spastic dysarthria. One patient developed features suggestive of AOS, and another became anarthric. All patients demonstrated progression in severity of spastic dysarthria between the initial and subsequent speech evaluations.
Eight patients were seen for neurologic evaluation six months to four years after the initial diagnosis. The medical diagnoses were unchanged at the time of final evaluation. Dysarthria and other pseudobulbar symptoms remained the most prominent features of the disorder.
MRI results
The MRI findings were non-specific (e.g., white matter hyperintensities and non-focal volume loss due to aging), and without any identifiable lesions (e.g. tumors or strokes) to account for the clinical presentations. The subgroup of patients included in volumetric MRI and FEG-PET analyses were representative of the full cohort with respect to severity of PSD and nonspeech neurologic impairment. This subgroup had longer symptom duration at the time the scans were completed compared to the full cohort.
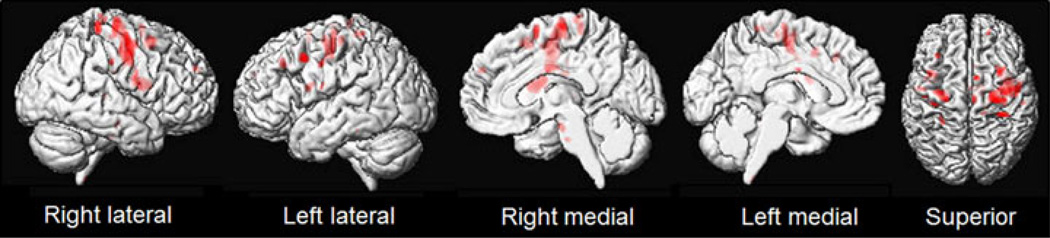
After correcting for multiple comparisons, the subgroup of seven PSD patients that underwent volumetric MRI showed bilateral patterns of white matter volume loss, but no regions of grey matter volume loss, when compared to controls (Figure 1). Regions of white matter volume loss were observed predominantly underlying the motor cortex (BA 4), including the frontoparietal operculum (BA 43), which appear to reflect involvement of the corticofugal projection fibers. White matter volume loss also extended anteriorly into premotor cortex and posterior prefrontal cortex, and was observed in the body of corpus callosum and anterior brainstem. Brainstem white matter loss particularly involved the middle third of the cerebral peduncle of the midbrain which reflects involvement of descending corticospinal and corticobulbar fibers. At the uncorrected threshold however we did observe grey matter loss affecting bilateral medial and lateral motor and premotor cortices.
Figure 1.
Three-dimensional renderings showing regions of white matter loss in a group of seven PSD patients compared to controls. An axial slice through the brainstem is also shown. Results are shown after correction for multiple comparisons using false discovery rate at p<0.05.
FDG-PET
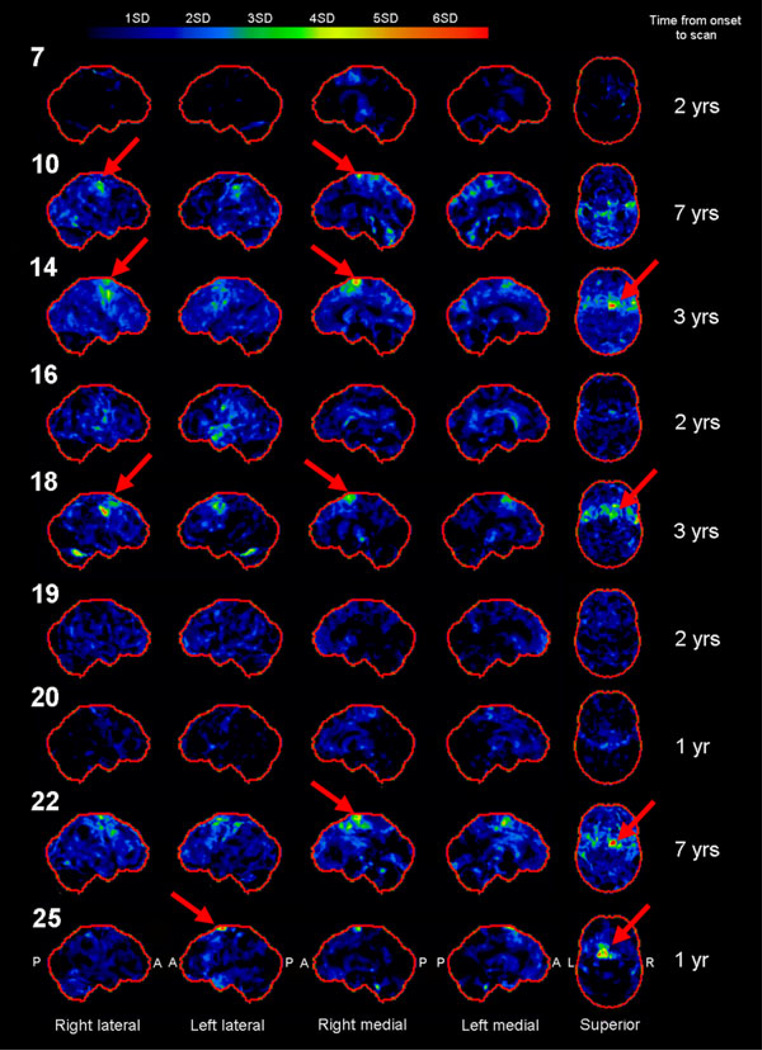
For the subgroup of nine patients who underwent FDG-PET, scans showed absent to subtle hypometabolism in the cortex (Figure 2). The majority of cases did not show focal abnormalities, although focal areas of hypometabolism affecting the premotor and motor cortices were observed in five patients (patients 10, 14, 18, 22 and 25; red arrows), four of whom had a time from onset to scan of longer than two years. Patterns of hypometabolism were bilateral in all patients, but greater in the right hemisphere in patients 14, 18 and 22.
Figure 2.
Three-dimensional stereotactic surface projections showing FDG-PET results for nine PSD patients. Patient numbers are shown on the left and time from onset to scan on the right. Red arrows highlight regions of premotor and motor hypometabolism.
Discussion
This study is the first to describe clinical and imaging features in a large cohort of patients with PSD. The findings were consistent with the classic description of spastic dysarthria with respect to predicted site of lesion, non-speech orofacial neurologic findings, and speech characteristics(2, 5). Darley, Aronson, and Brown(2) posited that the dysarthria associated with pseudobulbar palsy requires damage to bilateral corticobulbar pathways. Indeed, our MRI analysis revealed that PSD particularly targets the white matter, with degeneration observed beneath the motor cortex and mid cerebral peduncle, suggestive of involvement of descending corticospinal and corticobulbar fibers, bilaterally. Less striking grey matter loss and hypometabolism were also observed in the motor and premotor cortices.
The nonspeech oral movement characteristics associated with PSD(2) in our patients are consistent with those described for spastic dysarthria in general. These include reduction of force and speed on clinical measures of tongue, lip, and velar movements without evidence of atrophy; pathologic oral reflexes; and dysphagia. Neurological findings of an upper motor neuron pattern of weakness, spasticity, hyper-reflexia, unilateral Babinski and Hoffmann, and jaw clonus are consistent with imaging findings of motor cortex, corticospinal and corticobulbar tract involvement. Interestingly, pyramidal tract signs identified tended to be asymmetric and more prominent on left side, in keeping with greater volume loss in the right mid cerebral peduncle compared to the left mid cerebral peduncle.
Progressive spastic dysarthria may be the presenting symptom of motor neuron disease and may precede features of flaccid dysarthria (resulting in mixed spastic-flaccid dysarthria). The current sample demonstrates that spastic dysarthria, characterized by strained phonatory quality, slow rate, imprecise articulation, and slow, regular alternate motion rates may progress for an extended time without emergence of flaccid characteristics (e.g., phonatory flutter, prominent weakness, atrophy, and fasciculations). This mirrors the presentation of corticospinal symptoms in PLS. Descriptions of PLS suggest that bulbar symptoms rarely precede spinal symptoms(21–25). Moreover, reports describing progressive pseudobulbar syndromes have rarely characterized the nature of observed dysarthria, either with respect to the speech diagnosis (e.g., spastic dysarthria (23, 26, 27)) or specific speech features(24).
Patients with PLS with initial pseudobulbar symptoms typically ultimately develop limb symptoms(21–23, 25), but a small number do not(24, 26). Fourteen patients in the current study demonstrated limb involvement (3/8 with one year duration, 3/7 with 1<3 years duration, 8/9 with 3 or more years duration), even though dysarthria and other pseudobulbar symptoms remained the most prominent features of the disorder.
The patients in our study were initially diagnosed as PSD, pseudobulbar palsy or PLS. These are all appropriate terms given the clinical presentations. However, the most prominent symptom was progressive difficulty with speech and all patients were determined to have a spastic dysarthria. Patients with PLS(28) may present with bulbar symptoms including dysarthria and dysphagia, but the term PLS implies that there is limb involvement. Since some of our PSD patients had limb involvement initially, and others eventually developed limb involvement, it is our opinion that PSD could be considered a variant of, or a focal presentation of, PLS(29).
Conclusions
We have characterized a neurodegenerative condition that begins focally with spastic dysarthria and then spreads through a network (primarily motor) that may eventually affect speech praxis and limb movements, but in which the spastic dysarthria remains the most prominent deficit. This is not unlike other neurodegenerative conditions that may present as a focal language or speech deficit (e.g., primary progressive aphasia and/or primary progressive apraxia of speech) but ultimately evolve to include more widespread impairment of sensorimotor or cognitive functions. We propose that the term “progressive spastic dysarthria” best characterizes this pattern of deficit.
Acknowledgements
The study was partially funded by The National Institute of Health R01 DC010367.
Footnotes
Disclosures
Drs. Clark, Duffy, Ahlskog, and Sorenson have no financial relationships to disclose.
Dr. Whitwell is funded by NIH (NIDCD and NIA) and the Alzheimer’s Association, and has served a consultant for Bristol-Myers Squibb.
Dr. Josephs is funded by NIH R01 CD 10367 and R01 AG 37491 and by the Alzheimer’s Association.
References
- 1.Duffy J. Motor Speech Disorders: Substrates, Differential Diagnosis, and Management. 3 ed. St. Louis: Elsevier Mosby; 2013. [Google Scholar]
- 2.Darley FL, Aronson AE, Brown JR. Differential diagnostic patterns of dysarthria. J Speech Hear Res. 1969 Jun;12(2):246–269. doi: 10.1044/jshr.1202.246. [DOI] [PubMed] [Google Scholar]
- 3.Brown JR, Darley FL, Aronson AE. Ataxic dysarthria. Int J Neurol. 1970;7(2):302–318. [PubMed] [Google Scholar]
- 4.Kent RD, Duffy JR, Slama A, Kent JF, Clift A. Clinicoanatomic studies in dysarthria: review, critique, and directions for research. J Speech Hear Res. 2001 Jun;44(3):535–551. doi: 10.1044/1092-4388(2001/042). [DOI] [PubMed] [Google Scholar]
- 5.Darley FL, Aronson AE, Brown JR. Clusters of deviant speech dimensions in the dysarthrias. J Speech Hear Res. 1969 Sep;12(3):462–496. doi: 10.1044/jshr.1203.462. [DOI] [PubMed] [Google Scholar]
- 6.Turner MR, Scaber J, Goodfellow JA, Lord ME, Marsden R, Talbot K. The diagnostic pathway and prognosis in bulbar-onset amyotrophic lateral sclerosis. Journal of the Neurological Sciences. 2010 Jul 15;294(1–2):81–85. doi: 10.1016/j.jns.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Traynor BJ, Codd MB, Corr B, Forde C, Frost E, Hardiman OM. Clinical features of amyotrophic lateral sclerosis according to the El Escorial and Airlie House diagnostic criteria: A population-based study. Arch Neurol. 2000 Aug;57(8):1171–1176. doi: 10.1001/archneur.57.8.1171. [DOI] [PubMed] [Google Scholar]
- 8.Tomik B, Guiloff RJ. Dysarthria in amyotrophic lateral sclerosis: A review. Amyotroph Lateral Scler. 2010;11(1–2):4–15. doi: 10.3109/17482960802379004. [DOI] [PubMed] [Google Scholar]
- 9.Jiroutek P, Ruzicka E, Roth J, Jech R. Slowly progressive voluntary-automatic dissociation of facial movements (Foix-Chavany-Marie syndrome) Neuro Endocrinol Lett. 2007 Apr;28(2):137–140. [PubMed] [Google Scholar]
- 10.Sasaguri H, Sodeyama N, Maejima Y, Kanda T, Mizusawa H. Slowly progressive Foix-Chavany-Marie syndrome associated with chronic herpes simplex encephalitis. J Neurol Neurosurg Psychiatry. 2002 Aug;73(2):203–244. doi: 10.1136/jnnp.73.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uttner I, Brettschneider J, Unrath A, Riecker A. Slowly progressive Foix-Chavany-Marie syndrome as a precursor of a primary progressive aphasia. Journal of Clinical Neuroscience. 2012 May;19(5):765–767. doi: 10.1016/j.jocn.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 12.Jack CR, Jr, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain. 2008 Mar;131(Pt 3):665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. NeuroImage. 2006 Apr 1;30(2):436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 14.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE transactions on medical imaging. 1998 Feb;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 15.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. NeuroImage. 2000 Jun;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 16.Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005 Jul 1;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002 Apr;15(4):870–888. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 18.Josephs KA, Duffy JR, Fossett TR, Strand EA, Claassen DO, Whitwell JL, et al. Fluorodeoxyglucose F18 positron emission tomography in progressive apraxia of speech and primary progressive aphasia variants. Arch Neurol. 2010 May;67(5):596–605. doi: 10.1001/archneurol.2010.78. [DOI] [PubMed] [Google Scholar]
- 19.Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer's disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med. 1995 Jul;36(7):1238–1248. [PubMed] [Google Scholar]
- 20.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000 Dec;1(5):293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 21.Singer MA, Kojan S, Barohn RJ, Herbelin L, Nations SP, Trivedi JR, et al. Primary lateral sclerosis: clinical and laboratory features in 25 patients. J Clin Neuromuscul Dis. 2005 Sep;7(1):1–9. doi: 10.1097/01.cnd.0000176974.61136.45. [DOI] [PubMed] [Google Scholar]
- 22.Pringle CE, Hudson AJ, Munoz DG, Kiernan JA, Brown WF, Ebers GC. Primary lateral sclerosis. Clinical features, neuropathology and diagnostic criteria. Brain. 1992 Apr;115(Pt 2):495–520. doi: 10.1093/brain/115.2.495. [DOI] [PubMed] [Google Scholar]
- 23.Tomik B, Zur KA, Szczudlik A. Pure primary lateral sclerosis--Case reports. Clinical Neurology and Neurosurgery. 2008 Apr;110(4):387–391. doi: 10.1016/j.clineuro.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Becker A, Hardmeier M, Steck AJ, Czaplinski A. Primary lateral sclerosis presenting with isolated progressive pseudobulbar syndrome. European Journal of Neurology. 2007 Aug;14(8):e3. doi: 10.1111/j.1468-1331.2007.01699.x. [DOI] [PubMed] [Google Scholar]
- 25.Gordon PH, Cheng B, Katz IB, Pinto M, Hays AP, Mitsumoto H, et al. The natural history of primary lateral sclerosis. Neurology. 2006 Mar 14;66(5):647–653. doi: 10.1212/01.wnl.0000200962.94777.71. [DOI] [PubMed] [Google Scholar]
- 26.Beal MF, Richardson EP., Jr Primary lateral sclerosis: a case report. Arch Neurol. 1981 Oct 10;38:630–633. doi: 10.1001/archneur.1981.00510100058008. [DOI] [PubMed] [Google Scholar]
- 27.Santens P, Van Borsel J, Foncke E, Meire V, Merkx H, De Bleecker J, et al. Progressive dysarthria. Case reports and a review of the literature. Dement Geriatr Cogn Disord. 1999 May-Jun;10(3):231–236. doi: 10.1159/000017125. [DOI] [PubMed] [Google Scholar]
- 28.Younger DS, Chou S, Hays AP, Lange DJ, Emerson R, Brin M, et al. Primary lateral sclerosis. A clinical diagnosis reemerges. Arch Neurol. 1988 Dec;45(12):1304–1307. doi: 10.1001/archneur.1988.00520360022005. [DOI] [PubMed] [Google Scholar]
- 29.Kuipers-Upmeijer J, de Jager AE, Hew JM, Snoek JW, van Weerden TW. Primary lateral sclerosis: clinical, neurophysiological, and magnetic resonance findings. J Neurol Neurosurg Psychiatry. 2001 Nov;71(5):615–620. doi: 10.1136/jnnp.71.5.615. [DOI] [PMC free article] [PubMed] [Google Scholar]