Abstract
Background
Considerable controversy exists regarding the association between coffee consumption and cardiovascular disease (CVD) risk. A meta-analysis was performed to assess the dose-response relationship of long-term coffee consumption with CVD risk.
Methods and Results
Pubmed and EMBASE were searched for prospective cohort studies of the relationship between coffee consumption and CVD risk, which included coronary heart disease, stroke, heart failure, and CVD mortality. Thirty-six studies were included with 1,279,804 participants and 36,352 CVD cases. A non-linear relationship of coffee consumption with CVD risk was identified (P for heterogeneity = 0.09, P for trend < 0.001, P for non-linearity < 0.001). Compared with the lowest category of coffee consumption (median: 0 cups/d), the relative risk of CVD was 0.95 (95% CI, 0.87 to 1.03) for the highest (median: 5 cups/d) category, 0.85 (0.80 to 0.90) for the second highest (median: 3.5 cups/d), and 0.89 (0.84 to 0.94) for the third highest category (median: 1.5 cups/d). Looking at separate outcomes, coffee consumption was non-linearly associated with both CHD (P for heterogeneity = 0.001, P for trend < 0.001, P for non-linearity < 0.001) and stroke risks (P for heterogeneity = 0.07, P for trend < 0.001, P for non-linearity< 0.001) (P for trend differences > 0.05).
Conclusions
A non-linear association between coffee consumption with CVD risk was observed in this meta-analysis. Moderate coffee consumption was inversely significantly associated with CVD risk, with the lowest CVD risk at 3 to 5 cups/d, and heavy coffee consumption was not associated with elevated CVD risk.
Keywords: coffee, cardiovascular disease, meta-analysis
INTRODUCTION
Coffee is one of the most widely consumed beverages around the world; thus, investigating whether or not coffee consumption is associated with chronic disease risk has important public health implications. The relationship between coffee consumption and risk of coronary heart disease was first studied in the 1960s, given that the prevalence of coffee drinking and CHD were both high in western countries.1 Short-term metabolic studies found that caffeine ingestion acutely induces cardiac arrhythmias, and increases plasma renin activity, catecholamine concentrations, and blood pressure.2, 3 In the 1980s, cross-sectional studies found a positive association between coffee consumption and serum total cholesterol concentrations, which might be related to the coffee brewing method (i.e. boiled or unfiltered coffee).4 A later randomized trial showed that boiled coffee consumption increased the serum cholesterol.5 From the 1980s to the 2000s, many case-control studies, which are prone to recall and selection bias, showed a positive association between coffee consumption and CHD risk.6–8 In contrast, meta-analyses of prospective cohort studies tended to find no association, although results varied substantially across studies.9, 10
Since 2000, the association between coffee consumption and other cardiovascular disease (CVD) outcomes such as stroke, heart failure, and total CVD mortality has also been more frequently studied.11–13 Meta-analyses have been published to summarize the association between coffee and risk of CHD,14 stroke,15 and heart failure.16 These meta-analyses did not support an association between coffee consumption and a higher CVD risk, but the shape of the association remains uncertain. Moreover, a number of additional studies have been published since the publication of these meta-analyses,11, 13, 17–19 and one recent meta-analysis paper showed that heavy coffee consumption was not associated with risk of CVD mortality.20 To examine the dose response association of coffee consumption with cardiovascular disease risk, we conducted a systematic review and meta-analysis of coffee consumption and incidence of total CVD outcomes, including incidence of CHD, stroke, and heart failure, and CVD mortality.
Methods
We followed the Meta-Analysis of Observational Studies in Epidemiology21 protocol throughout the design, implementation, analysis, and reporting of our meta-analysis.
Search strategy and selection criteria
We searched the PubMed and EMBASE databases for prospective studies that had evaluated the association between coffee consumption and risk of CVD between January 1966 and March 2013. The computer-based searches included the key words “coffee”, “cardiovascular disease”, “coronary heart disease”, “stroke”, “mortality”, “heart failure”, “myocardial infarction”, “ischemic heart disease”, “sudden cardiac arrest”, and “acute coronary syndrome”. Reference lists of retrieved articles were manually scanned for all relevant additional studies and review articles. We restricted the search to studies on humans that were written in English.
Study Selection
Studies were included in this meta-analysis if they met the following criteria: 1) prospective cohort studies, including case-cohort studies and nested case-control studies with a prospective design; 2) the exposure was coffee consumption, including total coffee, caffeinated coffee, or decaffeinated coffee; 3) the outcome was risk of CVD, including incidence of CHD, stroke, and heart failure, and CVD mortality. Studies were excluded if 1) the study had a retrospective design; 2) the estimates were presented without standard errors or other information that allowed calculation of standard errors; 3) the outcome was atrial fibrillation, atherosclerosis, hypertension, aortic stiffness, or venous thrombus; 4) no confounders were adjusted for.
Data extraction and quality assessment
One author (M. D.) assessed study eligibility and extracted the data, the other author (A. S.) independently double-checked the available data. The following data were extracted from each study: first author's name, year of publication, geographical location, follow-up time, sex, age, number of CVD events, number of participants/person-years of follow up, categories of coffee consumption, mean/median coffee consumption in each category, CVD assessment method, covariates adjusted for in the multivariable analysis, and relative risks and the associated measure of variance for all categories of coffee consumption. For cohorts with published data on several CVD outcomes, we chose incidence instead of mortality or heart failure results. For studies with data on both CHD and stroke as the outcome, we included both in the meta-analysis. The correlation of CHD and stroke was accounted for in the main analysis (see below). In a sensitivity analysis, we analyzed one of the two outcomes. The Newcastle-Ottawa quality assessment scale (NOS) 22was used to evaluate the quality of the included studies. M. D. and S. B. developed the evaluation criteria (supplemental table 1). The score ranges from 0 to 9 points with a higher score indicating higher study quality.
To perform a dose-response meta-analysis, we assigned the median coffee consumption in each category of consumption to the corresponding relative risk for each study. We used means for this purpose if medians were not reported. If neither the mean nor the median consumption per category was reported, the midpoint of the upper and lower boundaries in each category was used to estimate median consumption. If the upper boundary for the highest category was not provided, the assigned median value was 25% higher than the lower boundary of that category. If the lower boundary for the lowest category was not provided, the assigned median value was half of the upper boundary of that category.
Data Synthesis and Analysis
To analyze the trend of coffee consumption and risk of CVD, we used both semi-parametric and parametric methods. For the semi-parametric method, four coffee consumption groups were generated, namely lowest, third highest, second highest, and highest. For each study that was included, the lowest and the highest coffee consumption categories corresponded to the lowest and highest groups, respectively. For studies with four exposure categories, the second and third categories corresponded to the second and third highest groups, respectively. For studies with three exposure categories, the middle category corresponded to either the second or the third highest group in the meta-analysis, depending on the similarity of the median coffee consumption to either the second or the third highest group of the meta-analysis. If the study had more than four exposure categories, two consumption groups, other than the lowest and highest, were chosen based on their similarity of the amount of coffee consumption in that category to the second and third highest groups of the meta-analysis. For each group, we computed correlation coefficients (ρ) between CHD and stroke outcomes in the same cohort. We imputed ρ =1 initially to obtain the most conservative effect estimates. A random-effects model was used first and was changed to a fixed-effects model if no between study heterogeneity was found for the random-effects model (tau-squared < 1). 23 Sensitivity analysis was conducted by imputing different ρ (0 < ρ ≤ 1) to evaluate the robustness of the effect estimates. We used the STATA command ROBUMETA to obtain the effect estimates.
For the parametric method, a dose-response meta-analysis was performed.24 The number of cases and participants in each coffee consumption category was extracted to estimate the covariance of the relative risk in each study. Together with the observed adjusted variance of the relative risk, we estimated the variance/covariance matrix of the data. The weight of each study was calculated as the inverse of the variance/covariance matrix. We used generalized least squares models (GLST) with the maximum likelihood method to estimate the coefficients for each study. We fit a fixed-effects generalized linear model first, and changed to a random-effects generalized linear model if the p value for the goodness of fit/heterogeneity of the previous model was < 0.05. Additionally, we tested for potential non-linearity in the association between coffee consumption and CVD risk using a fixed/random-effects restricted cubic spline model with 3 knots. In sensitivity analysis, we used two-stage fixed/random-effects dose response models to combine studies that reported results for categorized coffee consumption and studies with reported results for continuous coffee consumption. Specifically, the RR of CVD per unit increase of coffee consumption for each study was first estimated separately by GLST, and then the RRs from all of the studies were pooled together by a fixed/random-effects model. We used the STATA command GLST for model fitting, and the command LINCOME to obtain effect estimates for the fitted model.
We performed stratified analyses by baseline hypertension or MI of the study population, smoking status, publication year, NOS study quality score, dietary assessment method, evaluation of stroke or CHD as the outcome, country, sex, and type of coffee (caffeinated coffee or decaffeinated coffee). The interaction between categorized coffee consumption and the stratifying variable with the risk of CVD was tested by a likelihood ratio test comparing the models derived using GLST method with and without the interaction terms. We assessed the potential for publication bias using Egger's regression symmetry test.25 All analyses were conducted using STATA Version 11.2 (STATA Corp, College Station, Texas).
Results
Characteristics of studies
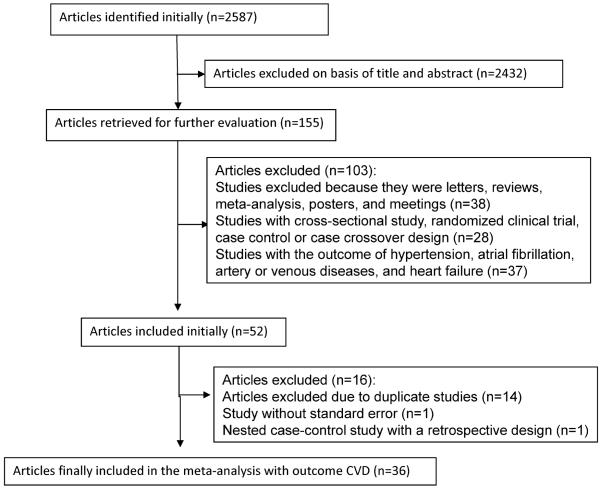
Our initial search identified 2587 potentially relevant citations. After screening titles and abstracts, we identified 53 studies for further evaluation. Of the 53 initially included studies, we excluded 14 studies due to duplicate publication, one study with point estimate without standard error, and one nested case control study with a retrospective design. Thirty-six studies remained in the meta-analysis (Figure 1). The included studies comprised approximately 1,283,685 study participants and 47,779 CVD cases, including 28,347 CHD cases, 12,030 stroke cases and 7,402 other CVD cases. Characteristics of these 36 studies are shown in Table 1. One study had a nested case-control study design, one had a case-cohort study design, and the rest of the studies were cohort studies. Duration of follow-up for incident CVD ranged from 6 to 44 years, with a median follow-up of 10 years. Twenty-one studies were conducted in Europe, 12 in the US, and 3 in Japan. Three studies assessed coffee consumption repeatedly during the course of the follow-up, and the rest of the studies assessed coffee consumption at baseline. Thirteen studies assessed coffee consumption without using a specific dietary assessment method, and the rest of the studies assessed coffee consumption by diet recalls, diet records or food frequency questionnaires (FFQ). One study modeled coffee consumption as a continuous variable, and the remaining studies modeled coffee consumption categorically. Nine studies assessed the association of caffeinated coffee consumption with CVD risk, and four studies assessed the association of decaffeinated coffee consumption with CVD risk. The outcome in 17 studies was risk of stroke, while the outcome in 22 studies was risk of CHD. The scores of the NOS quality assessment ranged from 3 to 8, and 31 studies had scores of 5 or higher. The corresponding results of each criteria of the NOS quality assessment for our meta-analysis are shown in Supplemental Table 1. The study modeling coffee as a continuous exposure was excluded in the following analysis due to the difficulty of combining the risk estimate with those of other studies and was only included in the sensitivity analysis.26 All of the remaining 35 studies were included in the main analysis, and 29 studies were included in the dose-response analysis between coffee consumption and risk of CVD.
Figure 1.
Study selection process of coffee consumption and risk of CVD.
Table 1.
Basic characteristics of included studies
| Author/Year/Country/Special annotation | Sex | Follow-up years | Age at start of follow-up (y) | No. of cases/Total No. of participants | Exposure(cup/d) Relative risk (95% CI) | Outcome | Exposure/outcome assessment | Confounders adjusted for |
|---|---|---|---|---|---|---|---|---|
| Wilhelmsen et al 1977 Europe |
Men | 12 | 50 | 60/834 | Per cup increase of coffee consumption: 1.11 (0.83–1.51) | CHD | Not specific diet questionnaire (baseline)/Hospital record | Smoking, cholesterol, SBP, dyspnea, registration by temperance board |
| Legrady et al 1987 US |
men | 19 | 40–56 | 220 CHD,57 stroke/1910 | Stroke mortality | stroke mortality | Not specific diet questionnaire (baseline)/Death certificates | age, diastolic blood pressure, serum cholesterol, and smoking status |
| 0–1 cup/d 1.00 (1.00–1.00) | ||||||||
| >1 cup/d 1.64 (0.80–3.38) | ||||||||
| Martin et al 1988 US Hypertensive population |
both | 4 | 30–69 | 336/10,064 | Stroke mortality | CVD mortality | Not specific diet questionnaire (baseline)/Death certificates | age, sex, race, type of care, marital status, month of interview, body weight, initial diastolic blood pressure, fasting plasma blood glucose and serum cholesterol, initial end organ damage, and location of the study center |
| 0 cup/d 1.00 (1.00–1.00) | ||||||||
| 0.1 mg–2 cups/d 0.73 (0.37–1.46) | ||||||||
| 2–4 cups/d 0.61 (0.26–1.44) | ||||||||
| >4 cups/d 1.30 (0.56–3.04) | ||||||||
| CHD mortality | ||||||||
| 0 cup/d 1.00 (1.00–1.00) | ||||||||
| 0.1–2 cups/d 0.93 (0.66–1.3) | ||||||||
| 2–4 cups/d 0.81 (0.53–1.23) | ||||||||
| >4 cups/d 0.80 (0.46–1.39) | ||||||||
| Grobbee et al 1990 US |
men | 2 | 40–75 | 411/45,589 | 0 cup/d 1.00 (1.00–1.00) | CVD | FFQ (baseline)/Confirmed cases | age, quintiles of Quetelet's index, smoking habits, history of diabetes, alcohol use, parental history of myocardial infarction, specific health profession, energy intake, cholesterol, and saturated, monounsaturated, and polyunsaturated fat |
| 0–1 cup/d 0.70 (0.51–0.97) | ||||||||
| 2–3 cups/d 1.00 (0.79–1.26) | ||||||||
| ≥4 cups/d 0.90 (0.67–1.22) | ||||||||
| Klatsky et al 1990 US Nested case-control study |
both | 8 (media n:5) | From <50 to >60 | 1914/1,01,774 | MI | CHD | Not specific diet questionnaire (baseline)/Hospitalization for coronary disease | age, race, cigarette smoking, alcohol intake, education, baseline disease, and tea use. |
| 0 cup/d 1.00 (1.00–1.00) | ||||||||
| <1 cup/d 0.78 (0.56–1.07) | ||||||||
| 1–3 cups/d 1.16 (0.93–1.45) | ||||||||
| ≥4 cups/d 1.42 (1.11–1.81) | ||||||||
| Other coronary cases | ||||||||
| 0 cup/d 1.00 (1.00–1.00) | ||||||||
| <1 cup/d 0.90 (0.72–1.11) | ||||||||
| 1–3 cups/d 0.89 (0.76–1.04) | ||||||||
| ≥4 cups/d 1.03 (0.85–1.24) | ||||||||
| Tverdal et al 1990 Europe |
both | 6.4 | 35–54 | 184/38564 | no sugar in coffee | CHD mortality | Not specific diet questionnaire (baseline)/Confirmed cases | age, high density lipoprotein, total cholesterol, systolic blood pressure, no of cigarettes/day |
| <1 cup/d 1.00 (1.00–1.00) | ||||||||
| ≥9 cups/d 4.10 (1.30–13.20) | ||||||||
| sugar in coffee | ||||||||
| <1 cup/d 1.00 (1.00–1.00) | ||||||||
| ≥9 cups/d 1.60 (0.60–4.30) | ||||||||
| Rosengren et al 1991 Europe |
men | 7.1 | 51–59 | 399/6765 | 0 cup/d 1.00 (1.00–1.00) | CHD | Not specific diet questionnaire (baseline)/National registries | age, systolic blood pressure, body mass index, diabetes, registration for alcohol abuse, family history of myocardial infarction, mental stress, physical activity, and occupational class, smoking |
| ≥9 cups/d 1.40 (0.80–2.40) | ||||||||
| Lindsted et al 1992 Europe |
men | 15 | ≥30 | NA/9484 | <1 cup/d 1.00 (1.00–1.00) | CVD mortality | FFQ (baseline)/Confirmed cases | Body mass index, stroke, heart disease, hypertension, race, exercise, sleep, marital status, education, smoking history, dietary pattern |
| 1–2 cups/d 1.38 (1.18–1.62) | ||||||||
| ≥3 cups/d 1.44 (1.18–1.76) | ||||||||
| Klag et al 1994 US |
men | 32 | 26 | 111/1040 | 0 cup/d 1.00 (1.00–1.00) | CHD | Not specific diet questionnaire (baseline)/National registries | age at graduation, baseline serum cholesterol, calendar time, time-dependent hypertension status, number of cigarettes, diabetes, and body mass index |
| 1–2 cups/d 1.70 (0.78–3.68) | ||||||||
| 3–4 cups/d 3.02 (1.37–6.65) | ||||||||
| ≥5 cups/d 2.94 (1.27–6.81) | ||||||||
| Gyntelberg et al 1995 Europe |
men | 6 | 53–74 | 184/2975 | 1–4 cups/d 1.00 (1.00–1.00) | CHD | Not specific diet questionnaire (baseline)/Confirmed cases | age, alcohol, blood pressure, serum selenium level, social class, and triglycerides |
| 5–8 cups/d 1.00 (0.70–1.40) | ||||||||
| ≥9 cups/d 0.60 (0.30–1.00) | ||||||||
| Hart et al 1997 Europe |
men | 21 | 35–64 | 625/5766 | 0 cup/d 1.00 (1.00–1.00) | CHD mortality | Not specific diet questionnaire (average)/National registries | age, diastolic blood pressure, cholesterol, smoking, social class, age leaving full time education, body mass index, angina, and ECG ischaemia |
| 0.5–1 cup/d 1.20 (0.87–1.64) | ||||||||
| 1.5–2 cup/d 1.17 (0.83–1.65) | ||||||||
| 2.5–4 cup/d 1.16 (0.81–1.66) | ||||||||
| >4.5 cup/d 1.49 (0.89–2.47) | ||||||||
| Hakim et al 1998 US Hypertensive population |
men | 25 | 55–68 | 76/499 | 0 cup/d 1.00 (1.00–1.00) | Stroke | 24h diet recall (baseline)/Confirmed cases | age, systolic blood pressure, total cholesterol, triglycerides, diabetes, alcohol use, and the physical activity index as measured at the time of study enrollment |
| ≥6 cups/d 2.1 (1.2–3.7) | ||||||||
| Woodward et al 1999 Europe |
both | 7.7 | 40–59 | 567/11000 | Men | CHD | Food consumption table (baseline)/Confirmed cases | age, housing tenure, activity at work, activity in leisure, cigarette smoking status, body mass index, Bortner score, cotinine, systolic blood pressure, fibrinogen, total cholesterol, HDL-cholesterol, triglycerides, alcohol, vitamin C, and tea. |
| 0 cup/d 1.00 (1.00–1.00) | ||||||||
| 1–2 cups/d 0.68 (0.42–1.10) | ||||||||
| 3–4 cups/d 0.39 (0.21–0.73) | ||||||||
| ≥5 cups/d 0.68 (0.37–1.24) | ||||||||
| women | ||||||||
| 0 cup/d 1.00 (1.00–1.00) | ||||||||
| 1–2 cups/d 0.54 (0.22–1.34) | ||||||||
| 3–4 cups/d 0.56 (0.20–1.56) | ||||||||
| ≥5 cups/d 0.55 (0.18–1.66) | ||||||||
| Kleemola et al 2000 Europe |
both | 10 | 30–59 | 1645/20179 | men with nonfatal MI | CHD, CHD mortality | Not specific diet questionnaire (baseline)/National registries | age, smoking status, serum cholesterol level, blood pressure, and history of MI |
| <1 cup/d 1.09 (0.78–1.54) | ||||||||
| 1–3 cups/d 1.00 (1.00–1.00) | ||||||||
| 4–7 cups/d 0.95 (0.79–1.15) | ||||||||
| >7 cups/d 0.79 (0.64–0.98) | ||||||||
| women with nonfatal MI | ||||||||
| <1 cup/d 1.72 (1.01–2.92) | ||||||||
| 1–3 cups/d 1.00 (1.00–1.00) | ||||||||
| 4–7 cups/d 0.84 (0.62–1.13) | ||||||||
| >7 cups/d 0.93 (0.63–1.36) | ||||||||
| men with CHD mortality | ||||||||
| <1 cup/d 1.88 (1.20–2.95) | ||||||||
| 1–3 cups/d 1.00 (1.00–1.00) | ||||||||
| 4–7 cups/d 1.23 (0.93–1.62) | ||||||||
| >7 cups/d 1.22 (0.90–1.65) | ||||||||
| women with CHD mortality | ||||||||
| <1 cup/d 0.00 (0.00–0.00) | ||||||||
| 1–3 cups/d 1.00 (1.00–1.00) | ||||||||
| 4–7 cups/d 0.67 (0.41–1.07) | ||||||||
| >7 cups/d 0.57 (0.28–1.16) | ||||||||
| Jazbec et al 2003 Europe |
both | 10 | 35–59 | 435/3364 | men | CVD mortality | Not specific diet questionnaire (baseline)/Confirmed cases | age, number of cigarettes consumed per day, diastolic blood pressure, ulcer, feeling of well-being, region |
| 0 cup/d 1.00 (1.00–1.00) | ||||||||
| <1 cups/d 0.70 (0.49–1.00) | ||||||||
| 1–2 cups/d 0.82 (0.58–1.16) | ||||||||
| >2 cups/d 0.72 (0.44–1.18) | ||||||||
| women | ||||||||
| 0 cup/d 1.00 (1.00–1.00) | ||||||||
| <1 cup/d 0.88 (0.56–1.39) | ||||||||
| 1–2 cups/d 0.67 (0.43–1.05) | ||||||||
| >2 cups/d 0.62 (0.30–1.28) | ||||||||
| Happonen et al 2004 Europe |
Men | 14 | 42–60 | 269/1971 | None 0.84 (0.41–1.72) | CHD mortality | Diet record (baseline)/National registries | Age, packyears of smoking, ischemia in exercise test, diabetes, income, and serum insulin concentration. Physical activity; family history of CHD; intake of alcohol, tea, saturated fat, total energy, and total water; serum glucose and plasma vitamin C concentration |
| Light 1.22 (0.90–1.64) | ||||||||
| Moderate 1.00 (1.00–1.00) | ||||||||
| Heavy 1.43 (1.06–1.94) | ||||||||
| Lopez-Garcia et al 2006 US |
both | 20 | men 53 women 46 | 4427/128493 | Women | CHD | FFQ (baseline)/National registries | age, smoking status, serum cholesterol level, blood pressure, and history of MI |
| <0.033 cup/d 1.00 (1.00–1.00) | ||||||||
| 0.033–0.57 cup/d 0.97 (0.83–1.14) | ||||||||
| 0.57–1 cup/d 1.02 (0.90–1.17) | ||||||||
| 2–3 cups/d 0.84 (0.74–0.97) | ||||||||
| 4–5 cups/d 0.99 (0.83–1.17) | ||||||||
| ≥6 cups/d 0.87 (0.68–1.11) | ||||||||
| Men | ||||||||
| <0.033 cup/d 1.00 (1.00–1.00) | ||||||||
| 0.033–0.57 cup/d 1.04 (0.91–1.17) | ||||||||
| 0.57–1 cup/d 1.02 (0.90–1.15) | ||||||||
| 2–3 cups/d 0.97 (0.86–1.11) | ||||||||
| 4–5 cups/d 1.07 (0.88–1.31) | ||||||||
| ≥6 cups/d 0.72 (0.49–1.07) | ||||||||
| Andersen et al 2006 US |
wo men | 15 | 55–69 | 1411/27312 | 0 cup/d 1.00 (1.00–1.00) | CVD mortality | FFQ (baseline)/National registries | age, smoking, and intake of alcohol, BMI, waist-hip ratio, education, physical activity, use of estrogens, use of multivitamin supplements, energy intake, and intakes of whole and refined grain, red meat, fish and seafood, and total fruit and vegetables |
| <1 cup/d 0.85 (0.68–1.06) | ||||||||
| 1–3 cups/d 0.76 (0.64–0.91) | ||||||||
| 4–5 cups/d 0.81 (0.66–0.99) | ||||||||
| ≥6 cups/d 0.87 (0.69–1.09) | ||||||||
| Bidel et al 2006 Europe Type 2 diabetic population |
both | 20.8 | 25–74 | 909/3837 | 0–2 cups/d 1.00 (1.00–1.00) | CVD mortality | Not specific diet questionnaire (baseline)/National registries | age, sex, study year, BMI, systolic blood pressure, total cholesterol, education, alcohol and tea consumption, and smoking status |
| 3–4 cups/d 0.79 (0.64–0.97) | ||||||||
| 5–6 cups/d 0.70 (0.57–0.86) | ||||||||
| ≥7 cups/d 0.71 (0.56–0.90) | ||||||||
| Greenberg et al 2007 US |
both | 8.8 | 32–86 | 426/6594 | <65y | CVD mortality | FFQ (baseline)/Confirmed cases | age, smoking, BMI, sex, race, physical activity, alcohol consumption, per capita income, educational level, and American-style diet |
| <0.5 cup/d 1.00 (1.00–1.00) | ||||||||
| 0.5–2 cups/d 0.95 (0.38–2.35) | ||||||||
| 2–4 cups/d 0.79 (0.34–1.85) | ||||||||
| ≥4 cups/d 0.86 (0.38–1.06) | ||||||||
| ≥65y | ||||||||
| <0.5 cup/d 1.00 (1.00–1.00) | ||||||||
| 0.5–2 cups/d 0.72 (0.52–0.99) | ||||||||
| 2–4 cups/d 0.69 (0.52–0.92) | ||||||||
| >4 cups/d 0.53 (0.38–0.75) | ||||||||
| Silletta et al 2007 Europe Myocardial infarction population |
both | 3.5 | 52–63 | 1167/11231 | 0 cup/d 1.00 (1.00–1.00) | CVD | FFQ (average)/Confirmed cases | Age, gender, smoking, BMI, dietary habits, cardiovascular risk factors, history of MI before the index MI, time from the index MI to enrollment, post-MI complications, and pharmacological therapies, with inclusion of the allocation treatments |
| <2 cups/d 1.02 (0.87–1.20) | ||||||||
| 2–4 cups/d 0.91 (0.75–1.09) | ||||||||
| >4 cups/d 0.88 (0.64–1.2) | ||||||||
| Greenberg et al 2008 US |
both | 10.1 | 65–97 | 523/1354 | 0 cup/d 1.00 (1.00–1.00) | CVD events | FFQ (baseline)/Confirmed cases | age, gender, smoking, body mass index, alcohol consumption, physical activity, marital status, BP, history of CVD, and antihypertensive medication use |
| ≥1 cup/d 1.00 (0.84– 1.20) | ||||||||
| Happonen et al 2008 Europe |
both | 14.5 | 70–94 | 344/817 | 0 cups/d 0.80 (0.47–1.35) | CVD mortality | Not specific diet questionnaire (baseline)/National registries | sex, current age, calendar period, marital status, educational level, previous occupational group, current smoking, BMI, history of myocardial infarction, presence of diabetes mellitus, cognitive impairment, physical disability, and self-rated health |
| 1–2 cups/d 1.00 (1.00–1.00) | ||||||||
| 3–4 cups/d 0.96 (0.72–1.27) | ||||||||
| 5–6 cups/d 0.89 (0.64–1.24) | ||||||||
| ≥7 cups/d 0.84 (0.48–1.47) | ||||||||
| Larsson et al 2008 Europe |
men | 13.6 | 50–69 | 2702/26556 | <2 cup/d 1.00 (1.00–1.00) | Stroke | FFQ (baseline)/National registries | age, supplementation group, No. of cigarettes smoked daily, body mass index, systolic and diastolic blood pressure, serum total cholesterol, serum HDL cholesterol, histories of diabetes and coronary heart disease, leisure-time physical activity, alcohol intake, and tea consumption |
| 2–3 cups/d 0.91 (0.79–1.06) | ||||||||
| 4–5 cups/d 0.88 (0.77–1.02) | ||||||||
| 6–7 cups/d 0.77 (0.66–0.90) | ||||||||
| ≥8 cups/d 0.77 (0.66–0.90) | ||||||||
| Mukamal et al 2009 Europe Myocardial infarction population |
both | 6.9–9.9 | 45–70 | 331 (MI), 135(stroke)/1369 | MI | CHD, stroke | FFQ (baseline)/National registries | age, sex, diabetes, smoking, obesity, physical inactivity, alcohol consumption, tea consumption, education, and intake of boiled coffee |
| 0–1 cup/d 1.00 (1.00–1.00) | ||||||||
| 1–3 cups/d 0.97 (0.65–1.45) | ||||||||
| 3–5 cups/d 0.75 (0.50–1.13) | ||||||||
| 5–7 cups/d 0.94 (0.61–1.44) | ||||||||
| ≥7 cups/d 0.84 (0.51–1.40) | ||||||||
| Stroke | ||||||||
| 0–1 cup/d 1.00 (1.00–1.00) | ||||||||
| 1–3 cups/d 1.08 (0.57–2.02) | ||||||||
| 3–5 cups/d 0.94 (0.49–1.78) | ||||||||
| 5–7 cups/d 1.17 (0.59–2.29) | ||||||||
| ≥7 cups/d 0.74 (0.31–1.75) | ||||||||
| Sugiyama et al 2010 Japan |
both | 10.3 | 40–64 | 426/37742 | men CVD mortality | CVD mortality | FFQ (baseline)/Mortality certificates at the public health center | age in years, sex, past history of hypertension and diabetes, education level, BMI, walking time, cigarette smoking , consumption of alcohol, green tea, oolong tea, black tea, intake of rice, miso soup, total meat, total dairy products, total fish, total vegetables, total fruits, and energy |
| 0 cup/d 1.00 (1.00–1.00) | ||||||||
| 0–1 cup/d 1.09 (0.79–1.51) | ||||||||
| 1–2 cups/d 0.85 (0.56–1.23) | ||||||||
| ≥3 cups/d 0.88 (0.56–1.39) | ||||||||
| women CVD mortality | ||||||||
| 0 cup/d 1.00 (1.00–1.00) | ||||||||
| 0–1 cup/d 0.56 (0.36–0.86) | ||||||||
| 1–2 cups/d 0.48 (0.29–0.80) | ||||||||
| ≥3 cups/d 0.45 (0.20–1.03) | ||||||||
| Ahmed et al 2009 Europe |
men | 9 | 45–79 | 784/37315 | ≤1 cup/d 1.00 (1.00–1.00) | Heart failure | FFQ (baseline)/Confirmed cases | age, body mass index, total activity score, smoking, history of high cholesterol, family history of MI before age 60, education level, marital status, aspirin use, alcohol, tea, energy-adjusted fat intake, and energy-adjusted daily sodium intake |
| 2 cups/d 0.87 (0.69–1.11) | ||||||||
| 3 cups/d 0.89 (0.70–1.14) | ||||||||
| 4 cups/d 0.89 (0.69–1.15) | ||||||||
| ≥5 cups/d 0.89 (0.69–1.15) | ||||||||
| Lopez-Garcia et al 2009 US |
women | 24 | 56 | 2280/83076 | <0.03 cup/d 1.00 (1.00–1.00) | Stroke | FFQ (average)/Confirmed cases | age, smoking status, body mass index, physical activity, alcohol intake, menopausal status and use of hormone replacement therapy, aspirin use; total caloric intake; quintiles of calcium, potassium, sodium, and folate intake; glycemic load; whole grain intake; and tertiles of fruits, vegetables, and fish consumption, high blood pressure, hypercholesterolemia, and type 2 diabetes mellitus |
| 0.03–0.57 cup/d 0.96 (0.82–1.13) | ||||||||
| 0.57–1 cup/d 0.88 (0.77–1.02) | ||||||||
| 2–3 cups/d 0.84 (0.72–0.98) | ||||||||
| ≥4 cups/d 0.85 (0.69–1.06) | ||||||||
| Leurs et al 2010 Europe Case-cohort study |
both | 10 | 55–69 | 1789(IHD deaths), 708(stroke deaths)/120852 | men with MI mortality | CHD mortality, stroke mortality | FFQ (baseline)/National registries | age, current smoking, number of cigarettes smoked, years of active smoking and total energy intake |
| 0–2 cups/d 1.00 (1.00–1.00) | ||||||||
| 2–4 cups/d 0.91 (0.71–1.16) | ||||||||
| 3–6 cups/d 1.02 (0.79–1.31) | ||||||||
| >6 cups/d 1.17 (0.86–1.59) | ||||||||
| women with MI mortality | ||||||||
| 0–2 cups/d 1.00 (1.00–1.00) | ||||||||
| 2–4 cups/d 0.75 (0.58–0.97) | ||||||||
| 3–6 cups/d 0.62 (0.46–0.84) | ||||||||
| >6 cups/d 0.71 (0.45–1.12) | ||||||||
| men with stroke mortality | ||||||||
| 0–2 cups/d 1.00 (1.00–1.00) | ||||||||
| 2–4 cups/d 0.84 (0.60–1.18) | ||||||||
| 3–6 cups/d 0.72 (0.50–1.04) | ||||||||
| >6 cups/d 1.15 (0.74–1.77) | ||||||||
| women with stroke mortality | ||||||||
| 0–2 cups/d 1.00 (1.00–1.00) | ||||||||
| 2–4 cups/d 0.79 (0.57–1.09) | ||||||||
| 3–6 cups/d 0.70 (0.48–1.02) | ||||||||
| >6 cups/d 1.10 (0.63–1.90) | ||||||||
| Gans et al 2010 Europe |
both | 13 | 20–69 | 1387(CHD cases), 563(stroke cases), 123(CHD deaths), 70(stroke deaths)/37514 | CHD morbidity | CHD, CHD mortality, Stroke, stroke mortality | FFQ (baseline)/National registries | sex; age; educational level; physical activity; smoking status; waist circumference; menopausal status; alcohol, tea; total energy; and saturated fat, fiber, and vitamin C level |
| <1 cup/d 1.00 (1.00–1.00) | ||||||||
| 1–2 cups/d 0.85 (0.70–1.04) | ||||||||
| 2–3 cups/d 0.79 (0.65–0.96) | ||||||||
| 3–4 cups/d 0.82 (0.68–0.98) | ||||||||
| 4–6 cups/d 0.86 (0.73–1.02) | ||||||||
| >6 cups/d 0.91 (0.74–1.11) | ||||||||
| stroke morbidity | ||||||||
| <1 cup/d 1.00 (1.00–1.00) | ||||||||
| 1–2 cups/d 1.08 (0.79–1.47) | ||||||||
| 2–3 cups/d 1.15 (0.85–1.57) | ||||||||
| 3–4 cups/d 1.10 (0.82–1.46) | ||||||||
| 4–6 cups/d 1.11 (0.84–1.46) | ||||||||
| >6 cups/d 1.22 (0.88–1.70) | ||||||||
| CHD mortality | ||||||||
| <1 cup/d 1.00 (1.00–1.00) | ||||||||
| 1–3 cups/d 1.06 (0.61–1.84) | ||||||||
| 3–6 cups/d 0.64 (0.37–1.11) | ||||||||
| >6 cups/d 0.73 (0.37–1.42) | ||||||||
| Stroke mortality | ||||||||
| <1 cup/d 1.00 (1.00–1.00) | ||||||||
| 1–3 cups/d 0.86 (0.39–1.87) | ||||||||
| 3–6 cups/d 1.20 (0.59–2.47) | ||||||||
| >6 cups/d 1.34 (0.49–3.64) | ||||||||
| Larsson et al 2011 Europe |
women | 10.4 | 49–83 | 1680/34670 | <1 cup/d 1.00 (1.00–1.00) | Stroke | FFQ (baseline)/National registries | age; smoking status and pack-years of smoking; education; body mass index; total physical activity; history of diabetes; history of hypertension; aspirin use; family history of myocardial infarction; and intakes of total energy, alcohol, red meat, fish, fruits, and vegetables |
| 1–2 cups/d 0.78 (0.66–0.91) | ||||||||
| 3–4 cups/d 0.75 (0.64–0.88) | ||||||||
| ≥5 cups/d 0.77 (0.63–0.92) | ||||||||
| Mineharu et al 2011 Japan |
both | 13.1 | 40–79 | 2012/76979 | men CVD mortality | CVD mortality | FFQ (baseline)/Mortality certificates at the public health center | body mass index (BMI), history of hypertension, history of diabetes, smoking status, alcohol intake, education, walking hours, hours of sports participation, perceived mental stress, multivitamin use, vitamin E supplement use, consumption of total fruits, total vegetable, total beans, total meat, total fish and seaweeds and total daily energy intake |
| <0.14 cup/d 1.00 (1.00–1.00) | ||||||||
| 0.14–1 cup/d 0.71 (0.53–0.96) | ||||||||
| 1–2 cups/d 0.84 (0.64–0.99) | ||||||||
| ≥3 cups/d 1.17 (0.77–1.76) | ||||||||
| women CVD mortality | ||||||||
| <0.14 cup/d 1.00 (1.00–1.00) | ||||||||
| 0.14–1 cup/d 0.87 (0.62–1.23) | ||||||||
| 1–2 cups/d 0.77 (0.55–0.99) | ||||||||
| ≥3 cups/d 2.30 (1.31–4.02) | ||||||||
| Floegel et al 2012 Europe |
both | 8.9 | 35–65 | 704/42659 | <1 cup/d 1.00 (1.00–1.00) | CVD | FFQ (baseline)/Confirmed self-reported | age at recruitment, center, sex, smoking, alcohol intake, physical activity, education, employment, vitamin and mineral supplement use during past 4 weeks, total energy intake, tea intake, and decaffeinated coffee intake, BMI, waist-to-hip ratio, and prevalent hypertension |
| 1–2 cups/d 0.94 (0.64–1.36) | ||||||||
| 2–3 cups/d 1.07 (0.81–1.42) | ||||||||
| 3–4 cups/d 1.02 (0.75–1.38) | ||||||||
| >4 cups/d 1.10 (0.84–1.44) | ||||||||
| Rautiainen et al 2012 Europe |
women | 9.9 | 49–83 | 1114/32561 | ≤2 cups/d 1.00 (1.00–1.00) | CHD | FFQ (baseline)/National registries | age, education, smoking, body mass index, physical activity, hypertension, hypercholesterolemia, family history of myocardial infarction, aspirin use, hormone replacement therapy use, dietary supplement use, and intakes of total energy and alcohol |
| 3 cups/d 0.87 (0.68–1.12) | ||||||||
| 4 cups/d 0.88 (0.69–1.13) | ||||||||
| ≥5 cups/d 0.96 (0.72–1.26) | ||||||||
| Freedman et al 2012 US |
both | 14 | 50–71 | 11828(CHD deaths), 2293(stroke deaths)/402260 | men CHD mortality | CHD mortality, stroke mortality | FFQ (baseline)/National registries | age; body-mass index; race or ethnic group; level of education; alcohol consumption; the number of cigarettes smoked per day, use or nonuse of pipes or cigars, and time of smoking cessation; health status; diabetes; marital status; physical activity; total energy intake; consumption of fruits, vegetables, red meat, white meat, and saturated fat; use or nonuse of vitamin supplements; and use or nonuse of postmenopausal hormone therapy |
| 0 cup/d 1.00 (1.00–1.00) | ||||||||
| <1 cup/d 0.93 (0.85–1.02) | ||||||||
| 1 cup/d 0.92 (0.84–1.01) | ||||||||
| 2–3 cups/d 0.86 (0.79–0.94) | ||||||||
| 4–5 cups/d 0.87 (0.79–0.96) | ||||||||
| ≥6 cups/d 0.88 (0.78–1.00) | ||||||||
| women CHD mortality | ||||||||
| 0 cup/d 1.00 (1.00–1.00) | ||||||||
| <1 cup/d 1.00 (0.89–1.13) | ||||||||
| 1 cup/d 0.91 (0.81–1.03) | ||||||||
| 2–3 cups/d 0.85 (0.76–0.95) | ||||||||
| 4–5 cups/d 0.78 (0.68–0.90) | ||||||||
| ≥6 cups/d 0.72 (0.59–0.88) | ||||||||
| men stroke mortality | ||||||||
| 0 cup/d 1.00 (1.00–1.00) | ||||||||
| <1 cup/d 0.99 (0.79–1.24) | ||||||||
| 1 cup/d 0.92 (0.73–1.15) | ||||||||
| 2–3 cups/d 0.84 (0.68–1.02) | ||||||||
| 4–5 cups/d 0.65 (0.51–0.84) | ||||||||
| ≥6 cups/d 0.83 (0.61–1.14) | ||||||||
| women stroke mortality | ||||||||
| 0 cup/d 1.00 (1.00–1.00) | ||||||||
| <1 cup/d 1.15 (0.91–1.45) | ||||||||
| 1 cup/d 0.89 (0.70–1.13) | ||||||||
| 2–3 cups/d 0.93 (0.75–1.15) | ||||||||
| 4–5 cups/d 0.82 (0.62–1.09) | ||||||||
| ≥6 cups/d 0.84 (0.56–1.25) | ||||||||
| Kokubo et al 2013 Japan |
both | 13 | 45–74 | 4335/82369 | Total CVD | CVD, CHD, stroke | FFQ (baseline)/Confirmed cases | age; sex; smoking; alcohol; body mass index; history of diabetes mellitus; medication of antihypercholesterolemia and antihypertension; sports; dietary intake of fruits, vegetables, fish, and energy; public health centers; and green tea consumption |
| 0 cup/week 1.00 (1.00–1.00) | ||||||||
| 1–2 cups/week 0.93 (0.86–1.01) | ||||||||
| 3–6 cups/ week 0.89 (0.81–0.98) | ||||||||
| 1 cups/d 0.84 (0.76–0.92) | ||||||||
| ≥2 cups/d 0.89 (0.80–0.99) | ||||||||
| Stroke | ||||||||
| 0 cup/week 1.00 (1.00–1.00) | ||||||||
| 1–2 cups/week 0.94 (0.85–1.02) | ||||||||
| 3–6 cups/week 0.89 (0.80–0.99) | ||||||||
| 1 cup/day 0.80 (0.72–0.90) | ||||||||
| ≥2 cups/day 0.81 (0.72–0.91) | ||||||||
| CHD | ||||||||
| 0 cup/week 1.00 (1.00–1.00) | ||||||||
| 1–2 cups/week 0.91 (0.76–1.10) | ||||||||
| 3–6 cups/week 0.92 (0.75–1.14) | ||||||||
| 1 cups/d 0.99 (0.81–1.23) | ||||||||
| ≥2 cups/d 1.21 (0.98–1.50) |
CHD: coronary heart disease; CVD: cardiovascular disease; FFQ: food frequency questionnaire
Coffee consumption and risk of CVD
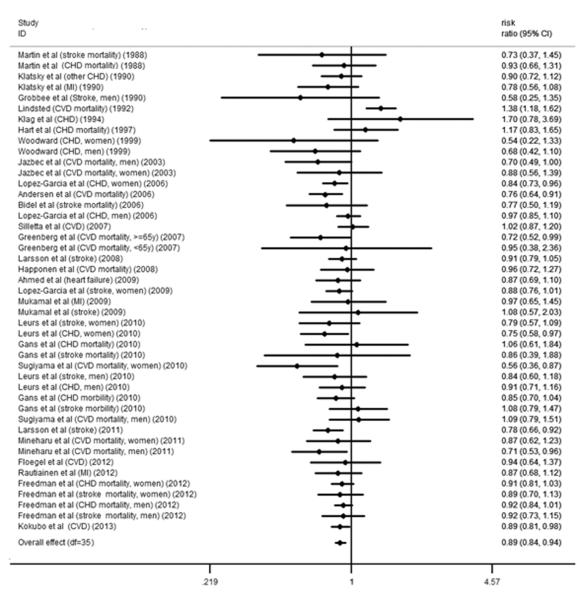
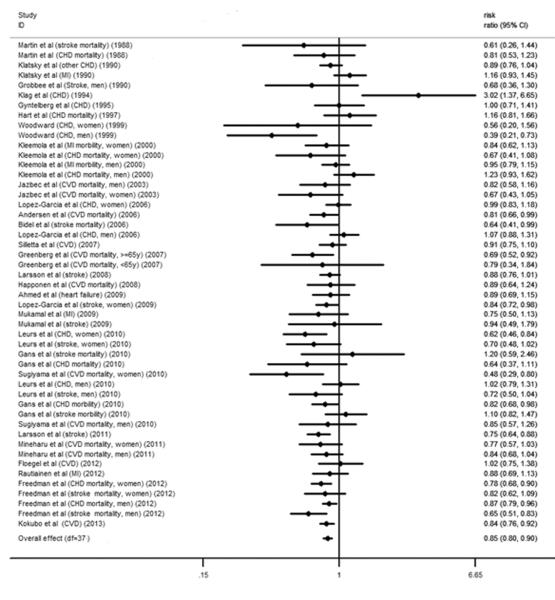
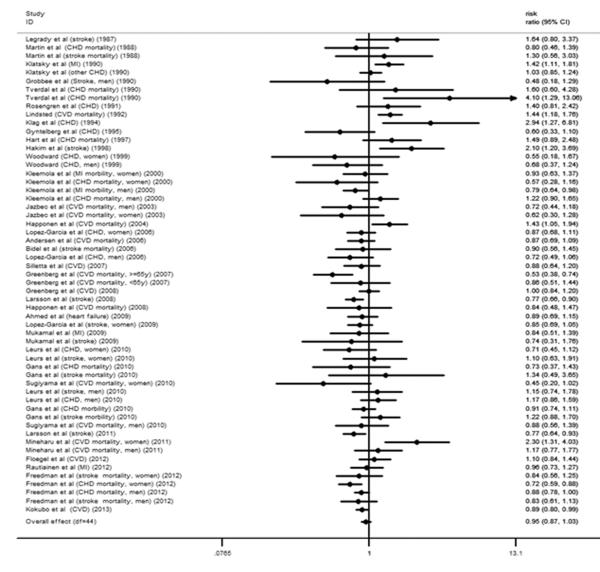
The relative risks for CVD with different coffee consumption categories relative to the lowest category are shown in Figure 2. Of the 35 studies, 6 cohorts presented the outcome of stroke and CHD simultaneously. Compared with the lowest category of coffee consumption (median and mean: 0 cups/d), the pooled RR for incident CVD was 0.89 (95% CI, 0.84 to 0.94) for the third highest (median: 1.5 cups/d; mean: 1.48 cups/d), 0.85 (95% CI, 0.80 to 0.90) for the second highest (median: 3.5 cups/d; mean: 3 cups/d) and 0.95 (95% CI, 0.87 to 1.03) for the highest (median: 5 cups/d; mean: 5.5 cups/d) category of coffee consumption (Figure 2). Low between-study variances of CVD risk were found for each category of coffee consumption (tau-squared = 0.00 for the random-effects models), and the imputed correlation coefficient between the risks of stroke and CHD within the same cohort (0 < ρ ≤ 1) did not have an effect on the relative risk of CVD for each category of coffee consumption.
Figure 2.
A. Forest plot of the association between third highest level of coffee consumption (median consumption: 1.5 cups/d) and risk of CVD compared to the lowest level (median consumption: 0 cup/d). The overall effect was obtained from a fixed-effects model that accounted for correlated outcomes. MI means myocardial infarction incidence; CVD means cardiovascular disease incidence; stroke means stroke incidence. B. Forest plot of the association between second highest level of coffee consumption (median consumption: 3.5 cups/d) and risk of CVD compared to the lowest level (median consumption: 0 cups/d). The overall effect was obtained from a fixed-effects model that accounted for correlated outcomes. MI means myocardial infarction incidence; CVD means cardiovascular disease incidence; stroke means stroke incidence. C. Forest plot of the association between highest level of coffee consumption (median consumption: 7 cups/d) and risk of CVD compared to the lowest level (median consumption: 0 cups/d). The overall effect was obtained from a fixed-effects model that accounted for correlated outcomes. MI means myocardial infarction incidence; CVD means cardiovascular disease incidence; stroke means stroke incidence.
Stratified analyses
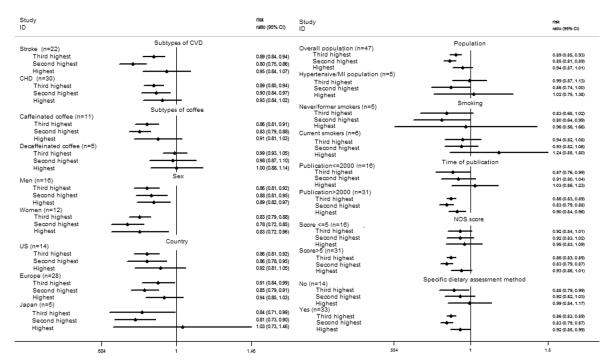
Stratified analyses were conducted according to baseline hypertension or MI of the study population, smoking status, publication year, NOS study quality score, dietary assessment method (24-h diet recall/diet record/FFQ versus other methods), stroke versus CHD as the outcome, country, sex, and type of coffee (caffeinated coffee or decaffeinated coffee). No interactions between categorized coffee consumption and stratification variables in relation to CVD risk were observed (all P for interactions >0.05) (Figure 3). Only 4 studies provided the stratified results by age.27–30 The summarized results showed that, comparing the highest with the lowest intakes, the RR of CVD was 0.96 (95% CI, 0.65 to 1.42) for age < 65 years, and the RR was 0.91 (95% CI, 0.59 to 1.40) for age ≥ 65 years.
Figure 3.
Stratified analysis of the association between coffee consumption and risk of CVD. The included studies for the stratified analysis were the same as that for the dose response analysis. n was the number of comparisons for the highest level of coffee consumption. NOS score: the score using the Newcastle-Ottawa scale; specific dietary assessment method: diet that was assessed by 24h diet recall, diet record or food frequency questionnaire.
For the risk of CHD, compared with the lowest category of coffee consumption, the RRs of CHD were 0.89 (95% CI, 0.85 to 0.94; P for heterogeneity = 0.83; I2 = 0.0%) for the third highest category, 0.90 (95% CI, 0.84 to 0.97; P for heterogeneity = 0.02; I2 = 40.3%) for the second highest category, and 0.93 (95% CI, 0.84 to 1.02; P for heterogeneity < 0.001; I2 = 52.8%) for the highest category of coffee consumption. The corresponding RRs of stroke were 0.89 (95% CI, 0.84 to 0.94; P for heterogeneity = 0.58; I2 = 0.0%) for the third category, 0.80 (95% CI, 0.75 to 0.86; P for heterogeneity = 0.37; I2 = 6.5%) for the second category, and 0.95 (95% CI, 0.84 to 1.07; P for heterogeneity = 0.001; I2 = 54.5%) for the highest category.
Dose-response analysis of coffee consumption with risk of CVD
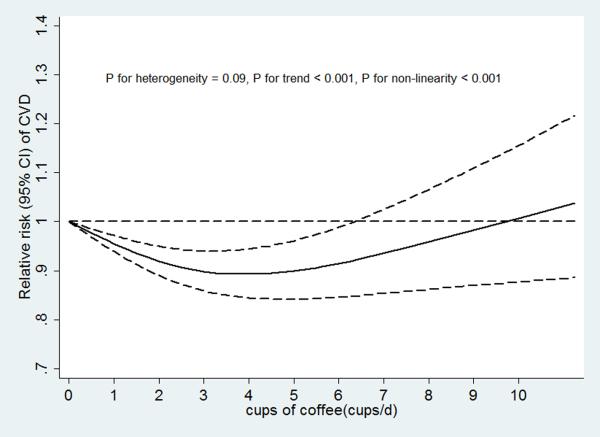
In our dose-response analysis, we observed a non-linear association between coffee consumption and risk of CVD (P for non-linearity < 0.001) with a significant trend (P for trend <0.001) and limited heterogeneity in study results (P for heterogeneity = 0.09) (Figure 4a). Compared to those with no coffee consumption, the RR estimated directly from the cubic spline model was 0.95 (95% CI, 0.93 to 0.97) for 1 cup/d, 0.92 (95% CI, 0.88 to 0.95) for 2 cups/d, 0.89 (95% CI, 0.85 to 0.93) for 3 cups/d, 0.88 (95% CI, 0.83 to 0.93) for 4 cups/d, 0.89 (95% CI, 0.83 to 0.95) for 5 cups/d, 0.91 (95% CI, 0.84 to 0.99) for 6 cups/d, and 0.93 (95% CI, 0.85 to 1.03) for 7 cups/d.
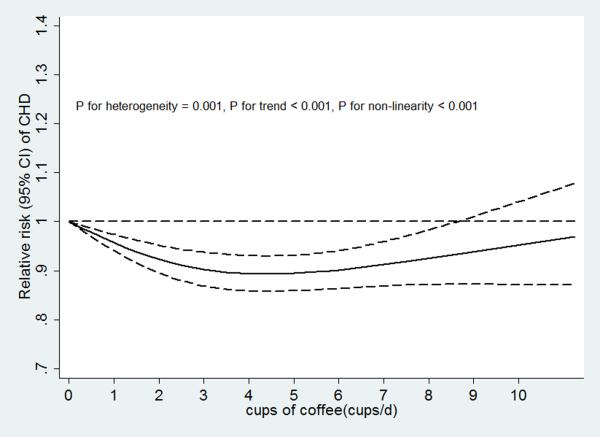
Figure 4.
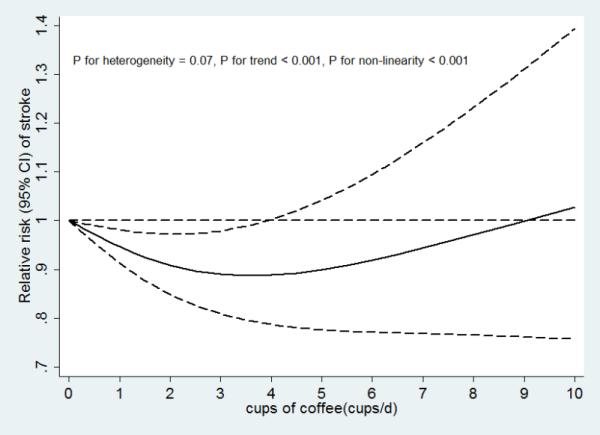
A. Coffee consumption and risk of CVD (n = 47). B. Coffee consumption and risk of CHD (n = 31). C. Coffee consumption and risk of stroke (n = 22). D. Dose response relationships of coffee consumption with risk of CVD. n was the number of comparisons.
Non-linear (p values for non-linearity <0.001) associations between coffee consumption and disease risk with significant trends (p values for trend <0.001) were found for both CHD and stroke (Figures 4b and 4c). There was stronger evidence for heterogeneity in study results for the association of coffee consumption with CHD risk (P heterogeneity=0.001) than for the association with stroke risk (P heterogeneity=0.07).
We further explored the reason for the heterogeneity between coffee consumption and CHD risk by stratifying the studies by publication year (≤ 2000 or > 2000). We found that in studies published in year 2000 or earlier, coffee consumption was not significantly associated with CHD risk (n=13, P for heterogeneity = 0.20), whereas in later studies, coffee consumption was nonlinearly associated with CHD risk (n= 18, P for heterogeneity = 0.08). We didn't perform a similar analysis for stroke because very few studies on stroke were published prior to 2000.
Sensitivity analysis
We tested the robustness of our results in sensitivity analyses. Because the RRs of stroke and CHD from the same cohort were correlated and a total of 6 studies included both CHD and stroke results, we conducted a sensitivity analysis by including only one outcome at a time. Our results remained largely unchanged and non-linear curves were found with including either CHD or stroke as the outcome (Supplemental Figure 1a and 1b).
One study with coffee consumption modeled as a continuous variable was excluded from the main analysis;26 we added the RR from this study to the dose-response analysis by a two stage method and the results did not substantially change.
To test whether the association between coffee consumption and risk of CVD was different for unadjusted and multivariable adjusted models, we performed a dose-response meta-analysis of the only age-adjusted data including 34 comparisons (Supplemental Figure 2). Multivariate adjustment strengthened the inverse association between moderate consumption and CVD risk, most likely due to adjustment for smoking.
Publication bias
The Egger test did not suggest publication bias for associations for any category of coffee consumption and risk of CVD (Supplemental Figure 3 and Table 2).
DISCUSSION
The findings from this systematic review and meta-analysis, based on approximately 1,283,685 study participants and 47,779 CVD cases, including about 28,347 CHD cases, 12,030 stroke cases and 7,402 other CVD cases, demonstrate a non-linear association between coffee consumption and risk of CVD. Moderate coffee consumption (3–5 cups/day) was associated with lower CVD risk, and heavy coffee consumption (≥6 cups/day) was neither associated with a higher nor a lower risk of CVD.
In contrast to our results, a previous meta-analysis summarizing 21 prospective cohort studies31 found no association between moderate coffee consumption and CHD risk in the overall population. One possible reason is that the previous meta-analysis included 7 studies without adjustment for confounders, which might have biased the relative risks upwards because of confounding by factors such as smoking.
A recent cohort study by Liu et al32 found that 4 cups per day of coffee consumption was associated with increased mortality, but the association was only significant for participants under 55 years old. The results from this study contradict those from this meta-analysis and the majority of studies in the literature. Possible reasons for this discrepancy include a relatively small size, lack of updated dietary assessment, and subgroup analysis. In our meta-analysis, stratified analysis by age revealed no significant differences in the association across age groups.
The debate about the relation between coffee consumption and CVD risk mainly stemmed from inconsistent results according to different study designs. Case-control studies, which are prone to recall bias and selection bias, tended to show a positive association, whereas cohort studies generally showed a null association.10 Still, findings from prospective cohort studies on coffee consumption and CVD risk have remained inconsistent. Differences among studies in sample sizes, the characteristics of the study populations, the assessment methods for coffee consumption, and statistical adjustments may have contributed to divergent results. Since the true association between coffee consumption and CVD risk is likely to be modest and nonlinear, the differences in coffee assessments and covariate adjustments may result in changes the magnitude and even the direction of the associations and thus lead to different conclusions.
The U-shaped association between coffee consumption and CVD risk observed in this meta-analysis need to be considered from both methodological and biological points of view. First, individuals with hypertension or other conditions related to CVD risk might have changed their coffee consumption before baseline. Thus, baseline disease, especially hypertension, as a confounder could result in reverse causation. However, we observed no significant difference in the association between coffee consumption and CVD risk between cohorts with hypertensive and MI patients and the general population cohorts. Second, smoking is likely to be an important confounder for the association between coffee consumption and CVD risk, and could bias the relative risks upwards. Heavy coffee consumption was associated with higher risk of CVD in age-adjusted analyses, but this is likely due to confounding by smoking. After adjustment for smoking and other covariates, heavy coffee was consumption was not significantly associated with CVD and the inverse association between moderate consumption and CVD became stronger.
The non-linear U-shaped between coffee consumption and CVD risk might also be true based on plausible biological mechanisms. Coffee is a complex chemical mixture with hundreds of compounds including the phenolic compound chlorogenic acid, caffeine, minerals such as potassium and magnesium, niacin and its precursor trigonelline, and lignans. Coffee consumption has been associated with higher insulin sensitivity, a lower risk of type 2 diabetes, and lower concentrations of inflammatory markers such as C-reactive protein and E-selectin.33, 34 However, short-term metabolic studies have shown that caffeine can acutely increase blood pressure by antagonizing the adenosine A1 and A2A receptor,35–37 and could also acutely adversely affect arterial stiffness and endothelium dependent vasodilation.38, 39 Long-term heavy coffee consumption has been associated with a slightly elevated risk of hypertension,40 and a higher level of plasma homocysteine.41, 42 In addition, cafestol in unfiltered coffee increases serum total cholesterol concentrations.43 The non-linear U-shaped between coffee consumption and risk of CVD might be due to a combination of beneficial and detrimental effects: for moderate coffee consumption, beneficial effects may be greater than adverse effects; whereas for heavy consumption, detrimental effect may counterbalance beneficial effects. Results from case crossover studies suggest that coffee consumption transiently increases risk of nonfatal myocardial infarction, ischemic stroke onset, and sudden cardiac death.44–46 However, we could not differentiate acute effects from long-term effects of habitual coffee consumption in this study.
No significant association between decaffeinated coffee consumption with CVD risk was observed in this meta-analysis. There were several potential explanations. First, the consumption of decaffeinated coffee was much lower than caffeinated coffee, diminishing the power to detect any association. Second, the null association might be due to a reverse causation problem in that individuals with hypertension or other CVD-related conditions might switch from regular coffee to decaffeinated coffee. This reverse causation may mitigate an inverse association between decaffeinated coffee consumption and CVD risk.
We did not observe a significant association between coffee consumption and CHD risk for earlier publications (2000 or earlier). There are two potential reasons for this finding. First, coffee brewing methods have changed over time and nowadays the filter method has become more popular, effectively replacing unfiltered forms of coffee such as boiled coffee that was more widely consumed by participants in earlier studies. It has been shown that drinking boiled coffee increases serum cholesterol, an important risk factor for CVD5. Second, in earlier studies, the sample size was typically small; the measurement of baseline characteristics was typically crude; statistical control of confounders such as diet was inadequate; and the average NOS study quality score was lower. Our stratified analysis showed that coffee consumption was not associated with CVD risk in subgroups with a lower NOS score.
A study by Cornelis MC et al.47 showed that CYP1A2 genotype was an effect modifier between coffee consumption and risk of myocardial infarction: coffee consumption was related to higher risk of myocardial infarction for the slow caffeine metabolizer, and was not related to myocardial infarction for the fast caffeine metabolizer. However, this analysis was based on a case-control study conducted in Costa Rico and the results have not been replicated in prospective cohort studies yet.
Recently, a genome-wide association study (GWAS) found a highly significant association between a variant on CYP1A2 and coffee intake48. However, this variant explains only a very small population variance. Since the vast majority of our participants were Caucasians, the allele frequency was expected to be consistent across various cohorts. Ideally, the meta-analyses should be done according to different genotypes of CYP1A2. However, none of the included cohorts assessed the genotypes and thus we were unable to conduct such a stratified analysis.
Our meta-analysis has several strengthens. First, our meta-analysis included 35 cohort studies and 1,283,685 participants, which provided sufficient power to detect modest associations. Second, because of the prospective design of all included studies, differential misclassification of coffee consumption due to recall bias was minimized and the likelihood of selection bias is reduced. Third, we used both semi-parametric and parametric methods, and both analyses indicated a U-shaped relationship between coffee consumption and CVD risk. Finally, we conducted stratified analyses according to disease endpoints, geographic locations of the studies, type of coffee, and baseline characteristics of the study populations. The subgroup results are highly consistent and robust.
Our study also has several limitations. Given the observational nature of the studies, the possibility of residual confounding cannot be excluded. However, since higher coffee consumption was generally associated with a less healthy lifestyle such as a higher prevalence of cigarette smoking, less physical activity, and a less healthy diet, the observed association between moderate coffee consumption and a lower CVD risk is unlikely to be explained by these confounders. In addition, residual confounding by smoking may have biased the association for heavy coffee consumption upward, which may explain our finding that adjustment for smoking and other covariates actually strengthened the inverse association. Nonetheless, because of the observational nature of the included studies, a causal relationship cannot be established with these data alone. In addition, coffee brewing methods were not assessed in the included studies. However, given coffee consumption habits in the studied populations most consumed coffee is likely to have been filtered coffee. As a result, our results may not apply to unfiltered coffee (e.g. French press, Scandinavian boiled, or Turkish/Greek coffee).
In conclusion, our meta-analysis suggests a non-linear relationship between coffee consumption and CVD risk. Moderate coffee consumption was associated with lower CVD risk, with the lowest CVD risk at 3 to 5 cups/d of coffee consumption, and heavy coffee consumption was not associated with CVD risk. This non-linear association with coffee consumption was observed for both the risk of CHD and stroke.
Supplementary Material
Clinical perspective.
Coffee is one of the most widely consumed beverages around the world, and its association with cardiovascular disease has been investigated in numerous epidemiologic studies. However, a key issue that remains to be resolved is the dose response relationship of long-term coffee consumption with cardiovascular disease (CVD) risk, including incidence of CHD, stroke, and heart failure, and CVD mortality. In the current meta-analysis, we summarized results from 36 prospective cohort studies on coffee consumption and CVD risk with 1,279,804 study participants and 36,352 CVD cases. We found a non-linear relationship of coffee consumption with CVD risk: moderate coffee consumption was associated with lower risk of CVD, with the lowest CVD risk at 3 to 5 cups/d, and heavy coffee consumption was not associated with risk of CVD. Looking at separately, we also found non-linear relationships of coffee consumption with CHD and stroke risks. The present study provides strong evidence that long-term heavy consumption of coffee is not associated with CVD risk, and also provides insight into the potential mechanism of the non-linear relationship between coffee consumption and CVD risk. We believe that this report will be of significant interest to clinicians involved in the prevention and treatment of CVD.
Acknowledgments
Funding Sources: This study received support from NIH grant HL60712. Dr. van Dam received a research grant from the Nestec Company.
Footnotes
Conflict of Interest Disclosures: No potential conflicts of interest relevant to this article were reported.
References
- 1.Paul O, Lepper MH, Phelan WH, Dupertuis GW, Macmillan A, Mc KH, Park H. A longitudinal study of coronary heart disease. Circulation. 1963;28:20–31. doi: 10.1161/01.cir.28.1.20. [DOI] [PubMed] [Google Scholar]
- 2.Robertson D, Frolich JC, Carr RK, Watson JT, Hollifield JW, Shand DG, Oates JA. Effects of caffeine on plasma renin activity, catecholamines and blood pressure. N Engl J Med. 1978;298:181–186. doi: 10.1056/NEJM197801262980403. [DOI] [PubMed] [Google Scholar]
- 3.Dobmeyer DJ, Stine RA, Leier CV, Greenberg R, Schaal SF. The arrhythmogenic effects of caffeine in human beings. N Engl J Med. 1983;308:814–816. doi: 10.1056/NEJM198304073081405. [DOI] [PubMed] [Google Scholar]
- 4.Thelle DS, Heyden S, Fodor JG. Coffee and cholesterol in epidemiological and experimental studies. Atherosclerosis. 1987;67:97–103. doi: 10.1016/0021-9150(87)90270-x. [DOI] [PubMed] [Google Scholar]
- 5.Bak AA, Grobbee DE. The effect on serum cholesterol levels of coffee brewed by filtering or boiling. N Engl J Med. 1989;321:1432–1437. doi: 10.1056/NEJM198911233212103. [DOI] [PubMed] [Google Scholar]
- 6.Coffee drinking and acute myocardial infarction. Report from the boston collaborative drug surveillance program. Lancet. 1972;2:1278–1281. [PubMed] [Google Scholar]
- 7.Jick H, Miettinen OS, Neff RK, Shapiro S, Heinonen OP, Slone D. Coffee and myocardial infarction. N Engl J Med. 1973;289:63–67. doi: 10.1056/NEJM197307122890203. [DOI] [PubMed] [Google Scholar]
- 8.Hennekens CH, Drolette ME, Jesse MJ, Davies JE, Hutchison GB. Coffee drinking and death due to coronary heart disease. N Engl J Med. 1976;294:633–636. doi: 10.1056/NEJM197603182941203. [DOI] [PubMed] [Google Scholar]
- 9.Kawachi I, Colditz GA, Stone CB. Does coffee drinking increase the risk of coronary heart disease? Results from a meta-analysis. Br Heart J. 1994;72:269–275. doi: 10.1136/hrt.72.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenland S. A meta-analysis of coffee, myocardial infarction, and coronary death. Epidemiology. 1993;4:366–374. doi: 10.1097/00001648-199307000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Freedman ND, Park Y, Abnet CC, Hollenbeck AR, Sinha R. Association of coffee drinking with total and cause-specific mortality. N Engl J Med. 2012;366:1891–1904. doi: 10.1056/NEJMoa1112010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kokubo Y, Iso H, Saito I, Yamagishi K, Yatsuya H, Ishihara J, Inoue M, Tsugane S. The impact of green tea and coffee consumption on the reduced risk of stroke incidence in japanese population: The japan public health center-based study cohort. Stroke. 2013;44:1369–1374. doi: 10.1161/STROKEAHA.111.677500. [DOI] [PubMed] [Google Scholar]
- 13.Floegel A, Pischon T, Bergmann MM, Teucher B, Kaaks R, Boeing H. Coffee consumption and risk of chronic disease in the european prospective investigation into cancer and nutrition (epic)-germany study. Am J Clin Nutr. 2012;95:901–908. doi: 10.3945/ajcn.111.023648. [DOI] [PubMed] [Google Scholar]
- 14.Sofi F, Conti AA, Gori AM, Eliana Luisi ML, Casini A, Abbate R, Gensini GF. Coffee consumption and risk of coronary heart disease: A meta-analysis. Nutr Metab Cardiovasc Dis. 2007;17:209–223. doi: 10.1016/j.numecd.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Larsson SC, Orsini N. Coffee consumption and risk of stroke: A dose-response meta-analysis of prospective studies. Am J Epidemiol. 2011;174:993–1001. doi: 10.1093/aje/kwr226. [DOI] [PubMed] [Google Scholar]
- 16.Mostofsky E, Rice MS, Levitan EB, Mittleman MA. Habitual coffee consumption and risk of heart failure: A dose-response meta-analysis. Circ Heart Fail. 2012;5:401–405. doi: 10.1161/CIRCHEARTFAILURE.112.967299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugiyama K, Kuriyama S, Akhter M, Kakizaki M, Nakaya N, Ohmori-Matsuda K, Shimazu T, Nagai M, Sugawara Y, Hozawa A, Fukao A, Tsuji I. Coffee consumption and mortality due to all causes, cardiovascular disease, and cancer in japanese women. J Nutr. 2010;140:1007–1013. doi: 10.3945/jn.109.109314. [DOI] [PubMed] [Google Scholar]
- 18.de Koning Gans JM, Uiterwaal CS, van der Schouw YT, Boer JM, Grobbee DE, Verschuren WM, Beulens JW. Tea and coffee consumption and cardiovascular morbidity and mortality. Arterioscler Thromb Vasc Biol. 2010;30:1665–1671. doi: 10.1161/ATVBAHA.109.201939. [DOI] [PubMed] [Google Scholar]
- 19.Larsson SC, Virtamo J, Wolk A. Coffee consumption and risk of stroke in women. Stroke. 2011;42:908–912. doi: 10.1161/STROKEAHA.110.603787. [DOI] [PubMed] [Google Scholar]
- 20.Malerba S, Turati F, Galeone C, Pelucchi C, Verga F, La Vecchia C, Tavani A. A meta-analysis of prospective studies of coffee consumption and mortality for all causes, cancers and cardiovascular diseases. Eur J Epidemiol. 2013;28:527–39. doi: 10.1007/s10654-013-9834-7. doi: 10.1007/s10654-013-9834-7. Epub 2013 Aug 11. [DOI] [PubMed] [Google Scholar]
- 21.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (moose) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 22.Wells GA SB, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The newcastle-ottawa scale (nos) for assessing the quality of nonrandomized studies in meta-analysis. 2011 www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 23.Hedges LV TE, Johnson MC. Robust variance estimation in meta-regression with dependent effect size estimates. Research Synthesis Methods. 2010;1:39–65. doi: 10.1002/jrsm.5. [DOI] [PubMed] [Google Scholar]
- 24.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilhelmsen L, Tibblin G, Elmfeldt D, Wedel H, Werko L. Coffee consumption and coronary heart disease in middle-aged swedish men. Acta Med Scand. 1977;201:547–552. doi: 10.1111/j.0954-6820.1977.tb15745.x. [DOI] [PubMed] [Google Scholar]
- 27.Klatsky AL, Friedman GD, Armstrong MA. Coffee use prior to myocardial infarction restudied: Heavier intake may increase the risk. Am J Epidemiol. 1990;132:479–488. doi: 10.1093/oxfordjournals.aje.a115684. [DOI] [PubMed] [Google Scholar]
- 28.Lindsted KD, Kuzma JW, Anderson JL. Coffee consumption and cause-specific mortality. Association with age at death and compression of mortality. J Clin Epidemiol. 1992;45:733–742. doi: 10.1016/0895-4356(92)90051-n. [DOI] [PubMed] [Google Scholar]
- 29.Bidel S, Hu G, Qiao Q, Jousilahti P, Antikainen R, Tuomilehto J. Coffee consumption and risk of total and cardiovascular mortality among patients with type 2 diabetes. Diabetologia. 2006;49:2618–2626. doi: 10.1007/s00125-006-0435-9. [DOI] [PubMed] [Google Scholar]
- 30.Greenberg JA, Dunbar CC, Schnoll R, Kokolis R, Kokolis S, Kassotis J. Caffeinated beverage intake and the risk of heart disease mortality in the elderly: A prospective analysis. AmJ Clin Nutr. 2007;85:392–398. doi: 10.1093/ajcn/85.2.392. [DOI] [PubMed] [Google Scholar]
- 31.Wu JN, Ho SC, Zhou C, Ling WH, Chen WQ, Wang CL, Chen YM. Coffee consumption and risk of coronary heart diseases: A meta-analysis of 21 prospective cohort studies. Int JCardiol. 2009;137:216–225. doi: 10.1016/j.ijcard.2008.06.051. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Sui X, Lavie CJ, Hebert JR, Earnest CP, Zhang J, Blair SN. Association of coffee consumption with all-cause and cardiovascular disease mortality. Mayo Clin Proc. 2013;88:1066–74. doi: 10.1016/j.mayocp.2013.06.020. doi: 10.1016/j.mayocp.2013.06.020. Epub 2013 Aug 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Dam RM, Hu FB. Coffee consumption and risk of type 2 diabetes: A systematic review. JAMA. 2005;294:97–104. doi: 10.1001/jama.294.1.97. [DOI] [PubMed] [Google Scholar]
- 34.Williams CJ, Fargnoli JL, Hwang JJ, van Dam RM, Blackburn GL, Hu FB, Mantzoros CS. Coffee consumption is associated with higher plasma adiponectin concentrations in women with or without type 2 diabetes: A prospective cohort study. Diabetes Care. 2008;31:504–507. doi: 10.2337/dc07-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nurminen ML, Niittynen L, Korpela R, Vapaatalo H. Coffee, caffeine and blood pressure: A critical review. Eur J Clin Nutr. 1999;53:831–839. doi: 10.1038/sj.ejcn.1600899. [DOI] [PubMed] [Google Scholar]
- 36.Mesas AE, Leon-Munoz LM, Rodriguez-Artalejo F, Lopez-Garcia E. The effect of coffee on blood pressure and cardiovascular disease in hypertensive individuals: A systematic review and meta-analysis. Am J Clin Nutr. 2011;94:1113–1126. doi: 10.3945/ajcn.111.016667. [DOI] [PubMed] [Google Scholar]
- 37.Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 38.Karatzis E, Papaioannou TG, Aznaouridis K, Karatzi K, Stamatelopoulos K, Zampelas A, Papamichael C, Lekakis J. Mavrikakis M. Acute effects of caffeine on blood pressure and wave reflections in healthy subjects: Should we consider monitoring central blood pressure? Int JCardiol. 2005;98:425–430. doi: 10.1016/j.ijcard.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 39.Papamichael CM, Aznaouridis KA, Karatzis EN, Karatzi KN, Stamatelopoulos KS, Vamvakou G, Lekakis JP, Mavrikakis ME. Effect of coffee on endothelial function in healthy subjects: The role of caffeine. Clin Sci (Lond) 2005;109:55–60. doi: 10.1042/CS20040358. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Z, Hu G, Caballero B, Appel L, Chen L. Habitual coffee consumption and risk of hypertension: A systematic review and meta-analysis of prospective observational studies. Am JClin Nutr. 2011;93:1212–1219. doi: 10.3945/ajcn.110.004044. [DOI] [PubMed] [Google Scholar]
- 41.Verhoef P, Pasman WJ, Van Vliet T, Urgert R, Katan MB. Contribution of caffeine to the homocysteine-raising effect of coffee: A randomized controlled trial in humans. Am J Clin Nutr. 2002;76:1244–1248. doi: 10.1093/ajcn/76.6.1244. [DOI] [PubMed] [Google Scholar]
- 42.Olthof MR, Hollman PC, Zock PL, Katan MB. Consumption of high doses of chlorogenic acid, present in coffee, or of black tea increases plasma total homocysteine concentrations in humans. Am J Clin Nutr. 2001;73:532–538. doi: 10.1093/ajcn/73.3.532. [DOI] [PubMed] [Google Scholar]
- 43.Urgert R, Katan MB. The cholesterol-raising factor from coffee beans. Annu Rev Nutr. 1997;17:305–324. doi: 10.1146/annurev.nutr.17.1.305. [DOI] [PubMed] [Google Scholar]
- 44.Baylin A, Hernandez-Diaz S, Kabagambe EK, Siles X, Campos H. Transient exposure to coffee as a trigger of a first nonfatal myocardial infarction. Epidemiology. 2006;17:506–511. doi: 10.1097/01.ede.0000229444.55718.96. [DOI] [PubMed] [Google Scholar]
- 45.Selb Semerl J, Selb K. Coffee and alcohol consumption as triggering factors for sudden cardiac death: Case-crossover study. Croat Med J. 2004;45:775–780. [PubMed] [Google Scholar]
- 46.Mostofsky E, Schlaug G, Mukamal KJ, Rosamond WD, Mittleman MA. Coffee and acute ischemic stroke onset: The stroke onset study. Neurology. 2010;75:1583–1588. doi: 10.1212/WNL.0b013e3181fb443d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cornelis MC, El-Sohemy A, Kabagambe EK, Campos H. Coffee, cyp1a2 genotype, and risk of myocardial infarction. JAMA. 2006;295:1135–1141. doi: 10.1001/jama.295.10.1135. [DOI] [PubMed] [Google Scholar]
- 48.Cornelis MC, Monda KL, Yu K, Paynter N, Azzato EM, Bennett SN, Berndt SI, Boerwinkle E, Chanock S, Chatterjee N, Couper D, Curhan G, Heiss G, Hu FB, Hunter DJ, Jacobs K, Jensen MK, Kraft P, Landi MT, Nettleton JA, Purdue MP, Rajaraman P, Rimm EB, Rose LM, Rothman N, Silverman D, Stolzenberg-Solomon R, Subar A, Yeager M, Chasman DI, van Dam RM, Caporaso NE. Genome-wide meta-analysis identifies regions on 7p21 (ahr) and 15q24 (cyp1a2) as determinants of habitual caffeine consumption. PLoS Genet. 2011;7:e1002033. doi: 10.1371/journal.pgen.1002033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.