Abstract
Living organisms are exposed on a daily basis to widespread mixtures of toxic compounds. Mixtures pose a major problem in the assessment of health effects because they often generate substance-specific effects that cannot be attributed to a single mechanism. Two compounds often found together in the environment are the heavy metal chromium and the polycyclic aromatic hydrocarbon benzo[a]pyrene (B[a]P). We have examined how long-term exposure to a low concentration of Cr(VI) affects the transcriptional response to B[a]P, a second toxicant with an unrelated mechanism of action. Growth of mouse hepatoma cells for 20 passages in medium with 0.1 or 0.5 μM Cr(VI) increases DNA damage and apoptosis while decreasing clonogenic ability. Treated cells also show transcriptome changes indicative of increased expression of DNA damage response and repair genes. In them, B[a]P activates cancer progression pathways, unlike in cells never exposed to Cr(VI), where B[a]P activates mostly xenobiotic metabolism pathways. Cells grown in Cr(VI) for 20 passages and then cultured for an additional 5 passages in the absence of Cr(VI) recover from some but not all the chromium effects. They show B[a]P-dependent transcriptome changes strongly weighted towards xenobiotic metabolism, similar to those in B[a]P-treated cells that had no previous Cr(VI) exposure, but retain a high level of Cr(VI)-induced DNA damage and silence the expression of DNA damage and cancer progression genes. We conclude that the combined effect of these two toxicants appears to be neither synergistic nor additive, generating a toxic/adaptive condition that cannot be predicted from the effect of each toxicant alone.
Keywords: Chromium, heavy metals, B[a]P, Gene expression, complex mixtures, transcriptome
1. Introduction
Living organisms are exposed to complex mixtures of natural and man-made compounds for the length of their life. Mixtures of toxic and carcinogenic compounds, including heavy metals, polycyclic aromatic hydrocarbons (PAHs), and halogenated compounds, are ubiquitous and widespread in the environment. One of the major problems in current toxicology and its application to risk assessment is the lack of a sound methodology to deal with the health effects of such chemical mixtures (Sexton et al. 1995). Data are scarce, and this scarcity points out at the need to evaluate critically the assumptions used to determine risk from exposure to complex toxicant mixtures, ultimately leading to an understanding of the health effects of exposure. While many studies have dealt with the mechanisms of action and effect of individual classes of compounds, little work has examined how the individual responses to the single toxic constituents of a mixture bears on the response to a mixture of the components or how the mechanisms operative at one dose of exposure can be extrapolated to the biological responses at different doses. Two classes of carcinogenic molecules in particular, heavy metals and PAHs, are increasingly common as co-contaminants in many anthropogenic activities such as municipal waste incineration, fossil fuel burning, car exhaust, and industrial smelting activities. Assessing the effects of combined exposures to non-metabolizable halogenated aromatic hydrocarbons has been proposed based on the relative potency of the individual compounds (Birnbaum and DeVito 1995) and to PAHs based on their relative carcinogenic potency (Collins et al. 1998). These approaches, while valuable, are limited to mixtures of similar compounds (Cizmas et al. 2004) and are of little use when dealing with complex mixtures of multiple components, because combined exposures generate substance-specific changes in gene expression that cannot be attributed to a single mechanism.
The heavy metal chromium and the PAH benzo(a)pyrene (B[a]P) are ranked 17th and 8th, respectively, on the National Priority List of Hazardous Substances and can be found as co-contaminants in cigarette smoke, exhaust from automotive catalytic converters, and industrial waste (Saha R. et al. 2011). Cr(III), the most common form of chromium naturally found in the environment, is an essential nutrient involved in the metabolism of fats and sugars (Cefalu et al. 2002). Cr(VI) on the other hand, is a powerful carcinogen and mutagen, and the form most often produced from industrial and anthropogenic processes (Dayan and Paine 2001; Ding et al. 2000). A clear association between Cr(VI) exposure and health effects, including increased incidence of lung cancer, has been recognized for over a century (Barchowsky and O'Hara 2003; Dayan and Paine 2001; Gibb et al. 2000). Cr(VI) enters the cell via the sulfate ion transporter and once inside undergoes reduction through the Cr(V) and Cr(IV) intermediate oxidation states to the stable Cr(III) state. This reduction process generates reactive oxygen species and induces radical mediated DNA damage (Zhitkovich 2005). Additionally, the reduction process leads to the formation of stable complexes between chromium-III and DNA, including chromium-DNA adducts, DNA-chromium-DNA crosslinks, and protein-chromium-DNA crosslinks (Zhitkovich 2005). These chromium-DNA complexes occur preferentially in areas of high DNA replication, transcription, and RNA processing (Xu et al. 1994). Chromium cross-linking of proteins and DNA in these areas leads to disruption of chromatin remodeling and gene expression, which cause Cr(VI) down-regulation of inducible gene expression (Majumder et al. 2003; Manning et al. 1992; O'Brien et al. 2003; Shumilla et al. 1999; Wei et al. 2004; Wetterhahn and Hamilton 1989). Previous studies in our laboratory have shown that Cr(VI) cross-links DNA to complexes of histone deacetylase-1 (HDAC1) and DNA methyltransferase-1 (DNMT1), inhibiting B[a]P induced activation of Cyp1a1 transcription by blocking the establishment of epigenetic marks responsible for chromatin accessibility (Schnekenburger et al. 2007).
B[a]P, the prototypical PAH procarcinogen, is metabolized by the cytochromes p450 CYP1A1 and CYP1B1 into the ultimate carcinogen, benzo[a]pyrene-diol-epoxide (Conney et al. 1994). Induction of CYP1A1 in response to B[a]P is mediated transcriptionally by the aryl hydrocarbon receptor (AHR) (Nebert et al. 1991). AHR is a ligand activated transcription factor that in its inactive form is sequestered in the cytoplasm (Perdew 1988). Upon activation by ligand binding, AHR translocates to the nucleus and forms a heterodimer with the AHR-Nuclear-Translocator (ARNT) protein (Reyes et al. 1992). This complex recognizes cis-acting AHR Response Elements (AhREs, also termed DREs and XREs) in the regulatory region of genes in the AHR gene battery, including xenobiotic metabolism and detoxification genes like NQO1, CYP1B1, and CYP1A1 among many others (Puga et al. 2009).
The Maximum Contaminant Level Goal (MCLG) set by EPA for Cr(VI+III) is 100 ppb, or approximately 1 μM, which is 50-times the Cr(VI) level of 2 ppb or less found in drinking water (http://water.epa.gov/drink/contaminants/basicinformation/chromium.cfm). Most previous studies on the mechanism of action of chromium have commonly used cultured cells acutely treated for a short time with as much as 50-times the MCLG, a concentration clearly cytotoxic (Maier et al. 2000) and unrealistic for environmentally relevant exposures. In the present work, we have examined how treatment with a low concentration of Cr(VI) over a long period of time affects cellular homeostasis. Specifically, we have analyzed how the transcriptional response to B[a]P of cells continuously grown for 20-25 passages (60 to 75 generations) in the presence of 0.1 or 0.5 μM Cr(VI) differs from the B[a]P-induced transcriptional response of cells that have never been treated with Cr(VI). When maintained for a long time, treatment with Cr(VI) concentrations below the MCLG causes DNA damage, increased apoptosis incidence and decreased cell viability, disrupting the transcriptional response to B[a]P. After removal of the Cr(VI) challenge, cells recover from some but not all the chromium effects, for apoptosis and transcriptional disruption are minimized to the levels of untreated cells, but Cr(VI)-induced DNA damage remains at a heightened level.
2. Materials and Methods
2.1 Cells and treatments
Mouse hepatoma Hepa-1c1c7 (Hepa-1) cells from American Type Culture Collection were cultured in α-minimal essential medium (α-MEM, Gibco) supplemented with 5% (v/v) fetal bovine serum (Sigma), and 1% (v/v) antibiotic-antimycotic (Gibco) in 5% CO2 humidified atmosphere at 37°C. Cells were passaged at a 1:6 ratio when they attained 80% confluence, which typically occurred every 3 days, although by approximately the 10th passage, the growth rate of Cr-treated cells slowed down significantly, possibly due to a combination of increased apoptosis and cell cycle time and decreased viability. Each passage was considered to correspond to 3 generations or cell doublings. For Cr(VI) treatment, the medium was supplemented daily with either 0.1 μM or 0.5 μM CrK2O4 or left untreated as control. At every 5th passage, cells were treated with 5 μM B[a]P or an equivalent volume of DMSO for 8 hours for further analyses of gene expression and biological functions. This concentration of B[a]P is approximately 150 times above the daily level of human exposure for smokers or people living near hazardous waste sites (http://www.atsdr.cdc.gov/ToxProfiles) but consistent with doses used experimentally in rodents.
2.2 RNA isolation
Total RNA was isolated from experimental biological duplicates using the RNeasy™ kit from Qiagen® as per the manufacturer protocol. RNA was submitted to the Genomics Sequencing Core of the Department of Environmental Health at the University of Cincinnati for quality control analysis using the Agilent™ 2100 Bio-analyzer and next generation sequencing using the Illumina® Hiseq 1000™. RNA library construction was done usingTruSeq RNA sample prep kit from Illumina using 1μg of RNA from preps with an RNA integrity score greater than 7.0. Agencourt AMPure XP (Beckman Coulter) beads were used to purify cDNA fragments which were subsequently ligated to sample specific adapters which served as primer targets for enrichment by 10 cycles of PCR to enrich cDNA fragment copy numbers. 1 μl of purified PCR product was analyzed in the Agilent™ 2100 Bio-Analyzer to determine cDNA size and yield. Six individually indexed samples were then clustered using the TruSeq SR Cluster kit v3(Illumina) in an Illumina cBot system flow cell. Samples were sequenced on the Illumina HiSeq system using the TrueSeq SBS kit for 50 cycles resulting in approximately 30 million reads which were aligned to the genome using Illumina’s standard sequence alignment pipeline.
2.3 RNAseq data analysis
Sequence reads were de-multiplexed and exported to fastq files using Illumina’s CASAVA 1.8 software. The reads were then aligned to the reference genome (mm10 build) using TopHat aligner (Trapnell et al. 2009). The counts of reads aligning to each gene’s coding region were summarized using ShortRead (Morgan et al. 2009) and associated Bioconductor packages (GenomicFeatures, IRanges, GenomicRanges, Biostrings, Rsamtools) for manipulating and analysis of next-generation sequencing data and custom-written R (Ihaka and Gentleman 1996) programs. Statistical analysis to identify differentially expressed genes between treatment and control was performed using the negative-binomial model of read counts as implemented in DESeq Biocondoctor package (Anders and Huber 2010). To validate the RNA-seq data, several (4-6) B[a]P target and non-target genes from each RNA-seq determination were used for qRT-PCR analyses and comparison to the values determined by RNA-seq. The resulting comparisons are shown in Supplemental Figure S1, A-D. The data pertaining to significantly altered gene expression values, determined as having a differential p-value<0.05 and FDR<0.05, were then analyzed using Ingenuity Knowledge Base software (IPA; Ingenuity®Systems, http://www.ingenuity.com) to determine ontology and molecular pathways associated with response to treatment. Genome-wide RNA.seq data has been submitted to the GEO database with access URL http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE49571.
2.4 Immunofluorescence analyses of γH2A.X-pSer139
Hepa-1 cells were treated as described above and at every 5th passage were plated on glass coverslips. After 24 hours, cells were washed with PBS, fixed with 4% paraformaldehyde in PBS for 20 minutes and permeabilized with 1% NP-40 in PBS for 5 minutes. Non-specific immunoreactive epitopes were blocked with 2% BSA in a 10% goat serum PBS mixture for 1 hour at room temperature followed by overnight incubation at 4°C with a 1:1000 dilution of a γH2AX-pSer139 histone antibody (Millipore®, 05-636). After washing, the coverslips were incubated for 1 hour at room temperature with a 1:2000 dilution of Alexa fluor 568 conjugated goat anti-mouse IgG (Invitrogen®, A11004). Coverslips were mounted using DAPI containing mounting medium (Vector Labs®, H-1500) and imaged using a Zeiss Axio microscope.
2.5 Flow cytometry
DNA damage was evaluated using a cell-based assay system (Upstate Cell Signaling (EMD, 17-344)) for the detection of histone γH2A.X phosphorylated at Ser139 in fixed cells. After fixation, cells were incubated with FITC-conjugated anti-phospho-histone γH2A.X-pSer139 or negative control mouse IgG-FITC and analyzed by flow cytometry. Fluorescent signals were gated at an intensity such that less than 2% of the negative control cells would score as positive. For analysis of apoptotic cells, we used a flow cytometry kit to detect activated caspase-3 (BD Biosciences 550480). Cells were harvested by trypsinization, fixed, incubated with a phycoerythrin (PE)-conjugated monoclonal rabbit anti-active caspase-3 antibody and analyzed by flow cytometry.
2.6 Clonogenic colony formation
At the end of each 5th passage in the presence of Cr or control, 500 viable cells, as determined by Trypan Blue exclusion, were plated in a 10-cm dish in complete medium, without chromium or B[a]P for 2 weeks, after which time colonies were stained with 0.4% Methylene Blue and counted. Only colonies larger than 1 mm in diameter were recorded.
2.7 Statistical analyses
For group comparisons, an ANOVA adjusted p-value<0.05 was considered statistically significant.
3. Results
3.1 Long term exposure to low concentrations of chromium causes DNA damage and decreases cell viability
It is well established that acute Cr(VI) treatment at high concentration causes genomic instability in cultured cells and in rodents (Wise and Wise, Sr. 2012; Zhitkovich 2011). To determine if a similar biological effect could result from treatment in a range of concentrations closer to the level in drinking water and below the EPA target goal of 100 ppb, we grew Hepa-1 cells continuously for 20 passages in the presence of 0.1 μM or 0.5 μM Cr(VI), equivalent to 10 or 50 ppb, respectively, and assessed whether treatment caused decreased viability relative to untreated cells, as measured by colony-forming ability. As early as passage 5, both Cr(VI) treatments caused a significant 30% decrease in clonogenic colony formation relative to the maximum 25% colony formation capacity of untreated control cells (Fig. 1A). This decrease was uniformly maintained through subsequent passages in the presence of Cr(VI), reaching its lowest point of 36 - 45% below control at passage 15 and rebounding to 15 - 25% at passage 20 (Fig. 1A). The apparent differences between the two concentrations, albeit significantly different from control at both concentrations, do not seem to show a statistically significant concentration effect, suggesting that the effect has reached a plateau level and that lower concentrations than those used here might also be cytotoxic during long-term treatment.
Figure 1. Cr(VI) damages cellular DNA causing increased apoptosis and decreased viability.
A. Colonies formed after plating 500 cells were stained and counted to determine cellular viability. Both 0.1 and 0.5 μM Cr(VI) decreased cellular viability at all passages.
B. Cells stained for γH2A.X show an exposure related increase in the number of foci, indicative of DNA damage with higher concentrations of and longer treatment with Cr(VI).
C. Quantitation by flow cytometry of cells undergoing apoptosis, as determined by active caspase 3 staining, shows significant treatment and concentration dependent increases in the number of apoptotic cells in the Cr(VI) exposed groups.
D. Quantitation of γH2A.X staining by flow cytometry show increased DNA damage with increased exposure to either 0.1 or 0.5 μM Cr(VI).
DNA damage activates the ATM-CHK2-E2F1 pathway, leading to the phosphorylation of Ser-139 in histone H2A.X, which is often used as a surrogate for direct DNA damage assays and the formation of double strand DNA breaks (Tanaka et al. 2007). To determine if our low concentration treatments induced DNA damage we used immunofluorescence detection of phosphorylated γH2A.X nodes in nuclei of Cr(VI)-treated and control cells. Both 0.1 μM and 0.5 μM treatments significantly increased the number of γH2A.X-positive nuclei relative to untreated cells at all passages (Fig. 1B). Confirmation of this finding by flow cytometry showed a dose- and passage-dependent increase of γH2A.X-positive cells that by passage 20 reached nearly 50% of the total cell population, or 5-times the level in control cells (Fig. 1C), diagnostic of a higher level of DNA damage in these cells.
Consistent with the activation of the ATM-CHK2-E2F1 pathway, increased DNA damage and decreased cell viability suggest that cells treated with Cr(VI) undergo increased apoptosis, an expectation that we confirmed experimentally. Compared to an approximate 5% background apoptosis in control cells, long-term passage in the presence of 0.1 μM or 0.5 μM Cr(VI) increases apoptosis, as measured by the activation of caspase-3, in a concentration- and passage-dependent fashion that reaches as much as 30% apoptotic cells by passage 20 (Fig. 1D).
Increased DNA damage coupled with increased apoptosis and decreased cell viability are biological effects consistent with the known mechanism of action of Cr(VI). To our knowledge this is the first demonstration that these effects can occur by sustained treatment of cells with environmentally relevant chromium concentrations close to the levels commonly found in drinking water.
3.2 Low-concentration chromium treatment disrupts the transcriptome
RNA.seq analysis of cells treated with 0.5μM Cr(VI) showed that greater than 1000 genes were significantly differentially regulated from control after 20 passages of long-term treatment (Supplemental Table S1). To determine which biological pathways would be affected by the altered expression of these genes we used Ingenuity Systems™ IPA® software to analyze gene ontology groups. This analysis showed that more than 190 biological pathways were differentially regulated in Cr-treated cells. The top 20 biological pathways with the lowest p-values comprise pathways mostly associated with inflammation and cancer (Fig. 2). Ontological groups include genes involved in the p53, GADD45, and ATM signaling pathways among others important for cancer progression and DNA damage response. Additionally, several inflammatory response pathways are significantly altered after long term exposure to Cr(VI). Since this analysis gives no information as to whether pathways are up- or down-regulated, further information was obtained from the IPA® analysis of the predicted activation state of upstream regulators, which showed that genes that respond to p53 and to known DNA damaging agents, like cisplatin- and doxorubicin-responsive genes, are predicted to be activated (Table 1). This significant alteration in gene expression focused on mechanisms of cancer progression and inflammatory responses reinforces the suggestion from our previous data that, even at a concentration as low as 0.5 μM, cells continuously exposed to Cr(VI) accumulate DNA damage that causes an adverse cellular response.
Figure 2. Low-concentration Cr(VI) treatment for 20 passages alters gene expression.
Gene Ontology analysis of RNA-seq data shows that treatment with 0.5 μM Cr(VI) for 20 passages alters most significantly the expression of genes related to cancer progression and inflammatory pathways. The top-20 most significant GO terms representative of specific biological pathways are shown as a function of their statistical significance expressed as –log(p-value). Values next to each bar indicate the number of genes in the pathway shown to be statistically significantly different from control over the total number of genes in the database related to that specific biological pathway.
Table 1.
Predicted activation state of upstream regulators of genes altered by long term exposure to 0.5 μM Cr(VI)
| Upstream Regulator | Predicted Activation State | Activation z-score | p-value of overlap |
|---|---|---|---|
| TP53 | Activated | 4.16 | 5.55E-10 |
| cisplatin | Activated | 2.93 | 5.53E-09 |
| epicatechin gallate | 1.76E-08 | ||
| TOPBP1 | Inhibited | −2.21 | 1.97E-08 |
| BCL2L12 | Inhibited | −2.00 | 5.11E-08 |
| BRCA1 | Activated | 2.18 | 8.57E-08 |
| idarubicin | Activated | 2.24 | 1.06E-07 |
| cytarabine | Activated | 2.43 | 1.49E-07 |
| methyl methanesulfonate |
Activated | 2.40 | 4.38E-07 |
| hydrogen peroxide | Activated | 3.31 | 4.93E-07 |
| F0X03 | Activated | 2.19 | 1.14E-06 |
| TP73 | Activated | 3.07 | 1.22E-06 |
| CD24 | Inhibited | −2.00 | 1.27E-06 |
| RNASEH2B | 1.81E-06 | ||
| PADI4 | 1.81E-06 | ||
| RRM2B | 1.81E-06 | ||
| CDKN2A | Activated | 3.06 | 3.67E-06 |
| CHEK2 | 1.95 | 4.40E-06 | |
| BCAS2 | 4.49E-06 | ||
| RPL22 | 4.49E-06 | ||
| benzo(a)pyrene | 1.31 | 6.15E-06 |
The z-score predicts activaition state; the p-value measures the statistical significanc 3 of the prediction.
3.3 Low concentration Cr(VI) alters cellular response to B[a]P
To determine the effect of long-term growth in low Cr(VI) concentration on the gene regulation response to B[a]P we used RNAseq deep-sequencing followed by pathway analysis of the genes with significantly altered expression when compared to either control, untreated cells or to cells grown for 20 passages in the presence of 0.5 μM Cr(VI). When compared to control cells, B[a]P-treated cells showed changes in gene expression in 393 molecular pathways, of which the 20 most significant pathways were xenobiotic metabolism, oxidative stress response, mechanisms of cancer, and several inflammatory response pathways (Fig. 3). Many of the genes and signaling pathways are similar to those in the response to Cr(VI) treatment alone, including genes related to p53 and IL-8 signaling and genes that are part of the molecular mechanisms of cancer, but the p-values are less significant than those of the xenobiotic response pathways (Fig. 3 and Table 2). The predicted upstream regulators activated by B[p]P treatment are those responding to xenobiotics (Table 2). Interestingly, the prediction of the Ingenuity Systems™ IPA® software indicates that B[a]P would inhibit the disruption of gene expression resulting from chromium exposure (Table 2).
Figure 3. B[a]P treatment causes alterations of gene expression for genes related to metabolism and cancer progression pathways.
treatment of control cells with B[a]P alone for 8 hours leads to expression changes detectable by RNA-seq of genes related to oxidative stress response, xenobiotic metabolism, and cancer progression biological pathways. The top-20 most significant GO terms representative of specific biological pathways are shown as a function of their statistical significance expressed as –log(p-value). Values next to each bar indicate the number of genes in the pathway shown to be statistically significantly different from control over the total number of genes in the database related to that specific biological pathway.
Table 2.
Predicted upstream regulators of genes altered by B[a]P treatment.
| Upstream Regulator | Predicted Activation State | Activation z-score | p-value of overlap |
|---|---|---|---|
| benzo(a)pyrene | Activated | 4.15 | 6.90E-23 |
| cigarette smoke | Activated | 2.80 | 1.22E-18 |
| cisplatin | Activated | 3.14 | 3.91E-15 |
| chromium | Inhibited | −4.24 | 5.51E-15 |
| camptothecin | Activated | 6.20 | 5.97E-15 |
| arsenite | 1.69 | 4.51E-14 | |
| tert-butyl-hydroquinone | Activated | 3.90 | 1.05E-13 |
| TP53 | Activated | 4.35 | 4.60E-13 |
| sulforafan | Activated | 2.05 | 7.47E-13 |
| nitric oxide | Activated | 4.26 | 4.27E-11 |
| PDGFBB | Activated | 2.97 | 6.69E-11 |
| FGF1 | Activated | 4.11 | 9.82E-11 |
| hydrogen peroxide | Activated | 3.34 | 1.59E-10 |
| HGF | −0.56 | 1.78E-10 | |
| NUPR1 | Activated | 3.43 | 2.59E-10 |
| BRCA1 | Activated | 2.17 | 3.96E-10 |
| U0126 | −1.56 | 4.61E-10 | |
| TNF | 1.83 | 6.13E-10 | |
| doxorubicin | Activated | 2.06 | 8.63E-10 |
| beta-naphthoflavone | Activated | 3.56 | 1.06E-09 |
| AHR | Activated | 2.96 | 2.07E-09 |
| tetrachlorodibenzodioxin | Activated | 3.58 | 2.12E-09 |
The z-score predicts activation state; the p-value measures the statistical significance of the prediction.
In contrast, the transcriptional response to B[a]P in cells that had been grown for 20 passages in 0.5μM Cr(VI) showed a response that affected the expression of pathways related to cancer progression more-so than any other collection of biological responses (Fig. 4 and Table 3). Twelve of the 20 most significantly altered pathways in response to B[a]P were related to cancer compared to only 8 in cells that were grown in the absence of Cr(VI) (compare Figs. 3 and 4). Perhaps the most striking effect is that the genes more strongly related to B[a]P responses, such as the xenobiotic response and oxidative stress response genes, were not in the top 20 pathways altered by B[a]P if the cells had been grown in the presence of Cr(VI). The pathways related to aryl hydrocarbon response and xenobiotic metabolism signaling fell to 55th and 95th in rank-order of significance, respectively. Examining the predicted upstream regulators of transcriptomic responses, cells grown in low concentration Cr(VI) and then treated with B[a]P show responses that include activation of pathways common to those of agents that inhibit DNA transcription, like camptothecin and topotecan, and inhibition of many genes that respond to growth factor signaling such as HGF, VEGF and TGFβ3. These results demonstrate that low concentrations of Cr(VI) cause alterations in cells that drastically change how they respond to a different, unrelated, type of toxic insult, in this case, B[a]P. This response is strikingly different from the transcriptomic response initiated by B[a]P alone, and would be expected to lead to a drastically different biological outcome.
Figure 4. B[a]P treatment of cells previously treated with low-concentration of Cr(VI) for 20 passages, causes alterations in the transcription of genes primarily related to cancer progression and inflammation.
B[a]P treatment of cells previously treated with 0.5 μM Cr(VI) for 20 passages further changes the expression of genes primarily involved in pathways relating to cancer progression and inflammation. Pathways related to xenobiotic expression are not in the 20 most significant pathways altered by B[a]P when cells are previously exposed to Cr(VI). The top-20 most significant GO terms representative of specific biological pathways are shown as a function of their statistical significance expressed as –log(p-value). Values next to each bar indicate the number of genes in the pathway shown to be statistically significantly different from control over the total number of genes in the database related to that specific biological pathway.
Table 3.
Predicted upstream regulators of genes altered by B[a]P treatment in cells exposed to 0.5 μM Cr(VI) for 20 passages
| Upstream Regulator | Predicted Activation State | Activation z-score | p-value of overlap |
|---|---|---|---|
| CD24 | Inhibited | −6.00 | 1.11E-18 |
| HGF | Inhibited | −7.11 | 3.81E-18 |
| Vegf | Inhibited | −7.92 | 2.17E-17 |
| camptothecin | Activated | 4.16 | 6.67E-14 |
| miR-124-3p ( miRNAs w/seed AAGGCAC) | Activated | 6.96 | 1.21E-12 |
| NUPRl | Activated | 4.12 | 6.75E-12 |
| miR-21-5p (miRNAs w/seed AGCUUAU) | Activated | 3.53 | 1.27E-11 |
| FSH | −1.27 | 1.62E-11 | |
| TP53 | 0.18 | 5.04E-11 | |
| Sos | 8.32E-11 | ||
| GNA12 | Inhibited | −4.54 | 5.52E-10 |
| TGFBl | Inhibited | −5.56 | 3.25E-09 |
| LMNA | 4.35E-09 | ||
| triamcinolone acetonide | 0.86 | 7.10E-09 | |
| miR-1 ( miRNAs w/seed GGAAUGU) | Activated | 6.32 | 8.87E-09 |
| methylselenic acid | Activated | 2.21 | 1.19E-08 |
| TGFB3 | Inhibited | −2.01 | 2.03E-08 |
| miR-17-5p (miRNAs w/seed AAAGUGC) | Activated | 3.44 | 2.29E-08 |
| AGT | −1.59 | 3.05E-08 | |
| HOXA9 | −1.81 | 3.37E-08 | |
| topotecan | Activated | 4.27 | 3.58E-08 |
The z-score predicts activation state; the p-value measures the statistical significance of the prediction.
3.4 Some but not all effects of long term chromium exposure are reversible
To determine if the effects of sustained low-concentration Cr(VI) treatment were the result of persistent irreversible damage accumulated through growth in the presence of Cr(VI), we continued to passage cells for an additional 5 passages either with continued Cr(VI) treatment or switched to medium without Cr(VI). If the damage were persistent, we would expect that the cells would not be able to recover and continue to show Cr(VI)-dependent effects even when the agent was no longer present in the medium. We found that the levels of DNA damage, as determined by detection of γH2A.X-pSer139, either by immunofluorescence or flow cytometry, did not change in cells grown for 5 passages in the absence of Cr(VI) when compared to cells that continue to grow in Cr(VI) medium (Fig. 5A,B). Interestingly, however, despite the continued DNA damage, cells previously exposed to Cr(VI) for 20 passages and further grown for 5 passages without Cr(VI) had rates of apoptosis similar to cells that had never been exposed to Cr(VI) at all (Fig. 5C).
Figure 5. DNA damage but not apoptosis persists after Cr(VI) treatment for 20 passages is discontinued.
A. Cells stained for γH2A.X show a Cr(VI) concentration dependent increase in nuclear foci even if after the cells that had previously been exposed to Cr(VI) were grown for 5 additional passages without Cr(VI).
B. Quantitation of γH2A.X staining shows increased DNA damage with increased exposure to either 0.1 μM or 0.5 μM Cr(VI) which is not significantly decreased in cells allowed to recover for 5 passages in the absence of Cr(VI).
C. The number of apoptotic cells was quantified by flow cytometry of active caspase-3 staining. The Cr(VI) treatment dependent increase in the number of apoptotic cells in the Cr(VI) exposed groups is reversed when cells were allowed to recover for 5 passages in the absence of Cr(VI).
Further, by the 25th passage, cells appear to have changed their transcriptional response to Cr(VI). The gene expression profile of cells grown in 0.5μM Cr(VI) for 25 passages differ the profile of cells that have never been exposed to Cr(VI) in only 26 genes, with no significant pattern of pathway alterations related to this exposure (Table 4). This effect is even more striking in cells that had previously been grown in the presence of Cr(VI) for 20 passages and were switched for 5 additional passages to medium without Cr(VI): in these cells, only 2 genes were significantly different from control cells that had never been treated with Cr(VI) (Table 4).
Table 4.
Genes significantly altered by 0.5 μM Cr(VI) at passage 25 and after Cr(VI) removal for 5 passages
| Cr(VI) for 25 Passages vs Control at 25 passages | ||
|---|---|---|
| symbol | name | Fold Change |
| Gm347 | predicted gene 347 | 0.12 |
| Sftpb | surfactant associated protein B | 0.14 |
| Gm4303 | predicted gene 4303 | 0.16 |
| Gm4305 | predicted gene 4305 | 0.16 |
| Gm4307 | predicted gene 4307 | 0.16 |
| Mbnl3 | muscleblind-like 3 (Drosophila) | 0.17 |
| Sh3rf2 | SH3 domain containing ring finger 2 | 0.18 |
| Phactr1 | phosphatase and actin regulator 1 | 0.21 |
| Fras1 | Fraser syndrome 1 homolog (human) | 0.23 |
| Fcgr4 | Fc receptor, IgG, low affinity IV | 0.25 |
| Speer4b | spermatogenesis associated glutamate (E)-rich protein 4b | 0.26 |
| Krt8 | keratin 8 | 1.80 |
| Lars 2 | leucyl-tRNA synthetase, mitochondrial | 2.03 |
| Fkbp8 | FK506 binding protein 8 | 2.06 |
| H2afj | H2A histone family, member J | 2.37 |
| Capg | capping protein (actin filament), gelsolin-like | 2.52 |
| Nrtn | neurturin | 3.25 |
| Krt5 | keratin 5 | 4.39 |
| Dgkh | diacylglycerol kinase, eta | 4.54 |
| Ppm1n | protein phosphatase, Mg2+/Mn2+ dependent, 1N (putative) |
4.75 |
| Tex101 | testis expressed gene 101 | 5.08 |
| Krt6a | keratin 6A | 5.29 |
| Kcnf1 | potassium voltage-gated channel, subfamily F, member 1 | 5.66 |
| Alpk2 | alpha-kinase 2 | 6.45 |
| Col16a1 | collagen, type XVI, alpha 1 | 9.04 |
| Cr(VI) removed after 20 passages vs Control at passage 25 | ||
| Fgf9 | fibroblast growth factor 9 | 0.16 |
| 5430437J10Rik | RIKEN cDNA 5430437J10 gene | 0.11 |
Cells that have been maintained in Cr(VI) for 25 passages also respond differently to B[a]P than cells that were not treated at all with Cr(VI) or only treated for 20 passages. In Cr(VI) untreated cells, B[a]P elicits a transcriptomic response that affects the expression of xenobiotic metabolisim genes and genes involved in cancer progression (Fig. 3 and Table 2). In cells grown for 20 passages in low Cr(VI)concentration, B[a]P elicits a transcriptomic response that affects the expression of cell proliferation and DNA synthesis pathways genes (Fig. 4 and Table 3). However, in cells grown in Cr(VI) for 25 passages B[a]P affects fewer gene pathways related to cancer progression and more related to xenobiotic metabolism (Fig. 6). A similar effect is observed in cells grown in Cr(VI) for 20 passages plus 5 passages in the absence of Cr(VI); in these cells, the B[a]P response alters primarily the expression of genes related to pathways of metabolism, with only 2 of the top twenty pathways not being xenobiotic metabolism related (Fig. 7). These data suggest that an extended exposure to Cr(VI) eventually leads to an adaptive inhibition of the expression of genes that respond to DNA damage leading to the induction of apoptosis. It appears that chronic inhibition of the Cr(VI)-induced DNA damage response also silences a similar response initiated by B[a]P treatment. This finding is consistent with previous in vivo data showing that, compared to an 8-day exposure, mice exposed to Cr(VI) for 91 days showed less activation of DNA damage response and repair genes in their intestinal epithelium (Kopec et al. 2012).
Figure 6. Cells treated with low-concentration Cr(VI) for 25 passages show a transcriptional response to B[a]P which more heavily favors metabolic pathways.
Cells that had been treated with Cr(VI) for 25 passages respond to B[a]P treatment by altering the transcription of genes primarily related to metabolic pathways. Other biological pathways affected by Cr(VI) treatment, such as the molecular mechanisms of cancer, are no longer significantly altered by BaP treatment. The top-20 most significant GO terms representative of specific biological pathways are shown as a function of their statistical significance expressed as –log(p-value). Values next to each bar indicate the number of genes in the pathway shown to be statistically significantly different from control over the total number of genes in the database related to that specific biological pathway.
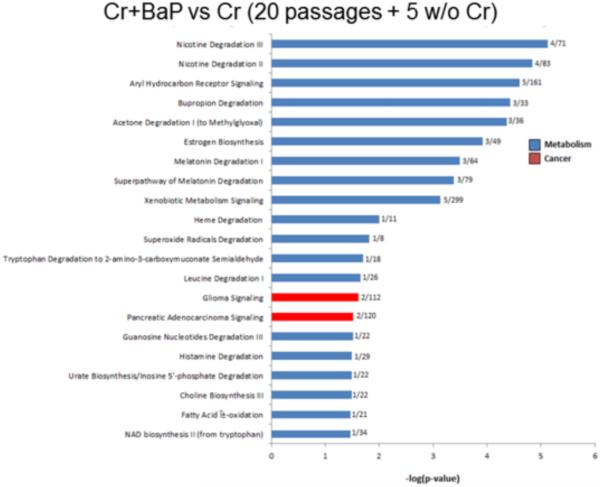
Figure 7. Cessation of Cr(VI) treatment for 5 passages after 20 passages in Cr(VI) retains the long term effects of Cr(VI) treatment on the transcriptomic response to B[a]P.
Cells treated with 0.5 μM Cr(VI) for 20 passages followed by 5 additional passages without Cr(VI) respond to B[a]P treatment by altering the expression of genes involved primarily in metabolism pathways. Compared to cell treated similarly but grown in Cr(VI) for 20 passages instead of 25, biological pathways related to inflammation are no longer among the top 20 most significantly altered pathways. Only 2 pathways related to cancer, (glioma signaling and pancreatic adenocarcinoma signaling) are present in the top 20 most significantly altered. Specific biological pathways are shown as a function of their statistical significance expressed as –log(p-value). Values next to each bar indicate the number of genes in the pathway shown to be statistically significantly different from control over the total number of genes in the database related to that specific biological pathway.
4. Discussion
The results from this study show that prolonged treatment of cells with environmentally relevant low concentrations of Cr(VI) have a significant effect on gene expression regulation. The resulting transcriptome changes cause cells to adjust their response to an additional, unrelated toxicant in ways that are unpredictable and distinct from their response to either agent alone. Five passages in the presence of 0.1 μM Cr(VI) is sufficient to generate increased DNA damage and cell death, consistent with previous work showing that higher concentrations of Cr(VI) generate DNA adducts and cross-links (Zhitkovich 2005; Zhitkovich et al. 2005). In our hands, DNA damage further increases with continued treatment for 20 passages and lingers on after the cells are no longer exposed to Cr(VI), indicating that, even at concentrations as low as 0.1 μM, cells may accumulate unrepaired DNA damage. In contrast, although, just as in the case of DNA damage, the number or cells undergoing apoptosis increases with passage, 5 passages in the absence of Cr(VI) is all that is necessary to return apoptosis incidence to control levels. It may be that, following long-term exposure to Cr(VI), a subset of cells continue to divide and proliferate despite having potentially deleterious DNA damage. Alternatively, Cr(VI) treatment may cause an irreversible state in which new DNA damage is generated at each replication event. In either case, maintenance of DNA damage constitutes a potentially mutagenic mechanism that can lead to carcinogenic deregulation of proliferation and additional DNA damage accumulation if not repaired. In fact, after 20 passages in 0.5 μM Cr(IV), expression of numerous genes involved in cancer progression is significantly altered, including genes involved in the ATM DNA damage response and p53 response pathways. Using the IPA® software to analyze the predicted activation state of potential upstream pathway regulators we determined that on the basis of the gene expression changes, the pathways that respond to p53 and cisplatin, a DNA damaging agent, are activated. We would conclude that extended Cr(VI) treatment over time, even at very low levels, induces adaptive responses that include DNA damage and are likely to activate carcinogenic mechanisms related to that damage.
The gene expression response to Cr(VI) in our studies using tissue culture cells does not differ significantly from the response previously seen in animal studies, although the amount of Cr(VI) needed to cause those responses differs widely. Mice exposed to Cr(VI) in drinking water in the range of 0 – 520 ppm ( equivalent to 0 – 2 mM) for 90 days showed several thousand significant gene expression profile changes in intestinal epithelium at the high doses, but very few changes at doses below 100 ppm (400 μM) (Kopec et al. 2012). At the 100 ppm, however, the transcriptome changes and the primary mechanisms for which gene expression was altered were the immune response, cell cycle, DNA damage repair, and oxidative stress responses, consistent with the responses that we find in cells treated with 0.5 μM Cr(VI) for 20 passages. It is remarkable that the transcriptomic changes are so consistent, even though the in vitro concentration is 800-times lower than the in vivo dose. Many caveats, however, need to be taken into consideration when comparing in vivo and in vitro data, including the diversity of cell types that respond in vivo compared to a single lineage in vitro, and the uncertainties associated with the difference between the exposure dose to a mouse and the concentration of treatment to a tissue culture cell.
Continuous treatment with Cr(VI) also causes significant changes in the cells response to B[a]P. In the absence of Cr(VI), while some pathways related to carcinogenicity and inflammatory responses are also affected, the ones most significantly altered by B[a]P treatment are the xenobiotic response pathways. This is not the response that cells exposed to Cr(VI) have to an additional treatment with B[a]P. Previous treatment with 0.5 μM Cr(VI) for 20 passages causes the response to B[a]P treatment to change the expression of genes mostly related to cancer progression pathways and much less the typical xenobiotic response, normally seen in B[a]P treated cells. The gene expression response activates the corresponding upstream regulators, leading to up-regulation of the DNA damage response due to activation of pathways related to the genotoxic topoisomerase inhibitors camptothecin and topotecan and down-regulation of growth factor signaling cascades, such as VEGF and HGF. This raises the central conclusion that the effects of Cr(VI) and B[a]P co-exposure are both different and more significant than just the sum of the effects of either single exposure.
Despite the fact that the DNA damaging effects due to Cr(VI) exposure are maintained even after the Cr(VI) is removed, the effect on transcriptome changes and increased apoptosis are not. While there were greater than 1000 genes with differential expression in cells treated with Cr(VI) for 20 passages, at 25 passages that difference dropped to only 26, and those 26 genes do not seem to significantly represent any specific biological pathway. Furthermore, when Cr(VI) treatment was removed for only 5 passages, the number of significantly altered genes was only 2. In either case, the cell transcriptome became remarkably similar to control. Possibly, the cells have responded to the increased DNA damage by adaptively silencing the transcriptomic responses, and this silencing carries over to the response to B[a]P as well. We would argue that long-term treatment with low concentration Cr(VI) might create a selective pressure that compels the cells to adapt to proliferate with steady levels of DNA damage, muting the response to DNA damage, including the adaptive alteration of the expression of genes related to cancer progression pathways, which are no longer observed after 25 passages. If this hypothesis were correct, it would explain why treatment with B[a]P no longer activates those same pathways, even in cells grown for 5 additional passages without Cr(VI) after long-term Cr(VI) treatment. Whatever the cause, it should be noted that similar responses have been observed in vivo. Most notably, the animal studies alluded to earlier have shown that DNA damage and repair genes were not as strongly activated when mice had been exposed to Cr(VI) for 91 days as when they were exposed for only 8 days (Kopec et al. 2012), suggesting that silencing is the result of more extended exposure. It is likely that these genes are suppressed by the same mechanisms through which acute high-concentration Cr(VI) treatment inhibits inducible gene expression (Schnekenburger et al. 2007).
In conclusion, we find that extended treatment with a very low concentration of Cr(VI) induces cellular damage and disrupts the cellular transcriptome. As a consequence, other transcriptomic responses, in this case the response induced by B[a]P, is also disrupted, causing an outcome unpredictable from the effect caused by that either single treatment. Furthermore, consistent with previous in vivo work, long treatments with low Cr(VI) concentration silence the activation of DNA damage repair pathways. It remains to be determined the mechanisms operative in the repression process.
Supplementary Material
Acknowledgments
We thank Chia-I Ko, Hisaka Kurita, Vinicius Carreira, Qin Wang and Francisco Javier Sánchez-Martín for critically reading the manuscript and providing helpful criticisms. This work was supported by NIH grants R01 ES010807, R01 ES006273and the NIEHS Center for Environmental Genetics grant P30 ES06096. The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchowsky A, O'Hara KA. Metal-induced cell signaling and gene activation in lung diseases. Free Radic. Biol. Med. 2003;34(9):1130–1135. doi: 10.1016/s0891-5849(03)00059-5. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, DeVito MJ. Use of toxic equivalency factors for risk assessment for dioxins and related compounds. Toxicology. 1995;105:391–401. doi: 10.1016/0300-483x(95)03237-a. [DOI] [PubMed] [Google Scholar]
- Cefalu WT, Wang ZQ, Zhang XH, Baldor LC, Russell JC. Oral chromium picolinate improves carbohydrate and lipid metabolism and enhances skeletal muscle Glut-4 translocation in obese, hyperinsulinemic (JCR-LA corpulent) rats. J. Nutr. 2002;132(6):1107–1114. doi: 10.1093/jn/132.6.1107. [DOI] [PubMed] [Google Scholar]
- Cizmas L, McDonald TJ, Phillips TD, Gillespie AM, Lingenfelter RA, Kubena LF, Phillips TD, Donnelly KC. Toxicity characterization of complex mixtures using biological and chemical analysis in preparation for assessment of mixture similarity. Environ. Sci. Technol. 2004;38(19):5127–5133. doi: 10.1021/es035287p. [DOI] [PubMed] [Google Scholar]
- Collins JF, Brown JP, Alexeeff GV, Salmon AG. Potency equivalency factors for some polycyclic aromatic hydrocarbons and polycyclic aromatic hydrocarbon derivatives. Regul Toxicol Pharmacol. 1998;28:45–54. doi: 10.1006/rtph.1998.1235. [DOI] [PubMed] [Google Scholar]
- Conney AH, Chang RL, Jerina DM, Wei S-JC. Studies on the metabolism of benzo[a]pyrene and dose-dependent differences in the mutagenic profile of its ultimate carcinogenic metabolite. Drug Metab. Rev. 1994;26:125–163. doi: 10.3109/03602539409029788. [DOI] [PubMed] [Google Scholar]
- Dayan AD, Paine AJ. Mechanisms of chromium toxicity, carcinogenicity and allergenicity: review of the literature from 1985 to 2000. Hum. Exp. Toxicol. 2001;20(9):439–451. doi: 10.1191/096032701682693062. [DOI] [PubMed] [Google Scholar]
- Ding M, Shi X, Castranova V, Vallyathan V. Predisposing factors in occupational lung cancer: inorganic minerals and chromium. J. Environ. Pathol. Toxicol. Oncol. 2000;19(1-2):129–138. [PubMed] [Google Scholar]
- Gibb HJ, Lees PS, Pinsky PF, Rooney BC. Lung cancer among workers in chromium chemical production. Am. J. Ind. Med. 2000;38(2):115–126. doi: 10.1002/1097-0274(200008)38:2<115::aid-ajim1>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Ihaka P, Gentleman RR. A language for data analysis and graphics. Journal of Computational and Graphical Statistics. 1996;5:299–314. [Google Scholar]
- Kopec AK, Kim S, Forgacs AL, Zacharewski TR, Proctor DM, Harris MA, Haws LC, Thompson CM. Genome-wide gene expression effects in B6C3F1 mouse intestinal epithelia following 7 and 90days of exposure to hexavalent chromium in drinking water. Toxicol. Appl. Pharmacol. 2012;259(1):13–26. doi: 10.1016/j.taap.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Maier A, Dalton TP, Puga A. Disruption of dioxin-inducible phase I and phase II gene expression patterns by cadmium, chromium, and arsenic. Mol. Carcinog. 2000;28(4):225–235. [PubMed] [Google Scholar]
- Majumder S, Ghoshal K, Summers D, Bai S, Datta J, Jacob ST. Chromium(VI) down-regulates heavy metal-induced metallothionein gene transcription by modifying transactivation potential of the key transcription factor, metal-responsive transcription factor 1. J. Biol. Chem. 2003;278(28):26216–26226. doi: 10.1074/jbc.M302887200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning FC, Xu J, Patierno SR. Transcriptional inhibition by carcinogenic chromate: relationship to DNA damage. Mol Carcinog. 1992;6:270–279. doi: 10.1002/mc.2940060409. [DOI] [PubMed] [Google Scholar]
- Morgan M, Anders S, Lawrence M, Aboyoun P, Pages H, Gentleman R. ShortRead: a bioconductor package for input, quality assessment and exploration of high-throughput sequence data. Bioinformatics. 2009;25(19):2607–2608. doi: 10.1093/bioinformatics/btp450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert DW, Petersen DD, Puga A. Human AH locus polymorphism and cancer: inducibility of CYP1A1 and other genes by combustion products and dioxin. Pharmacogenetics. 1991;1:68–78. doi: 10.1097/00008571-199111000-00003. [DOI] [PubMed] [Google Scholar]
- O'Brien TJ, Ceryak S, Patierno SR. Complexities of chromium carcinogenesis: role of cellular response, repair and recovery mechanisms. Mutat. Res. 2003;533(1-2):3–36. doi: 10.1016/j.mrfmmm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Perdew GH. Association of the Ah receptor with the 90-kDa heat shock protein. J. Biol. Chem. 1988;263(27):13802–13805. [PubMed] [Google Scholar]
- Puga A, Ma C, Marlowe JL. The aryl hydrocarbon receptor cross-talks with multiple signal transduction pathways. Biochem. Pharmacol. 2009;77(4):713–722. doi: 10.1016/j.bcp.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes H, Reiz-Porszasz S, Hankinson O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992;256:1193–1195. doi: 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- Saha R, Nandi R, Saha B. Sources and toxicity of hexavalent chromium. Journal of Coordination Chemistry. 2011;64:1782–1806. [Google Scholar]
- Schnekenburger M, Talaska G, Puga A. Chromium cross-links histone deacetylase 1-DNA methyltransferase 1 complexes to chromatin, inhibiting histone-remodeling marks critical for transcriptional activation. Mol Cell Biol. 2007;27(20):7089–7101. doi: 10.1128/MCB.00838-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton K, Beck BD, Bingham E, Brain JD, DeMarini DM, Hertzberg RC, O'Flaherty EJ, Pounds JG. Chemical mixtures from a public health perspective: the importance of research for informed decision making. Toxicology. 1995;105:429–441. doi: 10.1016/0300-483x(95)03240-g. [DOI] [PubMed] [Google Scholar]
- Shumilla JA, Broderick RJ, Wang Y, Barchowsky A. Chromium(VI) inhibits the transcriptional activity of nuclear factor- kappaB by decreasing the interaction of p65 with cAMP-responsive element-binding protein-binding protein [In Process Citation] J. Biol. Chem. 1999;274(51):36207–36212. doi: 10.1074/jbc.274.51.36207. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Huang X, Halicka HD, Zhao H, Traganos F, Albino AP, Dai W, Darzynkiewicz Z. Cytometry of ATM activation and histone H2AX phosphorylation to estimate extent of DNA damage induced by exogenous agents. Cytometry A. 2007;71(9):648–661. doi: 10.1002/cyto.a.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25(9):1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei YD, Tepperman K, Huang MY, Sartor MA, Puga A. Chromium inhibits transcription from polycyclic aromatic hydrocarbon-inducible promoters by blocking the release of histone deacetylase and preventing the binding of p300 to chromatin. J. Biol. Chem. 2004;279(6):4110–4119. doi: 10.1074/jbc.M310800200. [DOI] [PubMed] [Google Scholar]
- Wetterhahn KE, Hamilton JW. Molecular basis of hexavalent chromium carcinogenicity: effect on gene expression. Sci. Total Environ. 1989;86(1-2):113–129. doi: 10.1016/0048-9697(89)90199-x. [DOI] [PubMed] [Google Scholar]
- Wise SS, Wise JP., Sr. Chromium and genomic stability. Mutat. Res. 2012;733(1-2):78–82. doi: 10.1016/j.mrfmmm.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Manning FC, Patierno SR. Preferential formation and repair of chromium-induced DNA adducts and DNA--protein crosslinks in nuclear matrix DNA. Carcinogenesis. 1994;15(7):1443–1450. doi: 10.1093/carcin/15.7.1443. [DOI] [PubMed] [Google Scholar]
- Zhitkovich A. Importance of chromium-DNA adducts in mutagenicity and toxicity of chromium(VI) Chem. Res. Toxicol. 2005;18(1):3–11. doi: 10.1021/tx049774+. [DOI] [PubMed] [Google Scholar]
- Zhitkovich A. Chromium in drinking water: sources, metabolism, and cancer risks. Chem. Res. Toxicol. 2011;24(10):1617–1629. doi: 10.1021/tx200251t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhitkovich A, Peterson-Roth E, Reynolds M. Killing of chromium-damaged cells by mismatch repair and its relevance to carcinogenesis. Cell Cycle. 2005;4(8):1050–1052. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.