Abstract
Observations that Glioma-associated transcription factors Gli1 and Gli2 (Gli1/2), executers of the Sonic Hedgehog (Shh) signaling pathway and targets of the Transforming Growth Factor β (TGF-β) signaling axis, are involved in numerous developmental and pathological processes unveil them as attractive pharmaceutical targets. Unc-51-like serine/threonine kinase Ulk3 has been suggested to play kinase activity dependent and independent roles in the control of Gli proteins in the context of the Shh signaling pathway. This study aimed at investigating whether the mechanism of generation of Gli1/2 transcriptional activators has similarities regardless of the signaling cascade evoking their activation. We also elucidate further the role of Ulk3 kinase in regulation of Gli1/2 proteins and examine SU6668 as an inhibitor of Ulk3 catalytic activity and a compound targeting Gli1/2 proteins in different cell-based experimental models. Here we demonstrate that Ulk3 is required not only for maintenance of basal levels of Gli1/2 proteins but also for TGF-β or Shh dependent activation of endogenous Gli1/2 proteins in human adipose tissue derived multipotent stromal cells (ASCs) and mouse immortalized progenitor cells, respectively. We show that cultured ASCs possess the functional Shh signaling axis and differentiate towards osteoblasts in response to Shh. Also, we demonstrate that similarly to Ulk3 RNAi, SU6668 prevents de novo expression of Gli1/2 proteins and antagonizes the Gli-dependent activation of the gene expression programs induced by either Shh or TGF-β. Our data suggest SU6668 as an efficient inhibitor of Ulk3 kinase allowing manipulation of the Gli-dependent transcriptional outcome.
Abbreviations: AP, alkaline phosphatase; ASCs, adipose tissue derived stromal cells; GliACT, transcriptional activator form of Gli proteins; GliFL, full-length Gli proteins; Ptch, patched; RNAi, RNA interference; Shh, Sonic Hedgehog; Smo, Smoothened; shRNA, short hairpin RNA; siRNA, small interfering RNA; Sufu, Suppressor of Fused; TGF-β, Transforming Growth Factor β; Ulk3, unc-51-like kinase 3; WB, Western blot; HSC, Hedgehog signaling complex; qRT-PCR, quantitative real-time PCR
Keywords: Gli proteins, Signal transduction, Differentiation, Multipotent cells, Inhibitor
Highlights
-
•
Ulk3 is involved in the maintenance of Gli1/2 expression.
-
•
SU6668 prevents de novo expression of Gli1/2 proteins induced by Shh or TGF-β.
-
•
SU6668 inhibits up-regulation of Gli1/2 proteins via Ulk3.
-
•
Human ASCs differentiate towards osteoblasts in response to Shh.
1. Introduction
Misregulation of cellular signaling pathways, that are important in embryonic development and maintaining adult homeostasis, leads to inherited as well as sporadic diseases. One of such pathways, where a clear correlation between abnormal pathway activation and disease progression has been observed, is the Sonic Hedgehog (Shh) signaling pathway [1]. Disruption or misregulation of the Shh pathway results in various developmental abnormalities including holoprosencephaly, Pallister–Hall syndrome, Gorlin syndrome, Greig cephalopolysyndactyly, Rubinstein–Taybi syndrome and different types of cancer (basal cell carcinoma, medulloblastoma, glioma, breast, pancreatic, prostate cancers and more). Similarly important is the TGF-β signaling pathway, with its role in various types of cancer, vascular diseases and fibrosis [2], [3].
The Shh pathway utilizes Gli proteins (Gli1, 2, 3) as transcriptional effectors. According to the widely accepted paradigm, differentiated regulation of Gli proteins occurs in an Hh signal dependent way. In the absence of the ligand, Gli1 is transcriptionally repressed; full-length Gli2 and Gli3 (Gli2/3FL) proteins are bound by a putative cytoplasmic complex called Hedgehog signaling complex (HSC). HSC may consist of a number of proteins including Suppressor of Fused (Sufu), kinesin-like protein Kif7, unc-51-like kinase 3 (Ulk3), and Gli2/3FL transcription factors [4], [5], [6], [7], [8]. Gli2/3FL proteins bound by HSC are phosphorylated for degradation and processing into the transcriptional repressor forms (Gli2/3REP) [9], [10], [11], [12]. Activation of Shh pathway leads to rapid stabilization and activation of Gli2/3FL probably through yet uncharacterized phosphorylation events, their relocation to the nucleus and up-regulation of their target genes, for instance Ptch1 and self-amplifying Gli1 [7], [12]. Gli2 has been also suggested as a transcriptional target of Shh signaling in mouse CNS during embryonic development [13]. Although both proteins, Gli2 and Gli3, may be involved in primary mediation of Shh activities, the role of Gli2 activator is more crucial, whereas Gli3 acts mainly as a transcriptional repressor [14], [15], [16].
Gli proteins are known to be regulated independently of Hh ligands on both transcriptional and post-translational levels. Mouse Gli1 protein can be activated via Erk1/2 kinases, and Gli2 is shown to be up-regulated in the epidermis of mice over-expressing TGF-β1 [17], [18]. Also, the TGF-β1/SMAD3/TCF4/β-catenin signaling axis controls human GLI2, and consequently GLI1, expression [18], [19]. Regulation of Gli2 in bone metastases and tumor-induced osteolysis also occurs independently of the canonical Shh pathway [20].
Most of the small molecule inhibitors of the Shh pathway identified so far target trans-membrane SMO oncoprotein responsible for triggering the intracellular signaling cascade following the ligand binding to another trans-membrane protein PTCH1. In addition, several inhibitors of GLI proteins and Shh itself have been identified (reviewed in Ref. [21]). However, no inhibitors targeting the activity of either HSC complex or protein kinases required for activation of GLI proteins have been reported. The latter might be effective not only in Shh pathway inhibition, but also in alleviating TGF-β/GLI dependent signaling events.
SU6668 ((Z)-5-[(1,2-dihydro-2-oxo-3H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-propanoic acid; TSU68) has been shown to inhibit several tyrosine and serine/threonine protein kinases in an ATP competitive manner [22], [23]. The affinity chromatography experiment using a resin covalently bound with SU6668 has revealed that additionally to the previously known targets, SU6668 is capable to bind a number of other protein kinases including ULK3 [23]. We have recently identified Ulk3 as an important Gli regulator. However, a mechanism of regulation of the Ulk3 gene and possible interrelations between endogenous Ulk3 and Gli proteins remains unclear.
Adipose tissue derived stromal cells (ASCs, also known as mesenchymal stem or progenitor cells) have been extensively investigated during the last decade. These heterogeneous cell populations have evoked a great interest for regenerative medicine due to their non-immunogenic phenotype and capacity to respond to appropriate inducers by increasing expression of markers specific for different mesodermal lineages, such as adipocytes, chondrocytes or osteoblasts [24], [25], [26]. The Shh signaling pathway has not been extensively characterized in human ASCs, although one research group has reported that activation of Shh signaling negatively regulates differentiation of ASCs towards osteoblasts triggered by osteogenic cocktail [27]. However, these studies were conducted using Shh-conditional media or SMO agonists added to ASCs in the presence of osteogenic inductors, whereas influence of Shh itself on native ASCs has not been analyzed. In contrast, the osteogenic capacity of Shh in mouse ASCs and C3H10T1/2 is well documented [28], [29]. Differentiation of osteoprogenitors occurs under control of Runx2, a factor essential for bone formation and skeletal development [30], [31]. Runx2 is expressed from two alternative promoters at least in two isoforms. Both Runx2 isoforms are expressed in osteoblasts and participate in differentiation [30], [32]. Osteogenesis is characterized by expression of lineage-specific proteins, such as early markers Sp7 and alkaline phosphatase (AP) and late markers osteopontin (Opn) and osteocalcin (Bglap) [29], [33], [34]. Gli2/3 proteins as mediators of Hh activities participate not only in positive regulation of osteogenesis but also in early chondrogenesis in mice [35], [36], [37], whereas adipogenesis is inhibited by activation of the Shh signaling [28], [38]. Expression and activities of GLI1/2 proteins in human ASC tri-lineage differentiation programs have not been described.
The current study aims to investigate whether the mechanism of activation of Gli1 and Gli2 (Gli1/2) proteins has similarities regardless of signaling pathway evoking that. In answering this question, we examine SU6668 as a small molecule inhibitor able to prevent activation of Gli1/2 proteins in both Shh and TGF-β signaling pathways in an Ulk3 dependent manner. Finally, we provide novel data in the field of stem cell biology relating to possible roles of Shh signaling and GLI1/2 proteins in ASC differentiation programs.
2. Materials and methods
2.1. Ethic statement
Donors of the primary cells provided written informed consent to participate in this study in accordance with the approval for research with human materials No 159 from 14th of February, 2013 by Ethics Committee of National Institute for Health Development, Tallinn, Estonia.
2.2. Proteins and chemicals
FLAG-tagged ULK3 and GLI2, Shh and His-tagged ULK3-Ubi proteins were purified as previously described [6], [39], [40]. SU6668 was dissolved in DMSO (both from Sigma-Aldrich, Steinheim, Germany) and stored at − 70 °C prior use. Human recombinant TGF-β1 and TGF-β3 were purchased from PeproTech (Rock Hill, NJ, USA). Human insulin, dexamethasone (DEX), IBMX, indomethacin and ascorbate-2-phosphate were purchased from Sigma-Aldrich, while ITS supplement was purchased from Gibco, Invitrogen (Carlsbad, CA, USA).
2.3. Cell culture
Peripheral blood mononuclear cells (PBMCs) and human ASCs were isolated and characterized as previously described [41]. The donors of primary cells are described in Supplementary Table 1. Freshly isolated PBMCs were frozen in D-MEM containing 1 g/L glucose (Gibco), supplemented with 50% of Fetal Bovine Serum (FBS) (PAA, Pasching, Austria) inactivated at 56 °C for 30 min (HI-FBS) and 10% DMSO and stored in liquid nitrogen prior to use. The C3H10T1/2 cell line was a generous gift from Prof. Rune Toftgård's lab (Centre for Nutrition and Toxicology, Karolinska Institute, Sweden). The MDA-MB-231 and 3T3-L1 cell lines were purchased from ATCC. MDA-MB-231 and 3T3-L1 cells were propagated in D-MEM containing 4.5 g/L glucose (Gibco) supplemented with 10% FBS and 1% penicillin/streptomycin mix (PEST) (Gibco). C3H10T1/2 and ASCs were propagated in D-MEM containing 1 g/L glucose supplemented with 10% of HI-FBS and 1% PEST. The cells were grown at 37 °C and 5% CO2. All treatments of the cells except inductions of ASCs towards chondrocytes were conducted in the respective base growth medium supplemented with 3% of FBS or HI-FBS, 1% PEST, 12 nM Shh or 10 ng/ml of TGF-β3 and different concentrations of SU6668, if indicated. Adipogenic differentiation was induced by 10 μg/ml of human insulin, 1 μM dexamethasone, 0.5 mM IBMX and 10 μM indomethacin. Chondrogenic differentiation was conducted in DMEM-high glucose containing 10 ng/ml of TGF-β1, 1× ITS supplement, 100 μM ascorbate-2-phosphate, 1 μM DEX and 1% PEST. Media were replenished every 2 d.
2.4. Alkaline phosphatase activity
C2H10T1/2 cells were washed with PBS and lysed in Lysis Solution (Tropix, Bedford MA, USA). Alkaline phosphatase (AP) activity was measured using CSPD substrate with Sapphire-II™ Enhancer (Invitrogen) and Genios Pro combined fluoro-and luminometer (Tecan Group Ltd., Männedorf, Switzerland). Total protein concentrations were measured using BCA Protein Assay kit (Pierce Biotechnology Inc., Rockford, IL, USA) and used for normalization of AP activity values.
2.5. Over-expression studies
Synthetic Negative siRNA (Silencer Negative Control 2, Neg. siRNA), Silencer® Select siRNAs S89965 and S89966 against mouse Ulk3 and S24886 and S24887 against human ULK3 were purchased from Ambion (Austin, TX, USA). Synthetic siRNAs were delivered to cells using Lipofectamine RNAiMax reagent (Invitrogen). 3T3-L1 cells were transfected with 20 nM of siRNAs. Initial reverse and 24 h later forward transfection procedures were conducted using the same amounts of siRNAs and transfection reagent. ASCs were transfected with 10 nM siRNAs using forward transfection protocol. The cells were treated with DMSO, SU6668 or/and 12 nM Shh or 10 ng/ml TGF/β1 24 h after forward transfection. MDA-MB-231 cells were transfected with constructs encoding human FLAG-tagged GLI2, mouse GFP-tagged Gli2, pcDNA3.1 or pmax-GFP (Lonza Inc.) using Lipofectamine 2000 (Invitrogen) [6], [40]. To measure transfection efficiency, the cells were fixed in 4% PFA for 30 min, washed with PBS and analyzed using Accuri C6 flow cytometer.
2.6. Western blot
Western blot (WB) was performed as previously described [41]. WB analyses were done using 40–60 μg of total protein per lane. All immuno-blotting assays were performed at least in three replicates and representative images are shown. Commercially available antibodies and their dilutions are listed in Supplementary Table 2. Gli2 AF3635 antibody has been described previously [7]. The specificity of Gli2 antibodies was tested and representative images are shown in Supplementary Fig. 1A–C. Runx2 antibody was generated in Labas Ltd. (Tartu, Estonia) against a peptide STLSKKSQAGASELG and used in dilution 1:2000. WB images were quantified using ImageQuant software.
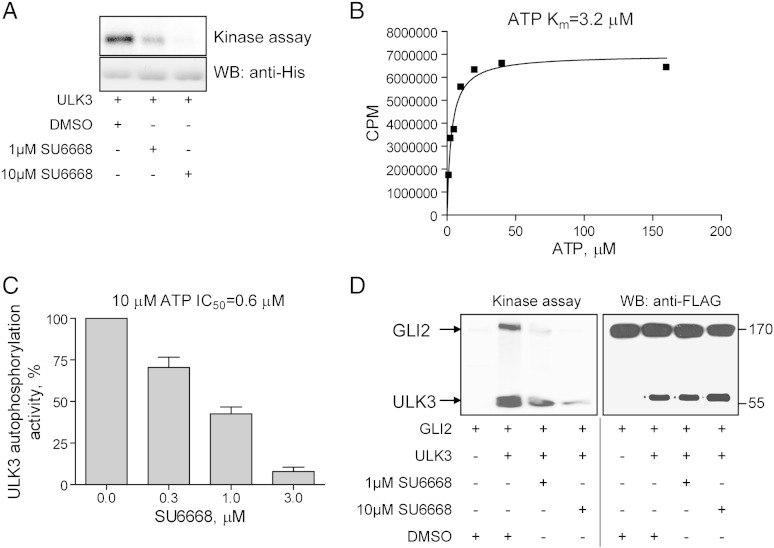
Fig. 1.
SU6668 inhibits ULK3 catalytic activity in vitro. (A) Bacterially expressed and purified His-tagged ULK3 protein was subjected to in vitro kinase assay in the presence of 2 μM ATP and vehicle or SU6668. The presence of ULK3 protein was verified by WB. (B) His-tagged ULK3 protein was subjected to in vitro kinase assay in the presence of indicated concentrations of ATP. The Michaelis–Menten curve was built using levels of ULK3 autophosphorylation activity normalized with amount of ULK3 protein identified by Coomassie staining (both quantified using ImageQuant software). (C) ULK3 protein was subjected to in vitro kinase assay in the presence of 10 μM ATP. Different concentrations of SU6668 were included into each reaction. The intensity of ULK3 autophosphorylation and the amount of ULK3 protein identified by Coomassie staining were quantified using ImageQuant software. The normalized level of ULK3 autophosphorylation activity in the presence of vehicle was set as 100%. (D) GLI2 and ULK3 were over-expressed and immuno-purified using a resin conjugated with FLAG antibody. The purified proteins were mixed and subjected to in vitro kinase assay (left panel). The proteins were verified by WB (right panel).
2.7. In vitro kinase assay and statistical analysis
In vitro kinase assay and statistical analysis were performed as described [6]. IC50, Km and Ki values were calculated using GraphPad Prism software.
2.8. Immunocytochemistry
Primary cilia and nuclei of ASCs were stained as previously described [42] using antibodies against acetylated tubulin (Sigma-Aldrich), Alexa Fluor 568 goat anti-mouse IgG (Invitrogen), and Mounting Medium with DAPI (Abcam). The cells were visualized using fluorescent microscope Olympus BX61 with UPLan SApo 40 × objective.
2.9. Nuclear extract preparation
Nuclear and cytoplasmic extracts were prepared using NE-PER® Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific). Approximately 40 μg of the extracts was used for WB analysis.
2.10. Quantitative real time PCR
Quantitative real time PCR (qPCR) was performed as described [41]. The data of qPCR analyses are expressed as the normalized with Hprt or GAPDH average means ± S.D. of three measurements obtained from at least three independent experiments. Expression of genes demonstrating Ct values above 37 were considered as undetectable. The primers used are listed in Supplementary Table 3.
3. Results
3.1. SU6668 inhibits ULK3 kinase activity in vitro
ULK3 is an active serine/threonine kinase able to phosphorylate itself and GLI proteins [40]. It has been previously shown that a tyrosine kinase inhibitor SU6668 can physically interact with ULK3 in a chromatographic pull-down assay [23]. In order to test, whether SU6668 is able to inhibit ULK3 kinase activity, bacterially expressed and purified His-tagged ULK3 protein was tested in the in vitro kinase assay in the presence of 1 or 10 μM SU6668. SU6668 inhibited ULK3 autophosphorylation activity in a concentration dependent manner (Fig. 1A).
In order to determine the Ki of SU6668 for ULK3, first the Km of ATP towards bacterially purified ULK3 was measured and found to be 3.2 μM under these conditions (Fig. 1B). Next, increasing concentrations of SU6668 were used in the presence of 10 μM ATP in the in vitro kinase assay (Fig. 1C). Under these conditions, SU6668 was able to inhibit the ULK3 autophosphorylation activity with an IC50 of 0.6 μM. The calculated Ki of SU6668 for ULK3 was found to be approximately 0.2 μM.
In order to exclude tag-specific phenomena and verify the effect of SU6668 using other ULK3 substrates, over-expressed and immuno-purified FLAG-tagged ULK3 and GLI2 proteins were subjected to in vitro kinase assay in the presence of 2 μM ATP and SU6668. Phosphorylation of GLI2 by ULK3 was completely inhibited by 10 μM SU6668; however, some residual catalytic activity of ULK3 was detectable in the presence of 1 μM SU6668 (Fig. 1D, left panel). The presence of the proteins tested in the in vitro kinase assay was confirmed by WB (Fig. 1D, right panel). These data demonstrate that SU6668 is an efficient inhibitor of ULK3 kinase in the in vitro kinase assay.
3.2. SU6668 inhibits the Shh signal transduction
Since Ulk3 is able to modulate the activity of Gli proteins and to interact with SU6668, the effects of SU6668 on the Shh pathway execution was tested in two Shh-responsive mouse cell lines C3H10T1/2 and 3T3-L1. Shh stimulates differentiation of C3H10T1/2 toward osteoblasts without significant increase in proliferation [29], [33]. In contrast, pre-adipocytes 3T3-L1 demonstrated increased proliferation rate in response to Shh (Supplementary Fig. 1D).
C3H10T1/2 and 3T3-L1 cells were treated with Shh and/or 5, 10 or 20 μM SU6668 for 42 h. Expression levels of Gli1, Ptch1, Gli2 and Ulk3 were analyzed by qPCR (Fig. 2 and Supplementary Fig. 2, for C3H10T1/2 and 3T3-L1, respectively). SU6668 alone had no significant effect on expression of Gli1, Ptch1 and Gli2 genes. Expression of Ulk3 was elevated approximately 1.5–2 times in the presence of 20 μM SU6668 (Fig. 2A d and Supplementary Fig. 2A d).
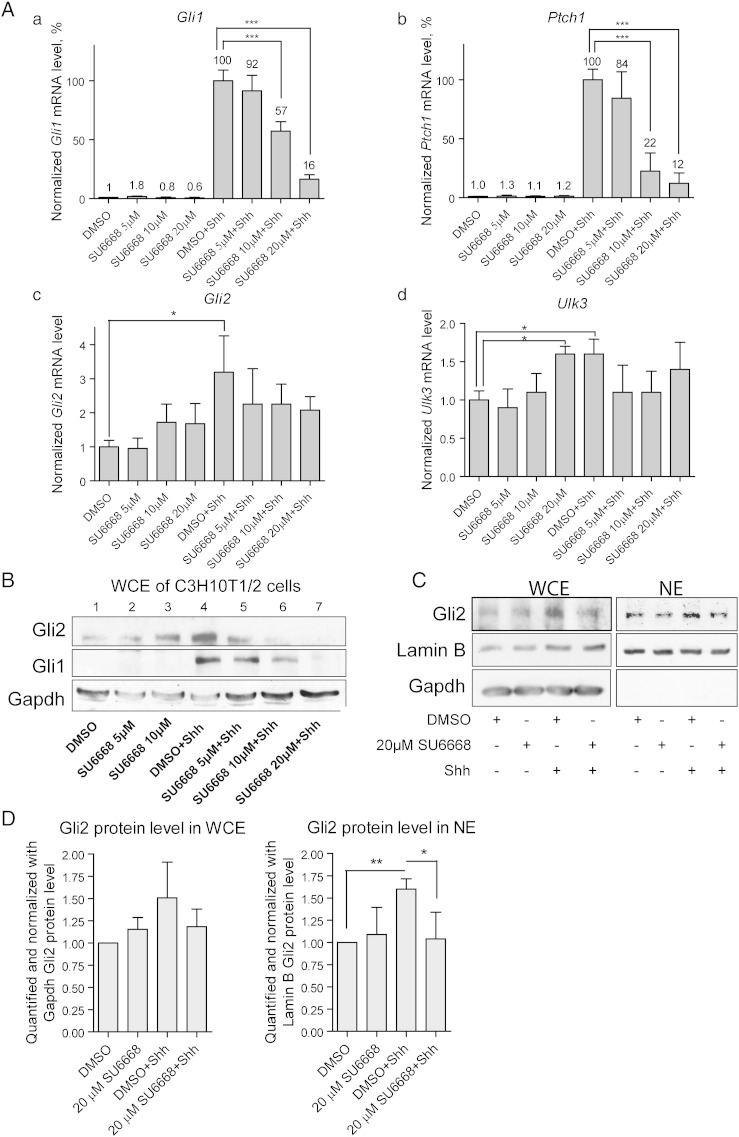
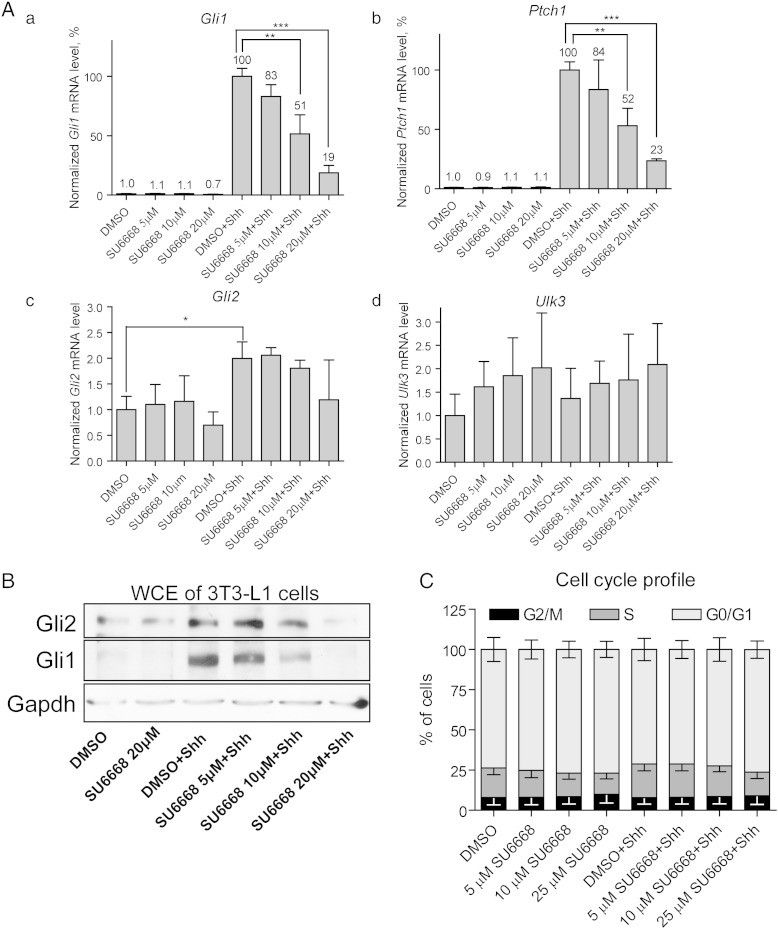
Fig. 2.
SU6668 hinders Shh signaling in C3H10T1/2 cells. (A, B) C3H10T1/2 cells were treated as indicated for 42 h. The levels of expression of Gli1, Ptch1, Gli2 and Ulk3 were analyzed by qPCR and normalized by the level of Hprt mRNA expression. The normalized level of the respective gene mRNA in the control sample treated with DMSO was set as 1. The data are presented as an average mean ± S.D.; *—p < 0.05, ***—p < 0.001, n = 4. (C) C3H10T1/2 cells were treated as indicated for 2 h. The cells were split for whole cell extract and nuclear extract preparation (WCE and NE, respectively). The proteins were analyzed by WB. Gli2 was detected using Gli2 G-20 (B) or AF3635 (C) antibodies. (D) Gli2, Lamin B and/or GAPDH images were quantified. Gli2 protein levels were normalized with Lamin B and/or GAPDH levels and set as 1 in the control cells treated with DMSO. Data are presented as an average mean ± S.D.; *—p < 0.05, **—p < 0.01, n = 3.
Shh pathway transcriptional targets Gli1 and Ptch1 became strongly induced in the Shh-stimulated cells: more than 100 and 30 times above the control, respectively. In order to synchronize data obtained from independent experiments, expression levels of Gli1 and Ptch1 in the cells treated with Shh and DMSO were set as 100%, and the data from samples treated with Shh and SU6668 were calculated relative to this (Fig. 2A a, b). SU6668 inhibited activation of Gli1 and Ptch1 expressions in a concentration dependent manner. In response to Shh, the levels of Gli2 and Ulk3 transcripts increased approximately 3 and 1.5 times, respectively (Fig. 2A c, d). In contrast to Gli1 and Ptch1, expression levels of Gli2 and Ulk3 were not significantly down-regulated by SU6668.
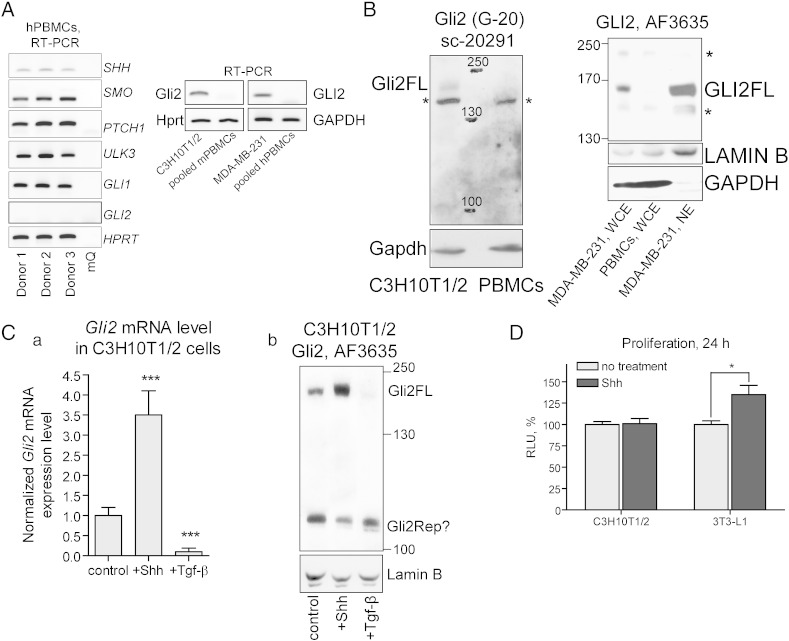
Along with RNA analysis, Gli1 and Gli2 protein levels were analyzed by WB (Fig. 2B and Supplementary Fig. 2B). Shh stimulated an increase of Gli2 protein level that correlated with up-regulation of Gli2 mRNA expression (Figs. 2B, upper panel, lanes 1 and 4 and A c). Nevertheless, the levels of Gli2 protein and mRNA did not correlate in the SU6668-treated cells. The level of Gli2 mRNA was similar in the cells treated with either 10 or 20 μM SU6668 alone or combined with Shh (Fig. 2A c). In contrast, cells treated with Shh and SU6668 expressed less Gli2 protein compared with the cells treated only with SU6668 (Fig. 2B, upper panel, lanes 3, 6 and 7). Generally, the levels of Gli2 protein, but not mRNA, correlated with the levels of Gli2 transcriptional target Gli1 in the cells treated with SU6668 and Shh (Fig. 2A a). The specificity of Gli2 antibodies was tested using C3H10T1/2, MDA-MB-231 and peripheral blood mononuclear cells (PBMCs) of mouse and human origin. In contrast to other cells analyzed, PBMCs do not express Gli2 mRNA, but express other components of the Shh signaling pathway (Supplementary Fig. 1A). Gli2FL migrating at approximately 165–170 kDa was detected using immuno-blotting in C3H10T1/2 and MDA-MB-231 cells but not in PBMCs (Supplementary Fig. 1B–C).
Although Gli1 transcripts were detected in the control cells, Gli1 protein was undetectable in the absence of Shh (Fig. 2B, middle panel). Similarly to Gli1 mRNA and Gli2 protein, Gli1 protein was up-regulated in response to Shh and down-regulated in the presence of SU6668.
It has been shown that stimulation of cells with Shh leads to rapid stabilization and nuclear translocation of Gli2FL [7]. Gli2FL protein was examined by WB in whole cell and nuclear extracts (WCE and NE, respectively) of C3H10T1/2 cells treated with 20 μM SU6668 and/or Shh for 2 h (Fig. 2C). The amount of Gli2FL increased in response to Shh in WCE and NE signifying physical stabilization and nuclear translocation of Gli2FL. SU6668 inhibited the positive effect of Shh on Gli2FL indicating that one of the possible mechanisms of SU6668 action may be prevention of stabilization and/or nuclear transport of Gli2FL. In order to confirm the visual observations, Gli2 and Lamin B and/or GAPDH immuno-blotting images obtained from 3 independent experiments were quantified. Gli2 protein levels were normalized using Lamin B or GAPDH protein levels and set as 1 in the control sample. Data from other samples were calculated relative to that (Fig. 2D). The level of Gli2 protein increased approximately 1.6 times in response to Shh in WCE and NE and remained similar to the control level in the presence of SU6668.
3.3. SU6668 acts via Ulk3
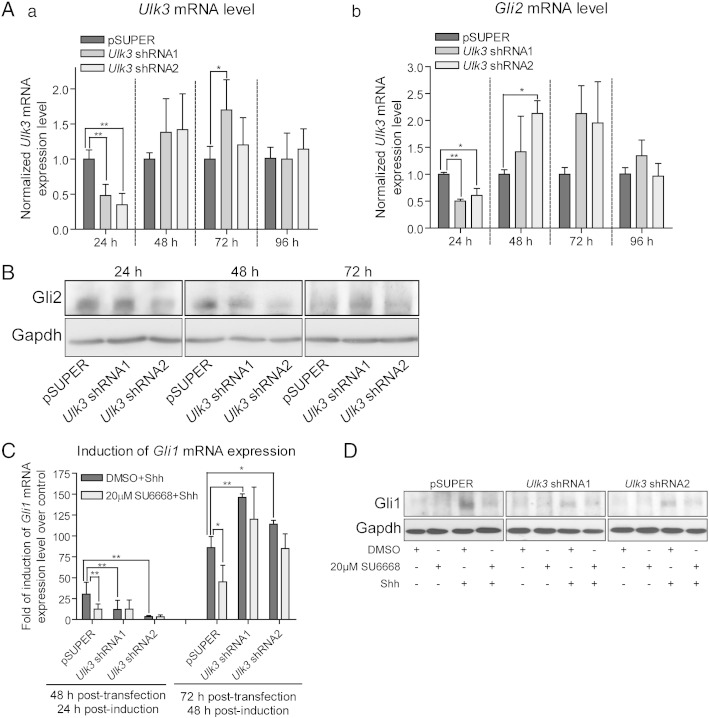
It has been shown that SU6668 induces G2/M phase arrest of HeLa cells [23]. Block of cell cycle in G2/M phase and subsequent inhibition of primary cilia formation may negatively influence the Shh pathway execution. Therefore, the cell cycle profile was analyzed in C3H10T1/2 and 3T3-L1 cells treated with Shh and/or different concentrations of SU6668. No significant change in G2/M phase of cell cycle was detected (Supplementary Fig. 2D). It has been shown that several other kinases apart of Ulk3 participate in regulation of Gli proteins, and SU6668 is not specific inhibitor for Ulk3 [22], [23]. In order to examine, whether the effects of SU6668 on Gli proteins are mediated via Ulk3 kinase, we suppressed Ulk3 mRNA expression in C3H10T1/2 and 3T3-L1 cells. For Ulk3 RNAi, the previously described pSUPER constructs encoding Ulk3 shRNAs [6] and Ulk3-specific synthetic siRNAs S89965 and S89966 (Ambion) were used. C3H10T1/2 cells were electroporated with the constructs encoding Ulk3 shRNAs, and 3T3-L1 cells were transfected with Ulk3 synthetic siRNAs using Lipofectamine. The obtained results are depicted in Fig. 3 and Supplementary Fig. 3 for 3T3-L1 and C3H10T1/2 cells, respectively.
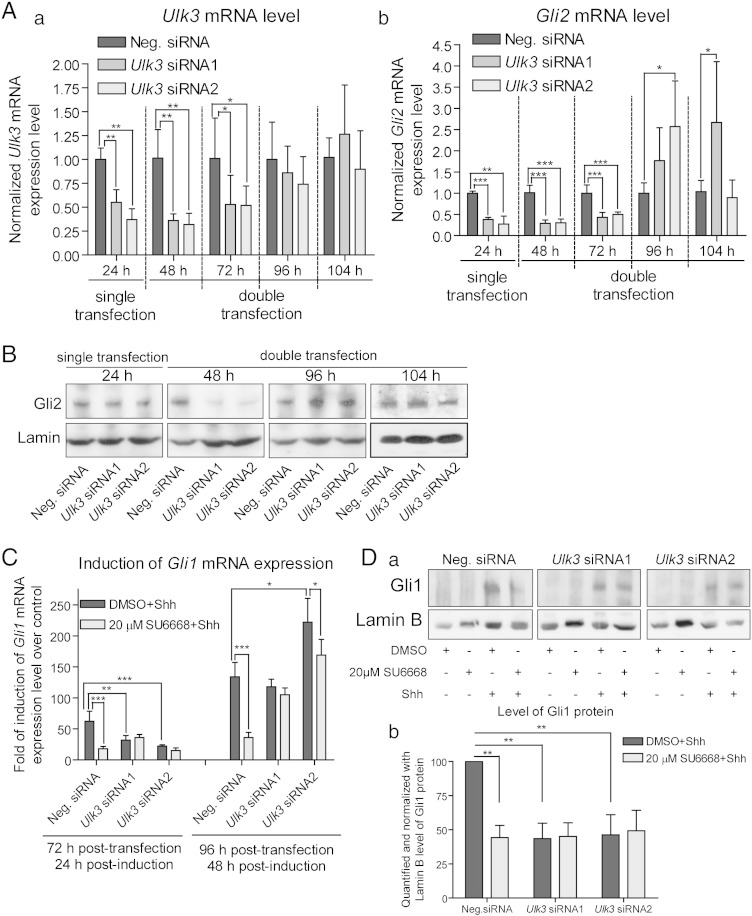
Fig. 3.
SU6668 inhibits Shh dependent up-regulation of Gli1 via Ulk3. (A, B, C, D) 3 T3-L1 cells were transfected with Ulk3-specific Silencer® Select siRNAs S89965 and S89966 using reverse followed by forward procedures resulted in single and double transfection, respectively. The cells were treated with DMSO, 20 μM SU6668 and Shh, if specified, and incubated during the indicated periods of time. (A) Levels of Ulk3 (a) and Gli2 (b) transcripts were measured by qPCR, normalized with Hprt mRNA expression level and set as 1 in the cells transfected with Negative siRNA. The data from samples transfected with Ulk3-specific siRNAs were calculated relative to the negative control. (B) The cells were subjected to WB analysis after the indicated periods of time of the siRNA delivery using Gli2 G-20 antibody. (C) The cells subjected to double transfection by siRNAs were treated with Shh, DMSO or SU6668 for 24 and 48 h. Gli1 mRNA expression level was measured by qPCR, normalized with Hprt expression level and set as 1 in the respectively transfected control sample treated with DMSO. Gli1 mRNA expression level in the Shh-treated samples were calculated relative to the control. The data are presented as fold of induction of Gli1 mRNA level. Data of qPCR analyses are presented as an average mean ± S.D.; *—p < 0.05, **—p < 0.01, ***—p < 0.001, n = 3. (D, a–b) Lysates of 78 h post-transfectional and 30 h post-inductional cells were analyzed by WB. Gli1 and Lamin B images were quantified. The normalized Gli1 protein level in the control cells treated with DMSO and Shh was set as 100%. Data from other samples were calculated relative to that. Data are presented as an average mean ± S.D.; **—p < 0.01, n = 4.
Ulk3 and Gli2 mRNA expression levels were measured using qPCR and set as 1 in the cells transfected with Neg. siRNA (Fig. 3A a, b). Ulk3 mRNA levels measured at 24 h post-transfection were reduced approximately 45% and 64% in the 3T3-L1 cells transfected with Ulk3 siRNA1 and siRNA2, respectively. The levels of Gli2 mRNA measured in the same cells were reduced approximately 62% and 73%. Cells transfected with Ulk3 siRNAs twice expressed approximately 70% less Ulk3 and Gli2 transcripts. Initial low Ulk3 and Gli2 mRNA levels increased gradually during propagation of cells reaching and even exceeding the control levels. Similar tendency was observed in C3H10T1/2 cells; however, the increase of Ulk3 and Gli2 mRNA levels was more rapid (already within 48 h after transfection) probably due to the single electroporation procedure applied (Supplementary Fig. 3A a–b).
Gli2 protein level was assessed by WB. Correlation between the levels of Gli2 protein and mRNA was observed with some temporal shift. Although 24 h post-transfectional cells contained similar amounts of Gli2 protein, cells transfected twice with Ulk3 siRNAs demonstrated reduced levels of Gli2 protein (Fig. 3B). Gli2 protein level gradually increased in the cells transfected with Ulk3 siRNAs during propagation exceeding that of control 96 h after initial transfection which correlated with up-regulation of Gli2 mRNA level.
Afterwards, 48 h post-transfectional cells expressing the lowest levels of Gli2 and Ulk3 were treated with Shh, 20 μM SU6668 or DMSO for 24 and 48 h. Gli1 mRNA levels were measured by qPCR. For the each particular set of data, Gli1 expression level was considered as 1 in control cells treated with DMSO and the data from other respectively transfected but differently treated samples were calculated as fold of Gli1 mRNA induction over control. SU6668 alone had no significant effect on Gli1 mRNA expression (data not shown). In response to Shh, Gli1 mRNA level initially increased approximately 75 times in the control cells and 32 and 22 times in the cells transfected with Ulk3 siRNAs (Fig. 3C). However, compared to the control cells, Gli1 mRNA level became similar or even higher in the cells transfected with Ulk3 siRNAs at the next time point (96 h post-transfection, 48 h post-induction with Shh). As the above-described analyses revealed, Gli2 mRNA and protein levels had been also increased at this time point in these cells (Fig. 3A b and B) that may be associated with increased transcriptional activity of Gli2. Up-regulation of Gli2 may explain higher Gli1 mRNA expression levels in the cells transfected with Ulk3 siRNAs and induced by Shh for 48 h. SU6668, added together with Shh, was active in the control cells but could not effectively inhibit Gli1 mRNA expression in the cells with reduced levels of Ulk3 and Gli2 (Fig. 3C). However, low negative effects of SU6668 on Shh dependent activation of Gli1 in the case of restored levels of Gli2 observed 96 h after Ulk3 RNAi may be caused by possible instability of SU6668 in cell culture conditions during 48 h of incubation and consequently, insufficient concentration of active SU6668 per Gli2/Ulk3 complex in the cells expressing increased levels of Gli2 protein.
In order to confirm the observations of the impact on Gli1 mRNA level, Gli1 protein was analyzed by WB in the cells transfected twice with Ulk3 or Neg. siRNAs and treated with Shh and/or 20 μM SU6668 for 32 h. Gli1 and Lamin B immuno-blotting images obtained from four independent experiments were quantified. Gli1 protein level was normalized with Lamin B and set as 100% in control sample. Data from other samples were calculated relative to this. Increase of Gli1 protein was stronger in the control cells, and SU6668 was not effective in the cells transfected with Ulk3 siRNAs (Fig. 3D). Similar results were obtained analyzing levels of Gli1 mRNA and protein in another experimental model (Supplementary Fig. 3C–D).
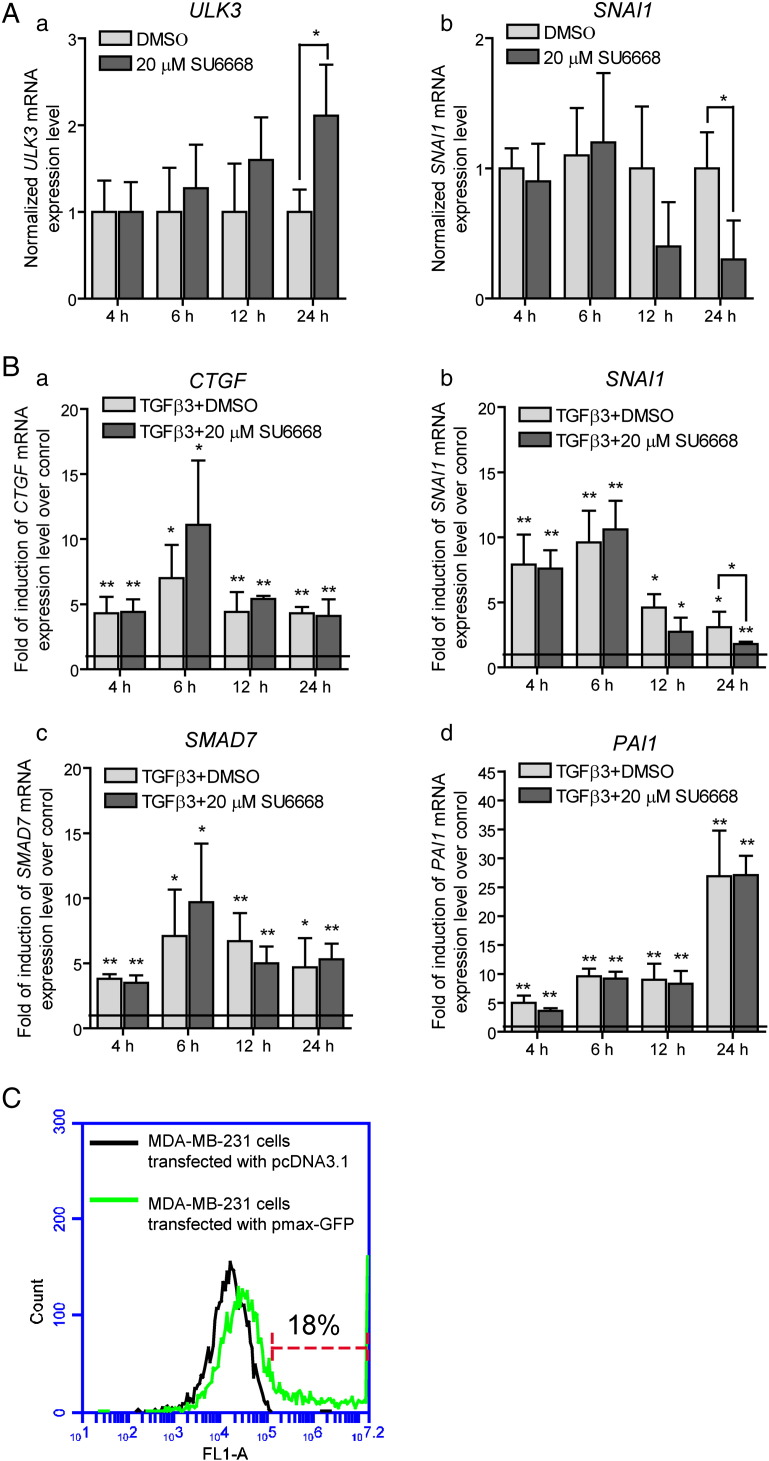
3.4. Regulation of GLI1/2 by TGF-β and SU6668 in human immortalized breast cancer cells
Apart from Shh, TGF-β regulates expression and transcriptional activity of GLI1/2 in a SMAD3, TCF4 and β-catenin dependent manner [18], [19]. In order to explore the effects of SU6668 on regulation of GLI2 in the context of TGF-β pathway, immortalized breast cancer cells MDA-MB-231 were used. These cells have been previously reported to express GLI1, GLI2 and PTCH1 and respond to TGF-β1 treatment with increased levels of GLI2 and GLI1 mRNAs [18], [43]. MDA-MB-231 cells were treated with TGF-β3, 20 μM SU6668 or DMSO for 4, 8, 12 and 24 h. The expression levels of “Shh mediators” GLI2, GLI1 and ULK3 and “classical” TGF-β target genes SMAD7, PAI1, CTGF and SNAI1 were measured by qPCR and normalized to 1 in the cells treated with DMSO (Fig. 4A and Supplementary Fig. 4).
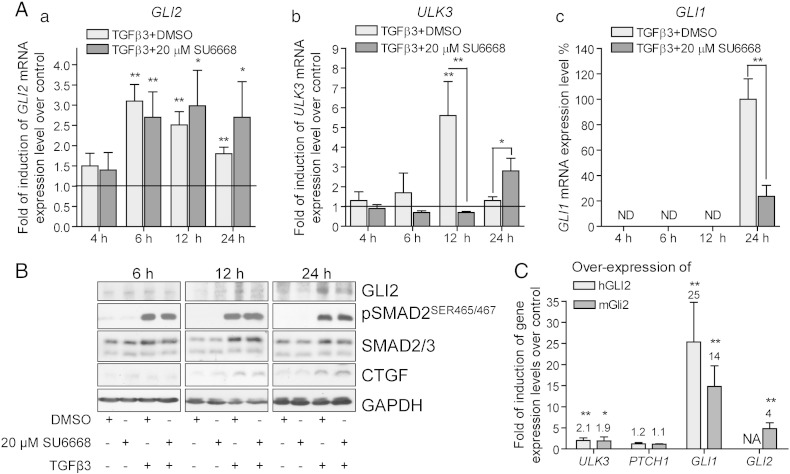
Fig. 4.
TGF-β induced positive regulation of GLI2 protein is inhibited by SU6668 in MDA-MB-231 cells. (A, B) MDA-MB-231 cells were treated with DMSO or 20 μM SU6668 in the presence of TGF-β3 during the indicated periods of time. (A) GLI2 (a), ULK3 (b) and GLI1 (c) mRNA levels were analyzed by qPCR, normalized with GAPDH expression level and set as 1 in the control cells treated with DMSO (indicated by baseline on the panels a and b). The level of GLI1 mRNA expression in the cells treated with TGF-β was set as 100%. The data are presented as an average fold of induction of gene expression level over a control ± S.D. (B) Whole cell lysates were subjected to WB analysis. GLI2 protein was detected using AF3635 antibody. (C) MDA-MB-231 cells were transfected either with plasmids encoding mouse mGli2 or human hGLI2. The levels of the indicated gene expression were measured by qPCR after 18 h. The data are presented as an average fold of induction ± S.D. of the particular gene expression level over a control level measured in the cells transfected with an empty vector; (A, C) *—p < 0.05, **—p < 0.01, ***—p < 0.001, n = 3, ND — not detected.
SU6668 had no statistically significant effect on expression of most genes analyzed except ULK3 and SNAI1 (data not shown and Supplementary Fig. 4A a, b). ULK3 expression gradually increased during exposure of cells to SU6668 reaching almost 2-fold induction within 24 h. Expression of SNAI1 was down-regulated by SU6668 approximately 65%.
TGF-β stimulated expression of all analyzed genes, however, the dynamic of up-regulation and effects of SU6668 varied for different genes (Fig. 4A and Supplementary Fig. 4A). Significant up-regulation of SMAD7, PAI1, CTGF and SNAI1 was observed already within 4 h of TGF-β treatment. Expression of GLI2 increased approximately 3 times during 6 h of TGF-β treatment and afterwards decreased gradually but remained elevated over control.
SU6668 did not significantly inhibit TGF-β induced up-regulation of GLI2, SMAD7, PAI1 and CTGF. It had statistically insignificant positive effect on GLI2 mRNA expression during prolonged propagation of cells in the presence of TGF-β (Fig. 4A a). Expression of ULK3 was induced 12 h after activation of TGF-β signaling (Fig. 4A b). Increase of ULK3 transcription was accompanied by augmentation of GLI2 protein level (Fig. 4A b and B, respectively). In contrast to “classic” TGF-β target genes, up-regulation of ULK3 expression was inhibited by SU6668 that correlated with depleted level of GLI2 protein in the respectively treated cells. However, ULK3 mRNA level increased in response to prolonged treatment with TGF-β and SU6668 similarly as it was observed in the case of SU6668 alone (Fig. 4A b and Supplementary Fig. 4A a). Also, TGF-β dependent induction of SNAI1 expression was down-regulated by SU6668 suggesting a positive correlation between GLI2 transcriptional activity and expression of SNAI1.
Contrary to the previously published reports, GLI1 expression was under the detection limit in native MDA-MB-231 cells. In order to compare GLI1 mRNA levels induced by TGF-β in the presence or absence of SU6668, expression level of GLI1 was set as 100% in the samples treated with TGF-β and the data from other samples were calculated relative to that. In contrast to GLI2, GLI1 expression was significantly down-regulated by SU6668 24 h after treatment with TGF-β, as it was observed for ULK3 at 12 h (Fig. 4A c).
WB analysis of cells treated with TGF-β combined with either DMSO or SU6668 showed similar levels of primary mediators of TGF-β signal — SMAD2/3 and activated form of SMAD2 phosphorylated at serine residues 465/467, as well as CTGF (Fig. 4B). However, GLI2 protein was not up-regulated in the presence of SU6668.
Increase of ULK3 mRNA expression correlated with up-regulation of GLI2 protein level at the respective time point. These data suggest a possible involvement of GLI2 in regulation of ULK3 gene expression. To test this hypothesis, mouse and human Gli2 were over-expressed in MDA-MB-231 cells for 16 h. The levels of ULK3, PTCH1, GLI1 and GLI2 mRNAs were measured by qPCR (Fig. 4C). GLI2 of human origin demonstrated slightly higher transcriptional activity than mouse Gli2 in MDA-MB-231 cells. Gli2 could up-regulate GLI2 expression, either directly or via induction of GLI1. Over-expressed Gli2 proteins could not induce PTCH1 expression in MDA-MB-231 cells, whereas ULK3 expression was induced approximately 2 times suggesting that GLI2 may play a co-activator role in regulation of ULK3 gene expression. Lower increase of ULK3 expression in response to over-expressed Gli2 proteins may be caused by poor transfection efficiency of MDA-MB-231 cells (approximately 18%) (Supplementary Fig. 4C).
3.5. ULK3 controls GLI1/2 proteins in human ASCs
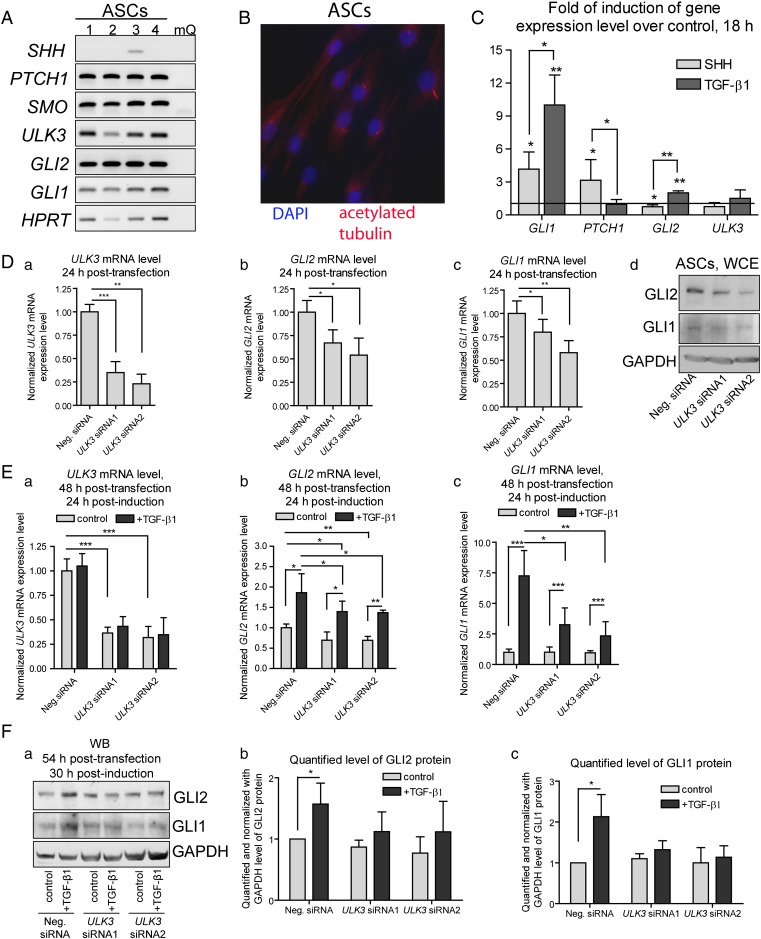
ASCs represent heterogeneous populations of cells able to respond to variable stimuli by expressing different lineage-specific markers and differentiate, at least under in vitro conditions, towards adipocytes, chondrocytes and osteoblasts [24]. Since human ASCs have not been systematically described in respect of the canonical Shh pathway, expression of the mediators of Shh signaling (SHH, PTCH1, SMO, ULK3, GLI2 and GLI1) was examined by RT-PCR in ASCs isolated from four donors. All genes except SHH were expressed in ASCs (Fig. 5A). Very low level of SHH expression was detected only in one sample suggesting that generally ASCs are not subjected to autocrine regulation by Shh. Also, ASCs were stained using an antibody against acetylated tubulin to visualize primary cilia required for the canonical Shh signal transduction. Approximately 50% of ASCs displayed primary cilia (Fig. 5B).
Fig. 5.
ULK3 participates in regulation of GLI1/2 transcriptional activators in ASCs. (A) Expression of genes encoding mediators of the Shh signaling pathway was analyzed using RT-PCR in ASCs isolated from four donors. (B) Primary cilia (red) and nuclei (blue) were stained using antibody against acetylated tubulin and DAPI, respectively. (C) Levels of GLI1 (a), PTCH1 (b), GLI2 (c) and ULK3 (d) mRNAs were measured by qPCR in ASCs stimulated with Shh or TGF-β1. (D) ASCs were transfected with Neg. or ULK3-specific siRNAs. The levels of ULK3 (a), GLI2 (b) and GLI1 (c) transcripts were measured by qPCR; (d) GLI1 and GLI2 proteins were analyzed by immuno-blotting. (E) ASCs were transfected with Neg. or ULK3-specific siRNAs and 24 h later treated with TGF-β1 for 24 h. The levels of ULK3 (a), GLI2 (b) and GLI1 (c) transcripts were measured by qPCR. (F) (a) GLI2 and GLI1 proteins were analyzed by WB in cells transfected with Neg. or ULK3-specific siRNAs and induced with TGF-β1 for 30 h. GLI2, GLI1 and GAPDH images were quantified. Normalized GLI2 (b) and GLI1 (c) protein levels were set as 1 in cells transfected with Neg. siRNA. Data of qPCR and quantified WBs are presented as an average mean ± S.D.; *—p < 0.05, **—p < 0.01, ***—p < 0.001, n = 3.
GLI proteins can be activated via Shh and TGF-β signaling axes. In order to examine canonical and non-canonical activation of GLI1/2 proteins in ASCs, the cells were treated with Shh or TGF-β1 for 18 h and the levels of expression of GLI1, PTCH1, GLI2 and ULK3 were examined by qPCR. The cells responded to Shh treatment by increased level of GLI1 and PTCH1 mRNA, whereas GLI2 mRNA level was elevated in response to TGF-β (Fig. 5C). In all cells analyzed, TGF-β activated GLI1 transcription approximately 2.2 times stronger than Shh did probably due to associated activation of GLI2. Up-regulation of GLI1 and PTCH1 expression in response to Shh, presence of primary cilia and expression of the key mediators of the Shh signaling together strongly indicate a subpopulation of ASCs possessing the functional Shh pathway.
Next, ULK3 was assessed as a regulator of GLI1/2 in ASCs. ULK3 mRNA expression was inhibited approximately 65 and 77% using two synthetic siRNAs (Fig. 5D a). Concurrently, GLI2 and GLI1 mRNA and protein levels were reduced in these cells (Fig. 5D b–d). Of note, in contrast to the mouse cells used, the level of GLI1 protein was high enough to be detected by WB in native ASCs (Fig. 5D d). Afterwards, 24 h post-transfectional ASCs were treated with TGF-β1 for additional 24 h and expression of ULK3, GLI2 and GLI1 were analyzed by qPCR. Gene expression levels in control cells transfected with Neg. siRNA were set as 1, and data from other samples were calculated relative to this (Fig. 5E a–c). The levels of ULK3 transcripts remained reduced 48 h post-transfection in cells treated with ULK3 siRNAs. GLI2 expression was induced in response to TGF-β treatment approximately 1.7 times in all cells analyzed. However, the absolute levels of GLI2 transcripts remained lower in the TGF-β1 treated cells transfected with ULK3-specific siRNAs and expressed lower levels of GLI2 mRNA and protein at treatment initiation. Substantial increase of GLI2 protein level in response to TGF-β1 was observed only in ASCs transfected with Neg. siRNA (Fig. 5F a, upper panel and b). GLI1 transcription was induced by TGF-β1 approximately 7.3, 2.6 and 2.4 times in ASCs transfected with Neg. siRNA, ULK3 siRNA1 and siRNA2, respectively (Fig. 5E c). Similarly to GLI2, significant increase of GLI1 protein in response to TGF-β treatment was observed only in the cell transfected with Neg. siRNA (Fig. 5F). Taken together, these data indicate that ULK3 does not participate in the TGF-β signaling cascade but is required for stability of GLI2 and GLI1 proteins.
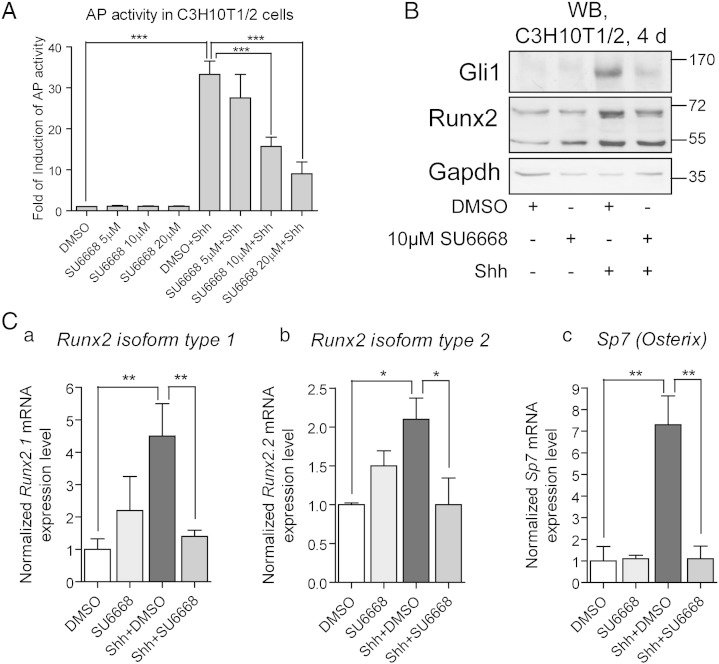
3.6. SU6668 inhibits osteo- and chondrogenic differentiation of ASCs
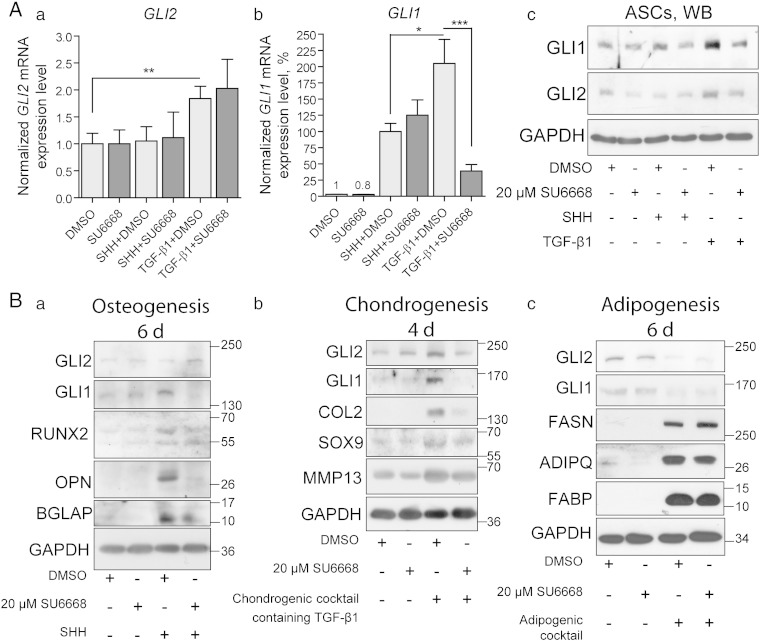
In order to explore the effect of SU6668 on GLI1/2 proteins in primary human cells, ASCs were treated with Shh or TGF-β1 in the presence of 20 μM SU6668 or DMSO. GLI1 and GLI2 expression levels were assessed by qPCR and the respective protein levels by immuno-blotting (Fig. 6A a–c). In order to synchronize the data obtained from different experiments, GLI1 expression level in control ASCs treated with Shh was set as 100% and data from other samples were recalculated relative to this. TGF-β stimulated GLI2 mRNA expression was not suppressed by SU6668, as it was observed in MDA-MB-231 cells (Figs. 6A a and 4A a, respectively). Also, SU6668 had no negative effect on Shh-induced GLI1 mRNA expression but inhibited expression of GLI1 up-regulated in response to TGF-β signaling (Fig. 6A b). However, immuno-blotting analysis showed that induction of GLI1 and GLI2 proteins in response to Shh or TGF-β was restrained by SU6668 (Fig. 6A c). The behavior of GLI1 in ASCs challenged with Shh and SU6668 resembled that of Gli2 in mouse C3H10T1/2 and 3T3-L1 cells: GLI1 mRNA and protein levels did not correlate in ASCs treated with Shh and SU6668 similarly to non-correlated Gli2 mRNA and protein levels in the respectively treated mouse progenitor cells (Figs. 6A b and c 1st panel, 2A c, B 1st panel, Supplementary Fig. 2A c and Supplementary Fig. 2B 1st panel). Similar phenomenon was observed in the case of GLI2 and TGF-β: GLI2 transcription was not inhibited by SU6668, but up-regulation of GLI2 protein and as a result its transcriptional activity was restrained. These data reveal that effects of SU6668 resemble effects of ULK3 RNAi in the case of GLI1/2 activation: neither SU6668 nor ULK3 knockdown interrupts Shh or TGF-β signaling cascades but both reduce stability of the de novo generated GLI1 and/or GLI2 proteins (Fig. 5 E–F and 6A). The difference between ULK3 RNAi and SU6668 treatments becomes apparent in the case of regulation of basal GLI proteins: SU6668 has no significant effect on their expression, but ULK3 knockdown leads to decrease of basal GLI1/2 levels (Fig. 5D and 6A c). This difference may be explained by possible dual role of ULK3: SU6668 inhibits only catalytic activity of ULK3 kinase, whereas both regulatory and catalytic activities are disrupted by ULK3 RNAi.
Fig. 6.
SU6668 prevents de novo generation of GLI1 and GLI2 proteins in ASCs. (A) ASCs were treated with Shh, TGF-β1 and 20 μM SU6668 or DMSO. (a) Levels of GLI2 and GLI1 transcripts were measured using qPCR. Data are presented as an average mean ± S.D.; *—p < 0.05, **—p < 0.01, ***—p < 0.001, n = 4. (b) WCEs were subjected to immuno-blotting analysis. (B) ASCs were treated with Shh (a), chondrogenic cocktail containing TGF-β1 (b) or adipogenic cocktail containing insulin, DEX, IBMX and indomethacin (c) in the presence of DMSO or 20 μM SU6668. GLI1/2 and lineage specific proteins were analyzed by WB.
To study the effects of SU6668 on tri-lineage differentiation of human ASCs, the respective treatments were conducted for 4 or 6 d, and WCEs were analyzed using immuno-blotting. Shh triggered expression of all osteogenic differentiation markers tested (both RUNX2 isoforms, OPN and BGLAP) and GLI1 (Fig. 6B a). Chondrocytes specific proteins COL2, SOX9, MMP13 as well as GLI1 and GLI2 were induced in response to prolonged exposure to TGF-β1 containing media (Fig. 6B b). SU6668 inhibited expression of the chondro- and osteo-lineage specific markers in ASCs, as it was observed in C3H10T1/2 cells (Fig. 6B and Supplementary Fig. 5, respectively). The inhibiting effect of SU6668 was noticeable in the case of the higher molecular weight RUNX2 isoform that was induced to higher extent by Shh (Fig. 6B a 3rd panel and Supplementary Fig. 5B–C). In contrast to osteo- and chondrogenic differentiation programs, adipogenic differentiation of ASCs was accompanied by down-regulation of GLI1/2 protein and was not inhibited by SU6668 (Fig. 6B c).
4. Discussion
Regulation of Gli1/2 transcription factors is one of the cross-talking points of Shh and TGF-β signaling pathways. However, mechanistic differences specific for these intracellular signaling cascades are obvious: TGF-β/SMAD axis controls GLI2 transcription, whereas post-translational regulation of Gli2 protein activity is the central event in the Shh pathway. In both cases, Gli2 is known to up-regulate Gli1 transcription. In this study we have identified SU6668 as a small molecular weight modulator of Gli protein activity and shown that there are common elements in regulation of Gli1/2 transcriptional activators by TGF-β and Shh.
4.1. SU6668 as an inhibitor of Ulk3 kinase activity and de novo generated Gli proteins
The existing inhibitors of the Shh pathway mostly target SMO oncoprotein, the trans-membrane signaling effectors of Shh. However, in many cases the somatic gain-of-function mutations in SMO occur in human cancers, making SMO inhibitors useless for therapeutic purposes [21]. Also, the fact that GLI transcription factors may be functioning independently of the canonical Shh pathway requires development of novel inhibitors targeting activity of GLI proteins [44], [45], [46]. This kind of inhibitors might be useful in treatment of specific GLI-dependent types of cancer. ULK3 has been suggested as a regulator of GLI1/2 transcriptional activators able to interact with the ATP-competitive tyrosine kinase inhibitor SU6668 [23], [40].
Here, SU6668 is demonstrated as an inhibitor of ULK3 kinase activity in vitro with Ki of 0.2 μM. Biochemical Ki of SU6668 towards receptor tyrosine kinases ranged from 0.008 μM for PDGFR to 2.1 μM for VEGFR2, and IC50 values for a number of other kinases were at least 10 μM [22]. The observed Ki value indicates that SU6668 is effective inhibitor also for ULK3.
Our results demonstrate that Gli1/2 transcriptional activators may be suppressed by SU6668 in different types of cells and independently of the signaling cascade involved in their activation. First, using two mouse cell lines and Ulk3 RNAi approach we have demonstrated that SU6668 inhibits the Shh pathway execution via Ulk3, as assessed by analyses of Gli1 and Ptch1 expression induced in a Gli2 dependent manner. Second, elevated levels of GLI1/2 proteins are down-regulated using SU6668 in ASCs. Third, SU6668 inhibits TGF-β mediated activation of GLI2 protein and subsequent GLI1 and ULK3 transcription in MDA-MB-231 cells. These findings are very important in the light of the established oncogenic properties of GLI1/2.
Interestingly, SU6668 does not inhibit TGF-β mediated transcriptional activation of GLI2 in ASCs or MDA-MB-231 cells as well as Shh induced expression of GLI1 mRNA in ASCs. A similar phenomenon was observed in Shh dependent up-regulation of Gli2 expression in the presence of SU6668 in mouse cells. However, Gli1/2 protein levels were substantially down-regulated in all above-mentioned cases. The observed negative effect of SU6668 might be explained by inhibition of post-transcriptional mechanisms regulating translation or stability of de novo generated Gli1/2 proteins in the context of active Shh or TGF-β signaling pathways. The mechanism of SU6668 action on activated GLI1/2 is similar to effects of ULK3 RNAi: activation of GLI2 transcription was not interfered, but the levels of GLI2 protein were not elevated in response to TGF-β. These results let to assume that SU6668 acts via ULK3. Also, our Ulk3 RNAi experiments revealed that SU6668 inhibits Gli proteins via Ulk3. Taken together these data suggest that Ulk3 is required not only for activation but also for stabilization of endogenous Gli1/2 proteins. This hypothesis is supported by data showing that Ulk3 is involved in maintenance of basal Gli2 expression. In view of the fact that SU6668 is not a very specific inhibitor of Ulk3, it cannot be formally ruled out that other kinase(s) regulating Gli1/2 may be also inhibited.
It is noteworthy that Ulk3 mRNA level increased during prolonged culturing of cells in the presence of SU6668. Because of lack of an Ulk3-specific antibody, we failed to examine a correlation between Ulk3 protein and mRNA levels and may only speculate that this increase could be caused by indirect compensatory transcriptional up-regulation of Ulk3, since our previous work has suggested that Ulk3 kinase is required for maintenance of cellular homeostasis [6].
4.2. Generation of Gli2ACT
Studies on the dynamics of the Shh pathway activation have suggested a mechanism of Gli2/3ACT formation: in response to ligand binding, Gli2/3FL are stabilized and transformed to Gli2/3ACT probably via yet uncharacterized phosphorylation event [7], [12], [47]. Our analysis of Gli2 in whole cell and nuclear extracts also shows that Shh induces rapid stabilization of Gli2FL and increase of nuclear Gli2FL, where it probably acts as a transcriptional activator. It is plausible that the observed nuclear translocation of Gli2FL is a consequence of specific phosphorylation resulted in generation of Gli2ACT. Several serine/threonine kinases, such as PKA, GSK3, CK1 and DYRK2, can directly phosphorylate Gli2, but the functional consequence of these phosphorylations is connected to processing and degradation of Gli2 [48], [49]. Phosphorylation of Gli2 by Ulk3 leads to increase of its transcriptional activity [40]. Our data show that SU6668 abolishes the Shh-induced nuclear translocation of Gli2FL and suggest that the nuclear form of Gli2FL may be the one phosphorylated by Ulk3 and this kind of phosphorylation is required for Gli2ACT generation.
4.3. Interrelationship between Gli2 and Ulk3 activities
The present work has revealed that Ulk3 and Gli2 are interconnected and probably subjected to cross-regulation on transcriptional and/or post-translational levels. Ulk3 RNAi resulted not only in reduction of Ulk3 transcripts but also in loss of Gli2 in mouse cells and human ASCs, whereas TGF-β-induced endogenous or over-expressed GLI2 proteins were associated with induction of ULK3 expression in MDA-MB-231 cells. These results indicate a possible positive feedback loop between Gli2 and Ulk3: Ulk3 mediated phosphorylation is required for stabilization and activation of Gli2, which in turn participates in positive regulation of Ulk3 transcription. Bioinformatic analysis of 600 bp of the ULK3 5′UTR revealed at least 3 non-consensus Gli binding sites: GTCCTCCCA(− 591 bp), GGCCTCCCA(− 471 bp) and AGCCACCCA(− 78 bp) (non-consensus nucleotides are underlined), which may be functional, since the sites with single substitutions have activities similar to the consensus site in luciferase reporter assay [50]. Nevertheless, the issue whether the Gli2 protein binds directly to Ulk3 promoter or works in an indirect manner remains to be investigated.
As it has been shown previously, inhibition of Ulk3 expression using plasmids expressing Ulk3-specific shRNA led to stronger increase of Gli1 mRNA expression in response to Shh than it was achieved in the control cells after 48 h of treatment [6]. The same phenomenon was observed in the present study in two experimental models and may be explained by up-regulation of Ulk3 and Gli2 to the levels higher than in the control cells at late stages of RNAi experiments. However, the present more detailed analysis has revealed that induction of Gli1 by Shh is attenuated in the cells with reduced expression levels of Ulk3 and Gli2 at initial stages of treatment. These results confirm a previously suggested, but not experimentally tested, model assuming a positive role of Ulk3 in regulation of endogenous Gli proteins.
4.4. Shh triggers the osteogenic differentiation program in ASCs
ASCs represent a promising tool for cell therapy and regenerative medicine, areas strictly depending on detailed understanding of the molecular regulatory mechanisms controlling behavior and fate of transplanted cells.
The present study shows that human ASCs possess functional Shh signaling axis. ASCs respond to Shh by increasing the levels of GLI1 and PTCH1 expressions, although the extent of their activation was at least 10 times lower compared with the mouse cell lines studied. This may be explained by relatively small and variable fractions of cells positive for Shh co-receptors PTCH/SMO within the heterogeneous ASCs isolated from different donors. An interesting point to make from these experiments is that GLI1 is found to be expressed at levels detectable by immuno-blotting in all ASCs analyzed. These data are in contrast to the mouse cells studied, where Gli1 protein cannot be detected without challenging the cells with Shh. The level of GLI1 protein was under the detection limit also in MDA-MB-231 cells even in the case of TGF-β treatment triggering up-regulation of GLI1 mRNA expression. Contrary to mouse cells, Shh has no significant positive effect on GLI2 expression or stability in ASCs that may be effectively induced by TGF-β. These results raise the possibility that the Shh signaling cascade may utilize GLI1 instead of GLI2 as a primary transcriptional activator because of high basal GLI1 expression in ASCs.
The Hh/Gli axis is known to control commitment of multipotent cells towards chondrocyte and osteocyte lineages in mice [28], [51]. This study shows that prolonged exposure to Shh triggers expression of osteocytic lineage specific markers in ASCs. These observations suggest Shh co-receptors PTCH/SMO as possible surface markers for isolation of a subpopulation of ASCs capable for osteogenic differentiation, which potentially could be very useful for the branch of regenerative medicine involved in treatment of disorders caused by loss of bone tissue.
Treatment with SU6668 attenuates both TGF-β induced chondrogenic and Shh mediated osteogenic differentiation programs. These effects are consistent with the putative roles of GLI proteins in these differentiation paradigms. In contrast, differentiation of ASCs towards adipocytes was effective in the presence of SU6668. Although we are aware of the fact that SU6668 is not a very specific inhibitor of Ulk3, our results open up possibilities to design novel and more specific inhibitors of Ulk3 kinase and Gli proteins using SU6668 as a starting compound.
The following are the supplementary data related to this article
Supplementary Fig. 1.
(A) Expression of Gli2 was analyzed in C3H10T1/2, MDA-MB-231 and peripheral blood mononuclear cells (PBMCs) of mouse or human origin using RT-PCR. Expression of SHH, SMO, PTCH1, ULK3 and GLI1 was detected using RT-PCR in PBMCs isolated from three different donors. (B) Mouse Gli2 and human GLI2 proteins were analyzed in C3H10T1/2, PBMCs and MDA-MB-231 cell lysates by immuno-blotting using GN2 antibodies G-20 (sc-20291, Santa Cruz Biotechnology) and AF3635 (R&D Systems). Approximately 60 μg of total protein of whole cell extracts (WCE) or 70 μg of nuclear extract (NE) were loaded per line; Gli2FL — full-length Gli2, * — unspecific bands. (C) C3H10T1/2 cell were treated with Shh or Tgf-βi for 48 h. Expression levels of Gli2 mRNA and protein were analyzed using RT-PCR and WB; *** — p < 0.001; Gli2Rep? — putative repressor form of Gli2. (D) C3H10T1/2 and 3 T3-L1 cells were treated with Shh for 24 h. Proliferation of the cells were tested using ViaLightTM plus kit and normalized with amount of total protein. The data are presented as an average mean of three independent experiments ± S.D., * — p < 0.05.
Supplementary Fig. 2.
(A, B) 3 T3-L1 cells were treated as indicated for 30 h, trypsinized and split for total RNA and protein isolation. (A) Expression levels of GIH, Ptchl, Gli2 and Ulk3 were analyzed by qPCR and normalized with the level of Hprt expression. The level of the respective gene mRNA expression in the control sample was set as 1, and the data from other samples were calculated relative to 1. The level of Gli1 and Ptch1 expressions in the samples treated with Shh and DMSO was set as 100%. Gli1 and Ptch1 expression levels in the samples treated Shh and SU6668 were calculated relative to 100%. The data are presented as an average mean ± S.D.; *—p < 0.05, **—p < 0.01, ***p < 0.001; n = 4. (B) Gli1, Gli2 and GAPDH proteins were analyzed by immuno-blotting. Approximately 60 μg of total protein of whole cell extracts (WCE) was loaded per line. (C) C3H10T1/2 cells were treated as indicated for 24 h. Cell cycle profile was analyzed using BrdU staining and BD FACSCalibur flow cytometer.
Supplementary Fig. 3.
(A) C3H10T1/2 cells were transfected with empty vector pSUPER or Ulk3 shRNAs and incubated during the indicated periods of time. Expression levels of Ulk3 (a) and Gli2 (b) were analyzed using qRT-PCR. The normalized with Hprt level of the respective gene expression at the indicated time point was set as 1 in the control cells transfected with pSUPER, and the data from the samples transfected with Ulk3 shRNAs were calculated relative to the control. The data are presented as an average mean ± S.D. (B) Expression of Gli2 and GAPDH proteins was analyzed by Western-blotting at the indicated time points in the post-transfectional C3H10T1/2 cells electroporated with empty vector pSUPER or Ulk3 shRNAs. Gli2 was detected using AF3635 antibody. (C) C3H10T1/2 cells were electroporated with pSUPER or Ulk3 shRNAs encoding constructs, incubated for 24 h and treated with DMSO or SU6668 in the presence or absence of Shh for additional 24 h or 48 h. Gli1 mRNA expression level was measured by qRT-PCR after 24 h and 48 h of treatment initiation (after 48 h and 72 h of transfection, respectively). For each data set obtained from the cells transfected with the same construct, the level of Gli1 mRNA expression was accepted as 1 in the control samples treated with DMSO. Data from other samples were calculated relative to their respectively transfected controls. The data are presented as an average mean of induction of Gli1 mRNA expression over control ± S.D. (D) C3H10T1/2 cells were transfected with pSUPER or Ulk3 shRNAs encoding constructs, incubated for 24 h and treated as indicated for additional 48 h. Levels of Gli1 and GAPDH proteins were examined by immuno-blotting (48 h post-treatment, 72 h post-transfection); *—p < 0.05, **—p < 0.01, n = 3.
Supplementary Fig. 4.
(A, B) MDA-MB-231 cells were treated with 10 ng/ml of TGF-β3 in the presence of DMSO or 20 μM SU6668 during the indicated periods of time. Expression levels of ULK3, SNAI1, CSMAD7 and PAI were analyzed by qPCR, normalized with GAPDH mRNA levels and set as 1 in the control cells treated with DMSO at the respective time point (indicated as a baseline on panel B). The data from other samples were calculated relative to control. The data are presented as an average mean ± S.D.; *—p < 0.05, **—p < 0.01. (C) MDA-MB-231 cells were transfected with pcDNA3.1 or pmax-GFP vectors using Lipofectamine 2000. A relative number of GFP-positive cells were measured using Accuri C6 flow cytometer.
Supplementary Fig. 5.
SU6668 prevents Shh-induced osteogenic differentiation of C3H10T1/2 cells. (A) C3H10T1/2 cells were treated as indicated for 3 d. Alkaline phosphatase (AP) activity was measured and normalized with the amount of total protein. The normalized level of AP activity in the control sample treated with DMSO was set as 1. The normalized data are expressed as an average mean ± S.D. ***—p < 0.001, n = 4. (B, C) C3H10T1/2 cells were treated for 4 d. Whole cell extracts were subjected to WB analysis. The levels of the respective gene mRNA expression were measured by qPCR, normalized with Hprt mRNA expression levels and set as 1 in the samples treated with DMSO. The data are presented as an average mean ± S.D. *—p < 0.05, **—p < 0.01, ***—p < 0.001, n = 3.
Donors of the cells used in the present study. ASCs — adipose tissue derived stromal cells; PBMCs — peripheral blood mononuclear cells.
List of antibodies.
List of primers.
Acknowledgements
We are thankful to Dr. Rune Toftgård and Dr. Csaba Finta (Center for Nutrition and Toxicology, Karolinska Institute, Sweden) for C3H10T1/2 cells. We thank Dr. Csaba Finta, Dr. Vello Tõugu, Arno Pihlak and Jekaterina Kazantseva for fruitful discussions and Torben Østerlund for critical reading of the manuscript. This study was supported by EAS grant EU42735, Estonian Science Foundation grants MJD266 to AP and ETF9478 to MP, and International Senior Research Fellowship No. 079014/Z/06/Z from the Wellcome Trust to ML. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jiang J., Hui C.C. Hedgehog signaling in development and cancer. Dev. Cell. 2008;15:801–812. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel P.M., Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat. Rev. Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 3.ten Dijke P., Arthur H.M. Extracellular control of TGFbeta signalling in vascular development and disease. Nat. Rev. Mol. Cell Biol. 2007;8:857–869. doi: 10.1038/nrm2262. [DOI] [PubMed] [Google Scholar]
- 4.Cooper A.F., Yu K.P., Brueckner M., Brailey L.L., Johnson L. Cardiac and CNS defects in a mouse with targeted disruption of suppressor of fused. Development. 2005;132:4407–4417. doi: 10.1242/dev.02021. [DOI] [PubMed] [Google Scholar]
- 5.Endoh-Yamagami S., Evangelista M., Wilson D., Wen X., Theunissen J.W. The mammalian Cos2 homolog Kif7 plays an essential role in modulating Hh signal transduction during development. Curr. Biol. 2009;19:1320–1326. doi: 10.1016/j.cub.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 6.Maloverjan A., Piirsoo M., Kasak L., Peil L., Osterlund T. Dual function of UNC-51-like kinase 3 (Ulk3) in the sonic hedgehog signaling pathway. J. Biol. Chem. 2010;285:30079–30090. doi: 10.1074/jbc.M110.133991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humke E.W., Dorn K.V., Milenkovic L., Scott M.P., Rohatgi R. The output of hedgehog signaling is controlled by the dynamic association between suppressor of fused and the Gli proteins. Genes Dev. 2010;24:670–682. doi: 10.1101/gad.1902910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen M.H., Wilson C.W., Li Y.J., Law K.K., Lu C.S. Cilium-independent regulation of Gli protein function by Sufu in hedgehog signaling is evolutionarily conserved. Genes Dev. 2009;23:1910–1928. doi: 10.1101/gad.1794109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Q., Shi Q., Chen Y., Yue T., Li S. Multiple Ser/Thr-rich degrons mediate the degradation of Ci/Gli by the Cul3-HIB/SPOP E3 ubiquitin ligase. Proc. Natl. Acad. Sci. U. S. A. 2009;106:21191–21196. doi: 10.1073/pnas.0912008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatia N., Thiyagarajan S., Elcheva I., Saleem M., Dlugosz A. Gli2 is targeted for ubiquitination and degradation by beta-TrCP ubiquitin ligase. J. Biol. Chem. 2006;281:19320–19326. doi: 10.1074/jbc.M513203200. [DOI] [PubMed] [Google Scholar]
- 11.Tempe D., Casas M., Karaz S., Blanchet-Tournier M.F., Concordet J.P. Multisite protein kinase A and glycogen synthase kinase 3beta phosphorylation leads to Gli3 ubiquitination by SCFbetaTrCP. Mol. Cell. Biol. 2006;26:4316–4326. doi: 10.1128/MCB.02183-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wen X., Lai C.K., Evangelista M., Hongo J.A., de Sauvage F.J. Kinetics of hedgehog-dependent full-length Gli3 accumulation in primary cilia and subsequent degradation. Mol. Cell. Biol. 2010;30:1910–1922. doi: 10.1128/MCB.01089-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruiz i Altaba A. Combinatorial Gli gene function in floor plate and neuronal inductions by sonic hedgehog. Development. 1998;125:2203–2212. doi: 10.1242/dev.125.12.2203. [DOI] [PubMed] [Google Scholar]
- 14.Aza-Blanc P., Lin H.Y., Ruiz i Altaba A., Kornberg T.B. Expression of the vertebrate Gli proteins in Drosophila reveals a distribution of activator and repressor activities. Development. 2000;127:4293–4301. doi: 10.1242/dev.127.19.4293. [DOI] [PubMed] [Google Scholar]
- 15.Bai C.B., Auerbach W., Lee J.S., Stephen D., Joyner A.L. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–4761. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki H., Nishizaki Y., Hui C., Nakafuku M., Kondoh H. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development. 1999;126:3915–3924. doi: 10.1242/dev.126.17.3915. [DOI] [PubMed] [Google Scholar]
- 17.Riobo N.A., Haines G.M., Emerson C.P., Jr. Protein kinase C-delta and mitogen-activated protein/extracellular signal-regulated kinase-1 control GLI activation in hedgehog signaling. Cancer Res. 2006;66:839–845. doi: 10.1158/0008-5472.CAN-05-2539. [DOI] [PubMed] [Google Scholar]
- 18.Dennler S., Andre J., Alexaki I., Li A., Magnaldo T. Induction of sonic hedgehog mediators by transforming growth factor-beta: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer Res. 2007;67:6981–6986. doi: 10.1158/0008-5472.CAN-07-0491. [DOI] [PubMed] [Google Scholar]
- 19.Dennler S., Andre J., Verrecchia F., Mauviel A. Cloning of the human GLI2 promoter: transcriptional activation by transforming growth factor-beta via SMAD3/beta-catenin cooperation. J. Biol. Chem. 2009;284:31523–31531. doi: 10.1074/jbc.M109.059964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson R.W., Nguyen M.P., Padalecki S.S., Grubbs B.G., Merkel A.R. TGF-beta promotion of Gli2-induced expression of parathyroid hormone-related protein, an important osteolytic factor in bone metastasis, is independent of canonical hedgehog signaling. Cancer Res. 2011;71:822–831. doi: 10.1158/0008-5472.CAN-10-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peukert S., Miller-Moslin K. Small-molecule inhibitors of the hedgehog signaling pathway as cancer therapeutics. Chem. Med. Chem. 2010;5:500–512. doi: 10.1002/cmdc.201000011. [DOI] [PubMed] [Google Scholar]
- 22.Laird A.D., Vajkoczy P., Shawver L.K., Thurnher A., Liang C. SU6668 is a potent antiangiogenic and antitumor agent that induces regression of established tumors. Cancer Res. 2000;60:4152–4160. [PubMed] [Google Scholar]
- 23.Godl K., Gruss O.J., Eickhoff J., Wissing J., Blencke S. Proteomic characterization of the angiogenesis inhibitor SU6668 reveals multiple impacts on cellular kinase signaling. Cancer Res. 2005;65:6919–6926. doi: 10.1158/0008-5472.CAN-05-0574. [DOI] [PubMed] [Google Scholar]
- 24.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 25.Tallone T., Realini C., Bohmler A., Kornfeld C., Vassalli G. Adult human adipose tissue contains several types of multipotent cells. J. Cardiovasc. Transl. Res. 2011;4:200–210. doi: 10.1007/s12265-011-9257-3. [DOI] [PubMed] [Google Scholar]
- 26.Gimble J.M., Katz A.J., Bunnell B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plaisant M., Fontaine C., Cousin W., Rochet N., Dani C. Activation of hedgehog signaling inhibits osteoblast differentiation of human mesenchymal stem cells. Stem Cells. 2009;27:703–713. doi: 10.1634/stemcells.2008-0888. [DOI] [PubMed] [Google Scholar]
- 28.James A.W., Leucht P., Levi B., Carre A.L., Xu Y. Sonic hedgehog influences the balance of osteogenesis and adipogenesis in mouse adipose-derived stromal cells. Tissue Eng. A. 2010;16:2605–2616. doi: 10.1089/ten.tea.2010.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura T., Aikawa T., Iwamoto-Enomoto M., Iwamoto M., Higuchi Y. Induction of osteogenic differentiation by hedgehog proteins. Biochem. Biophys. Res. Commun. 1997;237:465–469. doi: 10.1006/bbrc.1997.7156. [DOI] [PubMed] [Google Scholar]
- 30.Komori T., Yagi H., Nomura S., Yamaguchi A., Sasaki K. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 31.Nakashima K., Zhou X., Kunkel G., Zhang Z., Deng J.M. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 32.Xiao Z.S., Hjelmeland A.B., Quarles L.D. Selective deficiency of the “bone-related” Runx2-II unexpectedly preserves osteoblast-mediated skeletogenesis. J. Biol. Chem. 2004;279:20307–20313. doi: 10.1074/jbc.M401109200. [DOI] [PubMed] [Google Scholar]
- 33.Kinto N., Iwamoto M., Enomoto-Iwamoto M., Noji S., Ohuchi H. Fibroblasts expressing sonic hedgehog induce osteoblast differentiation and ectopic bone formation. FEBS Lett. 1997;404:319–323. doi: 10.1016/s0014-5793(97)00014-8. [DOI] [PubMed] [Google Scholar]
- 34.Nishio Y., Dong Y., Paris M., O'Keefe R.J., Schwarz E.M. Runx2-mediated regulation of the zinc finger Osterix/Sp7 gene. Gene. 2006;372:62–70. doi: 10.1016/j.gene.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 35.Amano K., Ichida F., Sugita A., Hata K., Wada M. MSX2 stimulates chondrocyte maturation by controlling Ihh expression. J. Biol. Chem. 2008;283:29513–29521. doi: 10.1074/jbc.M803681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joeng K.S., Long F. The Gli2 transcriptional activator is a crucial effector for Ihh signaling in osteoblast development and cartilage vascularization. Development. 2009;136:4177–4185. doi: 10.1242/dev.041624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mo R., Freer A.M., Zinyk D.L., Crackower M.A., Michaud J. Specific and redundant functions of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development. 1997;124:113–123. doi: 10.1242/dev.124.1.113. [DOI] [PubMed] [Google Scholar]
- 38.Spinella-Jaegle S., Rawadi G., Kawai S., Gallea S., Faucheu C. Sonic hedgehog increases the commitment of pluripotent mesenchymal cells into the osteoblastic lineage and abolishes adipocytic differentiation. J. Cell Sci. 2001;114:2085–2094. doi: 10.1242/jcs.114.11.2085. [DOI] [PubMed] [Google Scholar]
- 39.Maloveryan A., Finta C., Osterlund T., Kogerman P. A possible role of mouse Fused (STK36) in Hedgehog signaling and Gli transcription factor regulation. J. Cell Commun. Signal. 2007;1:165–173. doi: 10.1007/s12079-007-0014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maloverjan A., Piirsoo M., Michelson P., Kogerman P., Osterlund T. Identification of a novel serine/threonine kinase ULK3 as a positive regulator of hedgehog pathway. Exp. Cell Res. 2010;316:627–637. doi: 10.1016/j.yexcr.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 41.Kauts M.L., Pihelgas S., Orro K., Neuman T., Piirsoo A. CCL5/CCR1 axis regulates multipotency of human adipose tissue derived stromal cells. Stem Cell Res. 2013;10:166–178. doi: 10.1016/j.scr.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Rohatgi R., Milenkovic L., Corcoran R.B., Scott M.P. Hedgehog signal transduction by Smoothened: pharmacologic evidence for a 2-step activation process. Proc. Natl. Acad. Sci. U. S. A. 2009;106:3196–3201. doi: 10.1073/pnas.0813373106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mukherjee S., Frolova N., Sadlonova A., Novak Z., Steg A. Hedgehog signaling and response to cyclopamine differ in epithelial and stromal cells in benign breast and breast cancer. Cancer Biol. Ther. 2006;5:674–683. doi: 10.4161/cbt.5.6.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nolan-Stevaux O., Lau J., Truitt M.L., Chu G.C., Hebrok M. GLI1 is regulated through smoothened-independent mechanisms in neoplastic pancreatic ducts and mediates PDAC cell survival and transformation. Genes Dev. 2009;23:24–36. doi: 10.1101/gad.1753809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Desch P., Asslaber D., Kern D., Schnidar H., Mangelberger D. Inhibition of GLI, but not smoothened, induces apoptosis in chronic lymphocytic leukemia cells. Oncogene. 2010;29:4885–4895. doi: 10.1038/onc.2010.243. [DOI] [PubMed] [Google Scholar]
- 46.Jenkins D. Hedgehog signalling: emerging evidence for non-canonical pathways. Cell. Signal. 2009;21:1023–1034. doi: 10.1016/j.cellsig.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 47.Haycraft C.J., Banizs B., Aydin-Son Y., Zhang Q., Michaud E.J. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Varjosalo M., Bjorklund M., Cheng F., Syvanen H., Kivioja T. Application of active and kinase-deficient kinome collection for identification of kinases regulating hedgehog signaling. Cell. 2008;133:537–548. doi: 10.1016/j.cell.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 49.Pan Y., Wang C., Wang B. Phosphorylation of Gli2 by protein kinase A is required for Gli2 processing and degradation and the sonic hedgehog-regulated mouse development. Dev. Biol. 2009;326:177–189. doi: 10.1016/j.ydbio.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winklmayr M., Schmid C., Laner-Plamberger S., Kaser A., Aberger F. Non-consensus GLI binding sites in hedgehog target gene regulation. BMC Mol. Biol. 2010;11:2. doi: 10.1186/1471-2199-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimoyama A., Wada M., Ikeda F., Hata K., Matsubara T. Ihh/Gli2 signaling promotes osteoblast differentiation by regulating Runx2 expression and function. Mol. Biol. Cell. 2007;18:2411–2418. doi: 10.1091/mbc.E06-08-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Donors of the cells used in the present study. ASCs — adipose tissue derived stromal cells; PBMCs — peripheral blood mononuclear cells.
List of antibodies.
List of primers.