Abstract
For two-stage, implant-based, delayed-immediate reconstruction of the radiated breast, robotic-assisted latissimus dorsi harvest (RALDH) is a good option for patients who wish to avoid a traditional latissimus dorsi donor-site incision. The purpose of this study was to compare outcomes of RALDH and the traditional open technique (TOT) for patients undergoing delayed-immediate breast reconstruction following radiation therapy. A retrospective analysis of a prospective database of all consecutive patients undergoing latissimus dorsi harvest for radiated breast reconstruction between 2009 and 2013 was performed. Indications, surgical technique, complications, and outcomes were assessed. One hundred forty-six pedicled latissimus dorsi muscle flaps were performed for breast reconstruction and 17 were performed robotically during the study period (average follow-up 14.6 ± 7.3 mo). Latissimus dorsi breast reconstruction following radiation was performed in 64 patients using TOT and 12 using RALDH. Surgical complication rates were 37.5% in TOT versus 16.7% in RALDH (p = 0.31) including seroma (8.9% versus 8.3%), infection (14.1 versus 8.3%), delayed wound healing (7.8% versus 0), and capsular contracture (4.7% vs. 0). Robotic-assisted harvest of the latissimus dorsi muscle is associated with a low complication rate and reliable results for delayed reconstruction of the irradiated breast while eliminating the need for a donor-site incision.
Keywords: robotic assisted, delayed immediate, latissimus dorsi, radiated breast, breast reconstruction
Radiation therapy is associated with significant deleterious effects on implant-based breast reconstruction such as malposition, capsular contracture, and device extrusion. Therefore, many feel the standard of care for reconstruction of the irradiated breast is autologous tissue.1 2 3 Autologous reconstructions are typically delayed until after radiation therapy to prevent radiation sequelae such as fat necrosis and tissue fibrosis of the transferred tissue.4 Commonly utilized autologous reconstructive options include abdominal-based flaps and the latissimus dorsi muscle flap combined with an implant. Abdominal-based flaps can lead to a totally autologous reconstruction; however, certain patients may not be surgical candidates due to previous abdominal surgeries, failed free flaps, a paucity of abdominal tissue, or patient choice; consequently, these patients are best served by a pedicled latissimus dorsi muscle flap with an implant for their breast reconstruction.5
A two-stage delayed-immediate protocol has been well described and allows patients that require external beam radiation therapy (EBRT) to receive a skin-preserving mastectomy, while avoiding radiation effects associated with an immediate breast reconstruction.6 7 8 A tissue expander is placed at the time of mastectomy. After final pathology, if no radiation is required the patient can either proceed to pure autologous reconstruction or continue with implant-based reconstruction. If radiation is indicated, definitive reconstruction is delayed until after radiation therapy is complete. For the properly selected patient, delayed immediate breast reconstruction allows for optimal delivery of radiation therapy, while still providing patients with the aesthetic benefits of preserving the mastectomy skin envelope and decreasing the adverse effects of radiation therapy.
Robotic-assisted latissimus dorsi harvest (RALDH) was developed by the senior author (JCS) and has emerged as an integral part of the delayed immediate protocol at our institution for patients who have successfully completed EBRT with a tissue expander, but are not candidates or do not wish for abdominal-based flaps.9 10 Many patients who undergo radiation of the tissue expander achieve a reasonable re-expansion, but with an implant alone at stage 2, face very high long-term complication rates. The traditional open technique (TOT) of latissimus dorsi harvest can create an obvious donor site scar between 15 and 45 cm in length. Using only the muscle without a skin island provides the protection of autologous tissue without the additional incisions required to harvest it. Endoscopic latissimus dorsi harvest has been previously shown to result in less pain and allows for earlier and better shoulder movement.11 12
RALDH utilizes the da Vinci Robotic Surgical System (Intuitive Surgical Inc., Sunnyvale, CA) to assist in elevation of the latissimus dorsi flap and requires no additional incisions except for the mastectomy incision and three small ports that are used as exit sites for the drain. The robotic procedure is associated with improved visualization and surgical dexterity compared with endoscopic harvest and superior cosmetic results compared with the traditional open technique (TOT).
The purpose of this study is to describe our institutional protocol for delayed immediate breast reconstruction with RALDH, report a single institution's early experience, and to compare outcomes of RALDH versus traditional open technique (TOT) for patients undergoing breast reconstruction following radiation therapy.
Method
The University of Texas MD Anderson Cancer Center's Institutional Review Board approved the present study. A retrospective analysis of a prospective database of all consecutive patients undergoing latissimus dorsi harvest for breast reconstruction between 2009 and 2013 at MD Anderson Cancer Center was performed. Indications, comorbidities, and evolution of surgical technique were evaluated. Outcomes were compared including operative time, estimated blood loss, and surgical complications including infection, wound healing, and seroma rates between RALDH and TOT procedures. All patients underwent breast reconstruction for breast cancer-related defects.
Delayed-Immediate Protocol with RALDH
The MD Anderson Cancer Center institutional protocol for delayed-immediate breast reconstruction has been previously described6 and is presented as modified for inclusion of the RALDH technique (Fig. 1). Certain patient comorbidities were considered relative contraindications to implementation of the delayed immediate protocol such as severe obesity (body mass index [BMI] > 35), uncontrolled diabetes, and active smokers. All patients were evaluated by a multidisciplinary breast team, which included specialists in breast oncology, surgical oncology, radiation oncology, and plastic and reconstructive surgery.
Fig. 1.
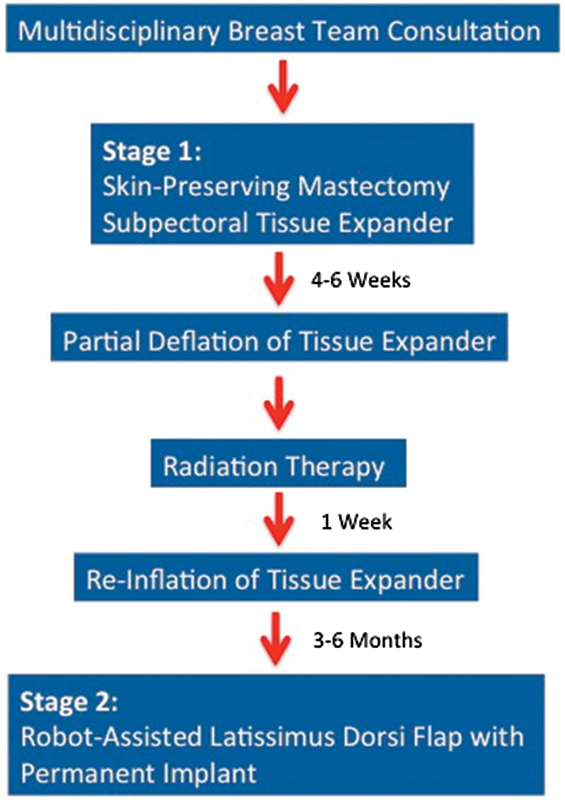
Delayed-immediate breast reconstruction protocol.
During surgical stage 1, patients underwent skin-sparing mastectomy and immediate placement of a tissue expander with or without bioprosthetic mesh. Patients were expanded weekly during the 4 to 6 weeks prior to radiation therapy and were then deflated to one-third total fill capacity just prior to initiation of EBRT as per radiation oncology protocol13 (Fig. 2). Within 1 week of the completion of EBRT, patients were reinflated to as close to original volume as possible. RALDH was performed 6 months after radiation therapy, at the time of the exchange of tissue expander to permanent implant to protect the radiated, re-expanded skin from long-term, implant-related complications.
Fig. 2.
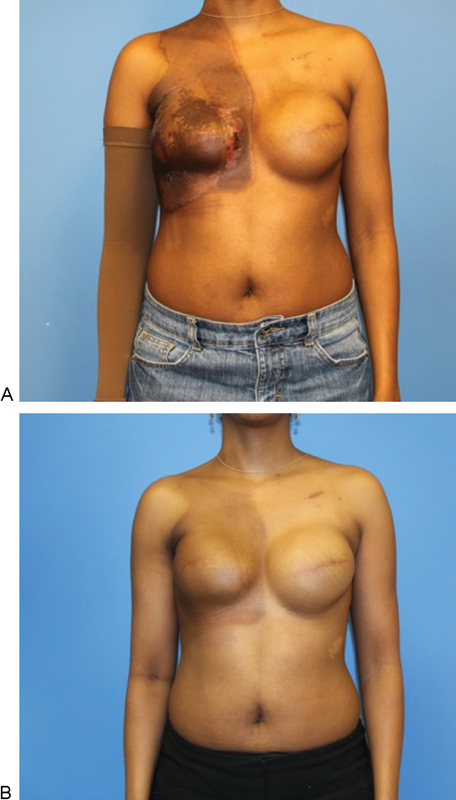
Delayed-Immediate reconstruction of an irradiated breast using a robotic-assisted latissimus dorsi harvest (RALDH). A 42-year-old woman was diagnosed with invasive ductal carcinoma of the right breast with positive lymph node metastasis. She was treated with bilateral mastectomies, right axillary dissection, and immediate reconstruction using tissue expanders (133MX 400 cc; Allergan Corp., Irvine, CA) followed by external beam radiation therapy (60 Gy) to the right chest wall. (A) Immediately and (B) 6 months following radiation therapy. Note radiation-induced constriction and elevation of the right inframammary fold, which must be corrected.
Robotic-Assisted Latissimus Dorsi Harvest Technique Modifications
The RALDH surgical technique has been previously described in detail.10 The following technical considerations are important for application of RALDH in the delayed-immediate breast reconstruction protocol. Tissue expansion must be sufficient to allow for the desired volume of final implant and muscle flap, which may require additional expansions after the completion of radiation therapy. If additional volume beyond pre-radiation levels is required, this should be accomplished at a slower rate (average every 2–3 wk) until the desired volume is met. For unilateral reconstructions, stage 2 (exchange of expander for implant) may be combined with a contralateral mastopexy or augmentation for symmetry.
A robotic harvest technique was performed completely through three access ports (used as drain sites) for robotic instrumentation with no additional incisions (Fig. 3). During muscle transposition, the thoracodorsal nerve is left intact, but the humeral insertion of the muscle is partially divided (50–80%) to allow for advancement of the muscle and to decrease animation deformity. The pectoralis major muscle that has been providing temporary expander coverage may be fibrosed or constricted from radiation therapy. Often, it can be released from the skin envelope and sutured back to the chest wall. Release of the pectoralis muscle from the mastectomy skin flap provides a noncapsular surface for the latissimus flap to adhere. If delaminating the mastectomy flap seems as if it would result in vascular compromise to the skin, it can be left in place.
Fig. 3.
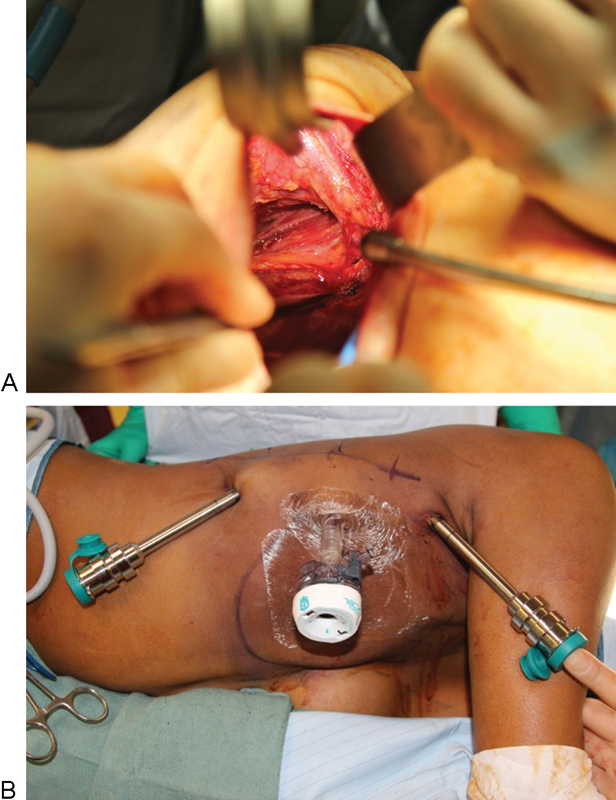
Intraoperative views during robotic-assisted latissimus dorsi harvest. (A) Predissection of latissimus dorsi with exposure of thoracodorsal artery and vein. Note all dissection is accomplished through anterior mastectomy incision with no additional skin incisions required. (B) 12- and two 8-French ports placed at the lateral border of the latissimus dorsi muscle.
For RALDH opposite a prosthetic reconstruction, the same-sized implant should be used for both breasts. Despite the transfer of the latissimus dorsi, the additional muscle volume is balanced by radiation-induced atrophy and tightening of the soft tissue envelope. If possible, total muscular coverage of the implant is desirable. Sometimes, the latissimus dorsi muscle alone can completely cover the implant, and the LD can be sutured to the pectoralis major as in biologic mesh reconstruction (Fig. 4). Radiation therapy tends to elevate the infra-mammary fold (IMF) and required lowering in almost all cases.
Fig. 4.
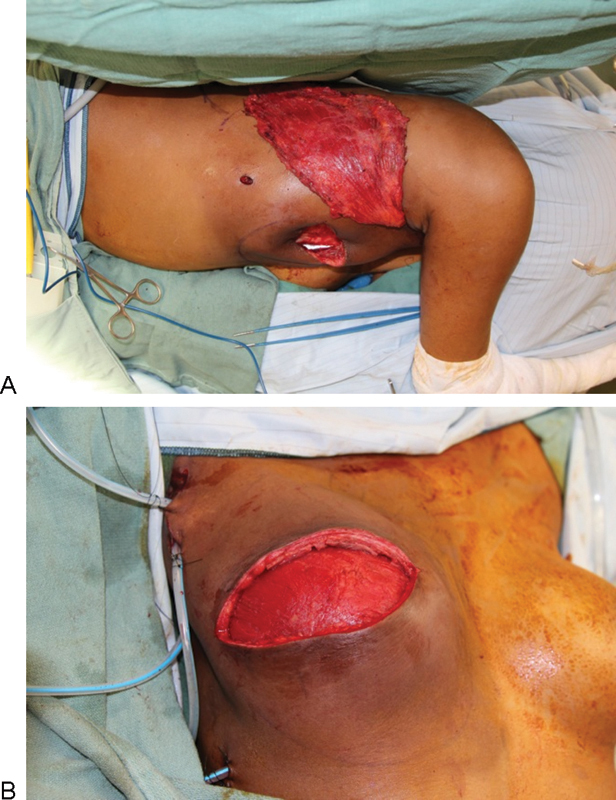
Intraoperative views during robotic-assisted latissimus dorsi harvest. (A) Transposition of latissimus dorsi muscle underneath a subcutaneous skin bridge. (B) Latissimus dorsi muscle achieves total muscle coverage over a permanent silicone shaped implant (410 FF 425 cc, Allergan Corp., Irvine, CA). Note previous port sites are utilized for drain placement.
Postoperative care included deep venous thrombosis prophylaxis with low-molecular-weight heparin initiated on postoperative day 1. In general, the hospital course was 2 to 3 days. Routine follow-up included physical examination in an outpatient clinic weekly until drain removal, then at 1 month and every 3 months for 1 year, and then annually thereafter (Fig. 5).
Fig. 5.
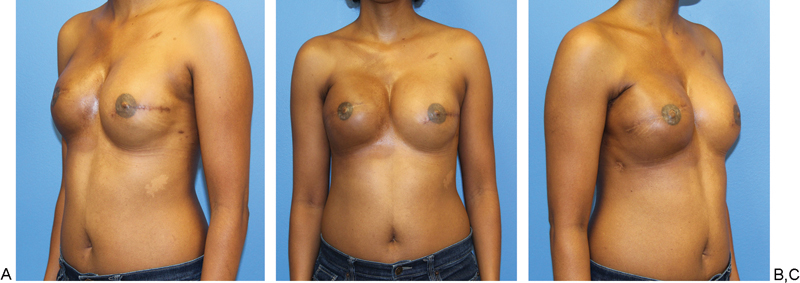
Postoperative results. (A) Patient is 10 months postoperative and has now received nipple construction with areolar tattooing. (B) Patient was noted to have a minor contour defect of her donor site. (C) Her postoperative course was without complication.
Definitions
Pre-existing comorbidities were defined as any preoperative systemic pathology that may have affected surgical outcomes, including pulmonary, renal, and cardiac disease. Patients who smoked within 1 month of surgery were considered active smokers. Delayed wound healing with skin necrosis was defined as full-thickness skin loss that required surgical excision, and abscess was defined as purulent fluid collection that required drainage. We defined cellulitis as erythema of the skin that required intravenous or oral antibiotics for resolution. Any unexpected adverse event directly related to the latissimus dorsi harvest including wound infection, dehiscence, seroma, hematoma, and flap loss was defined as a surgical complication.
Statistical Analysis
We analyzed differences in surgical outcomes between patients who underwent delayed immediate breast reconstruction with either TOT or RALDH technique. A Fisher exact test was used to compare categorical variables. All p values were two-tailed, and p values ≤ 0.05 were considered significant. The analyses were performed using the SAS 9.2 software program (SAS Institute Inc., Cary, NC) and R software program (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Characteristics
Of the 146 pedicled, latissimus dorsi muscle flaps performed for breast reconstruction, 17 were performed with the da Vinci robot during the study period (average follow-up 14.6 ± 7.3 mo) Latissimus dorsi breast reconstruction following radiation was performed in 76 patients, 64 (84.2%) using TOT (average follow-up 16.4 ± 6.9 mo) and 12 (15.8%) using RALDH (average follow-up 12.3 ± 8.3 mo) (Table 1). All patients received a stage 1 skin-sparing mastectomy with immediate tissue expander placement. Oncologic indications included invasive ductal (85.5%) and invasive lobular carcinoma (14.5%). Patients received an average of 2.8 (range 0–4) expansions initiated between 1 to 2 weeks postoperatively. Radiation therapy was on average 60 Gy with routine targeting of internal mammary nodes. Stage 2 reconstruction with latissimus dorsi muscle harvest and placement of a permanent implant was performed at an average of 7.1 months (range 3–11 mo) after the end of radiation. All pedicled flaps resulted in successful breast reconstructions. Average time of latissimus dorsi harvest in the TOT technique was 58 minutes (range 42 min–1 h 38 min) compared with 1 hour 32 minutes (range 1 h 5 min–2 h 35 min) for RALDH. Average length of hospital stay for the TOT patients was 3.4 days (range 3–6 d) compared with 2.7 (range 2–3 d) for RALDH patients.
Table 1. Patient characteristics and outcomes.
| Variable | RALDH (N = 12) | TOT (N = 64) |
|---|---|---|
| Average age (yr) | 54.3 | 56.1 |
| Previous radiation (%) | 100 | 100 |
| BMI | 25.4 | 25.9 |
| Comorbidities (%) | 16.6 | 18.8 |
| Smokers (%) | 25 | 21.9 |
| Stage 1 bioprosthetic mesh (%) | 100 | 71.2 |
| Surgical complication (%) | 16.7 | 37.5 |
| Seroma | 8.3 | 8.9 |
| Delayed healing | 0 | 7.8 |
| Infection | 14.1 | 8.3 |
| Unplanned reoperation | 8.3 | 12.5 |
| Capsular contracture | 0 | 4.7 |
| Average follow-up (mo) | 12.3 | 16.4 |
Abbreviations: BMI, body mass index; RALDH, robotic-assisted latissimus dorsi harvest; TOT, traditional open technique.
Surgical Complications
Surgical complication rates were statistically equivalent: 37.5% in TOT versus 16.7% in RALDH (p = 0.31) which included seroma (10.9% vs. 8.3%), infection (14.1 vs. 8.3%), delayed wound healing (7.8% vs. 0), and capsular contracture (4.7% vs. 0). No RALDH muscle flaps required conversion to an open technique and all flaps resulted in successful breast reconstructions. Formal donor-site muscle strength testing or functional assessment and was not performed, but subjectively assessed as normal for all RALDH patients.
Discussion
A successful aesthetic reconstruction of the irradiated breast is critically dependent upon timing and type of reconstruction.14 15 For the properly selected patient, delayed-immediate reconstruction enables creation of a stage 1 breast mound and preservation of the mastectomy skin envelope, which ultimately permits a more esthetically desirable outcome than if the reconstruction was completely delayed, and the chest wall was left completely flat until after radiation. Although autologous tissue is desirable at stage 2 because of the vulnerability of the radiated skin to long-term complications, if re-expansion is good, additional skin is not always needed. In these cases, the RALDH is an ideal solution. By providing muscle, the radiated skin is protected, but a donor-site incision is spared; a skin patch that does not match in color, texture, or thickness to the native breast skin is avoided. Our early experience reported here demonstrates that this protocol can be performed with a low complication rate comparable to traditional techniques. RALDH offers improved visualization and dexterity over other minimally invasive techniques without necessitating the large donor site scar on the back associated with TOT (Fig. 6).
Fig. 6.
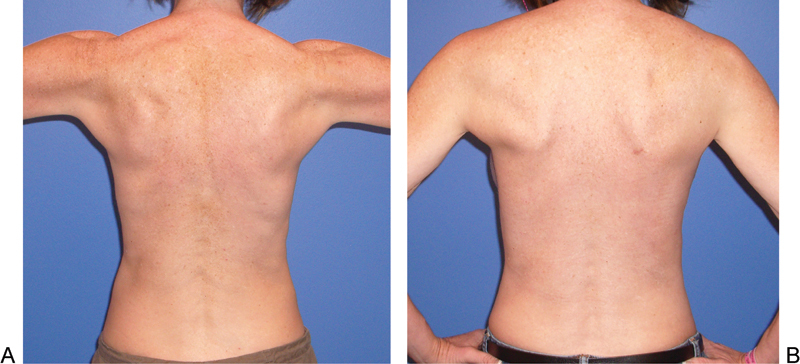
(A) Before and (B) after donor site for robotic-assisted latissimus dorsi harvest, demonstrating virtually no change in contour and no incision on the back. The muscle was taken from the patient's left side.
This represents the only series to date that compares radiated breast reconstruction outcomes with a TOT versus RALDH technique. Results with RALDH were reliable despite the presence of radiation therapy and could be performed without adding unreasonable duration to the procedure. The overall surgical complication, seroma, and capsular contracture rates were lower than in the traditional technique, but due to very small sample size at this point in the series, statistical significance was not reached. Long-term follow-up studies will be important to determine if these trends are sustained. Previously published series have shown evidence that nonirradiated autologous tissue flaps may provide cellular elements that can lead to repair of dermal fibrosis that results from postmastectomy radiation therapy.8 Although a formal histologic analysis was outside the scope of this study, within this limited experience, reconstructions remained soft and natural throughout the study period and radiated skin overlying the latissimus dorsi muscle appeared more healthy over time rather than less (which is typical of implant only reconstruction with radiation).
The present study had several limitations. The study population was small and therefore was not powered to detect differences observable in either the cohorts as a whole or subgroups of complication rates within each cohort. In this early clinical experience, patients were highly selected and may not represent an average breast reconstruction patient. Proper patient selection is critical to successful completion of the delayed-immediate protocol and morbidly obese patients and those with significant comorbidities should be considered with caution. The present study included only those patients who successfully completed radiation therapy and did not capture patients that lost their tissue expander prior to final reconstruction. Additionally, the mean follow-up time in the present study was only 14 months, which may not be long enough to capture the true rate of capsular contracture and unplanned reoperation secondary to radiation sequelae which may evolve over several years. Despite these limitations, early success with this protocol has led us to expand current indications for RALDH. Just as interest has grown for the use of bioprosthetic mesh for implant associated breast deformities,16 there may also be a role for RALDH for correction of thin skin/implant rippling, capsular contracture, and for segmental mastectomy defects. These are the subject of future investigations.
In conclusion, the present study's findings suggest that TOT and RALDH are associated with similar surgical complication rates, including seroma, capsular contracture, and wound healing at ∼1-year follow-up, and result in equivalent protective effects of autologous tissue without incisional morbidity in patients who undergo breast reconstruction following radiation therapy. Further studies that evaluate long-term outcomes in patients who undergo RALDH will be useful in determining potential selective indications and elucidating significant outcome differences.
References
- 1.Ascherman J A, Hanasono M M, Newman M I, Hughes D B. Implant reconstruction in breast cancer patients treated with radiation therapy. Plast Reconstr Surg. 2006;117(2):359–365. doi: 10.1097/01.prs.0000201478.64877.87. [DOI] [PubMed] [Google Scholar]
- 2.Spear S L, Onyewu C. Staged breast reconstruction with saline-filled implants in the irradiated breast: recent trends and therapeutic implications. Plast Reconstr Surg. 2000;105(3):930–942. doi: 10.1097/00006534-200003000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Kronowitz S J, Robb G L. Radiation therapy and breast reconstruction: a critical review of the literature. Plast Reconstr Surg. 2009;124(2):395–408. doi: 10.1097/PRS.0b013e3181aee987. [DOI] [PubMed] [Google Scholar]
- 4.Tran N V, Chang D W, Gupta A, Kroll S S, Robb G L. Comparison of immediate and delayed free TRAM flap breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2001;108(1):78–82. doi: 10.1097/00006534-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Spear S L, Boehmler, Clemens M W The latissimus flap in the irradiated breast Philadelphia, PA: Lippincott-Raven; 2011563–570. [Google Scholar]
- 6.Kronowitz S J, Hunt K K, Kuerer H M. et al. Delayed-immediate breast reconstruction. Plast Reconstr Surg. 2004;113(6):1617–1628. doi: 10.1097/01.prs.0000117192.54945.88. [DOI] [PubMed] [Google Scholar]
- 7.Kronowitz S J Immediate versus delayed reconstruction Clin Plast Surg 200734139–50., abstract vi [DOI] [PubMed] [Google Scholar]
- 8.Kronowitz S J. Delayed-immediate breast reconstruction: technical and timing considerations. Plast Reconstr Surg. 2010;125(2):463–474. doi: 10.1097/PRS.0b013e3181c82d58. [DOI] [PubMed] [Google Scholar]
- 9.Selber J C. Robotic latissimus dorsi muscle harvest. Plast Reconstr Surg. 2011;128(2):88e–90e. doi: 10.1097/PRS.0b013e31821ef25d. [DOI] [PubMed] [Google Scholar]
- 10.Selber J C, Baumann D P, Holsinger F C. Robotic latissimus dorsi muscle harvest: a case series. Plast Reconstr Surg. 2012;129(6):1305–1312. doi: 10.1097/PRS.0b013e31824ecc0b. [DOI] [PubMed] [Google Scholar]
- 11.Lin C H Wei F C Levin L S Chen M C Donor-site morbidity comparison between endoscopically assisted and traditional harvest of free latissimus dorsi muscle flap Plast Reconstr Surg 19991041070–1077.; quiz 1078; review erratum Plast Reconstr Surg 2000;105:823 [PubMed] [Google Scholar]
- 12.Pomel C, Missana M C, Lasser P. [Endoscopic harvesting of the latissimus dorsi flap in breast reconstructive surgery. Feasibility study and review of the literature] Ann Chir. 2002;127(5):337–342. doi: 10.1016/s0003-3944(02)00769-1. [DOI] [PubMed] [Google Scholar]
- 13.Motwani S B, Strom E A, Schechter N R. et al. The impact of immediate breast reconstruction on the technical delivery of postmastectomy radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66(1):76–82. doi: 10.1016/j.ijrobp.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 14.Spear S L, Ducic I, Low M, Cuoco F. The effect of radiation on pedicled TRAM flap breast reconstruction: outcomes and implications. Plast Reconstr Surg. 2005;115(1):84–95. [PubMed] [Google Scholar]
- 15.Schechter N R, Strom E A, Perkins G H. et al. Immediate breast reconstruction can impact postmastectomy irradiation. Am J Clin Oncol. 2005;28(5):485–494. doi: 10.1097/01.coc.0000170582.38634.b6. [DOI] [PubMed] [Google Scholar]
- 16.Spear S L, Seruya M, Clemens M W, Teitelbaum S, Nahabedian M Y. Acellular dermal matrix for the treatment and prevention of implant-associated breast deformities. Plast Reconstr Surg. 2011;127(3):1047–1058. doi: 10.1097/PRS.0b013e31820436af. [DOI] [PubMed] [Google Scholar]


