Abstract
Natural compounds that target microtubules and disrupt the normal function of the mitotic spindle have proven to be one of the best classes of cancer chemotherapeutic drugs available in clinics to date. There is increasing evidence showing that even minor alteration of microtubule dynamics can engage the spindle checkpoint, arresting cell cycle progression at mitosis and subsequently leading to cell death. Our improved understanding of tumor biology and our continued appreciation for what the microtubule target agents (MTAs) can do has helped pave the way for a new era in the treatment of cancer. The effectiveness of these agents for cancer therapy has been impaired, however, by various side effects and drug resistance. Several new MTAs have shown potent activity against the proliferation of various cancer cells, including resistance to the existing MTAs. Sustained investigation of the mechanisms of action of MTAs, development and discovery of new drugs, and exploring new treatment strategies that reduce side effects and circumvent drug resistance could provide more effective therapeutic options for cancer patients. This review focuses on the successful cancer chemotherapy from natural compounds in clinical settings and the challenges that may abort their usefulness.
Keywords: Natural products, microtubule-targeting agents, cancer, side effects
Microtubules: Important target for cancer therapy
Microtubules play an essential role in several eukaryotic cellular processes such as cell growth and division, motility, intracellular trafficking and the ability to adapt to a variety of shapes to interact with the environment (1). As a result of the great success of the mitotic agents in the treatment of cancer thereby, microtubules represent the best cancer target identified thus far. Also, the drugs of this class will continue to be important chemotherapeutic agents in the future as more selective approaches are developed (2).
The biological functions of microtubules in all cells are regulated in large part by their polymerization dynamics (3). Polymerization of microtubules occurs by a nucleation-elongation mechanism by the reversible, non-covalent addition of α and β tubulin dimers at both ends of microtubules (4).
Microtubules show in vitro and in vivo two kinds of non-equilibrium dynamics. The dynamic behavior that is highly prominent in cells, called dynamic instability, refers to the individual microtubule ends switching between phases of growth and shortening (1). The two ends of a microtubule are not equivalent; the plus end grows and shortens more rapidly and extensively than the minus end. When the microtubules neither grow nor shorten detectably, they undergo relatively long periods of slow lengthening, brief periods of rapid shortening and periods of attenuated dynamics or pause (4). Dynamic instability can be characterized by four main variables: the growth rate of microtubules; the rate of shortening; the frequency of transition from the growth or paused state to shortening, also called a ‘catastrophe’ and the frequency of transition from shortening to growth or pause, also called a ‘rescue’ (4). The second dynamic behavior is called treadmilling, which is the net growth at one microtubules end and balanced net shortening at the opposite end (5). It involves the intrinsic flow of tubulin subunits from the plus end of the microtubule to the minus end and is created by differences in the critical subunit concentrations at the opposite microtubule ends, and might be particularly important in mitosis (6). Both treadmilling and dynamic instability are compatible behaviors; a specific population of microtubules can display one behavior or a mixture of both behaviors. However, the mechanisms that control the degree to which a microtubule population displays one or the other behavior are poorly understood (7).
In interphase, microtubules exchange their tubulin with the soluble tubulin pool relatively slowly, with half-times that range from several minutes to several hours (8). The interphase microtubule network disassembles at the onset of mitosis and is replaced by a new population of spindle microtubules that are 4–100 times more dynamic than the microtubules in the interphase cytoskeleton (9). Although there is variation among the various spindle-microtubule subpopulations, the mitotic-spindle microtubules exchange their tubulin with tubulin in the soluble pool rapidly, with half-times on the order of 10–30 seconds (8).
Mitosis phase facilitates the equal partitioning of replicated chromosomes into two identical groups. The success of this process requires highly dynamic microtubules in the spindle (1, 8, 9). Microtubule dynamics are critical for the timely and correct attachment of chromosomes at their kinetochores to the spindle during pro-metaphase after nuclear-envelope breakdown, for the movements of the chromosomes to their properly aligned positions at the metaphase plate, and for the synchronous separation of the chromosomes in anaphase and telophase after the checkpoint of metaphase anaphase is complete (4). During pro-metaphase, microtubules originating from each of the two spindle poles grow to a maximum of typically 5–10 μm, and then shorten nearly completely. Then re-grow until they successfully become attached to chromosomes at their kinetochores (10). It is critical that each single chromosome should attach to a bipolar spindle of microtubule; failure to do so is sufficient to stop the cell from transitioning to anaphase; this blocks the cell in a pro-metaphase /metaphase-like state and it eventually undergoes apoptosis (programmed cell death) (11, 12).
Drugs that block mitosis seem to work by a common mechanism, which is suppress the dynamic of microtubules and kill tumor cells. Paclitaxel (Taxol) and Vinca alkaloids are the first class of anti-mitotic agents to be discovered and inhibit cancer cell proliferation. One possible explanation is that cancer cells are relatively sensitive to these drugs compared with normal cells because they divide more rapidly than normal cells and therefore frequently pass through a stage of vulnerability to mitotic poisons (13).
Microtubule-targeting agents
The microtubule-targeting agents (MTAs) are a very successful class of cancer drugs with therapeutic benefits in both hematopoietic and solid tumors (14). A large number of natural agents and/or their analogues bind to soluble tubulin and/or directly to tubulin in the microtubules. Most of these compounds are antimitotic agents that inhibit cell proliferation by acting on the polymerization dynamics of spindles, which are essential to proper spindle function of microtubules. The specific effects of MTAs on the polymer mass and the dynamic stability of the microtubules are complex (4).
Microtubule-targeted antimitotic drugs are classified into two main groups. The first group is microtubule-destabilizing agents, which inhibit microtubule polymerization at high concentrations and include several compounds such as the Vinca alkaloids (vinblastine, vincristine, vinorelbine, vindesine and vinflunine), cryptophycins, halichondrins, estramustine, colchicine and combretastatins, that are used clinically or are under clinical investigation for treatment of cancer (12). The second group is microtubule-stabilizing agents. These agents stimulate microtubule polymerization, and include paclitaxel (the first agent identified in this class), docetaxel (Taxotere), the epothilones, and discodermolide (12).
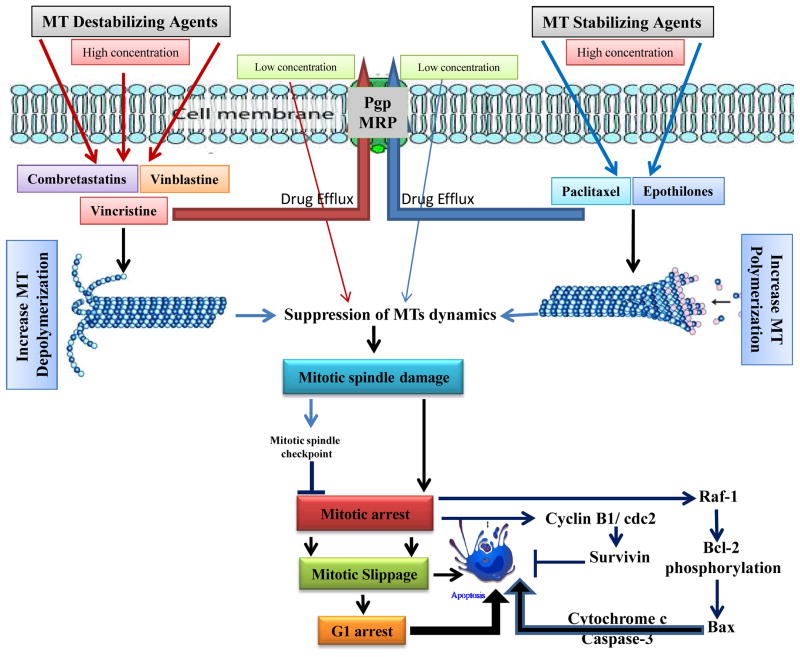
The classification of drugs as ‘stabilizers’ or ‘destabilizers’ of microtubule is that the drugs increase or decrease microtubule polymerization at high concentrations, powerfully suppress microtubule dynamics at 10–100-fold lower concentrations and, therefore, kinetically stabilize the microtubules, without changing the microtubule-polymer mass (4). The most important action of the two classes of drugs is the suppression of spindle-microtubule dynamics. This results in the slowing or blocking of mitosis at the metaphase–anaphase transition and induction of apoptotic cell death rather than their effect on microtubule polymer mass, which requires very high dosage levels to act primarily and continuously on microtubule-polymer mass (12, 15) (Figure 1).
Figure 1.
Schematic diagram of putative events involved in microtubule target agents (MTAs) -induced apoptosis. The interaction of chemotherapeutic agents that stabilize or destabilize microtubules results in suppression of microtubule dynamics that leads to damage to the mitotic spindle or to massive microtubule damage depending on drug concentration and time of exposure. These actions trigger apoptosis by inducing cell cycle arrest at the G2/M phase or a general failure in microtubule-related functions depending on the level of microtubule damage. These effects, together with the abnormal exit of mitosis leads to multinucleated cells and eventually to cell death, which are the major mechanisms involved in MTAs-induced apoptosis. However, cancer cells may become resistant to these drugs by activating drug efflux pump.
The ability of a drug to bind to soluble tubulin or directly to the microtubule depends on the location of the specific binding site in tubulin and the microtubule (15). It is important for the efficacy of these drugs in cancer chemotherapy to block the mitotic phase, slow cells and subsequently induce apoptosis (12). Although there are important differences in the mechanisms of actions of antimitotic drugs, the underlying mechanisms of the three classes of drugs are similar (Table 1).
Table 1.
Natural microtubule targeting agents: Source, binding site, side effects and clinical use
| BINDING DOMAIN | DRUG | ORIGIN | BINDING SITE ON TUBULIN | SIDE EFFECTS | CLINICAL USES | REFERENCES |
|---|---|---|---|---|---|---|
| Vinca Domain | Vinblastine (Velban) | Plant, Catharanthus roseus | β-tubulin subunit | Neutropenia (dose-limiting toxicity); MDR sensitive; Sensitive to βIII-tubulin | Hodgkin’s disease, testicular germ- cell cancer, breast cancer, head and neck cancer | [12, 33, 41] |
| Vincristine (Oncovin) | Plant, Catharanthus roseus | β-tubulin subunit | Peripheral neuropathy, hyponatremia, constipation, and hair loss | Leukaemia, lymphomas | [12, 33, 41] | |
| Taxanes site | Paclitaxel | Plant, Taxus brevifolia | β-tubulin subunit | Neutropenia (dose-limiting toxicity); MDR sensitive; Sensitive to βIII-tubulin | Head and neck, lung, breast, ovarian cancer and advanced Kaposi sarcoma | [46, 48, 51] |
| Epothilones | Bacteria, Sporangium cellulosum | β-tubulin subunit | MDR sensitive; Not sensitive to β-tubulin content | Ovarian, prostate, lung, breast cancers, refractory solid tumors, glioblastoma; Paclitaxel-resistant tumors | [57, 59, 60, 61] | |
| Colchicine Domain | Colchicine | Plant, Colchicum autumnale | β-tubulin subunit | Potent toxicity to normal cells | Non-neoplastic diseases (gout, familial Mediterranean fever) | [36, 37] |
| Combretastatins | Plant, Combretum caffrum | β-tubulin subunit | MDR insensitive; sensitive to β-tubulin content | Phase III trial of CA-4-P in combination with carboplatin for anaplastic thyroid cancer; Potential vascular- targeting agent | [41, 42] |
MTAs: Mechanisms of action
Microtubules serve as an intracellular cytoskeleton framework, and their unique polymerization dynamics are critical for many cellular functions (16). It is possible that the dysfunction of the cytoskeletal is an intracellular stress, which results in either a disrupted microtubule network or a stabilized microtubule cytoskeleton (17). MTAs, also known as antimitotic agents, exert their inhibitory effects on cell proliferation primarily by blocking mitosis, which requires an intense control of microtubule dynamics. Therefore, their actions on microtubule stability and dynamic parameters differ from each other. At relatively high concentrations, MTAs either inhibit microtubule polymerization, destabilizing microtubules and decreasing microtubule polymer mass, or promote microtubule polymerization, stabilizing microtubules and increasing the polymer mass (12, 18). At the cellular level both types of agents may lead to cell cycle arrest in mitosis and trigger cell death through apoptosis (12, 16). Mitotic arrest is associated with aberrant spindle formation, thus clearly linking interference with microtubule functionality to inhibition of cell proliferation. It is often assumed that apoptosis induced by microtubule-stabilizing agents is a direct consequence of G2/M arrest, which in turn would be a prerequisite for growth inhibition and cell death. However, Horwitz et al. documented in a series of experiments that the situation is more complex (19). So, the treatment of human cancer cells with low concentrations of microtubule-stabilizing drugs may lead to mitotic slippage, which is multipolar spindles formation, and subsequent cell cycle arrest in G1. Thus, cells arrested in (aneuploidic) G1 state and subsequent undergo apoptosis. On the other hand, higher drug concentrations lead to a protracted mitotic block from which the cells eventually exit without division, thus forming tetraploid G1 cells (19), which will then undergo apoptosis (Figure 1). These results supported that the entry of cells into mitosis is a fundamental prerequisite for cell killing by microtubule-stabilizing agents, but that apoptosis does not (necessarily) occur from a G2/M arrested state. It is important to note that the induction of tubulin polymerization in vitro requires μM or sub-μM concentrations of microtubule stabilizing agents (20). However, low nM or even sub-nM IC50 values for cancer cell growth inhibition is required.
Although at low but clinically relevant concentrations of microtubule stabilizing and destabilizing drugs potently suppress microtubule dynamics without affecting microtubule polymer mass, they have the potential to block mitotic progression and induce apoptosis (11, 12) (Figure 1). Thus, as our understanding of MTAs increases, we realize that the mechanism underlying the anti-mitotic and anti-cancer activities of MTAs may be due to their inhibitory effects on spindle microtubule dynamics, rather than their effects on microtubule polymer mass, as proposed previously.
Recent studies have shown that mammalian target of rapamycin (mTOR) activation is dependent on dynein-mediated transport that functions as the predominant minus end-directed microtubules motor in eukaryotic cells. It has been shown that molecular dynein is required for perinuclear localization of mTOR into the cells (21). The inhibition of microtubule function by MTAs causes inhibition of the AKT/mTOR signaling pathway and thus inhibits cancer cell proliferation. This mechanism is independent of MTAs induced mitotic arrest and could provide an alternative mechanism of drug action that can explain its clinical activity.
Currently there are three well established drug binding sites on β-tubulin. The vinca domain, located adjacent to the exchangeable GTP binding site in β-tubulin at the plus end interface (22). The taxane site resides in a deep hydrophobic pocket at the lateral interface between adjacent protofilaments, within the lumen of the microtubule (23). Finally, the colchicine site is located at the intra-dimer interface between β-tubulin and α-tubulin (24). Laulimalide is another drug that binds on β-tubulin site and stabilizes microtubules. This compound was isolated from marine sponge (Cacospongia mycofijiensis) and represents a first class of microtubule-stabilizing drug that binds at a site distinct from the taxane site on tubulin (25).
Microtubule destabilizing natural products
Vinca alkaloids and related drug
Vinca alkaloids, among the earliest developed for disruption of microtubule, were isolated from periwinkle leaves, Catharanthus roseus (L.) G. Don, (also known as Vinca rosea). These compounds have been successful in the clinic not only for the treatment of diabetes, high blood pressure, and as disinfectants but also for being cancer fighters (26). Vinca alkaloids were discovered in the 1950’s by Canadian scientists, Robert Noble and Charles Beer, for their antimitotic and, therefore, cancer chemotherapeutic potential. They were first used as single-agent in the treatment of childhood hematologic malignancies, then widely spread into use for solid malignancies and, shortly after, for adult haematological malignancies (Table 1) (27). Because of their great success in the treatment of childhood leukaemia, they were considered ‘wonder drugs’. Further development of the Vinca alkaloids has been motivated by the increased understanding of their mechanisms of action, their synergy in several combination therapies, and the desire to develop orally available analogues. This has led to the development of various novel semi-synthetic analogues, including vindesine, vinorelbine and vinflunine to overcome the side effects of peripheral neuropathy and reversible myelosuppression that commonly occur with these drugs (28).
Although the causes of the neurotoxicity are undefined, they definitely involve the effects of the drugs on microtubules, which are a key component of neurons (29). Neuropathy might result from disruption of axonal flow by bundling of microtubules by paclitaxel (30); from steric hindrance of motor-protein binding to microtubules; or from effects of altered microtubule dynamics in axonal processes or on transport in growth cones. Another cause of neurotoxicity might result from microtubule destabilization or from suppression of microtubule dynamics due to neuronal retraction and reduced arborization (30); also, from reduced responsiveness of neurons to incoming signals; or from demyelinization (29). Myelosuppression, also called bone marrow suppression or myelotoxicity, is a result of blockage of mitosis and proliferation of the rapidly cycling bone-marrow cells. This is a common side effect associated with Vinca alkaloids chemotherapy.
Jordan, et al reported that Vinca alkaloids destroy mitotic spindles at high concentrations (for example, 10–100 nM in HeLa cells) and depolymerize microtubules (4), therefore leaving the dividing cancer cells blocked in mitosis with condensed chromosomes. Also, the same authors stated that at low but clinically relevant concentrations, vinblastine does not depolymerize spindle microtubules, yet it powerfully blocks mitosis (for example, IC50 0.8 nM in HeLa cells) and cells die by apoptosisv (4). Studies from the same laboratory revealed that the mitotic-blocking action of low concentrations of Vinca alkaloid agent in living cancer cells is due to suppression of microtubule dynamics rather than microtubule depolymerization (12).
Vinblastine binds to the β-subunit of tubulin dimers at a distinct region called the Vinca-binding domain (31). Various other novel chemotherapeutic drugs also bind at this domain. The binding of vinblastine to soluble tubulin is rapid and reversible (12). This induces a conformational change in tubulin in connection with tubulin self-association (32).
In vitro studies revealed that Vinblastine also binds with high affinity to tubulin at the extreme microtubule ends; however, it binds with low affinity to tubulin that is buried in the tubulin lattice (12, 33). These studies show that binding of one or two molecules of vinblastine per microtubule plus end is sufficient to reduce both treadmilling and dynamic instability by ~ 50%, without significant microtubule depolymerization. This suppression of dynamics prevents the mitotic spindle from assembling normally and it reduces the tension at the kinetochores of the chromosomes. Mitotic progress is delayed with chromosomes often stuck at the spindle poles not capable of assembly at the spindle equator. The cell-cycle signal to the anaphase-promoting complex to pass from metaphase into anaphase is blocked and the cells eventually die by apoptosis (12, 33).
In addition, this group includes a large number of compounds that have not undergone clinical development for cancer therapy, including the anti-tussive noscapine (15), maytansine, rhizoxin, spongistatins, podophyllotoxin, steganacins and curacins (34); several herbicides that inhibit microtubule polymerization; antifungal and antihelmintic agents (35); and some psychoactive drugs (32) (Table 1).
Colchicine
Colchicine was first isolated in 1820 by French chemists P.S. Pelletier and J.B. Caventou from autumn crocus (Colchicum autumnale), and described for treatment of rheumatism and swelling (36). Although colchicine has been use clinically in the treatment of gout neither colchicine nor compounds that bind to the colchicine site on tubulin have been found to be of significant use in cancer treatment because of their potent toxicity (Table 1) (37).
As with Vinca alkaloids, Colchicine depolymerizes microtubules at high concentrations and stabilizes microtubule dynamics at low concentrations. Colchicine inhibits microtubule polymerization by binding to microtubule ends rather than to the soluble-tubulin pool. However, free colchicine itself probably does not bind directly to microtubule ends. Instead, it first binds to soluble tubulin, induces slow conformational changes in the tubulin, and ultimately forms a poorly reversible tubulin–colchicine complex (38). Which then copolymerize into the microtubule ends in small numbers along with large numbers of free tubulin molecules (39).
So, despite the differences between the effects at high concentrations of the Vinca/colchicine-like drugs and the taxane-like drugs, almost all of the microtubule-targeted antimitotic drugs stabilize microtubule dynamics at their lowest effective concentrations (4).
Several natural products that bind in the colchicine domain and disrupt microtubule assembly in vitro and in vivo are known, such as podophyllotoxin, from the mandrake herb Podophllum peltatum, and Combretastatins (40).
Combretastatins
Combretastatins are a class of natural cis-stilbenes (phenols) isolated from the South African tree Combretum caffrum (Combretacae). Combretastatins exhibit cytotoxic properties and tubulin polymerization inhibition in cancer cells in vitro (41). The most potent member of this group is combretastatin A-4 with IC50 of 7 nM (42). The combination of this compound with a phosphate pro-drug, called Combretastatin A4 phosphate (CA4P), also known Zybrestat™, fosbretabulin (9), progressed into clinical trials for the treatment of solid tumors (Table 1) (41, 42). The advantageous of this class of compounds over other anti-mitotic agents is that beside their anti-mitotic properties, they are also angiogenesis inhibitors and known as vascular disrupting agents (VDA) (42, 43). In addition, they are being evaluated for their efficacy in the treatment of diabetic retinopathy which is the biggest single cause of blindness (44).
Microtubule stabilizing natural products
Paclitaxel and related drugs
The Taxanes (paclitaxel, docetaxel), a novel class of anti-microtubule agents, are the most important addition to the chemotherapeutic armamentarium against cancer over the past several decades. Paclitaxel was discovered as part of a National Cancer Institute screening program which extracted thousands of plants for anticancer activity (45). Paclitaxel, Taxus brevifolia, was extracted from the stem bark of the Pacific yew tree in 1966 (46). The structure of paclitaxel was reported in 1971; however, the microtubule-stabilizing properties of this compound were discovered later by Horwitz et al in 1979 (47) (Table 1). Although taxanes bind poorly to soluble tubulin, they bind to tubulin along the length of the microtubule with high affinity (48). Paclitaxel binds to the β-tubulin subunit, and promotes microtubule stabilization by inducing conformational changes of the M-loop of β-tubulin that result in more stable lateral interactions between adjacent proto-filaments (48). In contrast to a high concentration of taxane required to increase microtubule polymerization, a study found that the binding of a very small number of paclitaxel molecules powerfully stabilizes the dynamics of the microtubules without increasing microtubule polymerization (11). Suppression of microtubule dynamics by paclitaxel leads to mitotic blocking in the absence of significant microtubule bundling (11). Jordan et al show that half of HeLa cells in mitosis are blocked at 8 nM paclitaxel, whereas there is no increase in microtubule-polymer mass below 10 nM paclitaxel (12). The polymer mass of paclitaxel is half increased at 80 nM (12). The suppression of spindle-microtubule dynamics by taxanes prevents the dividing cancer cells from progressing from metaphase into anaphase and the cells eventually die by apoptosis (11, 12, 49).
Because of the limited quantities of paclitaxel from the bark of Taxus brevifolia, , alternate sources, particularly an approved semi-synthesis of 10-deacetylbaccatin III were obtained to cover the need (4). The FDA approved Taxol® for the treatment of metastatic ovarian cancer in 1992. Today, taxol is one of the most important anticancer drugs and is widely used clinically for the treatment of ovarian, breast, and non-small cell lung cancer, either as monotherapy or in combination with cis-platin. It is also used for the treatment of acquired immunodeficiency syndrome -related Kaposi’s sarcoma.
Paclitaxel and its semisynthetic analogue docetaxel are widely prescribed antineoplastic agents for a broad range of malignancies (50). The side effects are neurotoxicity and myelosuppression similar to those associated with Vinca alkaloids (51). Treatment of prostate cancer with taxanes also inhibits androgen receptor signaling by inhibiting the androgen-dependent nuclear translocation of the androgen-receptor (AR) (52). In fact AR, in the absence of ligand dihydrotestosterone (DHT), is bound with heat shock protein 90 (Hsp90) chaperone, onto the cytoskeleton, while the link with the ligand results in AR homodimerisation and translocation in the nucleus, where binds to specific androgen-dependent genes with following proliferative and trophic effects (53). This findings is evidence that in addition to blocking cell division, microtubule targeting drugs also impair AR signaling
Paclitaxel was the first microtubule stabilizing agent that was investigated in an animal model of neurodegenerative tauopathies, the T44 tau Tg mouse. The ability of paclitaxel and docetaxel to cross the blood–brain barrier (BBB) is limited. This is believed to be caused at least in part by the P-glycoprotein (Pgp) efflux pump, which is highly expressed in the BBB (54). Thus, taxane analogues that are capable of overcoming Pgp-mediated transport may result in improving the effectiveness of the drugs. Several compounds of this type have been reported, such as cabazitaxel, an FDA approved semisynthetic taxane with weak Pgp substrates that can saturate the active transporter (55). Furthermore, pharmacokinetic (PK) studies with cabazitaxel show that administering the compound via rapid infusions in the brain significantly enhances the drug concentration, which results in plasma drug levels that are above the threshold needed to saturate Pgp (55). Other examples of taxanes that circumvent Pgp-mediated efflux are orally active BMS-275183 and milataxel (MAC-321) (56). In addition, other approaches with brain targeted delivery have been reported with favorable results, such as paclitaxel–peptide conjugate GRN1005, a Pgp-insensitive pro drug that exploits the low density lipoprotein receptor-related protein 1 (LRP-1). Albumin nanoparticle carriers proposed for taxanes not only enhance the microtubule inhibition, but also prevent the use of solubilizing cremophors which sometimes induce severe hypersensitivity (15). Other nanoparticle–based carriers, such as nanoporous silicon particles, for chemotherapeutic shutting, have been recently proposed.
Search for other drugs that enhance microtubule polymerization have yield several promising compounds, including the epothilones, discodermolide, the sarcodictyins, eleutherobin and laulimalide. Some of these compounds compete with paclitaxel for binding to microtubules and bind at or near the taxane site (epothilones, discodermolide, eleutherobins and sarcodictyins), but others, such as laulimalide, seem to bind to unique sites on microtubules (25).
Epothilones
Epothilones are a family of novel MTAs, stabilizing microtubules and inhibiting microtubule dynamic behavior at mitotic spindle and, therefore, preventing cancer cells from mitosis. Epothilones A and B were discovered by Höfle and Reichenbach as antifungal agents produced by the soil bacterium Sorangium cellulolus (57). These compounds compete with paclitaxel for the taxane binding site on β-tubulin, suggesting that this class of compounds may act on microtubules in a paclitaxel-like manner and promote microtubule assembly (58). This observation led to the suggestion that epothilones, taxanes, and other classes of microtubule-stabilizing natural products may share a similar pharmacophore (59) (Table 1). Many studies support this common pharmacophore model (60), however, other studies evaluated the complex of epothilone A with tubulin polymerized in zinc-stabilized sheets by electron crystallography and demonstrated that epothilone A and paclitaxel interact in substantially different ways within the same binding pocket in β-tubulin (61). Such differences in the binding modes of β-tubulin may provide a possible explanation of how the epothilones maintain their high antimitotic activity in cell lines that are resistant to taxanes because of point mutations in the β-tubulin subunit (62). Another unique feature of the epothilones is that unlike docetaxel and paclitaxel, epothilones maintain their cytotoxic performance even in cells overexpressing Pgp.
Currently there are five epothilone or epothilone-derived compounds undergoing clinical evaluation. One of the most advanced of these compounds is epothilone B (Patupilone; EP0-906). This drug is being evaluated by Novartis and is in Phase III clinical trials for a number of cancer types (63). Similarly, lactam analog (Ixabepilone; BMS-247550) of epothilone B is in Phase III trial targeting breast cancer the being developed by Bristol-Myers Squibb (BMS).
In addition to epothilones A and B, epothilone D has been isolated as a minor component of fermentation of myxobacteria (64). This compound revealed a greater therapeutic index as a chemotherapeutic agent, compared to epothilones B (65); however, severe side effects including CNS toxicities were reported from clinical trials (66).
Limitation of the MTAs in clinical setting
Toxicity and side effects
Although MTAs have proven to be highly effective for the treatment of cancer, the adverse side effects associated with their long and short term use, affects their applicability in the clinical setting. DNA damage and apoptosis are the main causes of drug-induced cytotoxicity (67). Neurological and hematological side effects are the main and often dose limiting toxicities, but several other side effects also occur during the treatment with each individual drug (68).
Neurotoxicity such as peripheral neuropathy which is characterized by the loss of deep tendon reflex at the ankle, numbness, and motor weakness is very common. Cranial neuropathy may also occur after treatment with the vinca alkaloids and taxanes and may cause different symptoms such as jaw pain and vocal cord dysfunction. Another common symptom is autonomic neuropathy causing constipation, abdominal cramping, and urinary retention. In addition, headache, dizziness, and mental depression are other symptom of neurotoxicity. Neurotoxicity typically occurs after prolonged treatment with MTAs. The inhibition of axonal microtubules, which are crucial for axonal transport in neurons, is at least in part the reason for such toxicity (29).
Hematological toxicity is another major side effect as a result of treatment with MTAs, and is referred to as myelosuppression (68). Severe neutropenia, in particular, occurs early after treatment. Myelosuppression may result from the inhibition of the rapidly dividing hematopoietic cells. Nausea, vomiting and diarrhea are other common symptoms besides the neurological and hematological toxicities (68) (Table 1).
Drug resistance
Drug resistance refers to the status of poor responsiveness of tumor cells to chemotherapeutic drugs. Multiple mechanisms of drug resistance in cancer have been described, but typically involve efflux mechanisms and membrane associated changes to prevent drug accumulation within tumor cells (4). Acquired drug resistance may be a result of the cellular response to MTAs, which modulate by adaptive changes of the cell. This is a common theme of anticancer drugs. Otherwise, because of the presence of specific resistance proteins, cells may be inherently protected from the anti-proliferative effects of growth inhibitors even upon first exposure (4). Drug efflux by ABC transporters such as Pgp, is one of the most frequent mechanisms of drug resistance encountered in cancer cells (4). Although Pgp-mediated drug efflux is a major resistance mechanism for taxol, some of the newer MTA’s have been found to be less vulnerable to Pgp action, and thus may offer more benefits over taxol (4). Concrete efforts to reveal the specific resistance mechanisms to MTA’s have been investigated over the last several years. Three major mechanisms have been proposed: first, overexpression of specific tubulin isotypes, especially of βIII-tubulin; second, inherit tubulin mutations that may affect microtubule stability and dynamics; third, the emergence of tubulin mutations, which lead to impaired ligand binding. Several groups have reported that the overexpression of class III β-tubulin is the cause of taxol-resistance in cancer cell lines and human tumors as well (32). In addition, in vitro studies reported that microtubules that assemble solely from αβIII dimers exhibit different assembly properties and significantly enhanced dynamicity over microtubules that assemble from αβII or αβIV dimers or unfractionated tubulin (63). Goncalves et al. demonstrated that taxol-resistant A549 lung carcinoma cells, which overexpress βIII-tubulin, in fact increased microtubule dynamics inherently. This phenomenon does not allow the cells to proceed from metaphase to anaphase (7). However, upon treatment with low doses of taxol the normal cell cycle progression is restored. These results support the theory that these cells are dependent on taxol to normalize their microtubule dynamics and to successfully proceed through mitosis. Another study showed the effects of βIII-tubulin overexpression on microtubule dynamics and taxol sensitivity by inducing either βI- or βIII-tubulin overexpression in CHO cells (51). Cells that harbor specific tubulin mutations may be resistant to MTAs as a result of microtubule stability having been reduced and/ or increased microtubule dynamics. This concept is supported by the observation that these cells often become dependent on the selecting agent and are hypersensitive to microtubule-destabilizing agents, thus indicating inherently reduced microtubule stability (69). None of these mechanisms, however, have been proven to be the cause of drug resistance in clinical settings (70). The importance of β-tubulin mutations in the clinical resistance to taxol in non-small-cell lung cancer patients by DNA sequence of the beta-tubulin gene was proposed (71). However, these findings were highly controversial and could not be confirmed in a number of subsequent prospective studies. Berrieman et al. investigated the possible role of β-tubulin mutations in the resistance to cancer chemotherapy and concluded that β-tubulin mutations in clinical samples are rare and unlikely to contribute to drug resistance (72). It should be noted that β-tubulin pseudogenes belong to a family of homologous DNA sequences that need further investigation (73).
Enhanced DNA repair by O6-alkylguanine-DNA alkyltransferase has been known as a common mechanism of resistance to drugs (74). Another mechanism of chemoresistance has been proposed by which the alteration in death-inducing signaling pathway may result in inhibition or prevention of apoptosis (75). Overexpression of anti-apoptotic genes such as Bcl-2, besides mutation or reduced expression of pro-apoptotic genes such as p53, has been shown to induce cancer cells to become resistant to anticancer treatment. The way that Pgp can protect tumor cells from apoptosis has recently been discussed in view of observations that inhibition of Pgp by chemosensitizing agents can restore the normal apoptotic cascade in cells with defective signaling pathways (75). Overall, there are multiple, complex and, possibly, interrelated mechanisms of drug resistance. These mechanisms are often found in combination, which can further complicate the chemotherapy of tumors (Table 1).
Conclusions and Future Developments
As our knowledge of MTAs increases, we realize that the mechanism underlying the anti-cancer activity of these agents may mainly lie in their inhibitory effects on spindle microtubule dynamics, rather than in their effects on microtubule polymer mass. More understanding of mechanisms of action of MTAs will enhance the use and effectiveness of these compounds. This knowledge of the mechanistic differences among this class is vital to understanding their tissue and cell specificity and the development of resistance. Our ability to understand resistance-mediated mechanisms in tumor biology has led to the creation of cabazitaxel as an alternative for those patients who become resistant to docetaxel-based chemotherapy regimens. The Identification and further elucidation of microtubule dependent tumor specific pathways not only may help us to better understand the molecular basis of clinical taxane resistance, but also help identify individual patients more likely to respond to treatment. Combination therapy at much lower doses than the doses already used are needed that will be non-toxic, yet effective in suppressing microtubule dynamics. Furthermore, the uses of adjuvants in chemotherapy that suppress microtubule dynamics need further investigation.
Acknowledgments
Financial support: The work is supported by United States Public Health Service Grant RO1 CA 160867 and RO1 CA 160867 S1 (H. Mukhtar).
Abbreviations
- MTAs
microtubule target agents
- AR
androgen-receptor
- Pgp
P-glycoprotein
- Cdc2
cell-division cycle 2
- Bcl-2
B-cell lymphoma 2
- MRP
multidrug resistance-related protein
- AIDS
acquired immunodeficiency syndrome
- mTOR
mammalian target of rapamycin
- Hsp90
heat shock protein 90
- BBB
blood–brain barrier
- DHT
dihydrotestosterone
Footnotes
Conflict of interest: The authors disclose no potential conflict of interest.
References
- 1.Mitchison TJ. Microtubule dynamics and kinetochore function in mitosis. Annu Rev Cell Biol. 1988;4:527–49. doi: 10.1146/annurev.cb.04.110188.002523. [DOI] [PubMed] [Google Scholar]
- 2.Giannakakou P, Sackett D, Fojo T. Tubulin/microtubules: still a promising target for new chemotherapeutic agents. J Natl Cancer Inst. 2000;92:182–3. doi: 10.1093/jnci/92.3.182. [DOI] [PubMed] [Google Scholar]
- 3.Waterman-Storer CM, Salmon ED. Microtubule dynamics: treadmilling comes around again. Curr Biol. 1997;7:R369–72. doi: 10.1016/s0960-9822(06)00177-1. [DOI] [PubMed] [Google Scholar]
- 4.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–65. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 5.Shaw SL, Kamyar R, Ehrhardt DW. Sustained microtubule treadmilling in Arabidopsis cortical arrays. Science. 2003;300:1715–8. doi: 10.1126/science.1083529. [DOI] [PubMed] [Google Scholar]
- 6.Chen W, Zhang D. Kinetochore fibre dynamics outside the context of the spindle during anaphase. Nat Cell Biol. 2004;6:227–31. doi: 10.1038/ncb1104. [DOI] [PubMed] [Google Scholar]
- 7.Wilson L, Panda D, Jordan MA. Modulation of microtubule dynamics by drugs: a paradigm for the actions of cellular regulators. Cell Struct Funct. 1999;24:329–35. doi: 10.1247/csf.24.329. [DOI] [PubMed] [Google Scholar]
- 8.Saxton WM, Stemple DL, Leslie RJ, Salmon ED, Zavortink M, McIntosh JR. Tubulin dynamics in cultured mammalian cells. J Cell Biol. 1984;99:2175–86. doi: 10.1083/jcb.99.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rusan NM, Fagerstrom CJ, Yvon AM, Wadsworth P. Cell cycle-dependent changes in microtubule dynamics in living cells expressing green fluorescent protein-alpha tubulin. Mol Biol Cell. 2001;12:971–80. doi: 10.1091/mbc.12.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayden JH, Bowser SS, Rieder CL. Kinetochores capture astral microtubules during chromosome attachment to the mitotic spindle: direct visualization in live newt lung cells. J Cell Biol. 1990;111:1039–45. doi: 10.1083/jcb.111.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yvon AM, Wadsworth P, Jordan MA. Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol Biol Cell. 1999;10:947–59. doi: 10.1091/mbc.10.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jordan MA. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr Med Chem Anticancer Agents. 2002;2:1–17. doi: 10.2174/1568011023354290. [DOI] [PubMed] [Google Scholar]
- 13.Shelby RD, Hahn KM, Sullivan KF. Dynamic elastic behavior of alpha-satellite DNA domains visualized in situ in living human cells. J Cell Biol. 1996;135:545–57. doi: 10.1083/jcb.135.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaefer KL. PPARgamma Inhibitors as Novel Tubulin-Targeting Agents. PPAR Res. 2008;2008:785405. doi: 10.1155/2008/785405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Q, Ching AK, Leung WK, Szeto CY, Ho SM, Chan PK, et al. Novel therapeutic potential in targeting microtubules by nanoparticle albumin-bound paclitaxel in hepatocellular carcinoma. Int J Oncol. 2011;38:721–31. doi: 10.3892/ijo.2011.902. [DOI] [PubMed] [Google Scholar]
- 16.Mollinedo F, Gajate C. Microtubules, microtubule-interfering agents and apoptosis. Apoptosis. 2003;8:413–50. doi: 10.1023/a:1025513106330. [DOI] [PubMed] [Google Scholar]
- 17.Wang TH, Wang HS, Ichijo H, Giannakakou P, Foster JS, Fojo T, et al. Microtubule-interfering agents activate c-Jun N-terminal kinase/stress-activated protein kinase through both Ras and apoptosis signal-regulating kinase pathways. J Biol Chem. 1998;273:4928–36. doi: 10.1074/jbc.273.9.4928. [DOI] [PubMed] [Google Scholar]
- 18.Checchi PM, Nettles JH, Zhou J, Snyder JP, Joshi HC. Microtubule-interacting drugs for cancer treatment. Trends Pharmacol Sci. 2003;24:361–5. doi: 10.1016/S0165-6147(03)00161-5. [DOI] [PubMed] [Google Scholar]
- 19.Chen JG, Yang CP, Cammer M, Horwitz SB. Gene expression and mitotic exit induced by microtubule-stabilizing drugs. Cancer Res. 2003;63:7891–9. [PubMed] [Google Scholar]
- 20.Altmann KH. Microtubule-stabilizing agents: a growing class of important anticancer drugs. Curr Opin Chem Biol. 2001;5:424–31. doi: 10.1016/s1367-5931(00)00225-8. [DOI] [PubMed] [Google Scholar]
- 21.Clippinger AJ, Alwine JC. Dynein mediates the localization and activation of mTOR in normal and human cytomegalovirus-infected cells. Genes Dev. 2012;26:2015–26. doi: 10.1101/gad.196147.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rai SS, Wolff J. Localization of the vinblastine-binding site on beta-tubulin. J Biol Chem. 1996;271:14707–11. doi: 10.1074/jbc.271.25.14707. [DOI] [PubMed] [Google Scholar]
- 23.Snyder JP, Nettles JH, Cornett B, Downing KH, Nogales E. The binding conformation of Taxol in beta-tubulin: a model based on electron crystallographic density. Proc Natl Acad Sci U S A. 2001;98:5312–6. doi: 10.1073/pnas.051309398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravelli RB, Gigant B, Curmi PA, Jourdain I, Lachkar S, Sobel A, et al. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature. 2004;428:198–202. doi: 10.1038/nature02393. [DOI] [PubMed] [Google Scholar]
- 25.Pryor DE, O’Brate A, Bilcer G, Diaz JF, Wang Y, Wang Y, et al. The microtubule stabilizing agent laulimalide does not bind in the taxoid site, kills cells resistant to paclitaxel and epothilones, and may not require its epoxide moiety for activity. Biochemistry. 2002;41:9109–15. doi: 10.1021/bi020211b. [DOI] [PubMed] [Google Scholar]
- 26.Noble RL, Beer CT, Cutts JH. Role of chance observations in chemotherapy: Vinca rosea. Ann N Y Acad Sci. 1958;76:882–94. doi: 10.1111/j.1749-6632.1958.tb54906.x. [DOI] [PubMed] [Google Scholar]
- 27.Johnson IS, Wright HF, Svoboda GH, Vlantis J. Antitumor principles derived from Vinca rosea Linn. I. Vincaleukoblastine and leurosine. Cancer Res. 1960;20:1016–22. [PubMed] [Google Scholar]
- 28.Gidding CE, Kellie SJ, Kamps WA, de Graaf SS. Vincristine revisited. Crit Rev Oncol Hematol. 1999;29:267–87. doi: 10.1016/s1040-8428(98)00023-7. [DOI] [PubMed] [Google Scholar]
- 29.Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249:9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- 30.Sahenk Z, Barohn R, New P, Mendell JR. Taxol neuropathy. Electrodiagnostic and sural nerve biopsy findings. Arch Neurol. 1994;51:726–9. doi: 10.1001/archneur.1994.00540190110024. [DOI] [PubMed] [Google Scholar]
- 31.Bai RL, Pettit GR, Hamel E. Binding of dolastatin 10 to tubulin at a distinct site for peptide antimitotic agents near the exchangeable nucleotide and vinca alkaloid sites. J Biol Chem. 1990;265:17141–9. [PubMed] [Google Scholar]
- 32.Lobert S, Correia JJ. Energetics of vinca alkaloid interactions with tubulin. Methods Enzymol. 2000;323:77–103. doi: 10.1016/s0076-6879(00)23362-4. [DOI] [PubMed] [Google Scholar]
- 33.Singer WD, Jordan MA, Wilson L, Himes RH. Binding of vinblastine to stabilized microtubules. Mol Pharmacol. 1989;36:366–70. [PubMed] [Google Scholar]
- 34.Hamel E, Covell DG. Antimitotic peptides and depsipeptides. Curr Med Chem Anticancer Agents. 2002;2:19–53. doi: 10.2174/1568011023354263. [DOI] [PubMed] [Google Scholar]
- 35.Lacey E, Gill JH. Biochemistry of benzimidazole resistance. Acta Trop. 1994;56:245–62. doi: 10.1016/0001-706x(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 36.Emmerson BT. The management of gout. N Engl J Med. 1996;334:445–51. doi: 10.1056/NEJM199602153340707. [DOI] [PubMed] [Google Scholar]
- 37.Borisy GG, Taylor EW. The mechanism of action of colchicine. Binding of colchincine-3H to cellular protein. J Cell Biol. 1967;34:525–33. doi: 10.1083/jcb.34.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hastie SB. Interactions of colchicine with tubulin. Pharmacol Ther. 1991;51:377–401. doi: 10.1016/0163-7258(91)90067-v. [DOI] [PubMed] [Google Scholar]
- 39.Skoufias DA, Wilson L. Mechanism of inhibition of microtubule polymerization by colchicine: inhibitory potencies of unliganded colchicine and tubulin-colchicine complexes. Biochemistry. 1992;31:738–46. doi: 10.1021/bi00118a015. [DOI] [PubMed] [Google Scholar]
- 40.Sackett DL. Podophyllotoxin, steganacin and combretastatin: natural products that bind at the colchicine site of tubulin. Pharmacol Ther. 1993;59:163–228. doi: 10.1016/0163-7258(93)90044-e. [DOI] [PubMed] [Google Scholar]
- 41.Pettit GR, Singh SB, Hamel E, Lin CM, Alberts DS, Garcia-Kendall D. Isolation and structure of the strong cell growth and tubulin inhibitor combretastatin A-4. Experientia. 1989;45:209–11. doi: 10.1007/BF01954881. [DOI] [PubMed] [Google Scholar]
- 42.Siemann DW, Chaplin DJ, Walicke PA. A review and update of the current status of the vasculature-disabling agent combretastatin-A4 phosphate (CA4P) Expert Opin Investig Drugs. 2009;18:189–97. doi: 10.1517/13543780802691068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Griggs J, Metcalfe JC, Hesketh R. Targeting tumour vasculature: the development of combretastatin A4. Lancet Oncol. 2001;2:82–7. doi: 10.1016/S1470-2045(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 44.Nambu H, Nambu R, Melia M, Campochiaro PA. Combretastatin A-4 phosphate suppresses development and induces regression of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3650–5. doi: 10.1167/iovs.02-0985. [DOI] [PubMed] [Google Scholar]
- 45.Rowinsky EK, Onetto N, Canetta RM, Arbuck SG. Taxol: the first of the taxanes, an important new class of antitumor agents. Semin Oncol. 1992;19:646–62. [PubMed] [Google Scholar]
- 46.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325–7. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 47.Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665–7. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 48.Nogales E, Wolf SG, Khan IA, Luduena RF, Downing KH. Structure of tubulin at 6. 5 A and location of the taxol-binding site. Nature. 1995;375:424–7. doi: 10.1038/375424a0. [DOI] [PubMed] [Google Scholar]
- 49.Kelling J, Sullivan K, Wilson L, Jordan MA. Suppression of centromere dynamics by Taxol in living osteosarcoma cells. Cancer Res. 2003;63:2794–801. [PubMed] [Google Scholar]
- 50.Yared JA, Tkaczuk KH. Update on taxane development: new analogs and new formulations. Drug Des Devel Ther. 2012;6:371–84. doi: 10.2147/DDDT.S28997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Markman M. Managing taxane toxicities. Support Care Cancer. 2003;11:144–7. doi: 10.1007/s00520-002-0405-9. [DOI] [PubMed] [Google Scholar]
- 52.Thadani-Mulero M, Nanus DM, Giannakakou P. Androgen receptor on the move: boarding the microtubule expressway to the nucleus. Cancer Res. 2012;72:4611–5. doi: 10.1158/0008-5472.CAN-12-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Solit DB, Scher HI, Rosen N. Hsp90 as a therapeutic target in prostate cancer. Semin Oncol. 2003;30:709–16. doi: 10.1016/s0093-7754(03)00346-4. [DOI] [PubMed] [Google Scholar]
- 54.Kemper EM, van Zandbergen AE, Cleypool C, Mos HA, Boogerd W, Beijnen JH, et al. Increased penetration of paclitaxel into the brain by inhibition of P-Glycoprotein. Clin Cancer Res. 2003;9:2849–55. [PubMed] [Google Scholar]
- 55.Bouchet BP, Galmarini CM. Cabazitaxel, a new taxane with favorable properties. Drugs Today (Barc) 2010;46:735–42. doi: 10.1358/dot.2010.46.10.1519019. [DOI] [PubMed] [Google Scholar]
- 56.Lockhart AC, Bukowski R, Rothenberg ML, Wang KK, Cooper W, Grover J, et al. Phase I trial of oral MAC-321 in subjects with advanced malignant solid tumors. Cancer Chemother Pharmacol. 2007;60:203–9. doi: 10.1007/s00280-006-0362-y. [DOI] [PubMed] [Google Scholar]
- 57.Gerth K, Bedorf N, Hofle G, Irschik H, Reichenbach H. Epothilons A and B: antifungal and cytotoxic compounds from Sorangium cellulosum (Myxobacteria). Production, physico-chemical and biological properties. J Antibiot (Tokyo) 1996;49:560–3. doi: 10.7164/antibiotics.49.560. [DOI] [PubMed] [Google Scholar]
- 58.Bollag DM, McQueney PA, Zhu J, Hensens O, Koupal L, Liesch J, et al. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res. 1995;55:2325–33. [PubMed] [Google Scholar]
- 59.Ojima I, Chakravarty S, Inoue T, Lin S, He L, Horwitz SB, et al. A common pharmacophore for cytotoxic natural products that stabilize microtubules. Proc Natl Acad Sci U S A. 1999;96:4256–61. doi: 10.1073/pnas.96.8.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reese M, Sanchez-Pedregal VM, Kubicek K, Meiler J, Blommers MJ, Griesinger C, et al. Structural basis of the activity of the microtubule-stabilizing agent epothilone a studied by NMR spectroscopy in solution. Angew Chem Int Ed Engl. 2007;46:1864–8. doi: 10.1002/anie.200604505. [DOI] [PubMed] [Google Scholar]
- 61.Nettles JH, Li H, Cornett B, Krahn JM, Snyder JP, Downing KH. The binding mode of epothilone A on alpha,beta-tubulin by electron crystallography. Science. 2004;305:866–9. doi: 10.1126/science.1099190. [DOI] [PubMed] [Google Scholar]
- 62.Giannakakou P, Sackett DL, Kang YK, Zhan Z, Buters JT, Fojo T, et al. Paclitaxel-resistant human ovarian cancer cells have mutant beta-tubulins that exhibit impaired paclitaxel-driven polymerization. J Biol Chem. 1997;272:17118–25. doi: 10.1074/jbc.272.27.17118. [DOI] [PubMed] [Google Scholar]
- 63.Mani S, Macapinlac M, Jr, Goel S, Verdier-Pinard D, Fojo T, Rothenberg M, et al. The clinical development of new mitotic inhibitors that stabilize the microtubule. Anticancer Drugs. 2004;15:553–8. doi: 10.1097/01.cad.0000131681.21637.b2. [DOI] [PubMed] [Google Scholar]
- 64.Hardt IH, Steinmetz H, Gerth K, Sasse F, Reichenbach H, Hofle G. New natural epothilones from Sorangium cellulosum, strains So ce90/B2 and So ce90/D13: isolation, structure elucidation, and SAR studies. J Nat Prod. 2001;64:847–56. doi: 10.1021/np000629f. [DOI] [PubMed] [Google Scholar]
- 65.Kolman A. Epothilone D (Kosan/Roche) Curr Opin Investig Drugs. 2004;5:657–67. [PubMed] [Google Scholar]
- 66.Beer TM, Higano CS, Saleh M, Dreicer R, Hudes G, Picus J, et al. Phase II study of KOS-862 in patients with metastatic androgen independent prostate cancer previously treated with docetaxel. Invest New Drugs. 2007;25:565–70. doi: 10.1007/s10637-007-9068-1. [DOI] [PubMed] [Google Scholar]
- 67.Au JL, Panchal N, Li D, Gan Y. Apoptosis: a new pharmacodynamic endpoint. Pharm Res. 1997;14:1659–71. doi: 10.1023/a:1012159208559. [DOI] [PubMed] [Google Scholar]
- 68.Rowinsky EK. The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu Rev Med. 1997;48:353–74. doi: 10.1146/annurev.med.48.1.353. [DOI] [PubMed] [Google Scholar]
- 69.Mastalerz H, Cook D, Fairchild CR, Hansel S, Johnson W, Kadow JF, et al. The discovery of BMS-275183: an orally efficacious novel taxane. Bioorg Med Chem. 2003;11:4315–23. doi: 10.1016/s0968-0896(03)00495-4. [DOI] [PubMed] [Google Scholar]
- 70.Kavallaris M, Verrills NM, Hill BT. Anticancer therapy with novel tubulin-interacting drugs. Drug Resist Updat. 2001;4:392–401. doi: 10.1054/drup.2002.0230. [DOI] [PubMed] [Google Scholar]
- 71.Monzo M, Rosell R, Sanchez JJ, Lee JS, O’Brate A, Gonzalez-Larriba JL, et al. Paclitaxel resistance in non-small-cell lung cancer associated with beta-tubulin gene mutations. J Clin Oncol. 1999;17:1786–93. doi: 10.1200/JCO.1999.17.6.1786. [DOI] [PubMed] [Google Scholar]
- 72.Berrieman HK, Lind MJ, Cawkwell L. Do beta-tubulin mutations have a role in resistance to chemotherapy? Lancet Oncol. 2004;5:158–64. doi: 10.1016/S1470-2045(04)01411-1. [DOI] [PubMed] [Google Scholar]
- 73.Belda-Iniesta C, Perona R, de Castro Carpeno J, Chattopadhyay S, Casado E, Cejas P, et al. Do beta-tubulin pseudogenes really matter? Lancet Oncol. 2004;5:271–2. doi: 10.1016/S1470-2045(04)01464-0. [DOI] [PubMed] [Google Scholar]
- 74.Gerson SL, Willson JK. O6-alkylguanine-DNA alkyltransferase. A target for the modulation of drug resistance. Hematol Oncol Clin North Am. 1995;9:431–50. [PubMed] [Google Scholar]
- 75.Haq R, Zanke B. Inhibition of apoptotic signaling pathways in cancer cells as a mechanism of chemotherapy resistance. Cancer Metastasis Rev. 1998;17:233–9. doi: 10.1023/a:1006075007857. [DOI] [PubMed] [Google Scholar]