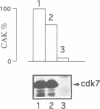
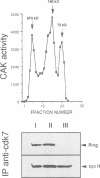
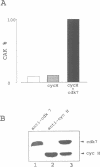
Abstract
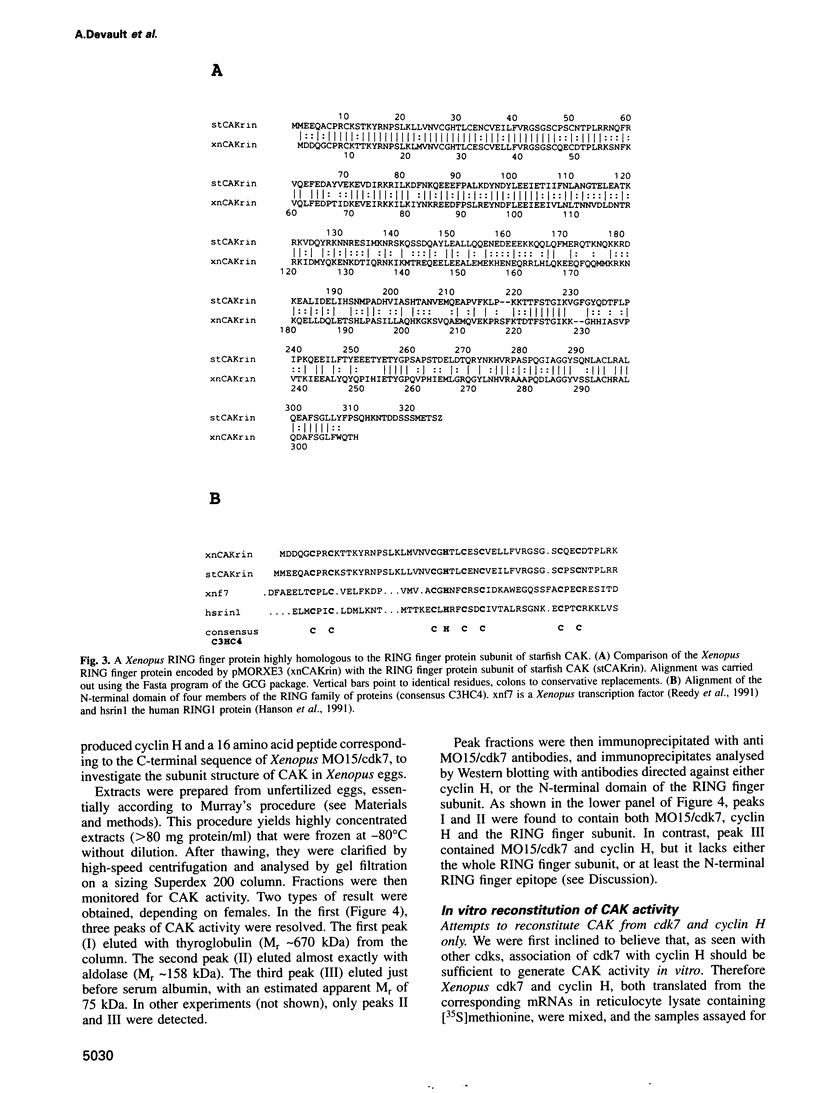
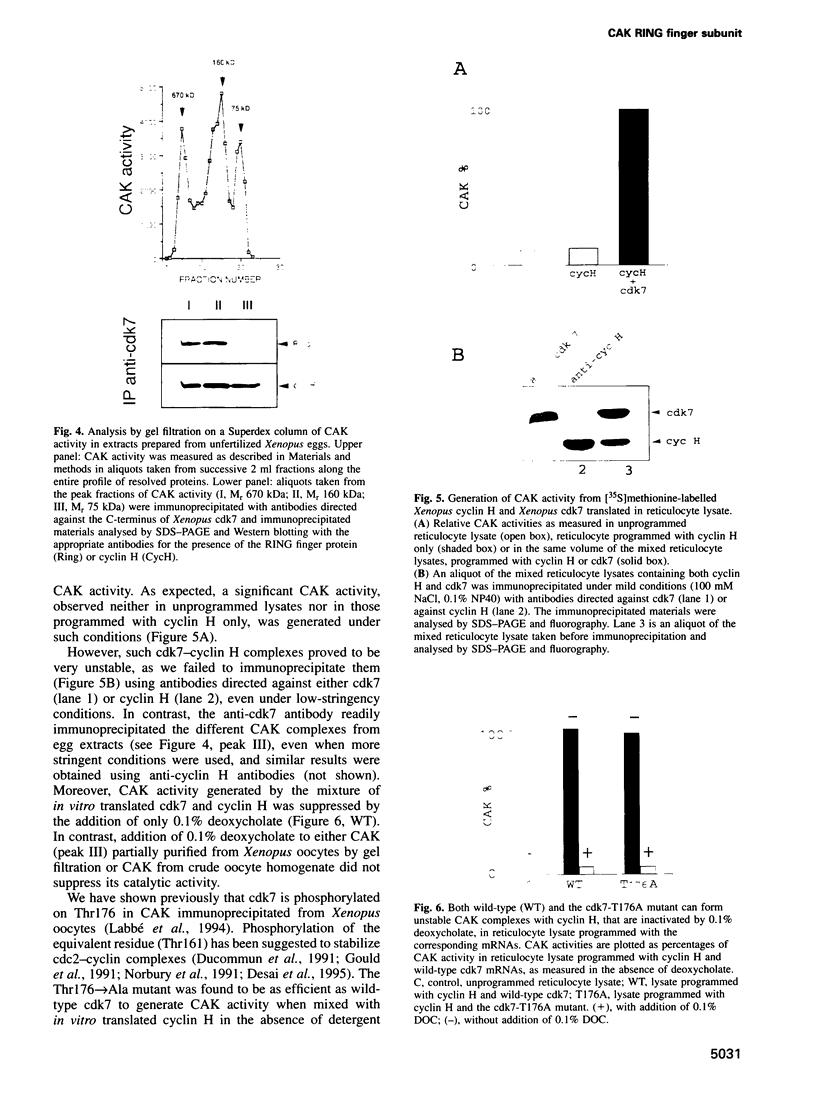
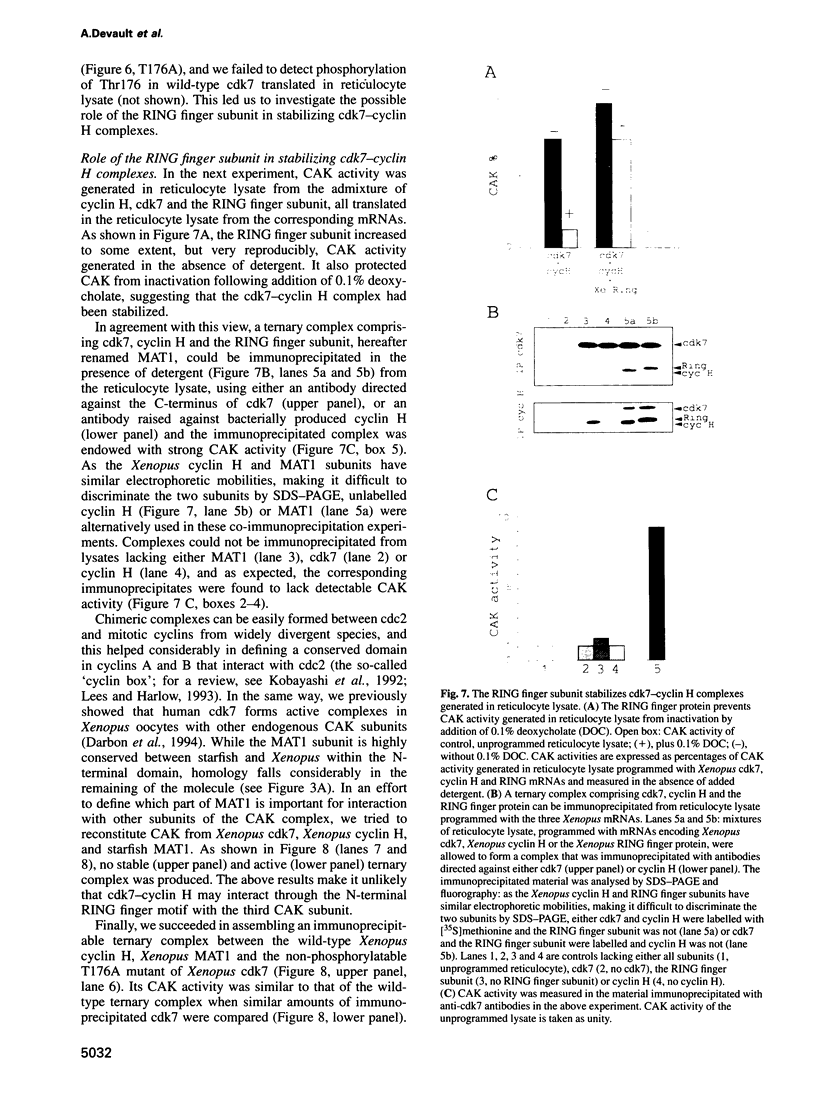
The kinase responsible for Thr161-Thr160 phosphorylation and activation of cdc2/cdk2 (CAK:cdk-activating kinase) has been shown previously to comprise at least two subunits, cdk7 and cyclin H. An additional protein co-purified with CAK in starfish oocytes, but its sequencing did not reveal any similarity with any known protein. In the present work, a cDNA encoding this protein is cloned and sequenced in both starfish and Xenopus oocytes. It is shown to encode a new member of the RING finger family of proteins with a characteristic C3HC4 motif located in the N-terminal domain. We demonstrate that the RING finger protein (MAT1: 'menage à trois') is a new subunit of CAK in both vertebrate and invertebrates. However, CAK may also exist in oocytes as heterodimeric complexes between cyclin H and cdk7 only. Stable heterotrimeric CAK complexes were generated in reticulocyte lysates programmed with mRNAs encoding Xenopus cdk7, cyclin H and MAT1. In contrast, no heterodimeric cyclin H-cdk7 complex could be immunoprecipitated from reticulocyte lysates programmed with cdk7 and cyclin H mRNAs only. Stabilization of CAK complexes by MAT1 does not involve phosphorylation of Thr176, as the Thr176-->Ala mutant of Xenopus cdk7 could engage as efficiently as wild-type cdk7 in ternary complexes. Even though starfish MAT1 is almost identical to Xenopus MAT1 in the RING finger domain, the starfish subunit could not replace the Xenopus subunit and stabilize cyclin H-cdk7 in reticulocyte lysate, suggesting that the MAT1 subunit does not (or not only) interact with cyclin H-cdk7 through the RING finger domain.
Full text
PDF

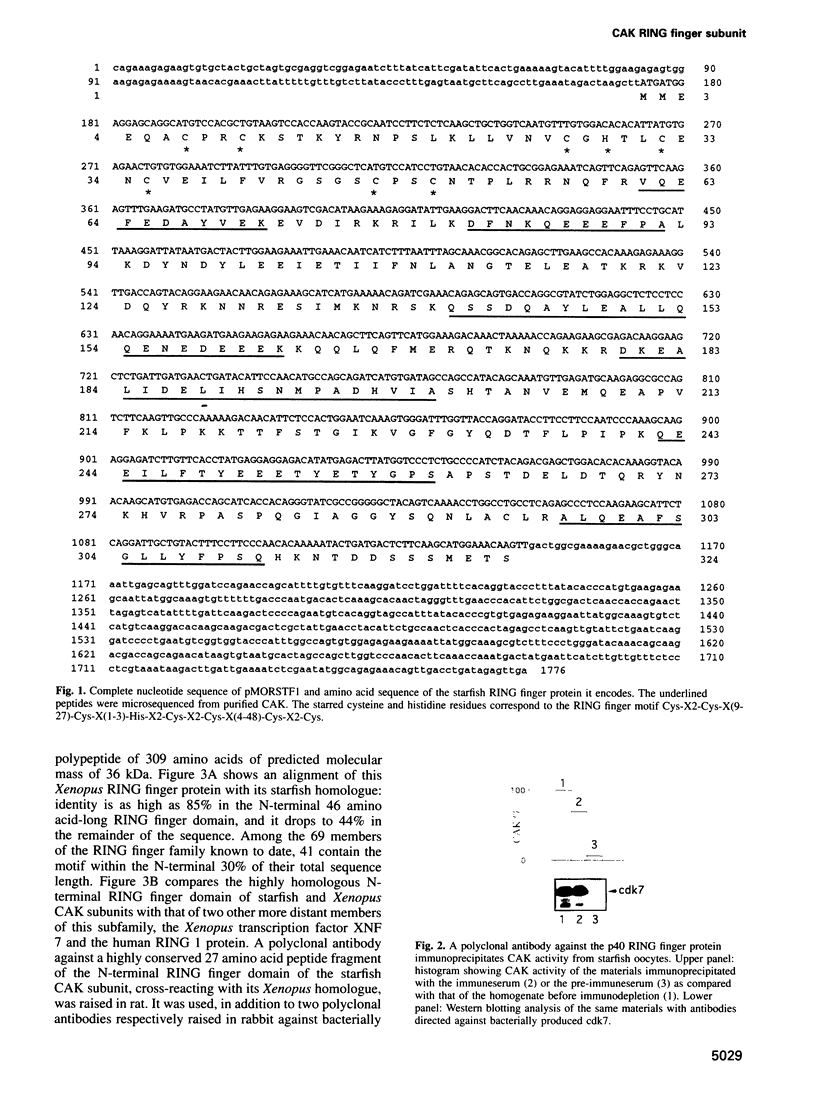




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Darbon J. M., Devault A., Taviaux S., Fesquet D., Martinez A. M., Galas S., Cavadore J. C., Dorée M., Blanchard J. M. Cloning, expression and subcellular localization of the human homolog of p40MO15 catalytic subunit of cdk-activating kinase. Oncogene. 1994 Nov;9(11):3127–3138. [PubMed] [Google Scholar]
- De Bondt H. L., Rosenblatt J., Jancarik J., Jones H. D., Morgan D. O., Kim S. H. Crystal structure of cyclin-dependent kinase 2. Nature. 1993 Jun 17;363(6430):595–602. doi: 10.1038/363595a0. [DOI] [PubMed] [Google Scholar]
- Desai D., Wessling H. C., Fisher R. P., Morgan D. O. Effects of phosphorylation by CAK on cyclin binding by CDC2 and CDK2. Mol Cell Biol. 1995 Jan;15(1):345–350. doi: 10.1128/mcb.15.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorée M., Galas S. The cyclin-dependent protein kinases and the control of cell division. FASEB J. 1994 Nov;8(14):1114–1121. doi: 10.1096/fasebj.8.14.7958616. [DOI] [PubMed] [Google Scholar]
- Ducommun B., Brambilla P., Félix M. A., Franza B. R., Jr, Karsenti E., Draetta G. cdc2 phosphorylation is required for its interaction with cyclin. EMBO J. 1991 Nov;10(11):3311–3319. doi: 10.1002/j.1460-2075.1991.tb04895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J. A., Nurse P. The cell cycle and suc1: from structure to function? Structure. 1995 Apr 15;3(4):321–325. doi: 10.1016/s0969-2126(01)00162-9. [DOI] [PubMed] [Google Scholar]
- Feaver W. J., Svejstrup J. Q., Henry N. L., Kornberg R. D. Relationship of CDK-activating kinase and RNA polymerase II CTD kinase TFIIH/TFIIK. Cell. 1994 Dec 16;79(6):1103–1109. doi: 10.1016/0092-8674(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Fesquet D., Labbé J. C., Derancourt J., Capony J. P., Galas S., Girard F., Lorca T., Shuttleworth J., Dorée M., Cavadore J. C. The MO15 gene encodes the catalytic subunit of a protein kinase that activates cdc2 and other cyclin-dependent kinases (CDKs) through phosphorylation of Thr161 and its homologues. EMBO J. 1993 Aug;12(8):3111–3121. doi: 10.1002/j.1460-2075.1993.tb05980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. P., Morgan D. O. A novel cyclin associates with MO15/CDK7 to form the CDK-activating kinase. Cell. 1994 Aug 26;78(4):713–724. doi: 10.1016/0092-8674(94)90535-5. [DOI] [PubMed] [Google Scholar]
- Freemont P. S. The RING finger. A novel protein sequence motif related to the zinc finger. Ann N Y Acad Sci. 1993 Jun 11;684:174–192. doi: 10.1111/j.1749-6632.1993.tb32280.x. [DOI] [PubMed] [Google Scholar]
- Fyrberg C., Ryan L., Kenton M., Fyrberg E. Genes encoding actin-related proteins of Drosophila melanogaster. J Mol Biol. 1994 Aug 19;241(3):498–503. doi: 10.1006/jmbi.1994.1526. [DOI] [PubMed] [Google Scholar]
- Gould K. L., Moreno S., Owen D. J., Sazer S., Nurse P. Phosphorylation at Thr167 is required for Schizosaccharomyces pombe p34cdc2 function. EMBO J. 1991 Nov;10(11):3297–3309. doi: 10.1002/j.1460-2075.1991.tb04894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwiger J. A., Wittenberg C., Mendenhall M. D., Reed S. I. The Saccharomyces cerevisiae CKS1 gene, a homolog of the Schizosaccharomyces pombe suc1+ gene, encodes a subunit of the Cdc28 protein kinase complex. Mol Cell Biol. 1989 May;9(5):2034–2041. doi: 10.1128/mcb.9.5.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 1995 May;9(8):576–596. [PubMed] [Google Scholar]
- Hanson I. M., Poustka A., Trowsdale J. New genes in the class II region of the human major histocompatibility complex. Genomics. 1991 Jun;10(2):417–424. doi: 10.1016/0888-7543(91)90327-b. [DOI] [PubMed] [Google Scholar]
- Hunt T. Cyclins and their partners: from a simple idea to complicated reality. Semin Cell Biol. 1991 Aug;2(4):213–222. [PubMed] [Google Scholar]
- Jessus C., Beach D. Oscillation of MPF is accompanied by periodic association between cdc25 and cdc2-cyclin B. Cell. 1992 Jan 24;68(2):323–332. doi: 10.1016/0092-8674(92)90473-p. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé J. C., Martinez A. M., Fesquet D., Capony J. P., Darbon J. M., Derancourt J., Devault A., Morin N., Cavadore J. C., Dorée M. p40MO15 associates with a p36 subunit and requires both nuclear translocation and Thr176 phosphorylation to generate cdk-activating kinase activity in Xenopus oocytes. EMBO J. 1994 Nov 1;13(21):5155–5164. doi: 10.1002/j.1460-2075.1994.tb06845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees E. M., Harlow E. Sequences within the conserved cyclin box of human cyclin A are sufficient for binding to and activation of cdc2 kinase. Mol Cell Biol. 1993 Feb;13(2):1194–1201. doi: 10.1128/mcb.13.2.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering R., Hanson I. M., Borden K. L., Martin S., O'Reilly N. J., Evan G. I., Rahman D., Pappin D. J., Trowsdale J., Freemont P. S. Identification and preliminary characterization of a protein motif related to the zinc finger. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2112–2116. doi: 10.1073/pnas.90.6.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M., Kato J. Y., Fisher R. P., Morgan D. O., Sherr C. J. Activation of cyclin-dependent kinase 4 (cdk4) by mouse MO15-associated kinase. Mol Cell Biol. 1994 Nov;14(11):7265–7275. doi: 10.1128/mcb.14.11.7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaille J. J., Gouy M., Blanchet S., Duret L. Isolation and characterization of a cDNA encoding a chicken actin-like protein. Gene. 1995 Mar 10;154(2):205–209. doi: 10.1016/0378-1119(94)00865-p. [DOI] [PubMed] [Google Scholar]
- Morgan D. O. Principles of CDK regulation. Nature. 1995 Mar 9;374(6518):131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- Mueller P. R., Coleman T. R., Dunphy W. G. Cell cycle regulation of a Xenopus Wee1-like kinase. Mol Biol Cell. 1995 Jan;6(1):119–134. doi: 10.1091/mbc.6.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W., Kirschner M. W. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989 May 25;339(6222):275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- Mäkelä T. P., Tassan J. P., Nigg E. A., Frutiger S., Hughes G. J., Weinberg R. A. A cyclin associated with the CDK-activating kinase MO15. Nature. 1994 Sep 15;371(6494):254–257. doi: 10.1038/371254a0. [DOI] [PubMed] [Google Scholar]
- Norbury C., Blow J., Nurse P. Regulatory phosphorylation of the p34cdc2 protein kinase in vertebrates. EMBO J. 1991 Nov;10(11):3321–3329. doi: 10.1002/j.1460-2075.1991.tb04896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury C., Nurse P. Animal cell cycles and their control. Annu Rev Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- Parge H. E., Arvai A. S., Murtari D. J., Reed S. I., Tainer J. A. Human CksHs2 atomic structure: a role for its hexameric assembly in cell cycle control. Science. 1993 Oct 15;262(5132):387–395. doi: 10.1126/science.8211159. [DOI] [PubMed] [Google Scholar]
- Poon R. Y., Yamashita K., Adamczewski J. P., Hunt T., Shuttleworth J. The cdc2-related protein p40MO15 is the catalytic subunit of a protein kinase that can activate p33cdk2 and p34cdc2. EMBO J. 1993 Aug;12(8):3123–3132. doi: 10.1002/j.1460-2075.1993.tb05981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy B. A., Kloc M., Etkin L. The cloning and characterization of a maternally expressed novel zinc finger nuclear phosphoprotein (xnf7) in Xenopus laevis. Dev Biol. 1991 Nov;148(1):107–116. doi: 10.1016/0012-1606(91)90321-s. [DOI] [PubMed] [Google Scholar]
- Richardson H. E., Stueland C. S., Thomas J., Russell P., Reed S. I. Human cDNAs encoding homologs of the small p34Cdc28/Cdc2-associated protein of Saccharomyces cerevisiae and Schizosaccharomyces pombe. Genes Dev. 1990 Aug;4(8):1332–1344. doi: 10.1101/gad.4.8.1332. [DOI] [PubMed] [Google Scholar]
- Roy R., Adamczewski J. P., Seroz T., Vermeulen W., Tassan J. P., Schaeffer L., Nigg E. A., Hoeijmakers J. H., Egly J. M. The MO15 cell cycle kinase is associated with the TFIIH transcription-DNA repair factor. Cell. 1994 Dec 16;79(6):1093–1101. doi: 10.1016/0092-8674(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Schwob E., Martin R. P. New yeast actin-like gene required late in the cell cycle. Nature. 1992 Jan 9;355(6356):179–182. doi: 10.1038/355179a0. [DOI] [PubMed] [Google Scholar]
- Serizawa H., Mäkelä T. P., Conaway J. W., Conaway R. C., Weinberg R. A., Young R. A. Association of Cdk-activating kinase subunits with transcription factor TFIIH. Nature. 1995 Mar 16;374(6519):280–282. doi: 10.1038/374280a0. [DOI] [PubMed] [Google Scholar]
- Sherr C. J. Mammalian G1 cyclins. Cell. 1993 Jun 18;73(6):1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- Shuttleworth J., Godfrey R., Colman A. p40MO15, a cdc2-related protein kinase involved in negative regulation of meiotic maturation of Xenopus oocytes. EMBO J. 1990 Oct;9(10):3233–3240. doi: 10.1002/j.1460-2075.1990.tb07522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M. J., Harper J. W., Shuttleworth J. CAK, the p34cdc2 activating kinase, contains a protein identical or closely related to p40MO15. EMBO J. 1993 Aug;12(8):3133–3142. doi: 10.1002/j.1460-2075.1993.tb05982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M. J. The function(s) of CAK, the p34cdc2-activating kinase. Trends Biochem Sci. 1994 Nov;19(11):496–500. doi: 10.1016/0968-0004(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Tagawa M., Sakamoto T., Shigemoto K., Matsubara H., Tamura Y., Ito T., Nakamura I., Okitsu A., Imai K., Taniguchi M. Expression of novel DNA-binding protein with zinc finger structure in various tumor cells. J Biol Chem. 1990 Nov 15;265(32):20021–20026. [PubMed] [Google Scholar]
- Tassan J. P., Schultz S. J., Bartek J., Nigg E. A. Cell cycle analysis of the activity, subcellular localization, and subunit composition of human CAK (CDK-activating kinase). J Cell Biol. 1994 Oct;127(2):467–478. doi: 10.1083/jcb.127.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Xiong Y., Beach D. Proliferating cell nuclear antigen and p21 are components of multiple cell cycle kinase complexes. Mol Biol Cell. 1993 Sep;4(9):897–906. doi: 10.1091/mbc.4.9.897. [DOI] [PMC free article] [PubMed] [Google Scholar]