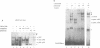Abstract
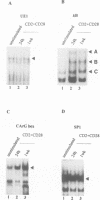
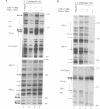
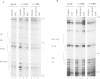
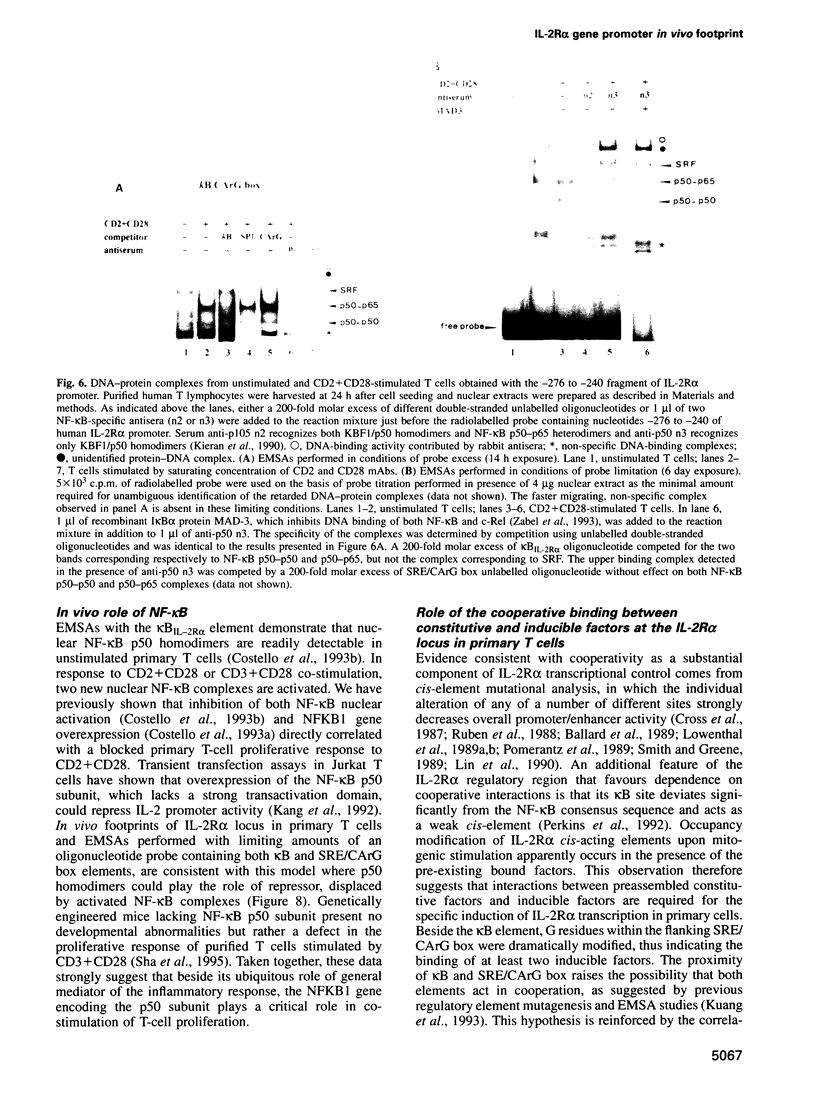
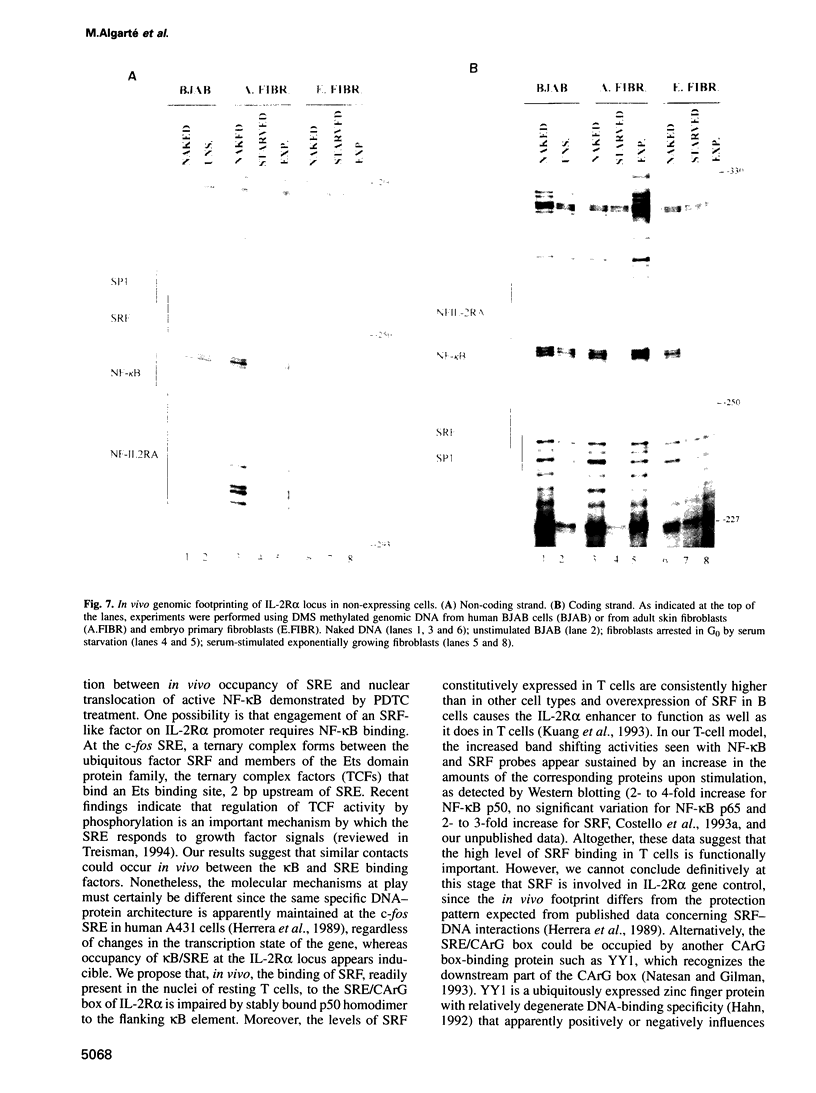
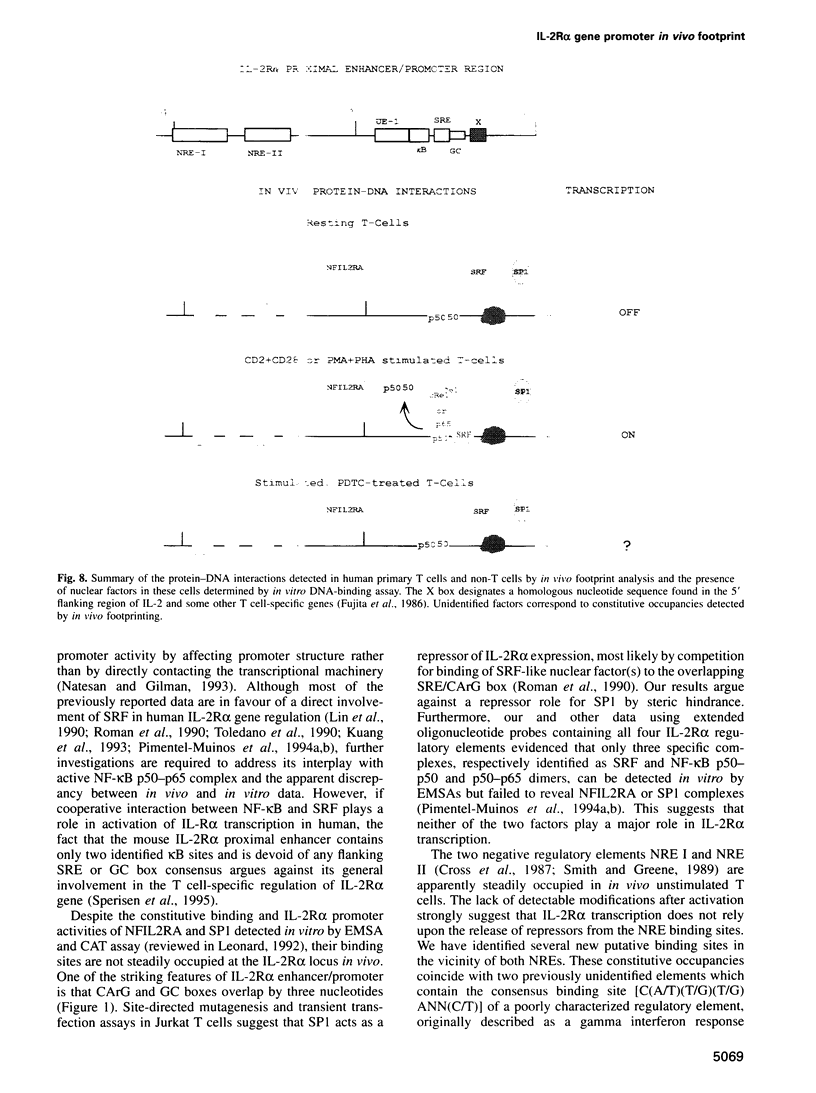
IL-2R alpha transcription is developmentally restricted to T cells and physiologically dependent on specific stimuli such as antigen recognition. To analyse the mechanisms used to activate IL-2R alpha transcription as well as those used to block it in non-expressing cells, we determined the protein-DNA interactions at the IL-2R alpha locus in three different cell types using the DMS/LMPCR genomic footprinting method. CD25/IL-2R alpha can be efficiently induced in primary human T cells since approximately 100% express this gene when receiving an appropriate combination of mitogenic stimuli. To understand why IL-2R alpha is not expressed in other haematopoietic cell types, we analysed BJAB B lymphoma cells which do not express the IL-2R alpha gene and contain constitutively active nuclear NF-kappa B. Primary fibroblasts from embryo and adult skin were selected to examine the mechanisms that may be used to keep the IL-2R alpha gene inactive in non-haematopoietic cells. The three main results are: (i) the stable in vivo occupancy of IL-2R alpha kappa B element in resting T cells, most probably by constitutive NF-kappa B p50 homodimer that could impair SRF binding to the flanking SRE/CArG box; (ii) its inducible occupancy by NF-kappa B p50-p65 associated with the binding of an SRE/CArG box DNA-binding factor upon mitogenic stimulation; and (iii) a correlation between the precommitment of T cells to activation and the presence of stable preassembled protein-DNA complexes in contrast with the bare IL-2R alpha locus in non-T cells.
Full text
PDF
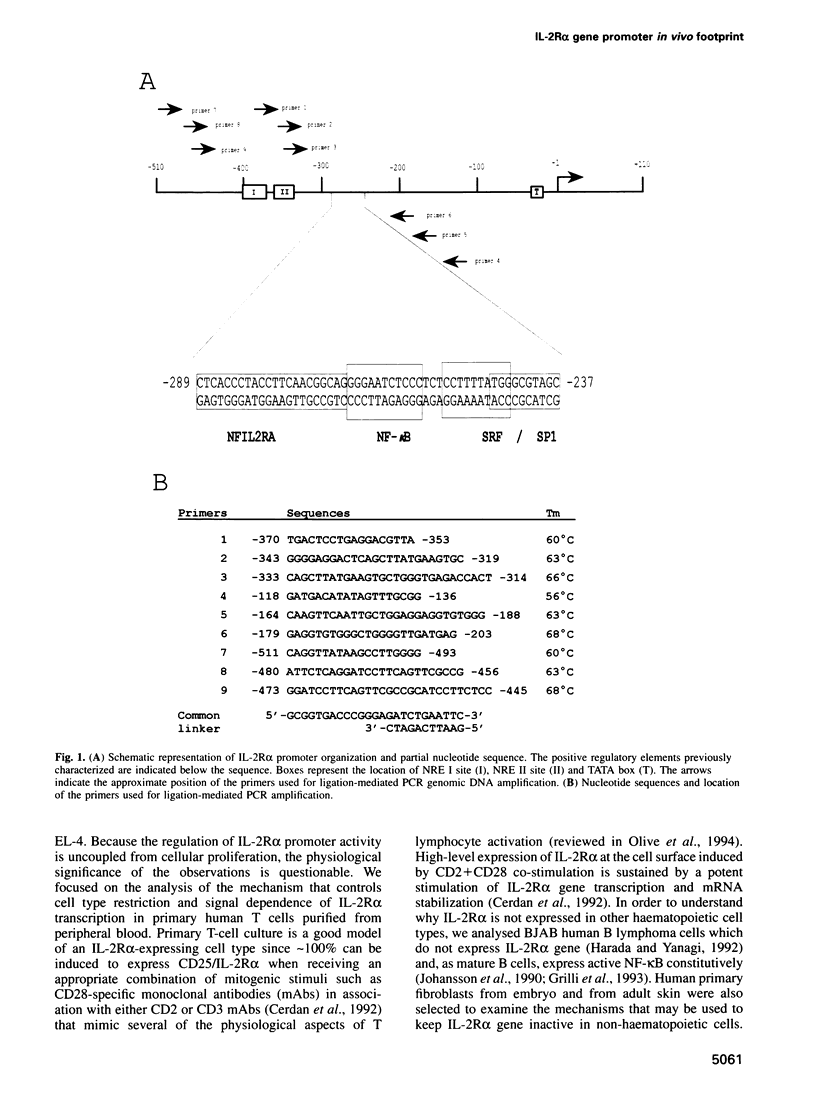
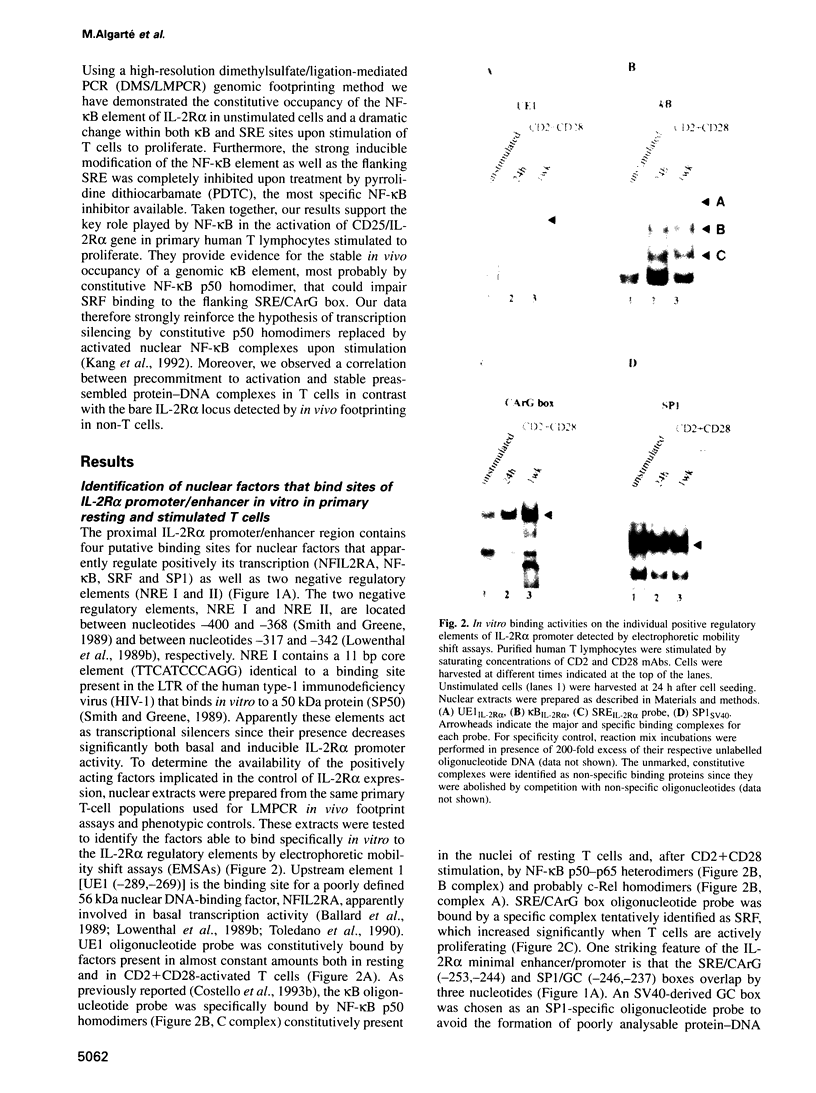

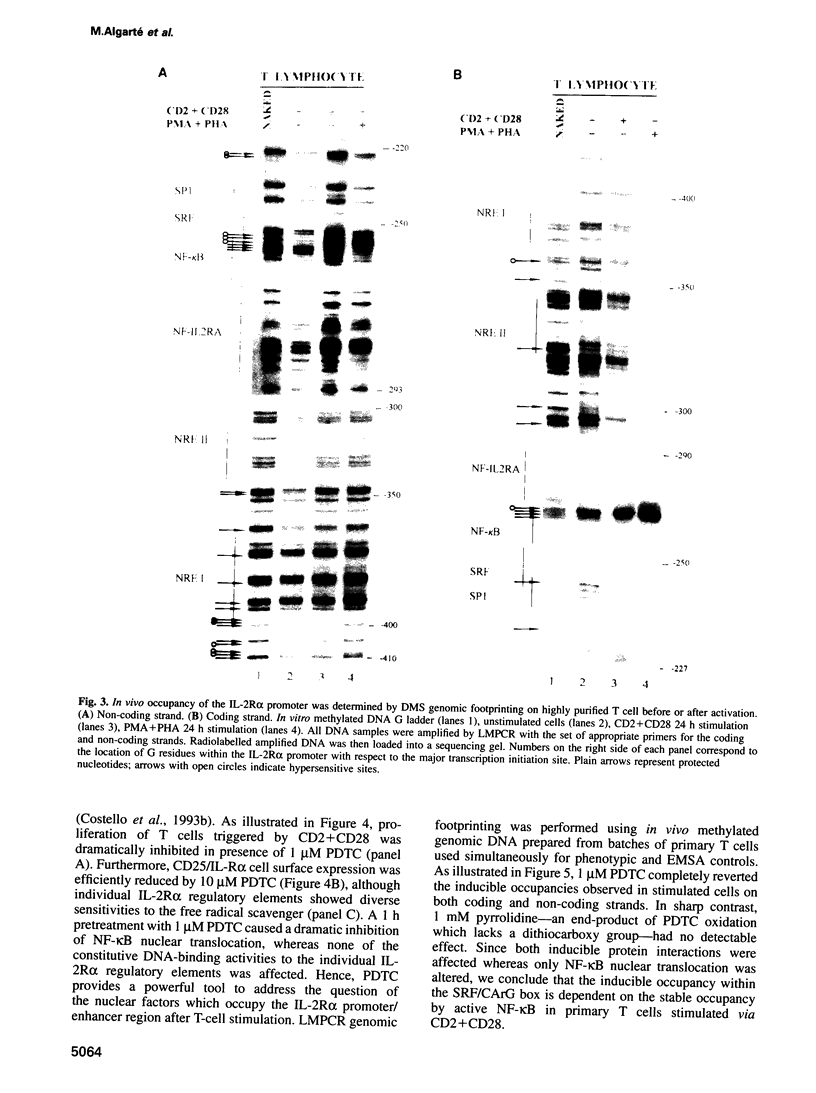
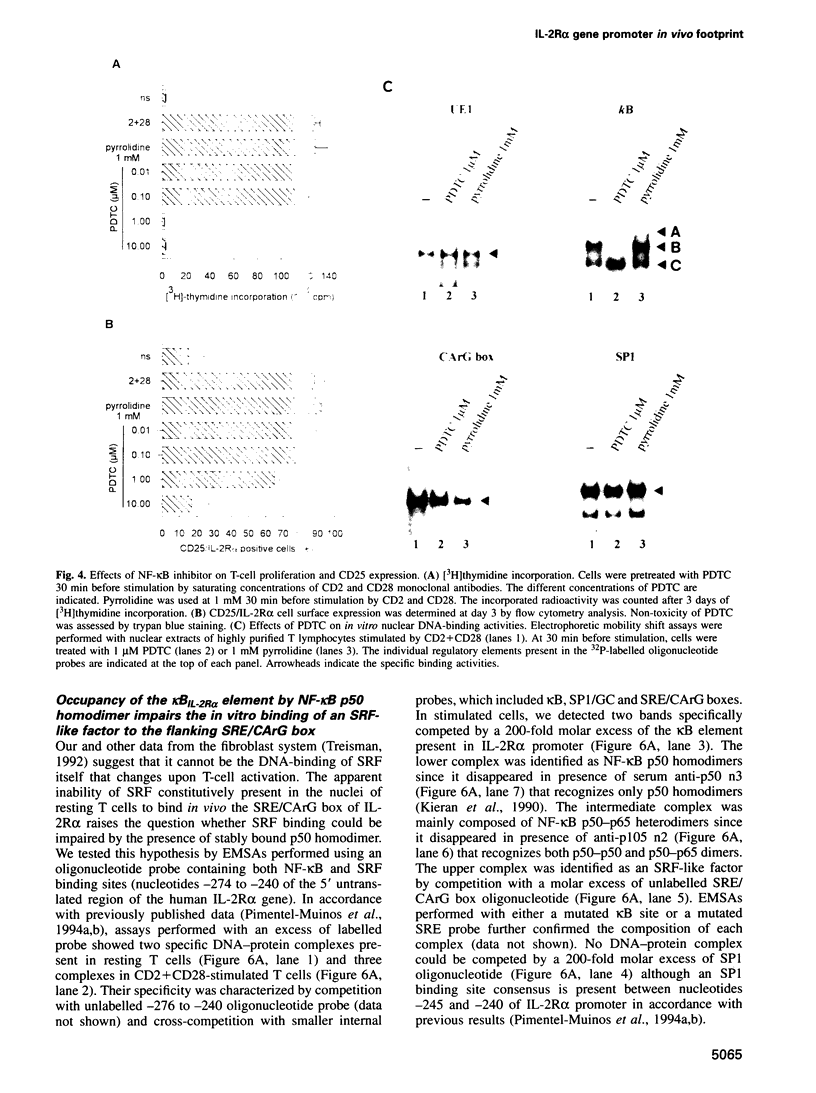




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Ballard D. W., Böhnlein E., Hoffman J. A., Bogerd H. P., Dixon E. P., Franza B. R., Greene W. C. Activation of the interleukin-2 receptor alpha gene: regulatory role for DNA-protein interactions flanking the kappa B enhancer. New Biol. 1989 Oct;1(1):83–92. [PubMed] [Google Scholar]
- Becker P. B., Ruppert S., Schütz G. Genomic footprinting reveals cell type-specific DNA binding of ubiquitous factors. Cell. 1987 Nov 6;51(3):435–443. doi: 10.1016/0092-8674(87)90639-8. [DOI] [PubMed] [Google Scholar]
- Brunvand M. W., Krumm A., Groudine M. In vivo footprinting of the human IL-2 gene reveals a nuclear factor bound to the transcription start site in T cells. Nucleic Acids Res. 1993 Oct 11;21(20):4824–4829. doi: 10.1093/nar/21.20.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhnlein E., Lowenthal J. W., Siekevitz M., Ballard D. W., Franza B. R., Greene W. C. The same inducible nuclear proteins regulates mitogen activation of both the interleukin-2 receptor-alpha gene and type 1 HIV. Cell. 1988 Jun 3;53(5):827–836. doi: 10.1016/0092-8674(88)90099-2. [DOI] [PubMed] [Google Scholar]
- Cerdan C., Martin Y., Brailly H., Courcoul M., Flavetta S., Costello R., Mawas C., Birg F., Olive D. IL-1 alpha is produced by T lymphocytes activated via the CD2 plus CD28 pathways. J Immunol. 1991 Jan 15;146(2):560–564. [PubMed] [Google Scholar]
- Cerdan C., Martin Y., Courcoul M., Brailly H., Mawas C., Birg F., Olive D. Prolonged IL-2 receptor alpha/CD25 expression after T cell activation via the adhesion molecules CD2 and CD28. Demonstration of combined transcriptional and post-transcriptional regulation. J Immunol. 1992 Oct 1;149(7):2255–2261. [PubMed] [Google Scholar]
- Chen D., Rothenberg E. V. Interleukin 2 transcription factors as molecular targets of cAMP inhibition: delayed inhibition kinetics and combinatorial transcription roles. J Exp Med. 1994 Mar 1;179(3):931–942. doi: 10.1084/jem.179.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. C., Oosterwegel M. A., Georgopoulos K. Transcription factors in early T-cell development. Immunol Today. 1993 Dec;14(12):591–596. doi: 10.1016/0167-5699(93)90198-T. [DOI] [PubMed] [Google Scholar]
- Costello R., Cerdan C., Lipcey C., Algarté M., Martin Y., Baeuerle P. A., Olive D., Imbert J. The role of NF-kappa B1 (p50/p105) gene expression in activation of human blood T-lymphocytes via CD2 and CD28 adhesion molecules. Cell Growth Differ. 1993 Nov;4(11):947–954. [PubMed] [Google Scholar]
- Costello R., Lipcey C., Algarté M., Cerdan C., Baeuerle P. A., Olive D., Imbert J. Activation of primary human T-lymphocytes through CD2 plus CD28 adhesion molecules induces long-term nuclear expression of NF-kappa B. Cell Growth Differ. 1993 Apr;4(4):329–339. [PubMed] [Google Scholar]
- Cross S. L., Feinberg M. B., Wolf J. B., Holbrook N. J., Wong-Staal F., Leonard W. J. Regulation of the human interleukin-2 receptor alpha chain promoter: activation of a nonfunctional promoter by the transactivator gene of HTLV-I. Cell. 1987 Apr 10;49(1):47–56. doi: 10.1016/0092-8674(87)90754-9. [DOI] [PubMed] [Google Scholar]
- Cross S. L., Halden N. F., Lenardo M. J., Leonard W. J. Functionally distinct NF-kappa B binding sites in the immunoglobulin kappa and IL-2 receptor alpha chain genes. Science. 1989 Apr 28;244(4903):466–469. doi: 10.1126/science.2497520. [DOI] [PubMed] [Google Scholar]
- Fujita T., Shibuya H., Ohashi T., Yamanishi K., Taniguchi T. Regulation of human interleukin-2 gene: functional DNA sequences in the 5' flanking region for the gene expression in activated T lymphocytes. Cell. 1986 Aug 1;46(3):401–405. doi: 10.1016/0092-8674(86)90660-4. [DOI] [PubMed] [Google Scholar]
- Garrity P. A., Chen D., Rothenberg E. V., Wold B. J. Interleukin-2 transcription is regulated in vivo at the level of coordinated binding of both constitutive and regulated factors. Mol Cell Biol. 1994 Mar;14(3):2159–2169. doi: 10.1128/mcb.14.3.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity P. A., Wold B. J. Effects of different DNA polymerases in ligation-mediated PCR: enhanced genomic sequencing and in vivo footprinting. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1021–1025. doi: 10.1073/pnas.89.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli M., Chiu J. J., Lenardo M. J. NF-kappa B and Rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- Hahn S. The Yin and the Yang of mammalian transcription. Curr Biol. 1992 Mar;2(3):152–154. doi: 10.1016/0960-9822(92)90268-f. [DOI] [PubMed] [Google Scholar]
- Harada S., Yanagi K. Induced CD25 expression in a human B-lymphoma cell line transfected with the Epstein-Barr virus nuclear antigen 2 gene. Microbiol Immunol. 1992;36(5):479–494. doi: 10.1111/j.1348-0421.1992.tb02046.x. [DOI] [PubMed] [Google Scholar]
- Herrera R. E., Shaw P. E., Nordheim A. Occupation of the c-fos serum response element in vivo by a multi-protein complex is unaltered by growth factor induction. Nature. 1989 Jul 6;340(6228):68–70. doi: 10.1038/340068a0. [DOI] [PubMed] [Google Scholar]
- Johansson K., Nilsson K., Leanderson T. Phorbol ester treatment down-regulates immunoglobulin RNA steady-state levels in B type chronic lymphocytic leukemia and non-Hodgkin's lymphoma cells. Leukemia. 1990 Sep;4(9):641–645. [PubMed] [Google Scholar]
- John S., Reeves R. B., Lin J. X., Child R., Leiden J. M., Thompson C. B., Leonard W. J. Regulation of cell-type-specific interleukin-2 receptor alpha-chain gene expression: potential role of physical interactions between Elf-1, HMG-I(Y), and NF-kappa B family proteins. Mol Cell Biol. 1995 Mar;15(3):1786–1796. doi: 10.1128/mcb.15.3.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S. M., Tran A. C., Grilli M., Lenardo M. J. NF-kappa B subunit regulation in nontransformed CD4+ T lymphocytes. Science. 1992 Jun 5;256(5062):1452–1456. doi: 10.1126/science.1604322. [DOI] [PubMed] [Google Scholar]
- Kara C. J., Glimcher L. H. In vivo footprinting of MHC class II genes: bare promoters in the bare lymphocyte syndrome. Science. 1991 May 3;252(5006):709–712. doi: 10.1126/science.1902592. [DOI] [PubMed] [Google Scholar]
- Kieran M., Blank V., Logeat F., Vandekerckhove J., Lottspeich F., Le Bail O., Urban M. B., Kourilsky P., Baeuerle P. A., Israël A. The DNA binding subunit of NF-kappa B is identical to factor KBF1 and homologous to the rel oncogene product. Cell. 1990 Sep 7;62(5):1007–1018. doi: 10.1016/0092-8674(90)90275-j. [DOI] [PubMed] [Google Scholar]
- Kuang A. A., Novak K. D., Kang S. M., Bruhn K., Lenardo M. J. Interaction between NF-kappa B- and serum response factor-binding elements activates an interleukin-2 receptor alpha-chain enhancer specifically in T lymphocytes. Mol Cell Biol. 1993 Apr;13(4):2536–2545. doi: 10.1128/mcb.13.4.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehming N., Thanos D., Brickman J. M., Ma J., Maniatis T., Ptashne M. An HMG-like protein that can switch a transcriptional activator to a repressor. Nature. 1994 Sep 8;371(6493):175–179. doi: 10.1038/371175a0. [DOI] [PubMed] [Google Scholar]
- Leung K., Nabel G. J. HTLV-1 transactivator induces interleukin-2 receptor expression through an NF-kappa B-like factor. Nature. 1988 Jun 23;333(6175):776–778. doi: 10.1038/333776a0. [DOI] [PubMed] [Google Scholar]
- Lin B. B., Cross S. L., Halden N. F., Roman D. G., Toledano M. B., Leonard W. J. Delineation of an enhancerlike positive regulatory element in the interleukin-2 receptor alpha-chain gene. Mol Cell Biol. 1990 Feb;10(2):850–853. doi: 10.1128/mcb.10.2.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenthal J. W., Ballard D. W., Bogerd H., Böhnlein E., Greene W. C. Tumor necrosis factor-alpha activation of the IL-2 receptor-alpha gene involves the induction of kappa B-specific DNA binding proteins. J Immunol. 1989 May 1;142(9):3121–3128. [PubMed] [Google Scholar]
- Lowenthal J. W., Ballard D. W., Böhnlein E., Greene W. C. Tumor necrosis factor alpha induces proteins that bind specifically to kappa B-like enhancer elements and regulate interleukin 2 receptor alpha-chain gene expression in primary human T lymphocytes. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2331–2335. doi: 10.1073/pnas.86.7.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Menezes J., Leibold W., Klein G., Clements G. Establishment and characterization of an Epstein-Barr virus (EBC)-negative lymphoblastoid B cell line (BJA-B) from an exceptional, EBV-genome-negative African Burkitt's lymphoma. Biomedicine. 1975 Jul;22(4):276–284. [PubMed] [Google Scholar]
- Minami Y., Kono T., Miyazaki T., Taniguchi T. The IL-2 receptor complex: its structure, function, and target genes. Annu Rev Immunol. 1993;11:245–268. doi: 10.1146/annurev.iy.11.040193.001333. [DOI] [PubMed] [Google Scholar]
- Molitor J. A., Ballard D. W., Greene W. C. Kappa B-specific DNA binding proteins are differentially inhibited by enhancer mutations and biological oxidation. New Biol. 1991 Oct;3(10):987–996. [PubMed] [Google Scholar]
- Muegge K., Durum S. K. From cell code to gene code: cytokines and transcription factors. New Biol. 1989 Dec;1(3):239–246. [PubMed] [Google Scholar]
- Mueller P. R., Wold B. In vivo footprinting of a muscle specific enhancer by ligation mediated PCR. Science. 1989 Nov 10;246(4931):780–786. doi: 10.1126/science.2814500. [DOI] [PubMed] [Google Scholar]
- Natesan S., Gilman M. Z. DNA bending and orientation-dependent function of YY1 in the c-fos promoter. Genes Dev. 1993 Dec;7(12B):2497–2509. doi: 10.1101/gad.7.12b.2497. [DOI] [PubMed] [Google Scholar]
- Olive D., Ragueneau M., Cerdan C., Dubreuil P., Lopez M., Mawas C. Anti-CD2 (sheep red blood cell receptor) monoclonal antibodies and T cell activation. I. Pairs of anti-T11.1 and T11.2 (CD2 subgroups) are strongly mitogenic for T cells in presence of 12-O-tetradecanoylphorbol 13-acetate. Eur J Immunol. 1986 Sep;16(9):1063–1068. doi: 10.1002/eji.1830160906. [DOI] [PubMed] [Google Scholar]
- Olive D., Raymond J., Dubreuil P., Charmot D., Jacques Y., Mawas C. Anti-interleukin 2 receptor monoclonal antibodies. Respective role of epitope mapping and monoclonal antibody-receptor interactions in their antagonist effects on interleukin 2-dependent T cell growth. Eur J Immunol. 1986 Jun;16(6):611–616. doi: 10.1002/eji.1830160605. [DOI] [PubMed] [Google Scholar]
- Perkins N. D., Schmid R. M., Duckett C. S., Leung K., Rice N. R., Nabel G. J. Distinct combinations of NF-kappa B subunits determine the specificity of transcriptional activation. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1529–1533. doi: 10.1073/pnas.89.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel-Muiños F. X., Mazana J., Fresno M. Regulation of interleukin-2 receptor alpha chain expression and nuclear factor.kappa B activation by protein kinase C in T lymphocytes. Autocrine role of tumor necrosis factor alpha. J Biol Chem. 1994 Sep 30;269(39):24424–24429. [PubMed] [Google Scholar]
- Pimentel-Muiños F. X., Muñoz-Fernández M. A., Fresno M. Control of T lymphocyte activation and IL-2 receptor expression by endogenously secreted lymphokines. J Immunol. 1994 Jun 15;152(12):5714–5722. [PubMed] [Google Scholar]
- Pomerantz J. L., Mauxion F., Yoshida M., Greene W. C., Sen R. A second sequence element located 3' to the NF-kappa B-binding site regulates IL-2 receptor-alpha gene induction. J Immunol. 1989 Dec 15;143(12):4275–4281. [PubMed] [Google Scholar]
- Roman D. G., Toledano M. B., Leonard W. J. Sp1 represses IL-2 receptor alpha chain gene expression. New Biol. 1990 Jul;2(7):642–647. [PubMed] [Google Scholar]
- Rothenberg E. V. The development of functionally responsive T cells. Adv Immunol. 1992;51:85–214. doi: 10.1016/s0065-2776(08)60487-3. [DOI] [PubMed] [Google Scholar]
- Ruben S., Poteat H., Tan T. H., Kawakami K., Roeder R., Haseltine W., Rosen C. A. Cellular transcription factors and regulation of IL-2 receptor gene expression by HTLV-I tax gene product. Science. 1988 Jul 1;241(4861):89–92. doi: 10.1126/science.2838905. [DOI] [PubMed] [Google Scholar]
- Schreck R., Meier B., Männel D. N., Dröge W., Baeuerle P. A. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992 May 1;175(5):1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991 Aug;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha W. C., Liou H. C., Tuomanen E. I., Baltimore D. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell. 1995 Jan 27;80(2):321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- Smith M. R., Greene W. C. The same 50-kDa cellular protein binds to the negative regulatory elements of the interleukin 2 receptor alpha-chain gene and the human immunodeficiency virus type 1 long terminal repeat. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8526–8530. doi: 10.1073/pnas.86.21.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldaini E., Pla M., Beermann F., Espel E., Corthésy P., Barangé S., Waanders G. A., MacDonald H. R., Nabholz M. Mouse interleukin-2 receptor alpha gene expression. Delimitation of cis-acting regulatory elements in transgenic mice and by mapping of DNase-I hypersensitive sites. J Biol Chem. 1995 May 5;270(18):10733–10742. doi: 10.1074/jbc.270.18.10733. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Minami Y. The IL-2/IL-2 receptor system: a current overview. Cell. 1993 Apr 9;73(1):5–8. doi: 10.1016/0092-8674(93)90152-g. [DOI] [PubMed] [Google Scholar]
- Thanos D., Maniatis T. The high mobility group protein HMG I(Y) is required for NF-kappa B-dependent virus induction of the human IFN-beta gene. Cell. 1992 Nov 27;71(5):777–789. doi: 10.1016/0092-8674(92)90554-p. [DOI] [PubMed] [Google Scholar]
- Toledano M. B., Roman D. G., Halden N. F., Lin B. B., Leonard W. J. The same target sequences are differentially important for activation of the interleukin 2 receptor alpha-chain gene in two distinct T-cell lines. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1830–1834. doi: 10.1073/pnas.87.5.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R. Identification and purification of a polypeptide that binds to the c-fos serum response element. EMBO J. 1987 Sep;6(9):2711–2717. doi: 10.1002/j.1460-2075.1987.tb02564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R. The serum response element. Trends Biochem Sci. 1992 Oct;17(10):423–426. doi: 10.1016/0968-0004(92)90013-y. [DOI] [PubMed] [Google Scholar]
- Ullman K. S., Northrop J. P., Verweij C. L., Crabtree G. R. Transmission of signals from the T lymphocyte antigen receptor to the genes responsible for cell proliferation and immune function: the missing link. Annu Rev Immunol. 1990;8:421–452. doi: 10.1146/annurev.iy.08.040190.002225. [DOI] [PubMed] [Google Scholar]
- Yang Z., Sugawara M., Ponath P. D., Wessendorf L., Banerji J., Li Y., Strominger J. L. Interferon gamma response region in the promoter of the human DPA gene. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9226–9230. doi: 10.1073/pnas.87.23.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel U., Henkel T., Silva M. S., Baeuerle P. A. Nuclear uptake control of NF-kappa B by MAD-3, an I kappa B protein present in the nucleus. EMBO J. 1993 Jan;12(1):201–211. doi: 10.1002/j.1460-2075.1993.tb05646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]