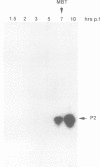
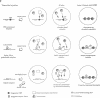
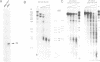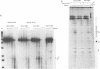Abstract
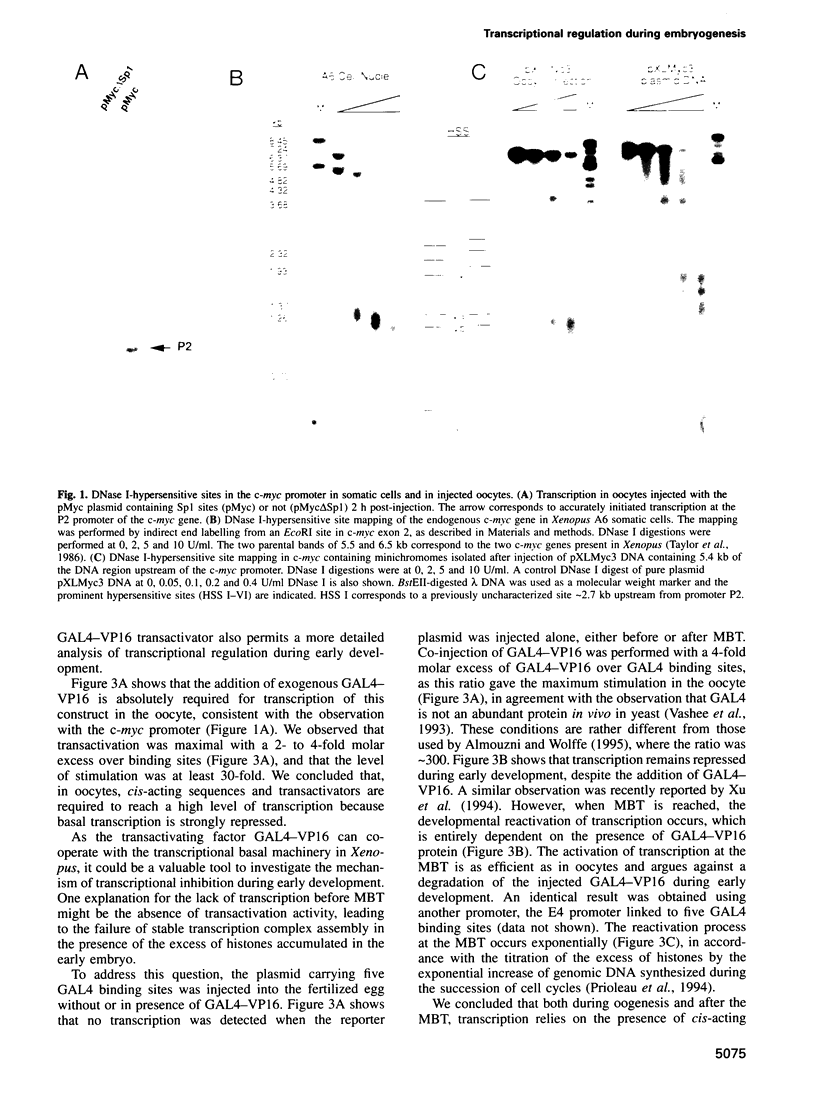
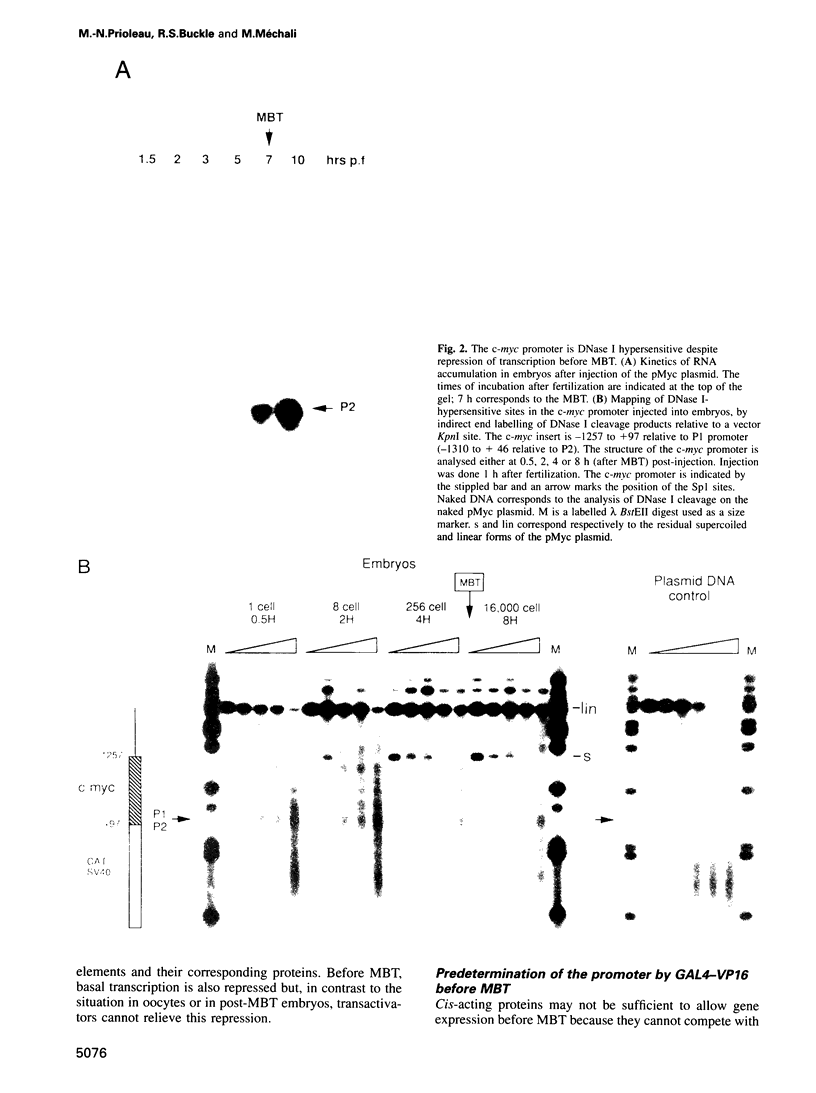
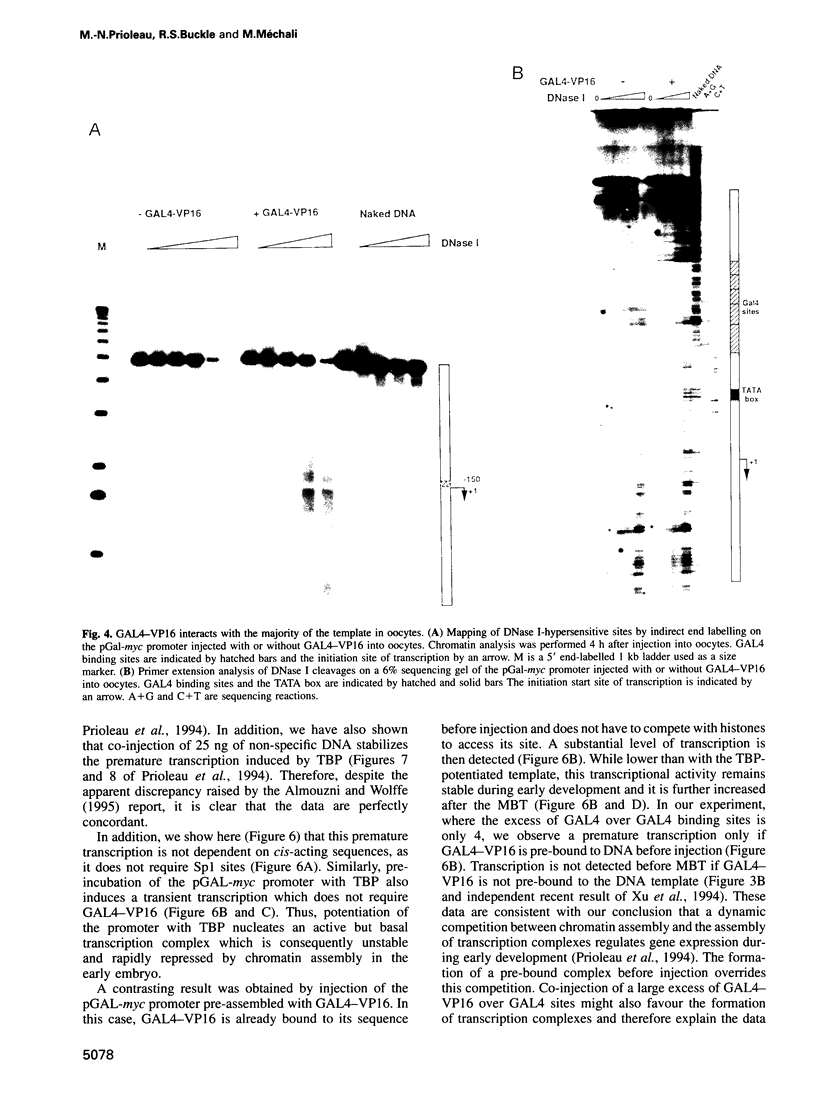
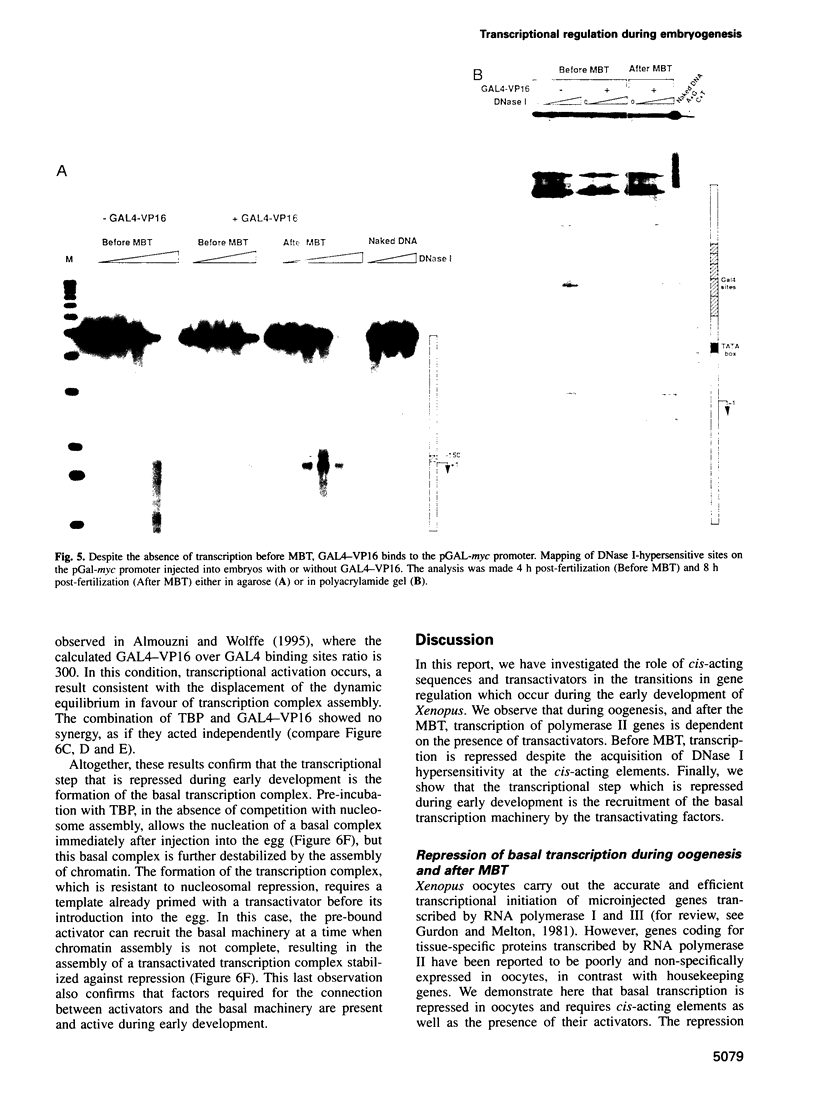
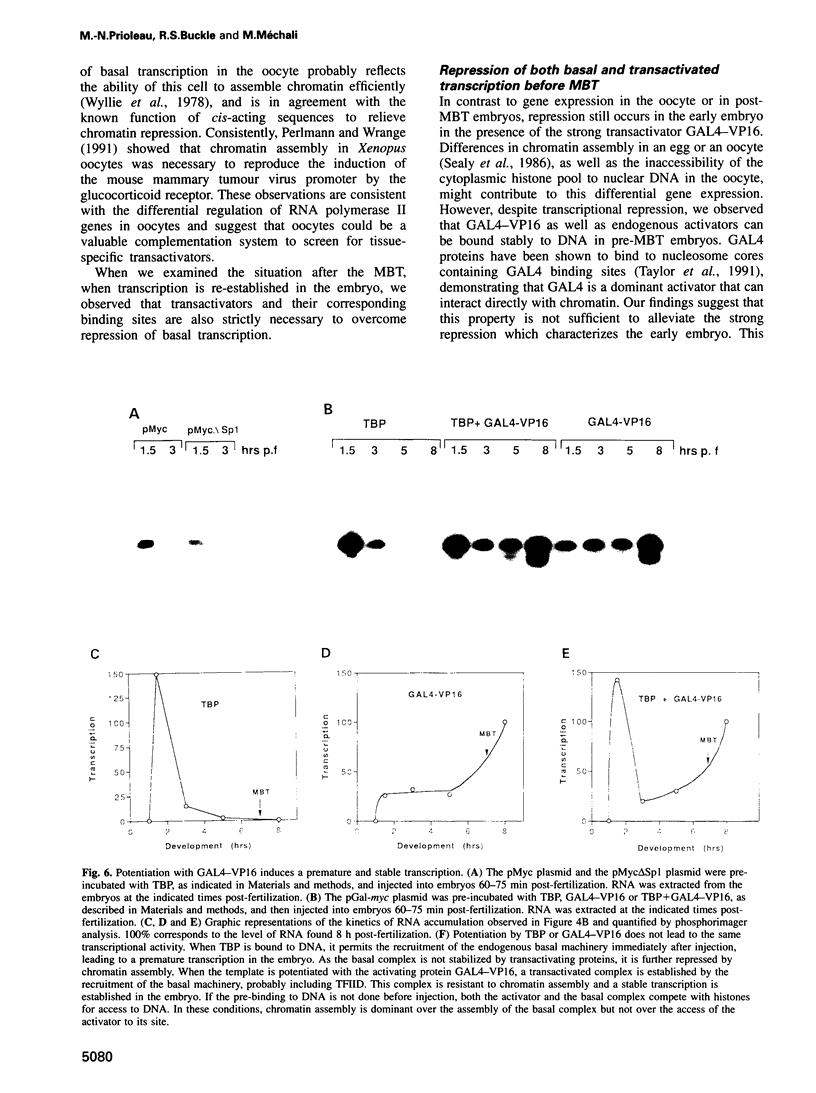
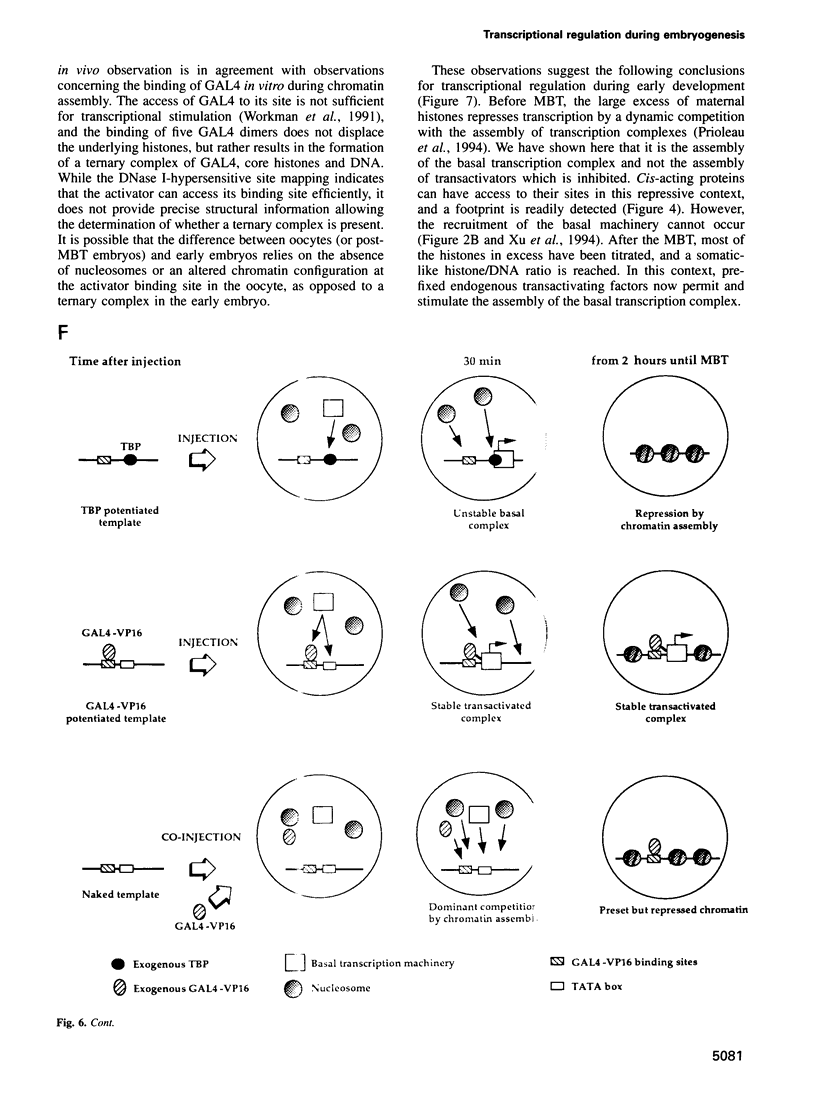
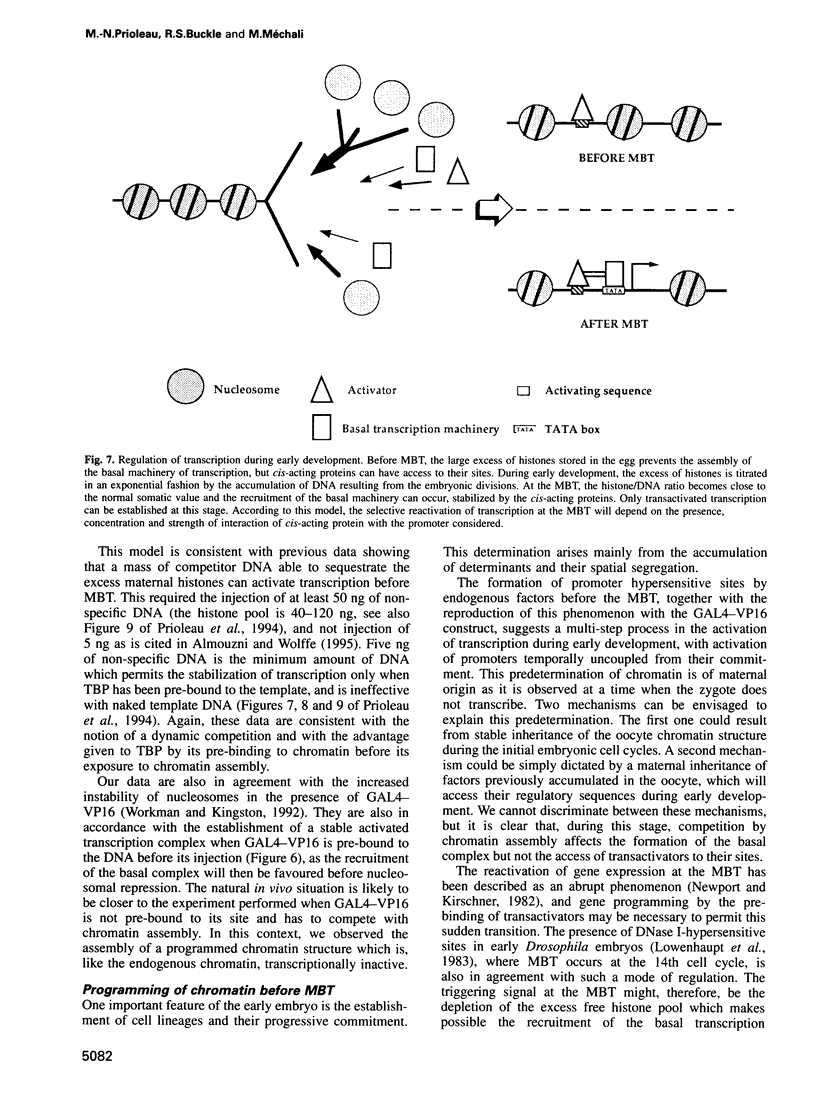
The determination of chromatin for transcription during early development as well as the requirement for trans-acting factors during this period has been analysed in Xenopus. Basal transcription is repressed both during oogenesis and after the mid-blastula transition (MBT), and transactivators are required to relieve this repression. In contrast, transactivators cannot overcome the generalized transcriptional repression which occurs in embryos before MBT. However, they do bind to promoters leading to a repressed but preset chromatin structure. Experiments involving the pre-binding of TATA binding protein (TBP) or of the strong transactivator GAL4-VP16 further show that there is no limiting factor before the MBT, and that it is the recruitment and stabilization of the basal transcription machinery and not of transactivators which is repressed during early development. This multi-step process in gene activation, with activation of promoters temporally uncoupled from their commitment, may be of importance in the regulation of early embryonic events by providing molecular signposts for future determinations.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson E. D., Woodland H. R. Histone synthesis in early amphibian development: histone and DNA syntheses are not co-ordinated. J Mol Biol. 1974 Sep 15;88(2):263–285. doi: 10.1016/0022-2836(74)90481-1. [DOI] [PubMed] [Google Scholar]
- Almouzni G., Wolffe A. P. Constraints on transcriptional activator function contribute to transcriptional quiescence during early Xenopus embryogenesis. EMBO J. 1995 Apr 18;14(8):1752–1765. doi: 10.1002/j.1460-2075.1995.tb07164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almouzni G., Wolffe A. P. Replication-coupled chromatin assembly is required for the repression of basal transcription in vivo. Genes Dev. 1993 Oct;7(10):2033–2047. doi: 10.1101/gad.7.10.2033. [DOI] [PubMed] [Google Scholar]
- Bendig M. M., Williams J. G. Fidelity of transcription of Xenopus laevis globin genes injected into Xenopus laevis oocytes and unfertilized eggs. Mol Cell Biol. 1984 Oct;4(10):2109–2119. doi: 10.1128/mcb.4.10.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley D. L., Groudine M. Sequence requirements for premature termination of transcription in the human c-myc gene. Cell. 1988 Apr 22;53(2):245–256. doi: 10.1016/0092-8674(88)90386-8. [DOI] [PubMed] [Google Scholar]
- Bienz M. A CCAAT box confers cell-type-specific regulation on the Xenopus hsp70 gene in oocytes. Cell. 1986 Sep 26;46(7):1037–1042. doi: 10.1016/0092-8674(86)90703-8. [DOI] [PubMed] [Google Scholar]
- Burch J. B., Weintraub H. Temporal order of chromatin structural changes associated with activation of the major chicken vitellogenin gene. Cell. 1983 May;33(1):65–76. doi: 10.1016/0092-8674(83)90335-5. [DOI] [PubMed] [Google Scholar]
- Burton N., Cavallini B., Kanno M., Moncollin V., Egly J. M. Expression in Escherichia coli: purification and properties of the yeast general transcription factor TFIID. Protein Expr Purif. 1991 Oct-Dec;2(5-6):432–441. doi: 10.1016/1046-5928(91)90105-r. [DOI] [PubMed] [Google Scholar]
- Chasman D. I., Leatherwood J., Carey M., Ptashne M., Kornberg R. D. Activation of yeast polymerase II transcription by herpesvirus VP16 and GAL4 derivatives in vitro. Mol Cell Biol. 1989 Nov;9(11):4746–4749. doi: 10.1128/mcb.9.11.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson P. J., Rabbitts T. H. Chromatin structure around the c-myc gene in Burkitt lymphomas with upstream and downstream translocation points. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1984–1988. doi: 10.1073/pnas.82.7.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin L. D., Maxson R. E., Jr The synthesis of authentic sea urchin transcriptional and translational products by sea urchin histone genes injected into Xenopus laevis oocytes. Dev Biol. 1980 Mar;75(1):13–25. doi: 10.1016/0012-1606(80)90140-2. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin as an essential part of the transcriptional mechanism. Nature. 1992 Jan 16;355(6357):219–224. doi: 10.1038/355219a0. [DOI] [PubMed] [Google Scholar]
- Gimlich R. L., Gerhart J. C. Early cellular interactions promote embryonic axis formation in Xenopus laevis. Dev Biol. 1984 Jul;104(1):117–130. doi: 10.1016/0012-1606(84)90042-3. [DOI] [PubMed] [Google Scholar]
- Green J., Brady J., Khoury G. 72-bp element contains a critical control region for SV40 late expression in Xenopus laevis oocytes. Virology. 1987 Aug;159(2):339–349. doi: 10.1016/0042-6822(87)90472-7. [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Birnstiel M. L. Identification of regulatory sequences in the prelude sequences of an H2A histone gene by the study of specific deletion mutants in vivo. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1432–1436. doi: 10.1073/pnas.77.3.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B., Melton D. A. Gene transfer in amphibian eggs and oocytes. Annu Rev Genet. 1981;15:189–218. doi: 10.1146/annurev.ge.15.120181.001201. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Mohun T. J., Fairman S., Brennan S. All components required for the eventual activation of muscle-specific actin genes are localized in the subequatorial region of an uncleaved amphibian egg. Proc Natl Acad Sci U S A. 1985 Jan;82(1):139–143. doi: 10.1073/pnas.82.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M. W., Roberts J. M., Eisenman R. N. Expression of the c-myc proto-oncogene during development of Xenopus laevis. Mol Cell Biol. 1986 Dec;6(12):4499–4508. doi: 10.1128/mcb.6.12.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D., Morris N. R. Assembly of SV40 chromatin in a cell-free system from Xenopus eggs. Cell. 1977 Feb;10(2):237–243. doi: 10.1016/0092-8674(77)90217-3. [DOI] [PubMed] [Google Scholar]
- Lin Y. S., Carey M. F., Ptashne M., Green M. R. GAL4 derivatives function alone and synergistically with mammalian activators in vitro. Cell. 1988 Aug 26;54(5):659–664. doi: 10.1016/s0092-8674(88)80010-2. [DOI] [PubMed] [Google Scholar]
- Lowenhaupt K., Cartwright I. L., Keene M. A., Zimmerman J. L., Elgin S. C. Chromatin structure in pre- and postblastula embryos of Drosophila. Dev Biol. 1983 Sep;99(1):194–201. doi: 10.1016/0012-1606(83)90267-1. [DOI] [PubMed] [Google Scholar]
- Mango S. E., Schuler G. D., Steele M. E., Cole M. D. Germ line c-myc is not down-regulated by loss or exclusion of activating factors in myc-induced macrophage tumors. Mol Cell Biol. 1989 Aug;9(8):3482–3490. doi: 10.1128/mcb.9.8.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj I. W., Lienhard S., Jiricny J., De Robertis E. M. An enhancer-like sequence within the Xenopus U2 gene promoter facilitates the formation of stable transcription complexes. Nature. 1985 Jul 11;316(6024):163–167. doi: 10.1038/316163a0. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Gavis E. R. Expression of the herpes thymidine kinase gene in Xenopus laevis oocytes: an assay for the study of deletion mutants constructed in vitro. Nucleic Acids Res. 1980 Dec 20;8(24):5931–5948. doi: 10.1093/nar/8.24.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modak S. P., Principaud E., Spohr G. Regulation of Xenopus c-myc promoter activity in oocytes and embryos. Oncogene. 1993 Mar;8(3):645–654. [PubMed] [Google Scholar]
- Mohun T. J., Garrett N., Gurdon J. B. Upstream sequences required for tissue-specific activation of the cardiac actin gene in Xenopus laevis embryos. EMBO J. 1986 Dec 1;5(12):3185–3193. doi: 10.1002/j.1460-2075.1986.tb04628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport J., Kirschner M. A major developmental transition in early Xenopus embryos: II. Control of the onset of transcription. Cell. 1982 Oct;30(3):687–696. doi: 10.1016/0092-8674(82)90273-2. [DOI] [PubMed] [Google Scholar]
- Nishikura K. Sequences involved in accurate and efficient transcription of human c-myc genes microinjected into frog oocytes. Mol Cell Biol. 1986 Nov;6(11):4093–4098. doi: 10.1128/mcb.6.11.4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovsenek N., Heikkila J. J. DNA sequence-specific binding activity of the heat-shock transcription factor is heat-inducible before the midblastula transition of early Xenopus development. Development. 1990 Oct;110(2):427–433. doi: 10.1242/dev.110.2.427. [DOI] [PubMed] [Google Scholar]
- Ovsenek N., Karn H. A., Heikkila J. J. Analysis of CCAAT box transcription factor binding activity during early Xenopus laevis embryogenesis. Dev Biol. 1991 Jun;145(2):323–327. doi: 10.1016/0012-1606(91)90130-u. [DOI] [PubMed] [Google Scholar]
- Paranjape S. M., Kamakaka R. T., Kadonaga J. T. Role of chromatin structure in the regulation of transcription by RNA polymerase II. Annu Rev Biochem. 1994;63:265–297. doi: 10.1146/annurev.bi.63.070194.001405. [DOI] [PubMed] [Google Scholar]
- Perlmann T., Wrange O. Inhibition of chromatin assembly in Xenopus oocytes correlates with derepression of the mouse mammary tumor virus promoter. Mol Cell Biol. 1991 Oct;11(10):5259–5265. doi: 10.1128/mcb.11.10.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prioleau M. N., Huet J., Sentenac A., Méchali M. Competition between chromatin and transcription complex assembly regulates gene expression during early development. Cell. 1994 May 6;77(3):439–449. doi: 10.1016/0092-8674(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Rougvie A. E., Lis J. T. The RNA polymerase II molecule at the 5' end of the uninduced hsp70 gene of D. melanogaster is transcriptionally engaged. Cell. 1988 Sep 9;54(6):795–804. doi: 10.1016/s0092-8674(88)91087-2. [DOI] [PubMed] [Google Scholar]
- Rozek D., Pfeifer G. P. In vivo protein-DNA interactions at the c-jun promoter: preformed complexes mediate the UV response. Mol Cell Biol. 1993 Sep;13(9):5490–5499. doi: 10.1128/mcb.13.9.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealy L., Cotten M., Chalkley R. Xenopus nucleoplasmin: egg vs. oocyte. Biochemistry. 1986 May 20;25(10):3064–3072. doi: 10.1021/bi00358a049. [DOI] [PubMed] [Google Scholar]
- Taylor I. C., Workman J. L., Schuetz T. J., Kingston R. E. Facilitated binding of GAL4 and heat shock factor to nucleosomal templates: differential function of DNA-binding domains. Genes Dev. 1991 Jul;5(7):1285–1298. doi: 10.1101/gad.5.7.1285. [DOI] [PubMed] [Google Scholar]
- Taylor M. V., Gusse M., Evan G. I., Dathan N., Mechali M. Xenopus myc proto-oncogene during development: expression as a stable maternal mRNA uncoupled from cell division. EMBO J. 1986 Dec 20;5(13):3563–3570. doi: 10.1002/j.1460-2075.1986.tb04683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. H., Elgin S. C. Protein/DNA architecture of the DNase I hypersensitive region of the Drosophila hsp26 promoter. EMBO J. 1988 Jul;7(7):2191–2201. doi: 10.1002/j.1460-2075.1988.tb03058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiyama T., Becker P. B., Wu C. ATP-dependent nucleosome disruption at a heat-shock promoter mediated by binding of GAGA transcription factor. Nature. 1994 Feb 10;367(6463):525–532. doi: 10.1038/367525a0. [DOI] [PubMed] [Google Scholar]
- Vashee S., Xu H., Johnston S. A., Kodadek T. How do "Zn2 cys6" proteins distinguish between similar upstream activation sites? Comparison of the DNA-binding specificity of the GAL4 protein in vitro and in vivo. J Biol Chem. 1993 Nov 25;268(33):24699–24706. [PubMed] [Google Scholar]
- Vriz S., Taylor M., Méchali M. Differential expression of two Xenopus c-myc proto-oncogenes during development. EMBO J. 1989 Dec 20;8(13):4091–4097. doi: 10.1002/j.1460-2075.1989.tb08593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallrath L. L., Lu Q., Granok H., Elgin S. C. Architectural variations of inducible eukaryotic promoters: preset and remodeling chromatin structures. Bioessays. 1994 Mar;16(3):165–170. doi: 10.1002/bies.950160306. [DOI] [PubMed] [Google Scholar]
- Wolffe A. P. Nucleosome positioning and modification: chromatin structures that potentiate transcription. Trends Biochem Sci. 1994 Jun;19(6):240–244. doi: 10.1016/0968-0004(94)90148-1. [DOI] [PubMed] [Google Scholar]
- Woodland H. R., Adamson E. D. The synthesis and storage of histones during the oogenesis of Xenopus laevis. Dev Biol. 1977 May;57(1):118–135. doi: 10.1016/0012-1606(77)90359-1. [DOI] [PubMed] [Google Scholar]
- Workman J. L., Buchman A. R. Multiple functions of nucleosomes and regulatory factors in transcription. Trends Biochem Sci. 1993 Mar;18(3):90–95. doi: 10.1016/0968-0004(93)90160-o. [DOI] [PubMed] [Google Scholar]
- Workman J. L., Kingston R. E. Nucleosome core displacement in vitro via a metastable transcription factor-nucleosome complex. Science. 1992 Dec 11;258(5089):1780–1784. doi: 10.1126/science.1465613. [DOI] [PubMed] [Google Scholar]
- Workman J. L., Roeder R. G. Binding of transcription factor TFIID to the major late promoter during in vitro nucleosome assembly potentiates subsequent initiation by RNA polymerase II. Cell. 1987 Nov 20;51(4):613–622. doi: 10.1016/0092-8674(87)90130-9. [DOI] [PubMed] [Google Scholar]
- Workman J. L., Taylor I. C., Kingston R. E. Activation domains of stably bound GAL4 derivatives alleviate repression of promoters by nucleosomes. Cell. 1991 Feb 8;64(3):533–544. doi: 10.1016/0092-8674(91)90237-s. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Laskey R. A., Finch J., Gurdon J. B. Selective DNA conservation and chromatin assembly after injection of SV40 DNA into Xenopus oocytes. Dev Biol. 1978 May;64(1):178–188. doi: 10.1016/0012-1606(78)90069-6. [DOI] [PubMed] [Google Scholar]
- Xu L., Rungger D., Georgiev O., Seipel K., Schaffner W. Different potential of cellular and viral activators of transcription revealed in oocytes and early embryos of Xenopus laevis. Biol Chem Hoppe Seyler. 1994 Feb;375(2):105–112. doi: 10.1515/bchm3.1994.375.2.105. [DOI] [PubMed] [Google Scholar]