Abstract
Introduction
Giant herniated thoracic discs (GHTD) remain a surgical challenge. When combined with calcification, these discs require altered surgical strategies and have only been infrequently described. Our objective was to describe our surgical approaches in the management of calcified GHTD.
Methods
This was a retrospective cohort study of all patients with calcified GHTD operated between 2004 and 2012. Data were collected from review of patients’ notes and radiographs and included basic demographic and radiological data, clinical presentation and outcome, operative procedure and complications.
Results
During the study period, there were 13 patients with calcified GHTD, including 6 males and 7 females (mean age 55 years, range 31–83 years). The average canal encroachment was 62 % (range 40–90 %); mean follow-up 37 months (12–98). All patients were treated with anterior thoracotomy, varying degrees of vertebral resection, removal of calcified disc and with or without reconstruction. The average time for surgery was 344 min (range 212–601 min) and estimated blood loss 1,230 ml (range 350–3,000 ml). Post-operatively, 8 patients improved by 1 Frankel grade (62 %), 2 improved by 2 grades (15 %) and 3 did not change their grade (23 %). The complication rate was 4/13 (31 %; 3 patients with durotomies (2 incidental, 1 intentional) and 1 with recurrence).
Discussion
Calcified GHTD remain a surgical challenge. Anterior decompression through a thoracotomy approach, and varying degrees of vertebral resection with or without reconstruction allowed us to safely remove the calcified fragment. All patients remained the same (23 %) or improved by at least 1 grade (77 %) neurologically, without radiographic failure at final follow-up.
Keywords: Giant calcified thoracic disc herniation, Surgical strategies, Complications, Mini-open thoracotomy, Vertebral resection
Introduction
Symptomatic herniated thoracic discs (HTDs) account for only 0.15–1.8 % of all intervertebral disc abnormalities treated surgically [1–3]. Hott et al. [4] defined giant herniated thoracic discs as those “occupying more than 40 % of the spinal canal based on pre-operative computed tomography (CT) myelography, magnetic resonance imaging (MRI) or both”. This subgroup displayed unique clinical presentation, surgical considerations and outcome as compared to small and medium size HTDs [4]. Until recently, there were no articles on this complex problem [4–6]. The surgical difficulty is greatly affected by the large volume and calcified nature of the herniated disc. Given that these patients tend to present with myelopathy with an already compromised spinal cord, absolute care in surgical planning and procedure is paramount [6].
Several approaches have been reported in the literature to treat thoracic disc herniations. There is no gold standard approach and each technique has unique advantages and disadvantages [7, 8]. These approaches include transpedicular, transfacet pedicle-sparing approaches, costotransversectomy, transthoracic transpleural approach, thoracoscopic approach [9–14]. Our objective was to review the outcomes of our patients and describe our surgical approach and management strategy in dealing with patients with a calcified giant thoracic herniated disc.
Materials and methods
We performed a retrospective cohort study of all patients operated for giant calcified thoracic disc excision between January 2004 and June 2012 in our academic tertiary referral spinal unit. Patients were identified from our electronic operative records database. Data were collected from review of patients’ notes and radiographs and included basic demographic and radiological data, clinical presentation and outcome, operative procedure and complications. All patients had pre-operative MRI which was evaluated to measure the amount of canal encroachment. The pre-operative CT scans were assessed to evaluate the calcification which was also confirmed as intra-operative finding during the operation (Fig. 1). The operative records were reviewed in detail to identify the duration and type of surgery, estimated blood loss, use of neurological monitoring and any intra-operative complications.
Fig. 1.
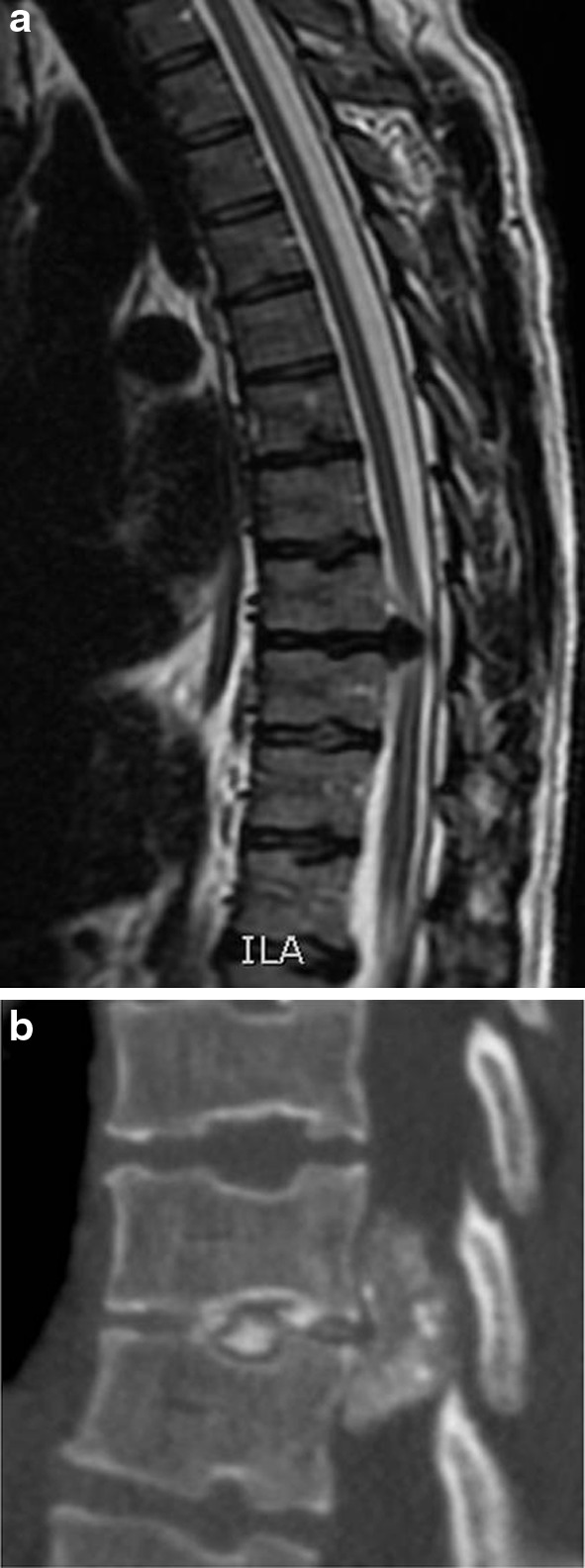
a T2-weighted sagittal MRI scan showing a giant thoracic disc and b CT scan confirming calcification
Outcome scores
Neurology was assessed by Frankel grading system and the Oswestry Disability Index (ODI) used to measure function. All scores were recorded immediately pre-operatively and post-operatively at regular intervals (with a minimum of 1 year follow-up).
Surgical procedure
A left or right sided approach for thoracotomy was used based on the location of the disc herniation, disc level and surgeon choice. Generally, the left sided approach avoids the risk of injury to the thoracic duct, inferior vena cava, and obstruction by the liver. Single lung ventilation was used only in patients with adequate pulmonary function and more cephalad disc levels. Intra-operative localisation of thoracic spine levels can be difficult due to anatomical constraints, such as scapular shadow, patient’s size and poor bone quality. However, it can be facilitated by percutaneous insertion of a ‘K’ wire into a pedicle as a marker in prone position [15]. This is our preferred method for identification of the correct thoracic level (Fig. 2).
Fig. 2.
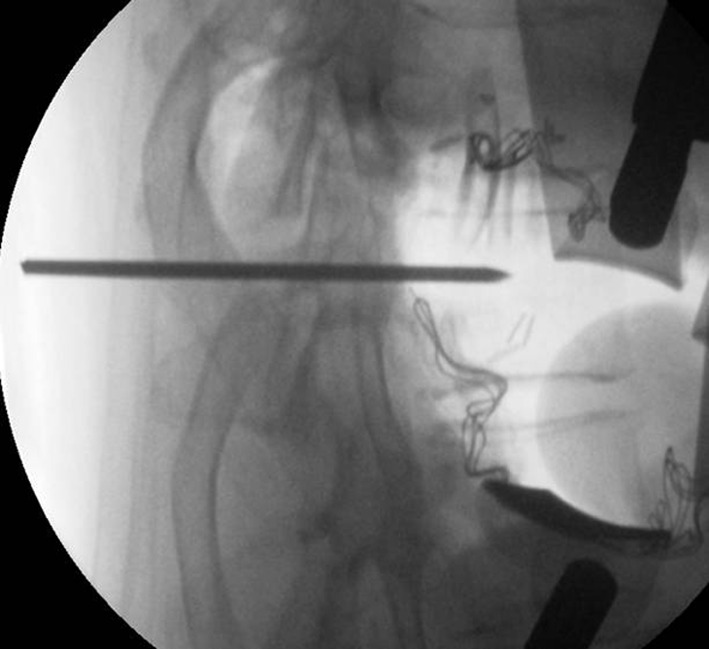
Insertion of pedicle K wire marker in prone position before the surgery
After positioning the patient in a true lateral position (we employ bean bags and side supports with straps), skin markings were made by confirming the level under fluoroscopy and based on the previously positioned K wire. An approximate 10 cm incision was made (2/3 anterior to the centre point of the disc space and 1/3 posterior) extending as far back as the angle of the rib.
The latissimus dorsi and serratus anterior muscles were split and periosteum was dissected off the rib with monopolar diathermy, taking care to preserve the neurovascular bundle. The rib was dissected free from the pleura and the rib resected. A trans or retropleural approach can be adopted to the spine. With transpleural (which we more commonly use), the parietal pleura was split and the rib cage retracted with a Finochetto retractor placed in cephalo-caudad direction. The spine is now exposed and the pleura was split longitudinally over the targeted disc and adjacent vertebrae, and a marker placed into the disc. After radiographic confirmation of the level, a malleable retractor was placed between the pleura and anterior border of the vertebral bodies adjacent to the target disc.
If necessary, the segmental vessels were ligated carefully and the corresponding rib head excised to identify the lateral wall of the pedicle. A high speed burr was then used under a surgical microscope to excise a 5–6 mm groove through the vertebral end plates on either side of the involved disc (Fig. 3). This groove was extended up to the vertebral cortex on the opposite side and until the posterior wall of the vertebral body was sufficiently thinned out. Curettes and Kerrison rongeurs were then used to remove this posterior wall. The calcified disc is usually found adhered to the dura and needs to be mobilised gently into the working corridor created by burring the endplates (Fig. 4). Failing this a transdural approach for resection of the intradural fragment needs to be performed. Once adequate decompression was completed, haemostasis was ensured using bone wax, diathermy or Floseal (Baxter Deerfiled, IL, USA).
Fig. 3.
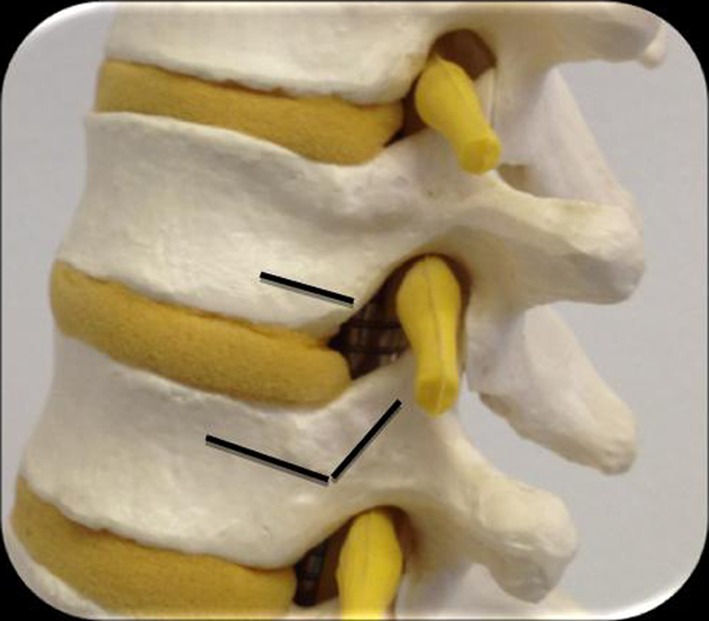
Planned resection of the end plates to provide a working corridor for resection of the calcified disc away from the dura
Fig. 4.
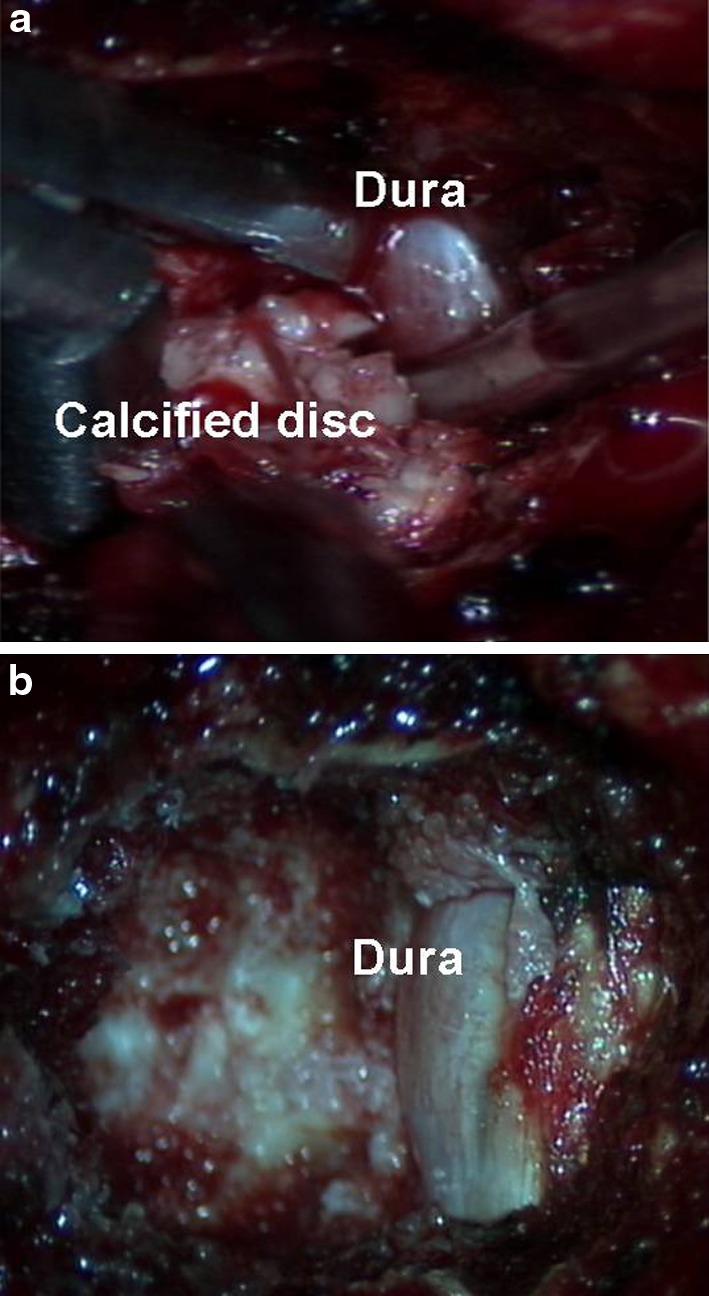
a, b Intra-operative pictures showing resection of the calcified disc into the working corridor and decompressed dura
In our experience (and knowledge from spinal oncological resections), if more than 50 % of the vertebral body is removed, it is necessary to support the anterior column with cage/instrumentation [16]. The pleura was sutured with vicryl, the tissue layers closed in sequence with the lung slowly reinflated during closure. A chest drain was used with underwater seal. The opinion with regards to use of intra-operative monitoring was largely surgeon dependent. A post-operative MRI scan was performed in all patients to confirm adequate decompression (Fig. 5).
Fig. 5.
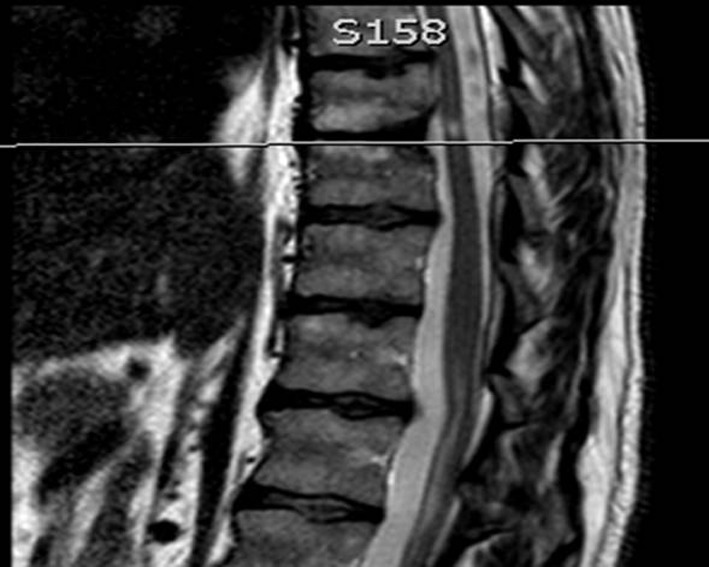
Post-operative MRI scan to confirm complete resection
Results
Between 2004 and 2012, a total number of 13 patients with calcified giant thoracic discs were treated (mean age 55 years, range 31–83 years). There were 6 male and 7 female patients. As described by Hott et al. [4], only discs occupying more than 40 % of the canal diameter were considered as giant and calcified and were included in this study. The average canal encroachment was 62 % (range 40–90 %). Clinical and radiological signs of myelopathy were seen in 12/13 (92 %) patients.
Thoracotomy was performed in all 13 patients (7 left and 6 right sided approaches). Intra-operative spinal cord monitoring utilised in six patients while the remaining seven did not have any spinal cord monitoring during the procedure. The average time for surgery was 344 min (range 212–601 min) and the estimated blood loss was 1,230 ml (range 350–3,000 ml). The post-operative length of stay was 11 days (range 5–28 days). Operative details are summarised in Table 1. Pre-operatively, the Frankel grades were C (n = 3) and D (n = 10). Post-operatively, eight patients improved by 1 grade (62 %), 2 improved by 2 grades (15 %) and three did not change their Frankel grades (23 %). There were no cases of neurological worsening. The mean follow-up was 37 months (12–98). There was 1 patient with post-operative transient intercostal neuralgia and no cases of pulmonary embolism or chest infection. At the final follow-up there were no cases of kyphosis or collapse at the discectomy site, clinically or radiologically.
Table 1.
Demographic and clinical details of individual patients included in the study
| Case | Age | Sex | Disc level | % Canal | Myelopathy | Type of surgery | Monitoring | Blood loss (ml) | Pre op Frankel |
Post op Frankel |
Pre op ODI |
Post op ODI |
Complication |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 58 | F | 9/10 | 50 | Y | Hemivertebrectomy and interbody fusion | Y | 1,500 | D | D | 68 | 24 | Nil |
| 2 | 35 | M | 5/6 | 40 | Y | Discectomy and interbody fusion (rib graft) | N | 1,000 | D | E | 56 | 22 | Nil |
| 3 | 58 | M | 8/9 | 50 | Y | Discectomy | N | 1,200 | C | E | 42 | 20 | Nil |
| 4 | 66 | F | 9/10 | 66 | N | Discectomy | N | 350 | C | D | 78 | 60 | Nil |
| 5 | 53 | M | 9/10 | 90 | Y | Discectomy and interbody fusion (rib graft) | N | 500 | D | E | 40 | 04 | Dural tear, CSF Hygroma |
| 6 | 68 | F | 7/8 | 70 | Y | Discectomy and interbody fusion | Y | 1,500 | D | E | 56 | 56 | Dural tear, Transient intercostal neuralgia |
| 7 | 50 | F | 11/1212/L1 | 80 | Y | Vertebrectomy and interbody fusion | Y | 1,200 | D | E | 66 | 50 | Nil |
| 8 | 45 | F | 9/10 | 70 | Y | Discectomy | N | 1,100 | D | E | 30 | 8 | Nil |
| 9 | 66 | M | 10/11 | 55 | Y | Discectomy and interbody fusion | N | 3,000 | C | E | 74 | 56 | Nil |
| 10 | 46 | M | 8/9 | 66 | Y | Hemi vertebrectomy and interbody fusion | Y | 2,500 | D | D | 72 | 56 | Dural tear, CSF Hygroma |
| 11 | 83 | F | 7/8 | 50 | Y | Discectomy | N | 350 | D | D | 56 | 30 | Recurrence |
| 12 | 31 | F | 11/12 | 50 | Y | Discectomy and interbody fusion | Y | 1,000 | D | E | 46 | 62 | Nil |
| 13 | 45 | M | 5/6 | 70 | Y | Discectomy | Y | 800 | D | E | 52 | 64 | Nil |
Complications were seen in 4 (31 %) patients—1 patient developed recurrence of disc herniation at the same level after initial resolution of the symptoms necessitating revision surgery from the opposite side; incidental durotomy occurred in 2 patients—one of these patients developed sudden worsening of headache and vision after a few days and was found to have a large but contained intra-pleural CSF hygroma. Another patient had a planned (intentional) durotomy for excision of the intradural disc fragment and this was repaired with Tachosil® and confirmed with a negative valsalva test intraoperatively. He represented to the unit after 2 weeks of discharge with headache and visual problems following an episode of severe coughing. Radiological investigations revealed a large intra-pleural CSF hygroma. The patient underwent a surgical repair of the dural defect. Both these patients with intra-pleural CSF hygromas developed an Abducens nerve palsy resulting in the visual symptoms suffered by the patients and have been reported by us previously [17].
Discussion
Giant calcified herniated thoracic discs remain a surgical challenge. Anterior decompression through a thoracotomy approach, and varying degrees of vertebral resection with or without reconstruction allowed us to safely remove the calcified fragment. All patients remained the same (23 %) or improved by at least one grade (77 %) neurologically, without radiographic failure at final follow-up.
Surgery for thoracic disc herniation can be challenging, more so if the disc is giant and calcified. Better clinical awareness and radiological advances have resulted in an increased diagnostic incidence of thoracic disc disease, which is commonly seen between 3rd and 6th decades [4, 18, 19]. The natural history of HTD suggests that it may remain asymptomatic for a long time [19], during which time it may enlarge but it rarely presents as acute myelopathy [19].
The thoracic spine and spinal cord have several unique features that make them vulnerable to anterior compression [20]. The thoracic spine is normally kyphotic and the spinal cord runs close to the posterior elements of the vertebral bodies [21]. In addition to its close proximity to anterior pathology, tethering of the spinal roots by the dentate ligaments also limits the mobility of the spinal cord to drift away from anterior impingement [22]. The spinal cord diameter to canal diameter ratio is higher in the thoracic spine than in the cervical or lumbar regions, leaving less room for the spinal cord in case of stenosis [20]. Finally, the thoracic spinal cord is vulnerable to ischemic injury due to the presence of an anatomic area of poor blood supply called the “watershed zone” [23]. In contrast with cervical and lumbar disc herniations, thoracic disc herniations are more frequently centrally located and known to calcify more often [24]. They may be adherent to and even erode through the dural sac over time. The development of clinical symptoms is attributed to local vascular compromise leading to spinal cord dysfunction [21]. Interestingly, in an animal study, Fujimaki et al. [25] reported that they had to interrupt at least five consecutive bilateral segmental arteries to show any spinal ischaemia. These unique characteristics of the thoracic spine and spinal cord are important in understanding the pathophysiology and the treatment approaches for symptomatic thoracic disc disease [21].
Both anterior and posterior surgical techniques have been described for the surgical management of thoracic disc herniations [4, 6, 26–28, 31]. The anterior approach provides good visualisation and surgical access but carries potential morbidity associated with thoracotomy. Thoracoscopic discectomy is associated with a steep learning curve and is best indicated for small lateral and uncalcified disc herniations only [32]. Posterior laminectomy for thoracic discectomy has been associated with unsatisfactory results since first reported by Logue [33]; however, Borm et al. [29] argue in a recent article that a tailored posterior approach based on the anatomic location and nature of the disc along with general health of the patient can yield satisfactory results.
Cho et al. [34] recently described a minimally invasive oblique paraspinal approach using 3-D navigation and tubular retractors with the aid of robotic holder. However, they do not recommend this approach for a sequestrated, calcified or hard disc herniation. Similarly, posterior transdural approach may offer an alternative surgical option for selected patients with thoracic paracentral soft discs [35]. This approach may also be deemed unsuitable for giant calcified herniated thoracic discs which may require extensive manipulation [35, 36].
Debnath et al. [30] have previously described a thoracotomy and hemivertebrectomy giving a better exposure and direct visualisation for decompression for thoracic disc herniations. This helps to develop a normal plane cephalad and caudad to the herniated disc and enables one to deal with two consecutive levels of herniated discs by one level vertebrectomy [30]. Hott et al. [4] recommend that an ideal procedure for a giant thoracic herniated disc is a two-level corpectomy and instrumented stabilisation through an open thoracotomy. They stated that the size and location of the disc is of paramount importance for the choice of the best surgical approach to minimise the morbidity. These reports however, are in our opinion, quite extensive procedures for excision of these calcified discs.
From our unit, Russo et al. [26, 37] reviewed our results with a mini thoracotomy approach for giant thoracic disc herniations—advantages of performing the procedure under direct vision and less potential peri-operative and post-operative morbidity were discussed. There was one post-operative pleural effusion in this small series of seven patients. Moran et al. [6] described a series of 17 patients with calcified giant thoracic disc herniations who underwent a mini-open thoracotomy and retropleural resection without the need for corpectomy or instrumentation. They had one post-operative PE and one patient with several co-morbidities who developed pneumonia (and later died). Others have reported thoracic access complications of up to 15 % with endoscopic [38] or Video-Assisted Thoracoscopic Surgery (VATS) [39]. We only had 1 patient who developed a thoracotomy related complication in our study of 13 patients—that of a post-operative intercostal neuralgia (transient). We accept that this small approach related complication rate may simply be due to the few number of patients in our study (rather than the use of a mini-open approach per se). However, it is worth noting that Uribe and colleagues [40] reviewed the literature on open (357 patients) versus minimally invasive approaches (466 patients) for thoracic disc herniations (not solely giant calcified) finding a complication rate of 36.7 % (0–182 %) and 28.4 % (0–92.3 %), respectively.
Even though the disc is large and well calcified on a pre-operative CT scan, it can be difficult to identify intra-operatively on fluoroscopic views and wrong level surgery for a calcified disc has previously been reported [5]. Pre-operative marking using a pedicle K wire remains our preferred method for level identification as described by Thambiraj and Quraishi [15]. Our surgical technique revolves around creating a working corridor by resection of the two contiguous endplates and a window for gentle excision of the calcified giant disc away from the spinal cord without any undue manipulation of the cord. This approach allows us to observe the fundamental principles of starting the decompression from normal tissue and ending with the abnormal calcified lesion.
The use of intra-operative monitoring is interesting and there remains no clear consensus in the literature. Those of us who do not use cord monitoring have the view that one is committed to decompressing the spinal cord and that spinal cord monitoring is not going to change the surgical objective. Those who use it, have a strategy in place should there be a deterioration in the neurophysiological function and this is not too dissimilar to worsening of neuromonitoring in scoliosis patients [41, 42]. For example, stopping the procedure in cases of significant drops in intra-operative monitoring, performing a wake-up test or even abandoning the anterior approach and performing a staged posterior decompression to allow the spinal cord to ‘float dorsally’ are strategies that we have employed in the past. In his review of treatment of thoracic disc, Vaccarro recommended that ‘the use intra-operative neurologic monitoring greatly reduces the risk of permanent neurologic injury and allows immediate intra-operative surgical correction if necessary’ [43]. In their recent large series of 60 patients from 5 institutions, Uribe and colleagues [40] used intra-operative neuromonitoring for all patients. However, we accept that the use of intra-operative monitoring is an area of debate; there is limited literature to provide guidance and this issue remains a contentious topic even within our own unit.
Myelopathy is seen in approximately 95 % of giant thoracic disc herniations in comparison to approximate 47 % of thoracic disc herniation [4, 38]. In our series, this was seen in 12/13 patients (92 %). Hott et al. [4] found 14/20 (70 %) of the GHTD to have an intra dural extension which was present in 2 (15 %) patients in our series. A lower incidence of intra dural extension of a thoracic disc herniation, ranging from 0 to 7 % has been noted by various authors previously [5, 13, 31]. However, overall a giant calcified herniated disc has increased chances of incorporation of the dura or intradural extension thus making its excision more difficult [4, 30, 32]. The calcified disc extrusion may result in damage to the ventral dura mater that may manifest as erosion, thinning and tearing of the ventral dura [5]. The rate of calcification of the herniated discs can vary between 26 and 90 % [4, 5, 13, 30, 31].
Barbanera et al. [5] reviewed subset of patients with a calcified giant thoracic herniated disc and found 71.4 % patients to show improvement of at least one grade on ASIA scale, 14.3 % were stabilised and 14.3 % worsened. Neurological worsening was seen in patient operated with pedunculo transversectomy which was performed as the disc level was lower thoracic (T10/11) [5]. The natural history of HTD suggests that majority occur in most caudal levels, with the highest frequency between T8 and T11 vertebra, located in central or centro-lateral position [18, 30, 44]. Barbanera et al. [5] recommend that even if the giant calcified herniated disc is located at the low thoracic levels an anterior approach should be considered. In the series by Hott et al. [4] reviewing giant thoracic herniated discs, 53 % patients improved neurologically, 42 % stabilised and 5 % worsened. They contrasted the outcomes in this patient group with better outcomes in smaller thoracic disc herniations in their series and concluded that the size of the disc herniation negatively affects the prognosis [4]. This was in variance to the finding by Le Roux et al. [13] and Stillerman and Weiss [31] in that there was no correlation between the size of the disc and the surgical outcome. The degree of pre-operative symptoms and presence of myelopathy, as usually seen in patients with a giant thoracic herniated disc may also affect the overall outcome [4, 5].
Conclusion
Giant calcified thoracic discs remain a surgical challenge. Anterior decompression through a thoracotomy approach and varying degrees of vertebral resection with or without reconstruction allowed us to safely remove the calcified fragment. All patients remained the same (33 %) or improved by at least 1 grade (77 %) neurologically, without radiographic failure at final follow-up. This approach provides adequate access and exposure for calcified giant thoracic herniated discs by creating a working corridor into which the disc fragments can be delivered away from the dura without any manipulation of the cord. It avoids (hemi) corpectomy and the need for reconstruction and instrumentation which we use selectively in cases of over 50 % vertebral body resection.
Conflict of interest
None.
References
- 1.Okada Y, Shimizu K, Ido K, Kotani S. Multiple thoracic disc herniations: case report and review of the literature. Spinal Cord. 1997;1997(35):183–186. doi: 10.1038/sj.sc.3100357. [DOI] [PubMed] [Google Scholar]
- 2.Stillerman CB, Chen TC, Couldwell WT, Zhang W, Weiss MH. Experience in the surgical management of 82 symptomatic herniated thoracic discs and review of the literature. J Neurosurg. 1998;88:623–633. doi: 10.3171/jns.1998.88.4.0623. [DOI] [PubMed] [Google Scholar]
- 3.Vollmer DG, Simmons NE. Transthoracic approaches to thoracic disc herniations. Neurosurg Focus. 2000;2000(9):e8. doi: 10.3171/foc.2000.9.4.8. [DOI] [PubMed] [Google Scholar]
- 4.Hott JS, Feiz-Erfan I, Kenny K, Dickman CA. Surgical management of giant herniated thoracic discs: analysis of 20 cases. J Neurosurg Spine. 2005;3(3):191–197. doi: 10.3171/spi.2005.3.3.0191. [DOI] [PubMed] [Google Scholar]
- 5.Barbanera A, Serchi E, Fiorenza V, Nina P, Andreoli A. Giant calcified thoracic herniated disc: considerations aiming a proper surgical strategy. J Neurosurg Sci. 2009;53(1):19–25. [PubMed] [Google Scholar]
- 6.Moran C, Ali Z, McEvoy L, Bolger C. Mini-open retropleural transthoracic approach for the treatment of giant thoracic disc herniation. Spine (Phila Pa 1976) 2012;37(17):E1079–E1084. doi: 10.1097/BRS.0b013e3182574657. [DOI] [PubMed] [Google Scholar]
- 7.Maiman DJ, Larson SJ, Luck E, et al. Lateral extracavitary approach to the spine for thoracic disc herniation: report of 23 cases. Neurosurgery. 1984;14:178–182. doi: 10.1227/00006123-198402000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Mulier S, Debois V. Thoracic disc herniations: transthoracic, lateral, or posterolateral approach? A review. Surg Neurol. 1998;49:606–608. doi: 10.1016/S0090-3019(98)00008-1. [DOI] [PubMed] [Google Scholar]
- 9.Bilsky MH. Transpedicular approach for thoracic disc herniations. Neurosurg Focus. 2000;9(4):e3. doi: 10.3171/foc.2000.9.4.4. [DOI] [PubMed] [Google Scholar]
- 10.Bransford R, Zhang F, Bellabarba C, Konodi M, Chapman JR. Early experience treating thoracic disc herniations using a modified transfacet pedicle-sparing decompression and fusion. J Neurosurg Spine. 2010;12(2):221–231. doi: 10.3171/2009.9.SPINE09476. [DOI] [PubMed] [Google Scholar]
- 11.Chi JH, Dhall SS, Kanter AS, Mummaneni PV. The Mini-Open transpedicular thoracic discectomy: surgical technique and assessment. Neurosurg Focus. 2008;25(2):E5. doi: 10.3171/FOC/2008/25/8/E5. [DOI] [PubMed] [Google Scholar]
- 12.Dinh DH, Tompkins J, Clark SB. Transcostovertebral approach for thoracic disc herniations. J Neurosurg. 2001;94(1 Suppl):38–44. doi: 10.3171/spi.2001.94.1.0038. [DOI] [PubMed] [Google Scholar]
- 13.Le Roux PD, Haglund MM, Harris AB. Thoracic disc disease: experience with the transpedicular approach in twenty consecutive patients. Neurosurgery. 1993;33:58–66. doi: 10.1227/00006123-199307000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Stillerman CB, Chen TC, Day JD, Couldwell WT, Weiss MH. The transfacet pedicle-sparing approach for thoracic disc removal: cadaveric morphometric analysis and preliminary clinical experience. J Neurosurg. 1995;83:971–976. doi: 10.3171/jns.1995.83.6.0971. [DOI] [PubMed] [Google Scholar]
- 15.Thambiraj S, Quraishi NA. Intra-operative localisation of thoracic spine level: a simple “‘K’-wire in pedicle” technique. Eur Spine J. 2012;21(Suppl 2):S221–S224. doi: 10.1007/s00586-012-2193-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis SJ, Kulkarni AG, Rampersaud YR, Jhaveri S, Quraishi N, Bacon SA, Magana SP. Posterior column reconstruction with autologous rib graft after en bloc tumor excision. Spine. 2012;37(4):346–350. doi: 10.1097/BRS.0b013e318220e89e. [DOI] [PubMed] [Google Scholar]
- 17.Khurana A, Brousil J, Russo A, Evans A, Quraishi NA, Boszczyk BM (2013) Intracranial hypotension with a sixth cranial nerve palsy subsequent to massive thoracic CSF hygroma: a rare complication of thoracic disc excision. Eur Spine J 22(9):2047–2054 [DOI] [PMC free article] [PubMed]
- 18.Cornips EM, Janssen ML, Beuls EA. Thoracic disc herniation and acute myelopathy: clinical presentation, neuroimaging findings, surgical considerations, and outcome. J Neurosurg (Spine) 2011;14(4):520–528. doi: 10.3171/2010.12.SPINE10273. [DOI] [PubMed] [Google Scholar]
- 19.Wood KB, Blair JM, Aepple DM, Schendel MJ, Garvey TA, Gundry CR, Heithoff KB. The natural history of asymptomatic thoracic disc herniations. Spine (Phila Pa 1976) 1997;22(5):525–529. doi: 10.1097/00007632-199703010-00011. [DOI] [PubMed] [Google Scholar]
- 20.Patterson RH, Jr, Arbit E. A surgical approach through the pedicle to protruded thoracic discs. J Neurosurg. 1978;48:768–772. doi: 10.3171/jns.1978.48.5.0768. [DOI] [PubMed] [Google Scholar]
- 21.Deviren V, Kuelling FA, Poulter G, Pekmezci M. Minimal invasive anterolateral transthoracic transpleural approach: a novel technique for thoracic disc herniation. A review of the literature, description of a new surgical technique and experience with first 12 consecutive patients. J Spinal Disord Tech. 2011;24(5):E40–E48. doi: 10.1097/BSD.0b013e318220af6f. [DOI] [PubMed] [Google Scholar]
- 22.Kahn E. The role of dentate ligaments in spinal cord compression and the syndrome of lateral sclerosis. J Neurosurg. 1944;4:191–199. doi: 10.3171/jns.1947.4.3.0191. [DOI] [PubMed] [Google Scholar]
- 23.Dommisse GF. The blood supply of the spinal cord: a critical vascular zone in spinal surgery. JBJS Br. 1974;56:225–235. [PubMed] [Google Scholar]
- 24.Severi P, Ruelle A, Andrioli G. Multiple calcified thoracic disc herniations: a case report. Spine. 1992;17:449–451. doi: 10.1097/00007632-199204000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Fujimaki Y, Kawahara N, Tomita K, et al. How many ligations of bilateral segmental arteries cause ischemic spinal cord dysfunction?: an experimental study using a dog model. Spine. 2006;31:E781–E789. doi: 10.1097/01.brs.0000238717.51102.79. [DOI] [PubMed] [Google Scholar]
- 26.Russo A, Balamurali G, Nowicki R, Boszczyk BM. Anterior thoracic foraminotomy through mini-thoracotomy for the treatment of giant thoracic disc herniations. Eur Spine J. 2012;21(Suppl 2):S212–S220. doi: 10.1007/s00586-012-2263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McInerney J, Ball PA. The pathophysiology of thoracic disc disease. Neurosurg Focus. 2000;9(4):e1. doi: 10.3171/foc.2000.9.4.2. [DOI] [PubMed] [Google Scholar]
- 28.McCormick WE, Will SF, Benzel EC. Surgery for thoracic disc disease Complication avoidance: overview and management. Neurosurg Focus. 2000;9(4):e13. doi: 10.3171/foc.2000.9.4.13. [DOI] [PubMed] [Google Scholar]
- 29.Börm W, Bäzner U, König RW, Kretschmer T, Antoniadis G, Kandenwein J. Surgical treatment of thoracic disc herniations via tailored posterior approaches. Eur Spine J. 2011;20(10):1684–1690. doi: 10.1007/s00586-011-1821-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Debnath UK, McConnell JR, Sengupta DK, Mehdian SM, Webb JK. Results of hemivertebrectomy and fusion for symptomatic thoracic disc herniation. Eur Spine J. 2003;12(3):292–299. doi: 10.1007/s00586-002-0468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stillerman CB, Weiss MH. Management of thoracic disc disease. Clin Neurosurg. 1992;38:325–352. [PubMed] [Google Scholar]
- 32.Horowitz MB, Moossy JJ, Julian T, Ferson PF, Huneke K. Thoracic discectomy using video assisted thoracoscopy. Spine (Phila Pa 1976) 1994;19(9):1082–1086. doi: 10.1097/00007632-199405000-00017. [DOI] [PubMed] [Google Scholar]
- 33.Logue V. Thoracic intervertebral disc prolapse with spinal cord compression. J Neurol Neurosurg Psychiatry. 1952;15(4):227–241. doi: 10.1136/jnnp.15.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho JY, Lee SH, Jang SH, Lee HY. Oblique paraspinal approach for thoracic disc herniations using tubular retractor with robotic holder: a technical note. Eur Spine J. 2012;21(12):2620–2625. doi: 10.1007/s00586-012-2438-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moon SJ, Lee JK, Jang JW, Hur H, Lee JH, Kim SH. The transdural approach for thoracic disc herniations: a technical note. Eur Spine J. 2010;19(7):1206–1211. doi: 10.1007/s00586-010-1294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coppes MH, Bakker NA, Metzemaekers JD, Groen RJ. Posterior transdural discectomy: a new approach for the removal of a central thoracic disc herniation. Eur Spine J. 2012;21(4):623–628. doi: 10.1007/s00586-011-1990-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayer HM. Microsurgical anterior approach to T5–T10 (Mini-TTA) In: Mayer HM, editor. Minimally invasive spine surgery. Berlin: Springer; 2006. pp. 129–137. [Google Scholar]
- 38.McAfee PC, Regan JR, Zdeblick T, et al. The incidence of complications in endoscopic anterior thoracolumbar spinal reconstructive surgery: a prospective multicenter study comprising the first 100 consecutive cases. Spine. 1995;20:1624–1632. doi: 10.1097/00007632-199507150-00012. [DOI] [PubMed] [Google Scholar]
- 39.Anand N, Regan JJ. Video-assisted thoracoscopic surgery for thoracic disc disease: classification and outcome study of 100 consecutive cases with a 2-year minimum follow-up period. Spine (Phila Pa 1976) 2002;27:871–879. doi: 10.1097/00007632-200204150-00018. [DOI] [PubMed] [Google Scholar]
- 40.Uribe JS, Smith WS, Pimenta L, et al. Minimally invasive lateral approach for symptomatic thoracic disc herniation:initial multicenter clinical experience. J Neurosurg Spine. 2012;16:264–279. doi: 10.3171/2011.10.SPINE11291. [DOI] [PubMed] [Google Scholar]
- 41.Hammett TC, Boreham B, Quraishi NA, Mehdian SM. Intraoperative spinal cord monitoring during the surgical correction of scoliosis due to cerebral palsy and other neuromuscular disorders. Eur Spine J. 2013;22(Suppl 1):S38–S41. doi: 10.1007/s00586-012-2652-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quraishi NA, Lewis SJ, Kelleher MO, Sarjeant R, Rampersaud YR, Fehlings MG. Intraoperative multimodality monitoring in adult spinal deformity: analysis of a prospective series of one hundred two cases with independent evaluation. Spine (Phila Pa 1976) 2009;34(14):1504–1512. doi: 10.1097/BRS.0b013e3181a87b66. [DOI] [PubMed] [Google Scholar]
- 43.Vanichkachorn JS, Vaccaro AR. Thoracic disk disease: diagnosis and treatment. J Am Acad Orthop Surg. 2000;8:159–169. doi: 10.5435/00124635-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Brown CW, Deffer PA, Jr, Akmakjian J, Donaldson DH, Brugman JL. The natural history of thoracic disc herniation. Spine (Phila Pa 1976) 1992;17(6 Suppl):S97–S102. doi: 10.1097/00007632-199206001-00006. [DOI] [PubMed] [Google Scholar]


