Abstract
Long-term monitoring data show that hard coral cover on the Great Barrier Reef (GBR) has reduced by >70 % over the past century. Although authorities and many marine scientists were in denial for many years, it is now widely accepted that this reduction is largely attributable to the chronic state of eutrophication that exists throughout most of the GBR. Some reefs in the far northern GBR where the annual mean chlorophyll a (Chl a) is in the lower range of the proposed Eutrophication Threshold Concentration for Chl a (~0.2–0.3 mg m−3) show little or no evidence of degradation over the past century. However, the available evidence suggests that coral diseases and the crown-of-thorns starfish will proliferate in such waters and hence the mandated eutrophication Trigger values for Chl a (~0.4–0.45 mg m−3) will need to be decreased to ~0.2 mg m−3 for sustaining coral reef communities.
Keywords: Coral reefs, Eutrophication, Corallivores, Coral skeletal disease, Coral bleaching
Introduction
Results from long-term monitoring by the Australian Institute of Marine Science (AIMS) show that the abundance of hermatypic (hard reef-building) corals in the Great Barrier Reef (GBR) has reduced by ~51 % since 1985 and that two-thirds of that decline has occurred since 1998 (De’ath et al. 2012). Further analysis of the available data (see below) suggests that >70 % reduction in hermatypic coral cover has occurred over the past century. The principal proximate causes of the loss of hermatypic corals have been attributed to storm damage, coral bleaching events, widespread growth of corallivores (e.g., crown-of-thorns starfish, COTS), and coral skeletal diseases (CSDs). A number of CSDs have been identified in the GBR, e.g., Black Band Disease (BBD), Brown Band syndrome (BrB), White Syndrome (WS), Atramentous Necrosis (AN), Skeletal Eroding Band (SEB), Pigmented Spots on Porites (PS) (Willis et al. 2004; Boyett 2006; Haapkyla et al. 2011). It is now widely accepted that the lack of recovery of the reefs and the proliferation of COTS are largely attributable to eutrophication (GBRMPA 2010; Brodie and Waterhouse 2012). Also evidence is emerging that CSDs and coral bleaching events are also promoted by eutrophication (see below).
An increase in the fertility/productivity of the sediments and water column of the GBR lagoon in recent times should be of no surprise when one considers that the annual exports of nutrients (e.g., nitrogen, N and phosphorus, P) from most coastal catchments, including many in the remote Cape York region, have probably increased severalfold since European settlement (Bell and Elmetri 1995; Kroon et al. 2012). In addition, there is evidence that Trichodesmium and other N-fixing organisms introduce large loads of N into the system; the magnitude of which is estimated to be far higher now than in the past and similar to that introduced by the rivers (Bell and Elmetri 1995; Bell et al. 1999). However, it is noted that many scientists were in denial for many years that significant impacts of eutrophication were manifest throughout the GBR (e.g., Walker 1991; Kinsey 1991; Wachenfeld 1995; McCook et al. 1997) although the available data suggested that the problem was approaching a chronic situation several decades ago (e.g., Bell 1991, 1992; Bell and Gabric 1990, 1991; Bell and Elmetri 1995).
As noted by Risk (1999), the misunderstanding/undervaluing the importance of eutrophication by coral reef scientists has contributed significantly to the ineffective monitoring, evaluation, and remediation protocols/procedures now generally adopted in coral reef regions. Indeed such misunderstandings led to the adoption of inappropriate management practices by the Great Barrier Reef Marine Park Authority (GBRMPA) for many years (e.g., see Brodie and Waterhouse 2012). Also such misunderstandings led GBRMPA to fund the poorly designed ENCORE project (Larkum and Steven 1994). This study came to the conclusion that increased nutrient loads would not promote algal growth in a pristine coral reef system (Koop et al. 2001) even though all reefs used in the experiment exhibited extensive macroalgal cover by the end of ENCORE (Bell et al. 2007).
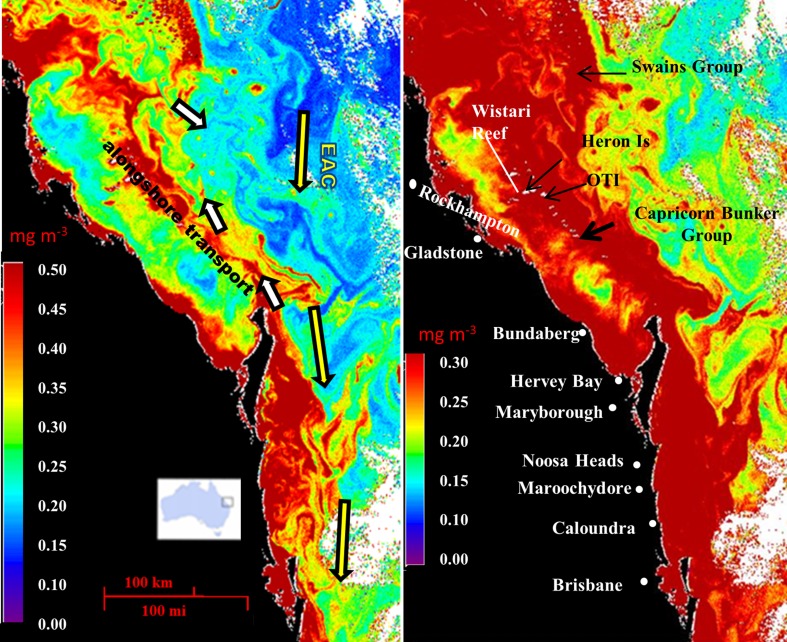
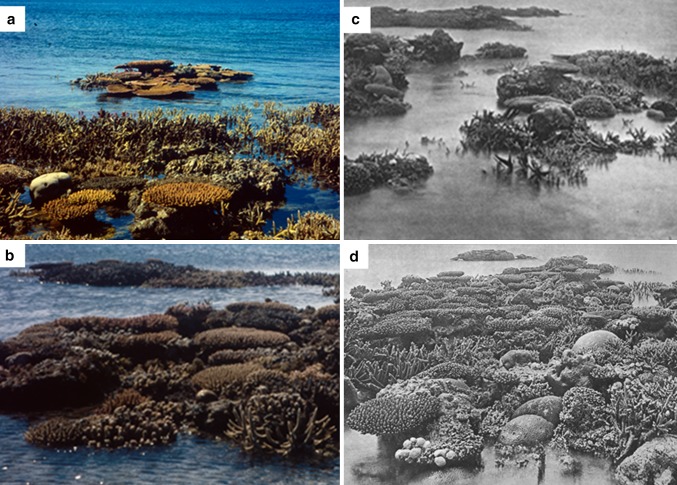
Despite the failure of ENCORE, GBRMPA has finally recognized the problems posed by eutrophication; GBRMPA has recently defined eutrophication Trigger values for various water quality parameters (GBRMPA 2010) and has implemented significant action on the management of nutrient loads associated with river runoff. However, insufficient attention has been paid to the management of point-source discharges and factors promoting N-fixation (Bell et al. 2007). Of particular concern are the large loads of P discharged from the waste-water treatment plants (WWTPs) located in coastal regions adjacent to the GBR and even from those located well to the south of the GBR region. For example ~300 tons of P per year are discharged from the Luggage Point WWTP in Brisbane (Wulff et al. 2011); most of this P load is readily bio-available phosphorus (i.e., soluble reactive phosphorus, P-PO4). This P-PO4 load is equivalent to about one-fourth of the total P-PO4 load in runoff emanating from all of the GBR coastal-river catchments (Kroon et al. 2012). The Luggage Point WWTP discharge and many others feed into the often Northerly flowing coastal plume which impacts directly onto the corals in the Capricorn Bunker Group (CBG) (located >70 km from the coast) and even the Swains Group (SG) (located 100–200 km from the coast) in the Southern GBR (see Fig. 1; Middleton et al. 1994; Bell et al. 2012). Many CBG and SG reefs and particularly those in less-well-flushed regions (e.g., see Fig. 2), are characterized by algal overgrowth, attacks by corallivores and CSDs. Extensive reef-degradation has occurred in this region over the past 50 years and it has been hypothesized that WWTP discharges have contributed significantly to this degradation (Bell et al. 2007).
Fig. 1.
SeaWiFS data (16-7-2000) showing variations of chlorophyll a (Chl a) concentrations in the Southern GBR and coastal plume that impinges on the GBR. The effects of the East Australian Current (EAC) and alongshore transport are shown
Fig. 2.
Coral and algal cover on coral mounds on Heron Is transect: a inner-lagoon; b mid-lagoon; c, d outer-lagoon; e outer Heron Is in Sykes Channel; f inner-lagoon Wistari Reef; g outer-lagoon Wistari Reef. Images captured from video taken by S. Bettridge January 2011
This paper reviews data pertaining to eutrophication and its effects on coral reefs and in particular, to its destructive effects on the GBR. The importance of factors such as grazing pressure, hydrodynamic/flushing conditions, and N-fixation on the effects of eutrophication and how eutrophication can promote the spread of CSDs and corallivores are also discussed. We also discuss the concept of threshold values for water quality parameters that best define the degree of eutrophication. We note that in the initial stages of eutrophication chlorophyll a (Chl a) is considered the best indicator of the degree of eutrophication of the water column; the reason being that the soluble inorganic nutrients are taken up rapidly by the algae and hence their concentrations will generally be quite low (Laws and Redalje 1979). A significant advantage in being able to use Chl a as the indicator of the degree of eutrophication is that it is relatively cheap and easy to measure and can be detected remotely, even by satellite.
Eutrophication and Coral Reefs
Unimpacted coral reef communities exhibit relatively high gross productivity even though the surrounding waters contain low concentrations of nutrients; the net import of N and P is usually low to negligible and, in the case of N is often negative due to N-fixation (e.g., see Webb et al. 1975; Bell et al. 2007). The maintenance of this high gross productivity therefore requires high rates of supply of recycled nutrients. The recycling of nutrients is achieved by the interaction of various symbiotic relationships within particular reef sectors and between sectors. The maintenance of the complex interactions between the various components of coral reef systems and hence ultimately their structure actually depends on the maintenance of the existing nutrient recycling pathways (Falkowski et al. 1993; Bell et al. 2007). The addition of nutrients to such a complex system will tend to breakdown the necessity for the natural symbiotic/recycling processes and in doing so will lead to a reduction in the stability of the system and leave it poised in a relatively unstable state of equilibrium and thus prone to sudden phase shifts.
Typical triggers for such phase shifts would be events that lead to the physical damage of the coral matrix, e.g., hurricanes/cyclones, bleaching events, and attacks by corallivores (e.g., COTS). If the system were not eutrophic the corals and the associated complex structure would most likely re-establish over time, i.e., the hermatypic corals in such a system could be considered robust (Bell and Elmetri 1995; Bell et al. 2007). However, if the system is eutrophic there will be increased competition for space by other organisms (e.g., algae and filter feeders such as soft corals, sponges, and bivalves) that will now flourish in the more fertile/productive waters. Under such a situation it is less likely that the hermatypic corals will re-establish to dominate the benthos as before. Hence in eutrophic conditions the hermatypic corals should be considered as being fragile and the system overall would exhibit a low resilience (Bell et al. 2007; Littler et al. 2009).
The impact of increased nutrient loads on hermatypic corals may be direct or indirect. Direct toxic effects will occur if the nutrient concentrations are high enough, however, these effects will not be discussed here. The main indirect effects result from increased growth of autotrophs (e.g., planktonic and benthic algae) and associated heterotrophs.
Effects of Increased Growth of Algae and Heterotrophs
The addition of nutrients to a pristine coral reef region will stimulate the growth of phytoplankton, benthic algae (including the symbiotic zooxanthellae), and heterotrophs (e.g., bacteria, viruses, and zooplankton including protozoa such as ciliates); this stimulated growth can cause significant changes in the coral reef community structure (e.g., see Smith et al. 1981; Tomascik and Sander 1985, 1987; Lapointe 1989, 1997; Bell 1992; Littler et al. 2009). For example, the phytoplankton compete with the symbiotic zooxanthellae for light and thus can interfere directly with coral growth. Also the stimulated growth of the zooxanthellae can lead to a reduction in calcification rates and promote coral bleaching (Marubini and Davies 1996; Nagelkerken 2006; Wooldridge and Done 2009). The benthic algae can act as sediment traps, compete directly with corals for space, inhibit settlement and survival of coral spats, weaken the coral structure by their boring into the coral matrix and alone, or together with a variety of heterotrophs, promote CSDs; further discussion on eutrophication as a promoter of CSDs is given below.
Algal growth also adds significantly to the production of dissolved organic matter (DOM) and particulate organic matter (POM). The sedimented POM will have a number of localized impacts, e.g., it will (i) promote the growth of other filter feeders which will compete directly with the hermatypic corals for space, (ii) through grazing and decay add to localized DOM and soluble nutrient loads which in turn will promote the localized growth of algae and heterotrophs, (iii) directly stress the corals by requiring them to increase their mucus production for cleansing purposes. Such increased mucus production together with the increased POM/DOM production will further stimulate the growth/virulence of various heterotrophs.
It is noted that phytoplankton growth in a polluted or eutrophic system is often accompanied by changes in the phytoplankton class structure (Greve and Parsons 1977; Ryther and Officer 1981). There is evidence that such changes have occurred in the GBR lagoon and that these changes have promoted the growth of jellyfish and corallivores such as the COTS (Bell and Elmetri 1995, 1998). Further discussion on eutrophication as a promoter of such growth is also given below.
Importance of the Hydrodynamic Regime
We hypothesize that corals growing in more sheltered regions will be more susceptible to the effects of eutrophication/sedimentation (including the proliferation of CSDs) and hence will require a better water quality (i.e., less eutrophic) than those in better-flushed regions. Results from a recent study in the GBR (Boyett 2006) support this hypothesis; laboratory results showed reduced water circulation encouraged the proliferation of BrB and field studies supported this finding. Also observations of the distribution of CSDs in the Red Sea (Antonius and Riegl 1997) showed that CSDs were more prevalent in less-well-flushed regions. It has been recorded that the building of causeways in the Palmyra Islands restricted flows and this led to the replacement of corals with algal communities dominated by Lyngbya sp. (Bell 1992). Our observations of reefs in the CBG namely, in the Heron Is. Lagoon (HIL) and Wistari Reef Lagoon (WRL), support this hypothesis (Fig. 2; Bell et al. 2012). The images in Fig. 2 show that:
coral cover is far less and algal cover is far greater in the less-well-flushed inner-lagoonal regions;
in the better-flushed outer regions the diversity of corals is far higher and in particular, plate-type corals are far more abundant.
These images also demonstrate that CSDs and overgrowth of hermatypic corals by filamentous and calcareous algae are significant in the outer better-flushed regions. The poor state of development of the outer HIL and WRL reefs suggests that this Southern GBR region as a whole is eutrophic. Indeed satellite images (e.g., see Fig. 1) and field-collected Chl a data (Fig. 3) support this finding.
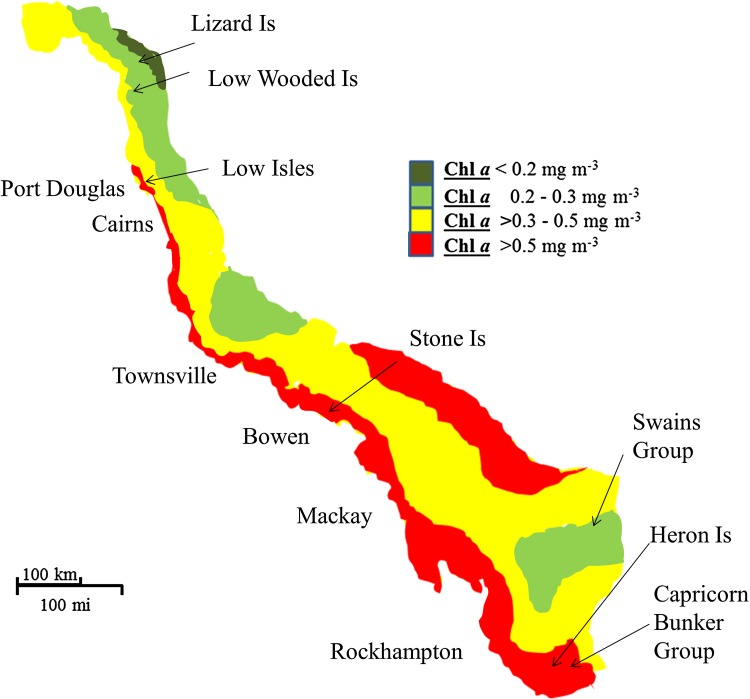
Fig. 3.
Summary of long-term GBR monitoring data (AIMS 2012); suggested region of chronic eutrophication is depicted by annual mean chlorophyll a (Chl a) values >0.2 mg m−3
Importance of Grazing Activity
The competition between hermatypic corals and other taxa is also influenced by the intensity of grazing fishes and invertebrates. For example, the relative-dominance model (RDM) (Littler et al. 2009) suggests that for eutrophic systems coralline algae will eventually dominate the hard substrate if highly grazed and that frondose macroalgae will eventually dominate if lowly grazed. Observations in the GBR lagoon at Low Isles and on the reefs in the Cairns region show that soft corals can also out-compete with hermatypic corals in a highly grazed eutrophic system (Endean and Stablum 1973; Bell and Elmetri 1995). It is well documented that there are far fewer herbivorous fish on the near-shore macroalgal-dominated reefs in the inner GBR (Williams and Hatcher 1983; McCook et al. 1997; Delean and De’ath 2008) and this observation led McCook et al. (1997) to adopt the simplistic “top-down” scenario, as was done by Hughes (1994) and Hughes et al. (1999) in Jamaica, for explaining the prolific algal growth there, i.e., the prolific growth of the algae results simply from the lack of herbivores and that nutrient enrichment did not play a role. However, the RDM suggests that both nutrient enrichment and reduced grazing pressure are important factors in promoting such prolific growth of benthic algae. It has generally been assumed that the low abundance of herbivores on the inner GBR reefs is a natural phenomenon but evidence from various studies suggests that this low abundance is a direct result of the changed habitat (Littler et al. 1983, 2009; Williams and Hatcher 1983; Bell and Elmetri 1998; Kerry 2011) which we propose has been promoted by the “bottom-up” effects of eutrophication. For example, increased growth of less palatable algae (e.g., Sargassum spp.) would reduce the production of the more palatable turf algae and hence would prove to be less attractive to grazing fish. Also the change from a coral-dominated habitat to a macroalgal-dominated habitat would be less supportive of the many reef fishes which require corals for food, protection, and recruitment (Kerry 2011). The recent underwater video data collected in the HIL support this direct “bottom-up” scenario on the effects of eutrophication on fish populations (see Fig. 2). Indeed these data show that coral cover and fish-population abundance/diversity both decrease dramatically as one moves from the better-flushed, more structurally complex, outer HIL to the inner-lagoon.
Importance of N-Fixation and Phosphorus
N-fixing bacteria and cyanobacteria (diazotrophs) fix a substantial load of “new” nitrogen in coral reef regions both in the water column and on a variety of benthic surfaces (e.g., see Webb et al. 1975; Bell et al. 1999). In the GBR region the “new” nitrogen input by Trichodesmium spp. is estimated to be far higher now than in the past and similar to that introduced by the rivers (Bell and Elmetri 1995; Bell et al. 1999). Satellite data show that GBR waters become progressively more productive as they flow through the reef complexes (Fig. 1); this increased production is attributed largely to benthic N-fixation (Bell et al. 2007).
Laboratory studies (Elmetri and Bell 2004; Fu et al. 2005) show that the N-fixation rates and growth rates of various diazotrophs common to the GBR are proportional to P-PO4 concentrations in the low concentration range (<0.3 μM). Such diazotrophs can also have high requirements for some trace compounds such as iron (Fe). Dissolved organics could also be important as they may increase the availability of trace elements, for example, through the chelation of Fe. Indeed various studies have shown that the addition of treated sewage or the chelating agent EDTA alone to coastal waters stimulates the growth of Lyngbya majuscula (Bell and Elmetri 2007). Thus relatively small increases in P-PO4 and/or trace components could lead to blooms of diazotrophs which in turn through decay/grazing mechanisms would supply bioavailable dissolved inorganic nitrogen (DIN ~ NH4-N + NOx-N) to the water column and thus lead to blooms of other taxa. Indeed there is evidence that elevated DIN concentrations in the GBR lagoon follow Trichodesmium blooms and that these increased concentrations lead to blooms of diatoms and dinoflagellates (Revelante and Gilmartin 1982; Bell et al. 1999). This scenario suggests that the situation in the GBR lagoon is potentially unstable in that large cyclical algal blooms, elevated productivity and thus widespread eutrophication could be maintained even if external N loads are reduced.
Eutrophication as a Promoter of CSDs
Results from various studies show that the prevalence of many CSDs positively correlates to effects of anthropogenic development and in particular, to eutrophication (Lapointe 1989; Bell 1992; Antonius and Riegl 1997; Bruckner et al. 1997; Goreau et al. 1998; Kuta and Richardson 2002; Aeby et al. 2011; Haapkyla et al. 2011). Also results from controlled laboratory and field-based studies show that addition of nutrients increases the rate of CSD progression and host tissue loss (Bruno et al. 2003; Voss and Richardson 2006). Voss and Richardson (2006) showed that relatively small increases in the background N and P concentrations to values around the proposed Nutrient Threshold Concentrations (NTCs; Bell 1992; Bell et al. 2007) increased the rate of expansion of BBD twofold. Kline et al. (2006) demonstrated that increases in DOM concentrations can increase the bacterial concentrations associated with coral surface mucopolysaccharide layer by an order of magnitude and this can lead to CSD-type conditions. Aeby et al. (2011) found that some CSDs positively correlated with the degree of anthropogenic development but also noted that Porites trematodiasis (PorTrm) was more prevalent in more pristine regions; they suggest that a more intact environment could be required for the spread of PorTrm. Haapkyla et al. (2011) found that the incidence of AN in a near-shore GBR reef is positively correlated to particulate organic carbon (and hence POM). The authors suggest that higher availability of nutrients and organic matter in runoff may increase pathogen virulence and hence facilitate disease outbreaks. It is noted that WWTP discharges not only promote eutrophication, they also provide a direct source of DOM/POM and pathogens that promote CSDs (Cervino et al. 2004; Nagelkerken 2006).
Eutrophication as a Promoter of Corallivores
Experimental evidence that demonstrated increased phytoplankton production due to terrestrial runoff, and in particular increased production of nanno (2–20 μm) phytoplankton, would promote the survival and development of COTS larvae was available decades ago but has only recently been accepted by the marine science community at large and by GBRMPA (e.g., see Brodie and Waterhouse 2012). For example, Lucas (1982) showed in laboratory studies with cultured nanno-phytoplankton that COTS larvae require an equivalent food source concentration corresponding to a Chl a value >0.4 mg m−3 to survive and develop. It is noted that such high concentrations of nanno-phytoplankton do not normally occur in GBR waters; the measured Chl a is normally dominated by pico (<2 μm) sized phytoplankton (Ayukai et al. 1997). However, there is evidence that the abundance of nanno-phytoplankton has increased severalfold in the GBR lagoon over the past century (Bell and Elmetri 1995) and that relatively high concentrations of nanno-phytoplankton do occur following river runoff events (Ayukai et al. 1997; Brodie et al. 2005). Also it is noted that other sources of food in addition to phytoplankton (e.g., POM and DOM; heterotrophs including bacteria, protozoans, and dinoflagellates) could be important food sources for COTS larvae (Lucas 1982). This observation suggests that the critical field-based Chl a value would be somewhat lower than 0.4 mg m−3. Indeed Olson (1987) showed that COTS could survive and develop unhindered at field-based Chl a levels ~0.25–0.28 mg m−3. More recent work (Fabricius et al. 2010) shows that survival and development of COTS larvae increases significantly above field-based Chl a values of 0.28 mg m−3. These results suggest the critical field-based annual mean concentration of Chl a for survival and growth of COTS larvae would be somewhat less than 0.3 mg m−3, i.e., towards the lower end of the proposed coral reef Eutrophication Threshold Concentration range for chlorophyll a (ETC-Chl a ~0.2–0.3 mg m−3; Bell and Elmetri 1995; Bell et al. 2007).
Eutrophication Threshold Model
Evaluations of time-series and spatial data from Kaneohe Bay (Hawaii) and Barbados were used to derive the Eutrophication Threshold Model (ETM) for coral reefs, i.e., Eutrophication Threshold Concentrations for Chl a and nutrients (Bell 1991, 1992). Initially an ETC-Chl a corresponding to an annual mean Chl a <0.5 mg m−3 was chosen (Bell 1992); the currently mandated Trigger value for Chl a (T-Chl a) ~0.4–0.45 mg m−3 (GBRMPA 2010) essentially agrees with this value. Further analysis of the Barbados data (Bell and Elmetri 1995; Bell et al. 2007) and application of the ETM to the demise of corals in the Florida Keys (Lapointe 1997; Lapointe et al. 2004) suggested that an even lower ETC-Chl a (~0.2–0.3 mg m−3) is applicable in regions that have a high proportion of coral species that are sensitive to settlement of POM (e.g., Acropora palmata and plate-type corals) and in particular to regions that are subject to a low flushing regime where settlement of POM and a build-up of DOM are promoted. Initially we defined a chronic state of eutrophication to exist in regions characterized by an annual mean Chl a >0.3 mg m−3 (Bell et al. 2012). However, the above discussed findings that COTS larval growth is promoted in the lower ETC-Chl a range 0.2–0.3 mg m−3 suggests that a chronic state would be better defined at the lower end of this range, i.e., >0.2 mg m−3. This value agrees with the ETC value suggested by data for the wider Caribbean (Lapointe et al. 2007; Lapointe and Mallin 2011).
The corresponding ETCs for soluble inorganic nutrient concentrations (annual mean), i.e., NTCs, are relatively low: the NTC for phosphorus (P-PO4), NTC-P, is 3–6 μg P L−1, i.e., 0.1–0.2 μM and that for DIN, NTC-N, is 14 μg N L−1, i.e., 1 μM (Bell 1992). The magnitudes of the proposed NTCs correspond to many reported values for the half-saturation constants (KS) for the Monod growth/N-fixation models for algae or saturation constants (KS) for the threshold/saturation algal growth model (Bell 1992; Bell et al. 2007). Thus a fundamental ecological property represented by the ETM is that the growth rates and N-fixation rates of various associated algae are significantly affected at concentrations around the NTCs. The relatively small magnitude of the NTCs, in comparison with the nutrient concentrations in wastewater discharges and even runoff, means that large nutrient discharges (e.g., rivers) can affect reefs over vast distances, and even very small discharges can affect nearby reefs (Bell 1992; Bell et al. 2007; Lapointe et al. 2007).
Evidence of Large-Scale Eutrophication in GBR
Previous applications of the ETM to the historical water quality data and ecological data of the GBR lagoon support the conclusion that the nutrient pool and hence productivity/fertility of the GBR lagoon had reached a critical level for the survival of coral reefs in most, if not all, near-shore regions some decades ago (Bell 1991, 1992; Bell and Gabric 1990, 1991; Bell and Elmetri 1995). The observed replacement of hermatypic corals with other benthos (e.g., algae, sponges, and soft corals) in these regions supports this conclusion. Further analysis of the available data (see below) suggests that many of the reefs in the mid-GBR lagoon and the outer-GBR lagoon have also been experiencing the negative impacts of eutrophication for some time.
Degraded Water Quality
Elevated Nutrient Concentrations
Water quality surveys during the 1970s and 1980s showed that elevated nutrient concentrations often corresponded to low salinity periods both in the inshore and offshore regions of the GBR demonstrating that riverine discharges are a probable cause of elevated nutrient concentrations (Bell 1991, 1992). For example, data collected off Townsville (Revelante and Gilmartin 1982) show a close correspondence between elevated P-PO4 in the inner to mid-GBR lagoon and low salinity. A comparison of these data with those collected during the 1928–1929 expedition to Low Isles suggests that the average P-PO4 values have more than doubled in the Central GBR in the intervening 50 years (Bell 1992). Also a comparison of these data with the suggested NTC-P range shows that the upper NTC-P value is exceeded at all stations (Bell 1992).
The historical data also demonstrate that DIN concentrations in the inner to mid-lagoon off Townsville often exceed the NTC-N value (Bell 1992). However, the elevated DIN values do not always correspond to low salinity periods which suggests that other DIN sources are important. For example, it has been noted that elevated DIN values occur following blooms of Trichodesmium spp. and that blooms of other phytoplankton taxa soon follow (Revelante and Gilmartin 1982; Bell 1992; Bell et al. 1999). Also there is evidence that wind resuspension of sediments is a significant source of DIN and hence could also lead to elevated DIN values and consequently to increased phytoplankton concentrations (Bell 1992). Indeed studies in the Northern GBR near to Low Isles have shown that there is a strong positive correlation between wind strength and diatom concentrations (Bell and Elmetri 1995).
Results from recent water quality monitoring surveys (Brodie et al. 2007, 2010) show that nutrient concentrations in riverine flood plumes in the GBR lagoon greatly exceed the suggested NTC-P and NTC-N values and the resultant Chl a values are 1–2 orders of magnitude higher than the suggested upper ETC-Chl a concentration. The results also demonstrate an inverse relation between salinity and P-PO4 and some DIN species demonstrating that the river flow is a probable source of readily bioavailable nutrients. However, it is noted that despite the large amount of funding that is now available for eutrophication related studies in the GBR there is no regular (say every 1–2 weeks) integrated water quality/ecological (e.g., phytoplankton–zooplankton/micro-heterotroph) cross-shelf monitoring program in place. Only two such studies have ever been conducted in the GBR: the 1928–1929 GBR Expedition study and that conducted by visiting scientists to AIMS (Ikeda et al. 1980; Revelante and Gilmartin 1982). Results from such studies not only provide for important ecological and water quality information on a seasonal basis but can also be used to evaluate important processes, e.g., the results can be used to (i) delineate the relative effects of river discharge, wind resuspension of sediments, and ocean upwellings on the input of nutrients and ultimately on algal blooms, (ii) determine the relative importance of N-fixing cyanobacteria as a source of N and the effect of this on the blooms of other algae (iii) provide information on the links between eutrophication and the proliferation of the COTS larvae, jellyfish, and CSD precursors.
Elevated Chl a Values
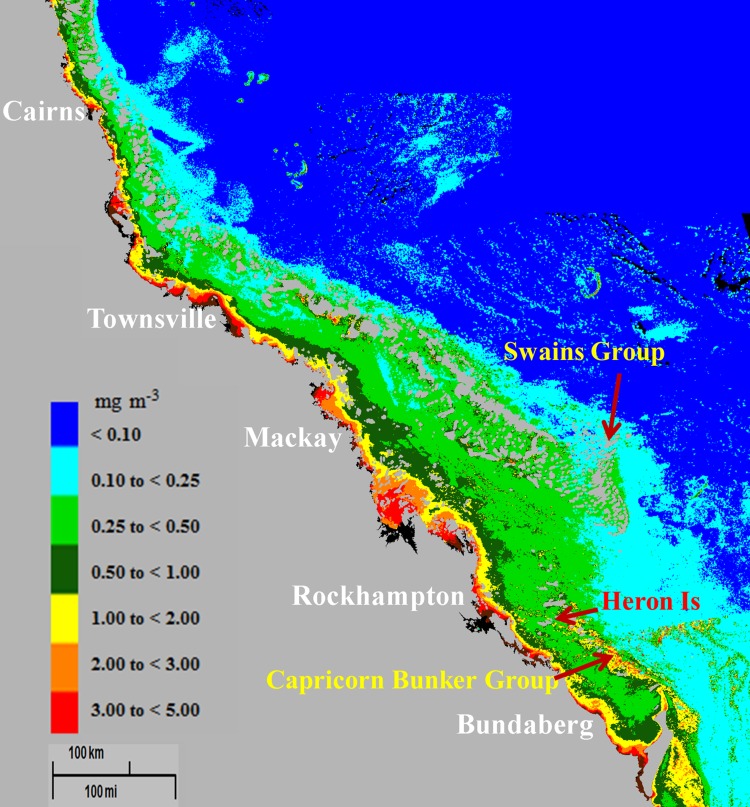
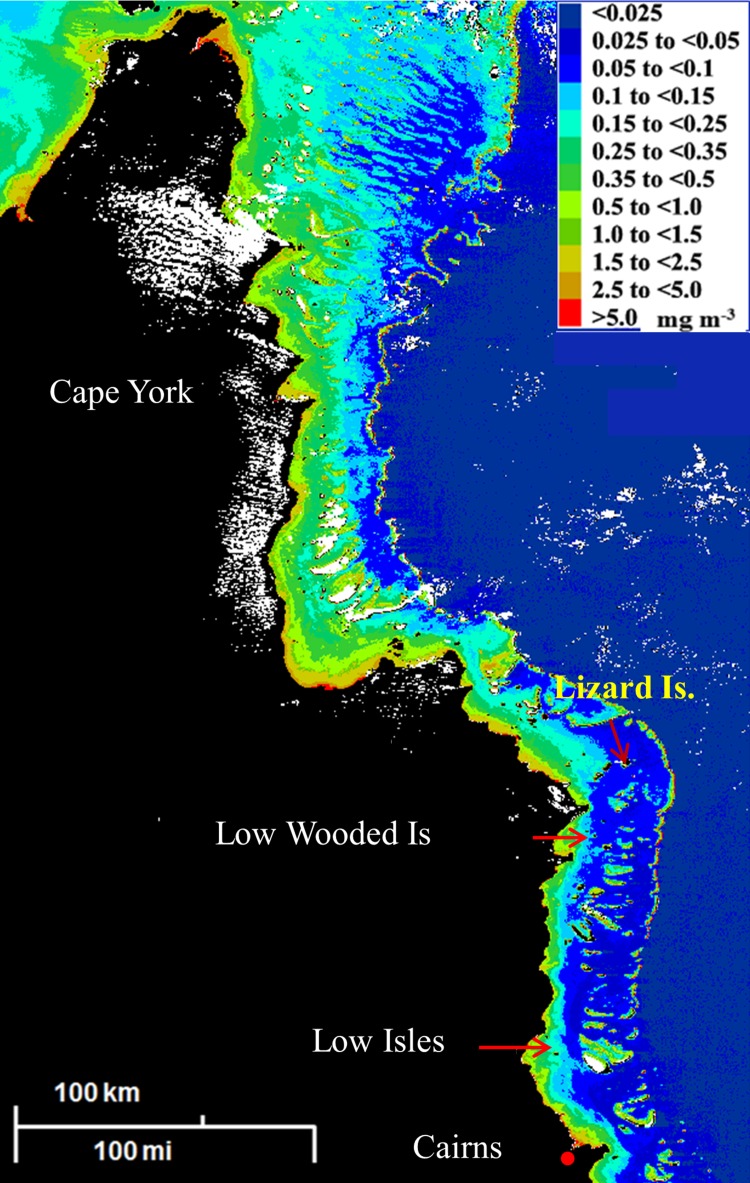
Previous analyses of historical data demonstrate that the suggested upper threshold value ETC-Chl a ~0.5 mg m−3 was exceeded some decades ago in the near-shore regions in both the Northern and Central GBR lagoon and that such elevated values often extend significant distances offshore (Bell 1992; Bell and Elmetri 1995, 1998). The data also show that the lower threshold range (ETC-Chl a ~0.2–0.3 mg m−3) was exceeded well beyond the mid-lagoon. Previous work using CZCS satellite imagery (Bell and Gabric 1990; Gabric et al. 1990; Bell and Elmetri 1998) demonstrated that similar cross-shelf gradients of Chl a occurred for the entire length of the GBR lagoon, including sections of the remote Cape York, SG, and CBG regions, more than 30 years ago (e.g., see Figs. 4, 5). More recent satellite data provided by the SeaWiFS and MODIS satellite sensors (e.g., see Fig. 1) confirm these earlier findings (Bell et al. 2007, 2012; Brodie et al. 2007; Brando et al. 2010).
Fig. 4.
Average of all 1981 CZCS images showing cross-shelf gradients of plant pigments (mg m−3) in Northern, Central, and Southern GBR lagoon
Fig. 5.
CZCS image 22 August 1979 showing the extent of plant pigments (mg m−3) in Northern GBR lagoon including the Cape York Region
As noted above, the ETM suggests that chronic eutrophic conditions will be initiated in waters with an annual mean Chl a >0.2 mg m−3. Results from long-term monitoring show that such conditions occur over most of the GBR lagoon (see Fig. 3). We propose, as discussed below, the widespread occurrence of CSDs, the proliferation of COTS (and probably Drupella spp.) and the >70 % reduction in coral cover, in the GBR lagoon are symptoms of this chronic eutrophic state.
Changes to Plankton Class Structure
A comparison of phytoplankton data collected during 1992–1993 from the mid-lagoon sampling station located 3 miles east of Low Isles (3ME) with those collected in 1928–1929 shows that this GBR region supports far higher micro (>20 μm) and nanno (2–20 μm) phytoplankton numbers now than in 1928–1929 (Bell and Elmetri 1995). The growth of the N-fixing blue-green algae Trichodesmium spp. has also increased dramatically in the Northern GBR since the 1928–1929 Expedition to Low Isles (Bell and Elmetri 1995; Bell et al. 1999). Even higher concentrations of Trichodesmium and the nanno + micro-phytoplankton are typical of the Central GBR near to Townsville (Revelante and Gilmartin 1982).
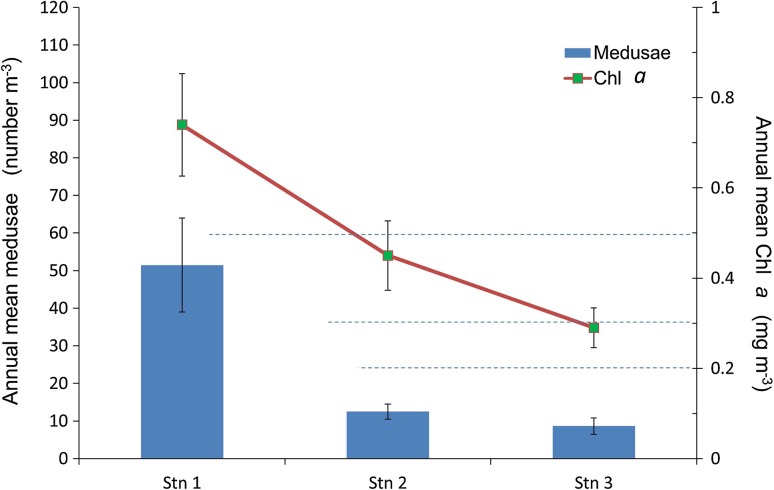
The results of Bell and Elmetri (1995) also suggest that a significant reduction in the diatom/flagellate ratio and a down-shift in the average size of the micro + nanno-phytoplankton have occurred in the Northern GBR lagoon since 1928–1929. Such changes in phytoplankton populations have been observed in other marine environments subjected to progressive anthropogenic eutrophication and other pollution (Bell and Elmetri 1995). It has been hypothesized that such changes would lead to the increased production of smaller secondary producers which could destabilize the food chain. One proposal is that such changes could lead to an increase in the number of jellyfish, because the smaller average food size and longer food chain, is more favorable for jellyfish than for fish (Greve and Parsons 1977; Richardson et al. 2009). There is some evidence that such ecological changes have occurred in the GBR lagoon. Indeed a comparison of the cross-shelf Chl a data with the net-zooplankton data collected off Townsville in 1977 (Fig. 6) shows that prolific growth of medusae occurs in the Chl a rich (i.e. eutrophic) nearshore waters (Ikeda et al. 1980; Revelante and Gilmartin 1982); these nearshore waters are characterised by an annual mean Chl a greater than the upper ETC-Chl a i.e. > 0.5 mg m−3. This finding provides supporting evidence of a link between the proliferation of jellyfish (and hence possibly the toxic box jellyfish) in the GBR lagoon and eutrophication. Also, as discussed above, there is evidence that the recorded changes in phytoplankton class structure will promote the growth of troublesome corallivores such as the COTS (and probably Drupella spp.).
Fig. 6.
Variation of annual mean medusae concentrations (Ikeda et al. 1980) and Chl a concentrations (Revelante and Gilmartin 1982) along a GBR inshore (Stn 1) offshore (Stn 3) transect off Townsville in 1977; bars denote Std. E
Proliferation of Corallivores
Significant damage has occurred to GBR reefs over the past 50 years due to the widespread proliferation of troublesome corallivores (e.g., COTS). Large-scale monitoring data show that COTS have impacted most regions of the GBR (Sweatman et al. 2008; Osborne et al. 2011); it is estimated that 42 % of all coral loss in the GBR since 1985 is due to COTS (De’ath et al. 2012). As noted above, there is experimental evidence that demonstrates the critical Chl a concentration for survival and growth COTS larvae is within the lower ETC-Chl a range ~0.2–0.3 mg m−3 and hence supports the hypothesis that the outbreaks of COTS can be linked directly to the degree of eutrophication and in particular to relatively low values around the now defined chronic eutrophic state i.e. an annual mean Chl a >0.2 mg m−3. It is noted that Drupella spp. which also appear to be enhanced in eutrophic conditions (Moyer et al. 1982; Bell 1988) is now quite common in a number of GBR regions (Sweatman et al. 2008) including some outer-GBR regions which have an annual mean Chl a < 0.3 mg m−3, e.g., Lizard Is. These observations suggest that the critical concentration for survival and growth of Drupella spp. larvae is also within the lower ETC-Chl a range ~0.2–0.3 mg m−3.
Widespread Occurrence of CSDs Links to Eutrophication
Large-scale monitoring has shown that CSDs are impacting most regions of the GBR (Sweatman et al. 2008); the fact that some CSDs are common in mid- to outer-GBR regions suggests a relatively low eutrophication threshold for CSDs is applicable. For example, a variety of CSDs (e.g., BBD, BrB, WS, SEB) have been identified at Lizard Is. The annual mean Chl a value for this region is ~0.25 mg m−3 (Brodie et al. 2007; AIMS 2012). These data suggest the annual mean Chl a for the proliferation CSDs would be in the lower ETC-Chl a range ~0.2–0.3 mg m−3 with the lower value, 0.2 mg m−3, being applicable to shallow less-well-flushed regions containing sensitive coral species. This analysis supports the setting of a chronic eutrophic state as being one with an annual mean Chl a >0.2 mg m−3.
It is important to note that evidence is emerging that a variety of corallivores, including COTS, play a role in CSD transmission, either by acting as vectors and/or stressors (Nugues and Bak 2009). Also associations between WS and Drupella spp. have been noted (Antonius and Riegl 1997). These findings suggest that the proliferation of corallivores by eutrophication in the mid- to outer-GBR regions could have promoted the spread of CSDs in these more remote GBR regions. These findings provide another probable link between eutrophication and the destruction of hermatypic corals by CSDs.
Degraded Coral Reefs
Long-term monitoring of the GBR by AIMS shows the average coral cover is ~14 %, a figure ~51 % lower than the presumed baseline of 28 % measured in 1985 (De’ath et al. 2012). However, the available data suggest that this proposed reduction is a significant underestimate of the total loss of coral cover over the past century because:
-
(i)
The AIMS monitoring is restricted to deeper reefs on the outside of coral complexes beyond the reef crest and hence does not include shallow-water/emergent reefs, those inside lagoons and those fringing the mainland. Various studies and photographic records (e.g., see Fig. 7a, b) show that the shallow-water reefs, many of which were exposed at low tides, that fringed both the mainland and many of the islands and cays were of a quality that could only be found in the outer-GBR today. There is evidence that many of these reefs exhibited high resilience in the past but are now severely degraded (Bell 1992; Bell and Elmetri 1995). Some reefs fringing the mainland are covered by macroalgal stands several meters high, resembling kelp forests (Fig. 7c). Also many shallow-water and lagoonal-reefs in outer-GBR regions (e.g., the CBG reefs located ~70 km from the coast) are characterized by extensive algal overgrowth (Fig. 2); the available evidence suggests that this algal overgrowth has occurred in recent times (Bell and Elmetri 2007).
-
(ii)
There is good evidence that many deeper mid to outer-GBR reefs had degraded significantly prior to the beginning of the AIMS monitoring in 1985 and hence the presumed baseline is far too low. For example the mid to outer reefs in the Cairns region that were not subjected to attacks by COTS in the 1960s had ~50 % coral cover in 1970 (Endean and Stablum 1973); overall the pre-European-development coral cover was probably ~50-60 % (Bruno and Selig 2007).
Fig. 7.
Comparison of shallow-water benthos in Madrepore Lagoon, Stone Island: a ca. 1892 showing extensive coral cover and diversity (Saville-Kent 1893) and b in 1994 (Photo: P.R.F. Bell), showing little or no live coral cover. c Sargassum sp. overgrowth of fringing reef, Port Douglas 1996 (image captured from B.E. Lapointe video)
These data suggest that there has been a >70 % loss in coral cover throughout the GBR since the development of the coastal catchments. In contrast to these observations some near-shore fringing-reef regions in the far northern GBR region (e.g., those at Low Wooded Is.; see Fig. 8) which are characterized by an annual mean Chl a in the lower range of the proposed ETC-Chl a (~0.2–0.3 mg m−3) show little or no evidence of degradation over the past century (Bell et al. 2012). These reefs would have suffered physical damage due to the effects of storms and cyclones many times over the past century and hence the observed quality of these reefs suggests they have exhibited a high degree of resilience since 1892. It is proposed that these reefs and their surrounding coastline should acquire the highest degree of protection that legislation allows as they can be used as “canaries in the coal-mine” to assess the effects of eutrophication in the GBR lagoon. These observations support the proposal that the Trigger value for Chl a be lowered to ~0.2–0.3 mg m−3 in order to support the dominance of hermatypic corals. However, the above discussions suggest a reduction to the lower end of this range (i.e., ~0.2 mg m−3) will probably be required to mitigate the proliferation of corallivores and CSDs.
Fig. 8.
Comparison of extensive shallow-water coral cover and diversity at Low Wooded Is. a, b in 1997 (Photo: P.R.F. Bell) and c, d ca. 1892 (Saville-Kent 1893)
Conclusions
The abundance of hard corals in the GBR region has reduced by >70 % since development of the coastal catchments. The principal causes of the loss of hard corals are attributed to lack of recovery following storm damage, the widespread growth of COTS, coral bleaching, and CSDs. It is now widely accepted that the lack of recovery of the reefs and the proliferation of COTS are largely attributable to eutrophication. Evidence is emerging that CSDs and coral bleaching are also promoted by eutrophication. Much of the increased fertility/eutrophication is due to the increased loads of nutrients exported via discharges from coastal developments. In addition, diazotrophs fix a substantial load of “new” nitrogen, the magnitude of which is estimated to be far higher now than in the past and similar to that introduced by the rivers. Authorities have recently taken significant action aimed at reducing runoff nutrient loads. However, further action is required to minimize the impacts of point-source discharges and particularly of P-PO4 rich discharges. Also further investigations on the links between eutrophication and the proliferation of CSDs, diazotrophs, and coral bleaching need to be conducted. Some reefs in regions characterized by annual mean Chl a concentrations in the lower range of the proposed ETCs namely ETC-Chl a ~0.2–0.3 mg m−3 show good resilience to physical damage but the available evidence suggests that CSDs and COTS will proliferate in such waters and hence the mandated eutrophication Trigger values for Chl a will need to be decreased to ~0.2 mg m−3 for sustaining coral reef communities.
Acknowledgments
Stafford Bettridge provided several underwater video clips. Paul Treloar, Anisul Islam, and Stephen Coombs prepared the SeaWiFS and CZCS images from data provided free of charge from the NASA archives. This support is gratefully acknowledged. Also, we wish to thank the reviewers of the manuscript who provided a number of constructive suggestions. This is contribution #1904 from the Harbor Branch Oceanographic Institute at Florida Atlantic University, Ft. Pierce, FL.
Biographies
Peter R. F. Bell
is Honorary Senior Fellow in the School of Chemical/Environmental Engineering at The University of Queensland and the Founding Director of the University of Queensland Low Isles Research station. His research work focuses on the causes and impacts of eutrophication in the marine environment. This work involves the use of satellite remote sensing, modeling and field/laboratory experimental determinations.
Ibrahim Elmetri
was Manager of the University of Queensland Low Isles Research station 1995–1999. Since then he has held Research Scientist positions in New Zealand at Massey University and the Cawthron Institute. His principal research interests are the evaluation of impacts of eutrophication on the marine environment and the development of management techniques to control such eutrophication. He is currently conducting environmental consulting work through AMZA Ltd.
Brian E. Lapointe
is a Research Professor with Harbor Branch Oceanographic Institute. His research interests include oceanography, algal physiology and biochemistry, seagrass and coral reef ecology, and marine eutrophication. Brian has extensive experience in water quality assessments of coral reef systems in the Pacific and the wider Caribbean regions, including areas of the Bahamas, Florida Keys, Jamaica, Tobago, Martinique, and Belize.
Contributor Information
Peter R. F. Bell, Phone: 61414170848, FAX: 61-7-365-4199, Email: p.bell@uq.edu.au
Ibrahim Elmetri, Email: ibrahim.elmetri@gmail.com.
Brian E. Lapointe, Email: belapointe@gmail.com
References
- Aeby GS, Williams GJ, Franklin EC, Kenyon J, Cox EF, Coles S, Work TM. Patterns of coral disease across the Hawaiian archipelago: Relating disease to environment. PLoS ONE. 2011;6:e20370. doi: 10.1371/journal.pone.0020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AIMS. 2012. Australian Institute of Marine Science, Townsville. Retrieved 10 November, 2012, from http://e-atlas.org.au/geoserver/www/map.html?v=2&z=5&ll=-18,148&n=1&m0=ea-Natural_Earth_2:V,Chlorophyll-Chlorophyll-micro_grams_per_litre:V.
- Antonius A, Riegl B. A possible link between coral diseases and a corallivorous snail (Drupella cornus) outbreak in the Red Sea. Atoll Research Bulletin. 1997;47:1–9. doi: 10.5479/si.00775630.447.1. [DOI] [Google Scholar]
- Ayukai, T., K. Okaji, and J.S. Lucas. 1997. Food limitation in the growth and development of crown-of-thorns starfish in the Great Barrier Reef. In Proceedings of the 8th International Coral Reef Symposium, vol. 1, 621–626. Panama: Smithsonian Tropical Research Institute.
- Bell JL. Optimal feeding by gastropod larvae: Patches and picoplankton. American Zoologist. 1988;28:167A. [Google Scholar]
- Bell PRF. Status of eutrophication in the Great Barrier Reef Lagoon. Marine Pollution Bulletin. 1991;23:89–93. doi: 10.1016/0025-326X(91)90655-C. [DOI] [Google Scholar]
- Bell PRF. Eutrophication and coral reefs—Some examples in the Great Barrier Reef Lagoon. Water Research. 1992;26:553–568. doi: 10.1016/0043-1354(92)90228-V. [DOI] [Google Scholar]
- Bell PRF, Elmetri I. Ecological indicators of large scale eutrophication in the Great Barrier Reef (GBR) Lagoon. AMBIO. 1995;24:208–215. [Google Scholar]
- Bell, P.R.F., and I. Elmetri. 1998. Large-scale eutrophication in the Great Barrier Reef Lagoon—Causes of the problem and some possible solutions. In Chemeca 98, Australasian Chemical Engineering Conference. Paper No. 305, ed. R.B. Newell, and C.J. Smith, Institution of Engineers, Australia, Barton, 12 pp.
- Bell PRF, Elmetri I. Some chemical factors regulating the growth of Lyngbya majuscula in Moreton Bay, Australia: Importance of sewage discharges. Hydrobiologia. 2007;592:359–371. doi: 10.1007/s10750-007-0773-8. [DOI] [Google Scholar]
- Bell, P.R.F., and A.J. Gabric. 1990. The use of field survey and satellite remote sensing in determining the extent and causes of eutrophication in the Great Barrier Reef Lagoon, Australia. In Proceedings of the Fourth Pacific Congress on Marine Science and Technology, vol. II, 25–32. Tokyo: PACON 90.
- Bell PRF, Gabric AJ. Must GBR pollution become chronic before management reacts? Search. 1991;22:117–119. [Google Scholar]
- Bell PRF, Elmetri I, Uwins P. Nitrogen fixation of Trichodesmium spp. in the Great Barrier Reef Lagoon-importance to the overall nitrogen budget. Marine Ecology Progress Series. 1999;186:119–126. doi: 10.3354/meps186119. [DOI] [Google Scholar]
- Bell PRF, Lapointe BE, Elmetri I. Reevaluation of ENCORE: Support for the eutrophication threshold model for coral reefs. AMBIO. 2007;36:416–424. doi: 10.1579/0044-7447(2007)36[416:ROESFT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bell, P.R.F., I. Elmetri, and B.E. Lapointe. 2012. Synoptic scale monitoring supports the coral reef eutrophication threshold model. Retrieved August 15, 2012, from http://www.icrs2012.com/eposters/P227.pdf.
- Boyett, H.V. 2006. The ecology and microbiology of black band disease and brown band syndrome on the Great Barrier Reef. MSc Thesis. Townsville, Australia: James Cook University.
- Brando, V.E., T. Schroeder, and A.G. Dekker. 2010. Reef rescue marine monitoring program: using remote sensing for GBR wide water quality. Retrieved January 5, 2013, from http://www.rrrc.org.au/publications/downloads/372b--378_CSIRO_final-report_15Apr2010.pdf.
- Brodie J, Waterhouse J. A critical review of environmental management of the ‘not so Great’ Barrier Reef. Estuarine, Coastal and Shelf Science. 2012;104–105:1–22. doi: 10.1016/j.ecss.2012.03.012. [DOI] [Google Scholar]
- Brodie J, Fabricius K, De’ath G, Okaji K. Are increased nutrient inputs responsible for more outbreaks of crown-of-thorns starfish? An appraisal of the evidence. Marine Pollution Bulletin. 2005;51:266–278. doi: 10.1016/j.marpolbul.2004.10.035. [DOI] [PubMed] [Google Scholar]
- Brodie J, De’ath G, Devlin M, Furnas M, Wright M. Spatial and temporal patterns of near-surface chlorophyll a in the Great Barrier Reef lagoon. Marine & Freshwater Research. 2007;58:342–353. doi: 10.1071/MF06236. [DOI] [Google Scholar]
- Brodie J, Schroeder T, Rohde K, Faithful J, Masters B, Dekker A, Brando V, Maughan M. Dispersal of suspended sediments and nutrients in the Great Barrier Reef lagoon during river-discharge events: Conclusions from satellite remote sensing and concurrent flood-plume sampling. Marine & Freshwater Research. 2010;61:651–664. doi: 10.1071/MF08030. [DOI] [Google Scholar]
- Bruckner, A.W., R.J. Bruckner, and E.H. Williams Jr. 1997. Spread of a black-band disease epizootic through the coral reef system in St. Ann’s Bay, Jamaica. Bulletin of Marine Science 61: 919–928.
- Bruno JF, Selig ER. Regional decline of coral cover in the Indo-Pacific: Timing, extent, and subregional comparisons. PLoS ONE. 2007;2:e711. doi: 10.1371/journal.pone.0000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno JF, Petes LE, Harvell CD, Hettinger A. Nutrient enrichment can increase the severity of coral diseases. Ecological Letters. 2003;6:1056–1061. doi: 10.1046/j.1461-0248.2003.00544.x. [DOI] [Google Scholar]
- Cervino JM, Hayes RL, Polson SW, Polson SC, Goreau TG, Martinez RJ, Smith GW. Relationship of Vibrio species infection and elevated temperatures to yellow blotch/band disease in Caribbean Corals. Applied and Environmental Microbiology. 2004;70:6855–6864. doi: 10.1128/AEM.70.11.6855-6864.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De’ath, G., K.E. Fabricius, H. Sweatman, and M. Puotinen. 2012. The 27-year decline of coral cover on the Great Barrier Reef and its causes, 1–5. Retrieved October 2, 2012, from www.pnas.org/cgi/doi/10.1073/pnas.1208909109. [DOI] [PMC free article] [PubMed]
- Delean, S., and G. De’ath. 2008. Spatial and temporal patterns of indicators of reef health on the Great Barrier Reef. Report to the Marine and Tropical Sciences Research Facility. Reef and Rainforest Research Centre Limited, Cairns, 116 pp.
- Elmetri, I., and P.R.F. Bell. 2004. Effects of phosphorus on the growth and nitrogen fixation rates of Lyngbya majuscula: implications for management in Moreton Bay, Queensland. Marine Ecology Progress Series 281: 27–35.
- Endean R, Stablum W. The apparent extent of recovery of reefs of Australia’s Great Barrier Reef devastated by the crown-of-thorns starfish. Atoll Research Bulletin. 1973;168:1–41. doi: 10.5479/si.00775630.168.1. [DOI] [Google Scholar]
- Fabricius, K.E., K. Okaji, and G. De’ath. 2010. Three lines of evidence to link outbreaks of the crown-of-thorns seastar Acanthaster planci to the release of larval food limitation. Coral Reefs 29: 593–605.
- Falkowski PG, Dubinsky Z, Muscatine L, McCloskey L. Population control in symbiotic corals. BioScience. 1993;43:606–611. doi: 10.2307/1312147. [DOI] [Google Scholar]
- Fu F, Zhang Y, Bell PRF, Hutchins D. Phosphate uptake and growth kinetics of Trichodesmium (Cyanobacteria) isolates from the North Atlantic Ocean and the Great Barrier Reef, Australia. Journal of Phycology. 2005;41:62–73. doi: 10.1111/j.1529-8817.2005.04063.x. [DOI] [Google Scholar]
- Gabric AJ, Hoffenberg P, Boughton W. Spatio-temporal variability in surface chlorophyll distribution in the central Great Barrier Reef as derived from CZCS imagery. Australian Journal of Marine and Freshwater Research. 1990;41:313–324. doi: 10.1071/MF9900313. [DOI] [Google Scholar]
- GBRMPA. 2010. Water Quality Guidelines for the Great Barrier Reef Marine Park Revised Edition. Great Barrier Reef Marine Park Authority. Retrieved May 1, 2012, from http://elibrary.gbrmpa.gov.au/jspui/handle/11017/432.
- Goreau TJ, Cervino J, Goreau M, Hayes R, Hayes M, Richardson L, Smith G, DeMeyer K, et al. Rapid spread of diseases in Caribbean coral reefs. Revista de Biología Tropical. 1998;46:157–171. [Google Scholar]
- Greve W, Parsons TR. Photosynthesis and fish production: Hypothetical effects of climate change and pollution. Helgoländer Wissenschaftliche Meeresuntersuchungen. 1977;30:666–671. doi: 10.1007/BF02207869. [DOI] [Google Scholar]
- Haapkyla J, Unsworth RKF, Flavell M, Bourne DG, Schaffelke B, Willis BL. Seasonal rainfall and runoff promote coral disease on an inshore reef. PLoS ONE. 2011;6:e16893. doi: 10.1371/journal.pone.0016893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, T. 1994. Catastrophes, phase-shifts and large-scale degradation of a Caribbean coral reef. Science 265: 1547–1551. [DOI] [PubMed]
- Hughes TP, Szmant AM, Steneck R, Carpenter R, Miller S. Algal blooms on coral reefs: What are the causes? Limnology and Oceanography. 1999;44:1583–1586. doi: 10.4319/lo.1999.44.3_part_2.0932. [DOI] [Google Scholar]
- Ikeda, T., M. Gilmartin, N. Revelante, A.W. Mitchell, J.H. Carleton, P. Dixon, S.M. Hutchinson, E. Fay Hing, et al. 1980. Biological, chemical and physical observations in inshore waters of the Great Barrier Reef, North Queensland 1975-1978. Data Report AIMS-OS-80-1, Australian Institute of Marine Science Townsville, 56 pp.
- Kerry, J. 2011. Relationship between corals and fishes on the Great Barrier Reef. Australian Institute of Marine Science (AIMS). Retrieved November 9, 2012, from http://e-atlas.org.au/content/relationship-between-corals-and-fishes-great-barrier-reef.
- Kinsey DW. Can we resolve the nutrient issue for the reef? Search. 1991;22:119–121. [Google Scholar]
- Kline, D.I., N.M. Kuntz, M. Breitbart, N. Knowlton, and F. Rohwer. 2006. Role of elevated organic carbon levels and microbial activity in coral mortality. Marine Ecology Progress Series 314: 119–125.
- Koop K, Booth D, Broadbent A, Brodie J, Bucher D, Capone D, Coll J, Dennison W, et al. ENCORE: The effect of nutrient enrichment on coral reefs. Synthesis of results and conclusions. Marine Pollution Bulletin. 2001;42:91–120. doi: 10.1016/S0025-326X(00)00181-8. [DOI] [PubMed] [Google Scholar]
- Kroon FJ, Kuhnert PM, Henderson BL, Wilkinson SN, Kinsey-Henderson A, Abbott B, Brodie JE, Turner RDR. River loads of suspended solids, nitrogen, phosphorus and herbicides delivered to the Great Barrier Reef lagoon. Marine Pollution Bulletin. 2012;65:167–181. doi: 10.1016/j.marpolbul.2011.10.018. [DOI] [PubMed] [Google Scholar]
- Kuta KG, Richardson LL. Ecological aspects of black band disease of corals: Relationships between disease incidence and environmental factors. Coral Reefs. 2002;21:393–398. [Google Scholar]
- Lapointe, B.E. 1989. Caribbean coral reefs: Are they becoming algal reefs. Sea Frontiers, March–April, 83–84.
- Lapointe BE. Nutrient thresholds–Bottom-up control of macroalgal blooms on coral reefs in Jamaica and South East Florida. Limnology and Oceanography. 1997;42:1119–1131. doi: 10.4319/lo.1997.42.5_part_2.1119. [DOI] [Google Scholar]
- Lapointe, B.E., and M.A. Mallin. 2011. Nutrient enrichment and eutrophication on fringing coral reefs of Bonaire and Curaçao, Netherlands Antilles. Report to the United Nations Environment Programme for the NACRI Coral Reef Monitoring Program, Harbor Branch Oceanographic Institute, Ft. Pierce Fl, 42 pp.
- Lapointe BE, Barile P, Matzie WR. Anthropogenic nutrient enrichment of seagrass and coral reef communities in the Lower Florida Keys: Discrimination of local versus regional nitrogen sources. Journal of Experimental Marine Biology and Ecology. 2004;308:23–58. doi: 10.1016/j.jembe.2004.01.019. [DOI] [Google Scholar]
- Lapointe B., B. Bedford, and R. Baumberger. 2007. Looe Key, FL: Nutrients and climate change pose threat to coral reefs. In Effects of Nutrient enrichment in the nation’s estuaries: A decade of change, 104–105. Retrieved August 30, 2013, from http://ccma.nos.noaa.gov/publications/eutroupdate/.
- Larkum AWD, Steven ADL. ENCORE: The effect of nutrient enrichment on coral reefs. 1. Experimental design and research programme. Marine Pollution Bulletin. 1994;29:112–120. doi: 10.1016/0025-326X(94)90434-0. [DOI] [Google Scholar]
- Laws EA, Redalje DG. Effect of sewage enrichment on phytoplankton population of a subtropical estuary. Pacific Science. 1979;33:129–144. [Google Scholar]
- Littler MM, Taylor PR, Littler DS. Algal resistance to herbivory on a Caribbean barrier reef. Coral Reefs. 1983;2:111–118. doi: 10.1007/BF02395281. [DOI] [Google Scholar]
- Littler MM, Littler DS, Brooks BL. Herbivory, nutrients, stochastic events, and relative dominances of benthic indicator groups on coral reefs: A review and recommendations. Smithsonian Contributions to the Marine Sciences. 2009;38:401–414. doi: 10.5479/si.01960768.38.401. [DOI] [Google Scholar]
- Lucas, J.S. 1982. Quantitative studies of feeding and nutrition during larval development of the coral reef asteroid Acanthaster planci (L.). Journal of Experimental Marine Biology and Ecology 65: 173–193.
- Marubini F, Davies PS. Nitrate increases zooxanthellae population density and reduces skeletogenesis in corals. Marine Biology. 1996;127:319–328. doi: 10.1007/BF00942117. [DOI] [Google Scholar]
- McCook, L.J., I.R. Price, and D.W. Klumpp. 1997. Macroalgae on the GBR: Causes or consequences, indicators or models of reef degradation. In Proceedings of the 8th International Coral Reef Symposium, vol. 2, 1851–1856. Panama: Smithsonian Tropical Research Institute.
- Middleton JH, Coutis P, Griffin DA, Macks A, McTaggart A, Merrifield MA, Nippard GJ. Circulation and water mass characteristics of the Southern Great Barrier Reef. Australian Journal of Marine and Freshwater Research. 1994;45:1–18. doi: 10.1071/MF9940001. [DOI] [Google Scholar]
- Moyer, J.T., W.K. Emerson, and M. Ross. 1982. Massive destruction of scleractinian corals by the muricid gastropod Drupella in Japan and the Philippines. Nautilis 96: 69–82.
- Nagelkerken I. Relationship between anthropogenic impacts and bleaching-associated tissue mortality of corals in Curaçao (Netherlands Antilles) Revista de Biologia Tropical. 2006;54:31–44. [Google Scholar]
- Nugues MM, Bak RPM. Brown-band syndrome on feeding scars of the crown-of-thorn starfish Acanthaster planci. Coral Reefs. 2009;28:507–510. doi: 10.1007/s00338-009-0468-x. [DOI] [Google Scholar]
- Olson RR. In situ culturing as a test of the larval starvation hypothesis for the Crown-of-Thorns Starfish, Acanthaster planci. Limnology and Oceanography. 1987;32:895–904. doi: 10.4319/lo.1987.32.4.0895. [DOI] [Google Scholar]
- Osborne K, Dolman AM, Burgess SC, Johns KA. Disturbance and the dynamics of coral cover on the Great Barrier Reef (1995–2009) PLoS ONE. 2011;6:e17516. doi: 10.1371/journal.pone.0017516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revelante N, Gilmartin M. Dynamics of phytoplankton in the Great Barrier Reef lagoon. Journal of Plankton Research. 1982;4:47–76. doi: 10.1093/plankt/4.1.47. [DOI] [Google Scholar]
- Richardson AJ, Bakun A, Hays GC, Gibbons MJ. The jellyfish joyride: Causes, consequences and management responses to a more gelatinous future. Trends in Ecology & Evolution. 2009;24:312–322. doi: 10.1016/j.tree.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Risk MJ. Paradise lost: How marine science failed the world’s coral reefs. Marine & Freshwater Research. 1999;50:831–837. doi: 10.1071/MF99067. [DOI] [Google Scholar]
- Ryther, J.H., and C.B. Officer 1981. Impact of nutrient enrichment on water uses. In Estuaries and nutrients, ed. B.J. Neilson, and L.E. Cronin, 247–261 Clifton: Humana Press.
- Saville-Kent, W. 1893. The Great Barrier Reef of Australia: Its products and potentialities, 387 pp. London: W.H. Allen & Co. Ltd. (reproduced Melbourne: John Currey, O’Neil Pty Ltd., 1972).
- Smith, S.V., W.J. Kimmerer, E.A. Laws, R.E. Brock, and T.W. Walsh. 1981. Kaneohe Bay sewage diversion experiment: Perspectives on ecosystem responses to nutritional perturbation. Pacific Science 35: 279–395.
- Sweatman, H., A. Cheal, G. Coleman, M. Emslie, K. Johns, M. Jonker, I. Miller, and K. Osborne. 2008. Long term monitoring of the Great Barrier Reef, status report number 8. Australian Institute of Marine Science, Townsville, 369 pp.
- Tomascik T, Sander F. Effects of eutrophication on reef building corals I. Growth rate of the reef-building coral Montastrea annularis. Marine Biology. 1985;87:143–155. doi: 10.1007/BF00539422. [DOI] [Google Scholar]
- Tomascik T, Sander F. Effects of eutrophication on reef building corals II. Structure of scleractinian coral communities on fringing reefs, Barbados, West Indies. Marine Biology. 1987;94:53–75. doi: 10.1007/BF00392900. [DOI] [Google Scholar]
- Voss JD, Richardson LL. Nutrient enrichment enhances black band disease progression in corals. Coral Reefs. 2006;25:569–576. doi: 10.1007/s00338-006-0131-8. [DOI] [Google Scholar]
- Wachenfeld, D.R. 1995. Long term trends in the status of coral reef-flat benthos—The use of historical photographs, State of the Great Barrier Reef World Heritage Area Workshop. GBRMPA Workshop Series 23: 134–148.
- Walker TA. Is the reef really suffering from chronic pollution? Search. 1991;22:115–117. [Google Scholar]
- Webb KL, DuPaul WD, Wiebe W, Sottille W, Johannes RE. Enewetak (Eniwetok) Atoll: Aspects of nitrogen cycle on a coral reef. Limnology and Oceanography. 1975;20:198–210. doi: 10.4319/lo.1975.20.2.0198. [DOI] [Google Scholar]
- Williams, D.M., and A.I. Hatcher. 1983. Structure of fish communities on outer slopes of inshore, mid-shelf and outer shelf reefs of the Great Barrier Reef. Marine Ecology Progress Series 10: 239–250.
- Willis, B.L., C.A. Page, and E.A. Dinsdale. 2004. Coral disease on the Great Barrier Reef. In Coral health and disease, ed. E. Rosenberg, and Y. Loya, 69–104. Berlin: Springer.
- Wooldridge SA, Done TJ. Improved water quality can ameliorate effects of climate change on corals. Ecological Applications. 2009;19:1492–1499. doi: 10.1890/08-0963.1. [DOI] [PubMed] [Google Scholar]
- Wulff F, Eyre BD, Johnstone R. Nitrogen versus phosphorus limitation in a subtropical coastal embayment (Moreton Bay; Australia): Implications for management. Ecological Modelling. 2011;222:120–130. doi: 10.1016/j.ecolmodel.2010.08.040. [DOI] [Google Scholar]