Abstract
This study examines the relationship between focused-stimulation thresholds, electrode positions, and speech understanding in deaf subjects treated with a cochlear implant (CI). Focused stimulation is more selective than monopolar stimulation, which excites broad regions of the cochlea, so may be more sensitive as a probe of neural survival patterns. Focused thresholds are on average higher and more variable across electrodes than monopolar thresholds. We presume that relatively high focused thresholds are the result of larger distances between the electrodes and the neurons. Two factors are likely to contribute to this distance: (1) the physical position of electrodes relative to the modiolus, where the excitable auditory neurons are normally located, and (2) the pattern of neural survival along the length of the cochlea, since local holes in the neural population will increase the distance between an electrode and the nearest neurons. Electrode-to-modiolus distance was measured from high-resolution CT scans of the cochleae of CI users whose focused-stimulation thresholds were also measured. A hierarchical set of linear models of electrode-to-modiolus distance versus threshold showed a significant increase in threshold with electrode-to-modiolus distance (average slope = 11 dB/mm). The residual of these models was hypothesized to reflect neural survival in each subject. Consonant–Nucleus–Consonant (CNC) word scores were significantly correlated with the within-subject variance of threshold (r2 = 0.82), but not with within-subject variance of electrode distance (r2 = 0.03). Speech understanding also significantly correlated with how well distance explained each subject’s threshold data (r2 = 0.63). That is, subjects with focused thresholds that were well described by electrode position had better speech scores. Our results suggest that speech understanding is highly impacted by individual patterns of neural survival and that these patterns manifest themselves in how well (or poorly) electrode position predicts focused thresholds.
Keywords: cochlear implants, electrode configuration, neural survival, psychophysics, spiral ganglion cell, speech reception
INTRODUCTION
Since the introduction of the first cochlear implant in the 1970s, implant recipients’ speech reception in quiet has improved to the degree that many recipients can converse confidently over the telephone. However, there is still much variability from listener to listener. One hypothesis concerning the wide range of outcomes across listeners is the difference in neural survival patterns across different listeners. Age, etiology, and duration of deafness are significant predictors of speech understanding with cochlear implants (Blamey et al. 1996; 1992; Holden et al. 2013), and they are also predictors of spiral ganglion cell (SGC) counts (Makary et al. 2011; Nadol et al. 1989). Yet, a firm connection between speech understanding and SGC counts was not found in the two small studies on the topic which examined cadaveric temporal bones of subjects whose speech understanding in life had been measured (Fayad and Linthicum 2006; Khan et al. 2005).
Assessing neural survival in living cochlear implant users presents several challenges. Pfingst and Xu (2004) and Bierer (2007) used psychophysical tests to determine the effectiveness of the electro-neural interface in living human subjects without information about electrode position, while Cohen et al. (2001) measured the impact of electrode position on threshold, comfort level, and dynamic range using information from X-rays. The present paper brings these approaches together in a study of a single group of subjects with the addition of high-resolution CT scans for determination of electrode position. Our conceptual model of the status of the auditory nerve and electrode positions is similar to that described by Bierer (2010). That is, the reference situation contains a full complement of SGCs and electrodes close to the modiolus. In this best case, one would expect to find low focused stimulation thresholds and good speech understanding. In contrast, electrode arrays with variable distances even with a full complement of SGCs would be expected to produce variable focused thresholds (explainable by distance) and good speech understanding. Finally, a sparse or patchy complement of SGCs could produce high focused thresholds not explainable by distance and poor speech understanding. In summary, we hypothesize that neural survival has a significant impact on threshold and speech understanding while variations in electrode to modiolus, medial–lateral (EMML) distance only affect thresholds significantly. This paper analyzes the applicability of such a conceptual model by examining the relationships between speech understanding, focused thresholds, and electrode positions.
In addition, we examine whether mean threshold alone or mean EMML distance alone predicts speech understanding. We also consider the potential contribution of bone and fibrous tissue growth and electrode scalar position to the threshold and speech understanding results.
METHODS
We studied ten listeners using three different techniques: psychophysical thresholds, CT scans, and speech understanding measures. Speech understanding was measured using Consonant–Nucleus–Consonant (CNC) word scores (Peterson and Lehiste 1962). The CNC word scores were obtained using a monopolar ACE strategy with two lists per test presented at 70 dB SPL. This was done at 12 months post-activation, after the scores typically have reached asymptote (Holden et al. 2013). The logit of the CNC word score was used in all correlations in order to correct for the non-normality of percent correct scores (Judd et al. 2009). We measured psychophysical thresholds to electric stimuli using a multipolar current focusing paradigm (van den Honert and Kelsall 2007). This multi-electrode configuration focuses the voltage in the cochlea and has been shown to provide psychophysically narrow regions of excitation (van den Honert et al. 2007) and to increase spectral resolution when incorporated into a full sound coding strategy (Smith et al. 2013). Subjects were adult users of an experimental cochlear prosthesis that contained no implanted electronic components. It consisted of a 22-contact intracochlear Nucleus® Contour Softip™ perimodiolar electrode array and two extracochlear electrodes located below the temporalis muscle. Wires from all electrodes were terminated directly to a connector housed in a percutaneous titanium pedestal mounted to the skull behind the ear. For take-home use, each subject was equipped with an externally worn receiver stimulator that was electrically equivalent to a Nucleus® Contour Advance™ implant. Through the percutaneous device we could present 24 simultaneous electrode currents with high precision using bench-top current sources. The focused thresholds were obtained with biphasic, pulse-train stimuli at a 250-pps rate with 100-μs phase duration, 0-μs interphase-gap, and 200-ms train duration. Thresholds were measured using a method of adjustment (MOA) procedure until adaptive software became available. Thresholds collected over time were averaged in order to obtain the best prediction of the true mean per electrode, per subject. An average of five thresholds per electrode per subject was obtained (see Table 2). In all cases, the mean within-subject, within-electrode standard error of the thresholds was less than 1 dB.
TABLE 2.
Summary of generalized linear modeling of focused PA threshold and medial–lateral electrode distance from the modiolar wall
| Research ID | Mean distance (mm) | Mean threshold (dB) | # of MOA Thrsh | # of Adptv Thrsh | Threshold at mean distance (dB) | r 2 | RMSE (dB) |
|---|---|---|---|---|---|---|---|
| S36 | 0.97 | 54.60 | 5 | 0 | 52.96 | 0.62 | 5.93 |
| S38 | 1.12 | 50.47 | 6 | 0 | 48.00 | 0.57 | 4.95 |
| S40 | 0.96 | 46.15 | 9 | 3 | 45.81 | 0.32 | 3.03 |
| S41 | 0.95 | 43.78 | 5 | 0 | 43.37 | 0.18 | 5.79 |
| S54 | 1.19 | 57.02 | 5 | 2 | 54.92 | 0.07 | 6.25 |
| S55 | 0.82 | 46.24 | 0 | 6 | 46.19 | 0.01 | 2.59 |
| S66 | 0.71 | 45.12 | 4 | 0 | 46.86 | 0.46 | 4.15 |
| S80 | 0.72 | 47.53 | 0 | 3 | 51.61 | 0.65 | 3.71 |
| S89 | 0.63 | 53.21 | 0 | 3 | 57.30 | 0.41 | 4.64 |
| S101 | 0.82 | 53.11 | 0 | 3 | 53.80 | 0.34 | 3.75 |
| Mean | 0.89 | 49.72 | 3.4 | 2.0 | 50.08 | 4.48 |
The table shows subject and mean values for mean distance from the modiolar wall (in mm), mean threshold (in dB re 1 μA), number of method of adjustment (MOA) thresholds per electrode, number of adaptive thresholds per electrode, threshold at the overall mean distance (at 0.89 mm in dB re 1 μA), r 2, and root mean square error of the linear fit (RMSE dB)
MOA threshold data were obtained using a subject-controlled interface and a one interval procedure. The subject had access to two large arrows (producing five current level [CL] steps) and two small arrows (producing 2CL steps). A 2CL step is equivalent to a 0.35-dB current change. Subjects were instructed to bring the loudness from inaudible up to medium, then to reduce the loudness until a small arrow step caused a transition between hearing something and hearing nothing. Subjects could repeat this as many times as they wished.
The adaptive procedure used a three alternative forced choice procedure with a two-down, one-up staircase (Levitt 1971). A set of eight reversals was used with the last six reversals used in the average. The steps were 5CLs for the first three reversals, 2CLs for the next three reversals, and 1CL for the last two reversals. The procedure converged on 70.7 % correct. Only the thresholds from the middle 20 channels were used in further analysis, as focusing is reduced at the most apical and basal channels. In addition, monopolar (MP), bipolar (BP), and tripolar (TP) thresholds were measured in a number of the subjects using the same methods. BP thresholds were measured in BP + 0 mode (on adjacent electrodes). TP thresholds were measured in full tripolar mode with no extra-cochlear return current. The average spacing between adjacent electrodes was 0.6 mm. The research received approval of the relevant ethical review body, Western IRB.
Analysis of Position of Implanted Electrodes from CT Scan Data
We also measured electrode positions and calculated the distance of each electrode from the modiolar wall of the cochlea using methods that overcome metal artifact contamination and correctly identify the position of implanted electrodes in the inner ear. This is described in more detail by Skinner et al. (2007) and validated by Teymouri et al. (2011). Using well-defined anatomical landmarks, we co-registered an individual’s pre-implant CT voxel space, optimized for anatomical detail, with their post-implant CT image space, optimized for resolution of the electrode (ANALYZE software, Mayo Clinic, Rochester, MN, USA; Robb 2001). The electrode lead wires and contacts were identified, segmented from the post-implant image data, and copied into the pre-implant image space to provide a composite image of electrode placement within an individual’s cochlea. To better visualize the scalar position of the segmented array and the individual electrode contacts, a high resolution cochlear atlas was then aligned with the aforementioned composite CT volume to estimate the location of fine and soft tissue intra-cochlear structures not resolved by CT, such as the basilar membrane. The atlas was based on an orthogonal-plane, fluorescence optical sectioning (OPFOS) microscopy scan of a single male donor with normal cochlear anatomy and illustrates details of both the soft tissue and bony structure of the cochlea (Voie 2002). The atlas was used to supplement information gathered from the pre-operative and post-operative CT scans of these subjects. Alignment and scaling of the atlas allow for good matching: 77 % of the variance in the length of the organ of Corti can be accounted for by a single measurement of the distance from the middle of the round window through the cochlea midpoint to the opposite side (Stakhovskaya et al. 2007). Thus, for this group of adults, with no cochlear dysplasia, a single scaled atlas was used.
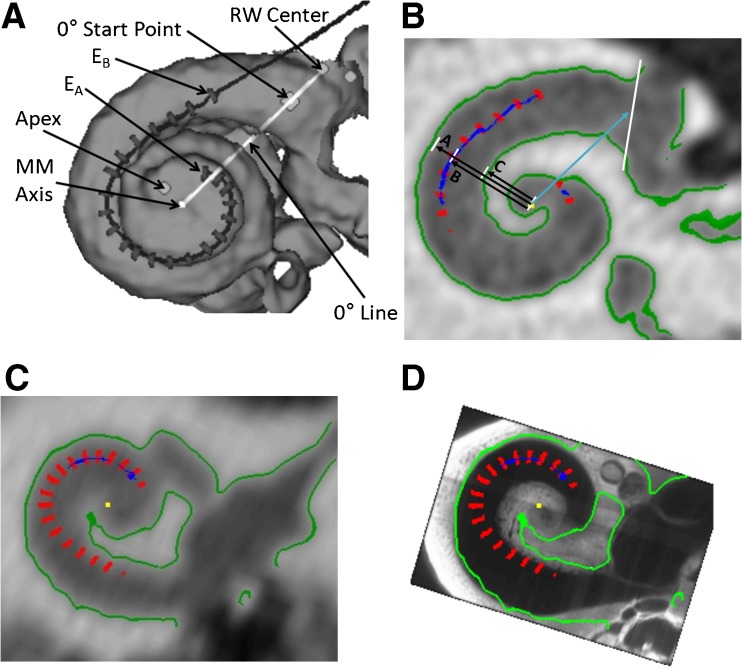
Figure 1A shows a composite CT volume rendering of subject S41’s cochlea and electrode array viewed along the mid-modiolar (MM) axis. The figure also shows the markers and lines used to measure the participant’s cochlear dimensions and array position. The dark line and gray hash marks in Figure 1A show the path of the array and the location of the 22 electrode contacts of a Contour Advance™ array (EA denotes apical-most electrode, EB denotes basal-most electrode).
FIG. 1.
Methods for distance measures. A The dark line and gray hash marks show the path of the array and the location of the 22 electrode contacts of a Contour Advance™ array (E A apical-most electrode, E B basal-most electrode). Shown are the center of the round window (RW), the cochleostomy site, the 0 ° start point, the apex of the cochlea, the mid modiolar axis and the 0 ° reference line. B The distance from the mid-modiolar (MM) axis to the inner wall "C", from the MM axis to the electrode "B", and from the MM axis to the outer wall "A" are shown here for S41’s electrode 6. This is a slice through the cochlea looking down the mid-modiolar axis. We use the information to determine the electrode to modiolus medial–lateral (EMML) distance, "B" minus "C". C The electrode positions (red) relative to the clinical CT in greyscale with boundaries derived from the clinical CT in green for a participant with a low resolution pre-operative scan. D The same electrode positions and boundaries relative to the aligned OPFOS cochlea. The boundaries of the cochlea from the clinical CT are less clear toward the apex (i.e., the green boundary stops), while the boundary identified by the OPFOS template continues. Using the OPFOS image allows EMML distance measures to be made at the apex.
Identifying Medial Wall Where Not Resolved by Pre-operative CT
High-resolution (i.e., 100-μm cubic voxels) post-operative CT scans were acquired using the same equipment and protocol as described in Skinner et al. (2007). Subjects for whom a high-resolution pre-operative CT was not available and who had an un-implanted contralateral cochlea had the post-operative scan of the contralateral ear flipped and used in place of a pre-operative CT image. This technique was based on results from a micro CT analysis of 14 sets of matching left and right cadaveric temporal bones, which demonstrated differences much less than the measurement error of clinical scans. For subjects with bilateral implants, their highest resolution pre-operative CT scan was upsampled if necessary and used for the analysis. Automatic thresholding of the position of cochlear canal walls fails where the inter-scalar septum or the modiolus itself is smaller than the resolution of the CT. Alternatively, automatic thresholding can fail when partial-volume averaging results in an intensity value that is below the threshold being used to differentiate the bone of the otic capsule from the fluid/tissue space of the cochlear canal. This is most problematic when measuring the inner canal wall for insertion angles greater than 300° (i.e., more apically) and for subjects without a high-resolution image set. For these measurements the OPFOS cochlear atlas was used to assist in identifying the unresolved medial wall and modiolus. Figure 1C shows an example of a subject’s pre-operative clinical CT scan that was not acquired at high-resolution and Figure 1D shows how the OPFOS image can be examined to estimate the location of the modiolar wall. For more apical electrodes, the cochlear boundaries from the pre-operative scan (in green) are less clear and the registered OPFOS image is more informative and is used to estimate the distance to the modiolar wall. Figure 1B shows an example measurement of EMML distance and insertion angle for subject S41’s electrode 6, where A is the distance to the lateral wall, B is the distance to the electrode, and C is the distance to the inner wall from the MM axis along a medial–lateral line through the electrode. In this paper we report the EMML distance: the distance in millimeters of the electrode from the inner wall (B–C). Given that the inner wall is not always orthogonal to the axis defined by the electrode to MM axis, some variability across electrodes is introduced into the distance measure. Therefore, all analyses were repeated using percent distance (100 × (B–C)/(A–C)), which removes this variability. The conclusions of this study were not altered by these analyses, so for clarity we only describe the results in detail using millimeter distances.
RESULTS
CNC word scores at 12 months post-activation ranged from 25 % to 94 %, with a mean of 69.6 % and standard deviation (std) of 21.5 % correct as shown in Table 1. Holden et al. (2013) reported an average CNC Final word score of 65.4 % (std 20.7 %) for 114 subjects, which is not significantly different from our group mean (two-tailed t-test; t(122) = 0.613; p = 0.541). Thus, our small group appears to have a similar distribution of word scores as a larger cochlear implant population.
TABLE 1.
Subject demographics
| Research ID | Age (years)a | CNC words (%)b | Sound processor | Etiology | Duration of Sev/Prof HL (years)c |
|---|---|---|---|---|---|
| S36 | 72 | 25 | Freedom | Infection | 65 |
| S38 | 26 | 53 | Freedom | Unknown | 26 |
| S40 | 34 | 94 | Freedom | Unknown | 12 |
| S41 | 32 | 74 | Freedom | Unknown | 22 |
| S54 | 43 | 61 | Freedom | Ushers Type II | 7 |
| S55 | 52 | 92 | CP810 | Ménière's Syndrome | 14 |
| S66 | 44 | 55 | Freedom | Unknown | 28 |
| S80 | 33 | 82 | CP810 | Unknown | 17 |
| S89 | 38 | 72 | CP810 | Unknown | 34 |
| S101 | 62 | 82 | CP810 | Unknown | 52 |
Speech reception data were collected at the subject's clinical site at 12 months post-activation. MAP and sound processor settings were deemed acceptable by the clinical audiologist prior to testing. In speech testing, all subjects used their percutaneous cochlear implant with a Contour Advance™ electrode array attached to a commercial sound processor providing MP ACE stimulation
aAge at percutaneous implant surgery
bCNC word score (Peterson and Lehiste 1962) at 12 months post-activation with two lists (100 words) presented at 70 dB SPL
cDuration of Severe/Profound Hearing Loss at candidacy evaluation
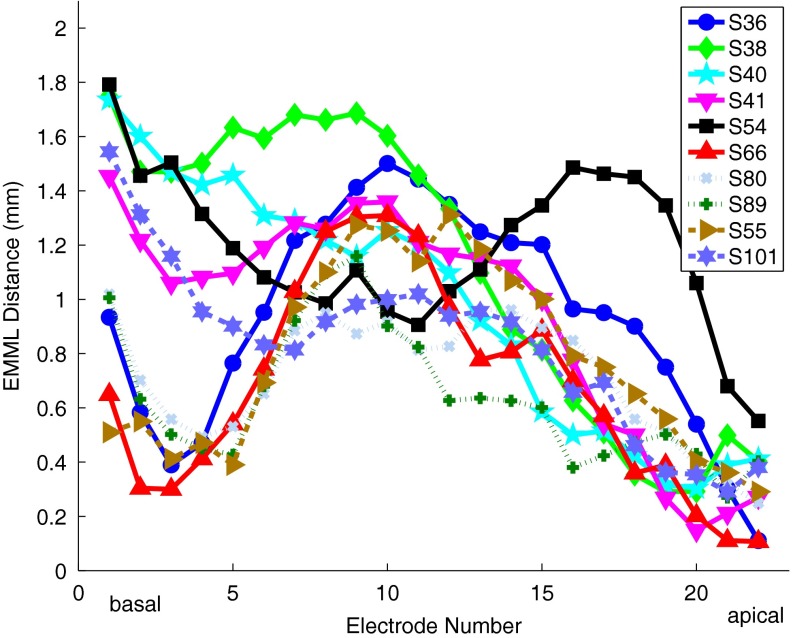
CT scan based distances between the electrodes and the modiolus are shown in Figure 2 for all ten subjects. There is a wide variability of distance both within and across subjects, with a total range of 0.1–1.8 mm. The seven most apical electrodes of the array are closer to the modiolus (mean = 0.53 mm, std = 0.23 mm, n = 10) than the eight at mid-array (mean = 1.09 mm, std = 0.17 mm, n = 10) and the seven at the base (mean = 0.99 mm, std = 0.39 mm, n = 10). The positions of the basal seven of the electrodes are more variable across subjects than the middle eight. The most apical electrode for subject S54 "folded over" and so the medial–lateral distances for many of this subject’s apical electrodes were elevated compared to the other subjects. Tip fold over can result in broken wires and an electrode that cannot be stimulated or an atypical pattern of distances of electrodes from the modiolus (Vanpoucke et al. 2012).
FIG. 2.
EMML distance for all electrodes for all subjects. The distances range from 0.1 to 1.8 mm. The electrodes at the apex are closest to the modiolus on average. S54’s apical pattern is different reflecting the fold-over of the most apical electrode.
Figure 3 shows Monopolar (MP) thresholds and Phased-Array (PA) thresholds (van den Honert and Kelsall 2007) for all subjects. MP thresholds are relatively homogeneous across the cochlea within a given subject compared to the PA thresholds, which can vary from as low as MP thresholds to greater than 20 dB higher. The within-subject variance of threshold is on average 2.25 dB2 across subjects for MP mode and 34.8 dB2 for PA mode. The variance of the PA thresholds was significantly correlated with the logit of the speech understanding score (r2 = 0.82; F(1,8) = 36.7; p = 0.0003; n = 10). The variance of the BP thresholds was significantly correlated with the logit of the speech understanding score (r2 = 0.82; F(1,5) = 22.7; p = 0.005; n = 7). The variance of TP thresholds was marginally significantly correlated with the logit of the speech understand score using the five subjects with thresholds (r2 = 0.72; F(1,3) = 7.7; p = 0.070; n = 5). The variance of the MP thresholds was not significantly correlated with the logit of the speech understanding score (r2 = 0.002; F(1,8) = 0.02; p = 0.893; n = 10).
FIG. 3.
Thresholds for all electrodes for all subjects in two of the modes under consideration. Monopolar (MP, blue circles) and Phased-Array (PA, red triangles) thresholds are shown as a function of electrode number at 250 pps, 100 μs/phase with each subject in a panel. The points indicate average thresholds over time per electrode. Notice that the PA thresholds ranged from as low as MP thresholds to many dB higher.
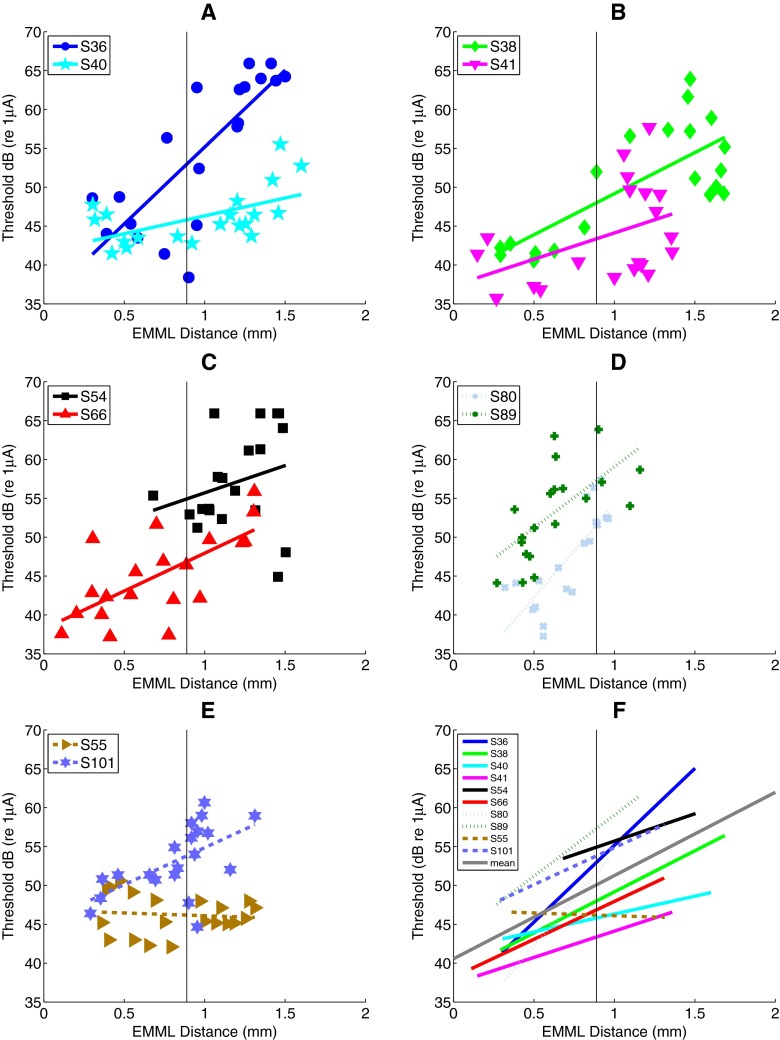
Figure 4A shows the raw data and linear regression lines for PA threshold and EMML distance for subjects S40 and S36, who had the best and worst CNC word scores, respectively. S40 had relatively low thresholds on average (46.15 dB re 1 μA), while S36 had higher ones on average (54.60 dB re 1 μA), but there is an overlap between the sets of thresholds. Thresholds for each of these subjects (expressed in dB) are well described by a linear relationship to medial–lateral distance (see Table 2). The PA threshold as a function of distance for S40 shows a significant correlation between distance and threshold (r2 = 0.32, p = 0.00949). S36 shows a different pattern, but a similar effect of distance on threshold (r2=0.62. p = 0.00004). Figure 4B,C,D,E shows the threshold-to-distance relationship for the eight other subjects. There was a significant correlation of threshold with distance for seven of the ten subjects (p < 0.01) as shown in Table 3. Distance explains between 32 % and 65 % of the threshold variance (for those seven significant subjects). Only subjects S41, S54, and S55 do not show a significant positive correlation of distance and threshold at a p = 0.05 level. Figure 4F shows the linear fits per subject without the raw data, where it is easy to see the similarity in slopes across most subjects. The average slope is 11 dB/mm and significantly different from zero (F(1,9) = 21.28; p = 0.0013; n = 10). The average slope in terms of percent distance is 0.25 dB/percent-distance and is significantly different from zero (F(1,9) = 26.18; p = 0.00063; n = 10).
FIG. 4.
PA threshold versus EMML distance for all subjects. A The PA threshold versus EMML distance for two example subjects (S36 and S40). B–E PA threshold versus distance for the eight other subjects. F Summary plot showing similarity of slopes. See text for more details.
TABLE 3.
Slopes (in dB/mm) and significance of relationships between thresholds and EMML distance for PA, TP, BP, and MP on a per subject basis
| Research ID | PA | TP | BP | MP | ||||
|---|---|---|---|---|---|---|---|---|
| Slope dB/mm | Significance | Slope dB/mm | Significance | Slope dB/mm | Significance | Slope dB/mm | Significance | |
| S36 | 19.75 | 0.00004 *** | DNT | 21.39 | 0.00004 *** | 2.72 | 0.00031 *** | |
| S38 | 10.57 | 0.00011 *** | DNT | DNT | 1.03 | 0.00270 ** | ||
| S40 | 4.59 | 0.00949 ** | 4.59 | 0.03516 * | 3.50 | 0.02279 * | 0.10 | 0.88755 |
| S41 | 6.77 | 0.05950 | DNT | DNT | 2.88 | 0.00154 ** | ||
| S54 | 7.03 | 0.27515 | DNT | 1.74 | 0.80292 | 1.94 | 0.14521 | |
| S55 | −0.70 | 0.69947 | −0.25 | 0.82623 | 7.12 | 0.03165 * | 1.58 | 0.07882 |
| S66 | 9.73 | 0.00098 *** | DNT | DNT | 1.58 | 0.14200 | ||
| S80 | 24.07 | 0.00002 *** | 25.06 | 0.00024 *** | 17.32 | 0.00015 *** | 4.13 | 0.08621 |
| S89 | 15.73 | 0.00225 ** | 22.76 | 0.00001 *** | 14.09 | 0.00019 *** | −1.04 | 0.34784 |
| S101 | 9.50 | 0.00755 ** | 11.44 | 0.00290 ** | 4.20 | 0.09962 | 3.63 | 0.00046 *** |
| Mean | 10.70 | 12.72 | 9.91 | 1.86 | ||||
| % significant | 70 % | 80 % | 71 % | 40 % | ||||
The table shows subject significance of the slope with p value listed as well as asterisks indicating different significance thresholds (***p < 0.001; **p < 0.01; *p < 0.05). The last row indicates what percent of subjects per condition met the significance threshold of 0.05
DNT did not test
We also obtained TP thresholds with a subset of five listeners and found an average slope of 13 dB/mm for TP thresholds and EMML distance which was marginally significantly different from zero (F(1,4) = 6.62; p = 0.062; n = 5). For BP thresholds, we found a significant 10 dB/mm average slope across the subjects tested (F(1,6) = 11.70; p = 0.014; n = 7). For MP thresholds, we found a significant, average slope of 2 dB/mm across ten subjects (F(1,9) = 13.78; p = 0.0048; n = 10). Table 3 shows the slopes in dB/mm and the significance level of the correlations on a per subject basis for each of the conditions and shows that MP mode produced the fewest subjects showing significance individually (40 % at p < 0.05). In contrast, with PA stimulation, the thresholds of 70 % of the subjects showed significant correlation with EMML distance at a 0.05 level (and 40 % did at a 0.001 level). The average increase in threshold with EMML distance was similar for PA, TP, and BP while that for MP was lower. The variance of PA thresholds was significantly correlated with the variance of TP (r2 = 0.98; F(1,4) = 138.91; p = 0.0013; n = 5) and BP (r2 = 0.84; F(1,6) = 25.76; p = 0.0039; n = 7) thresholds. No other significant correlations (p < 0.05) were found between the six possible pairings of the thresholds.
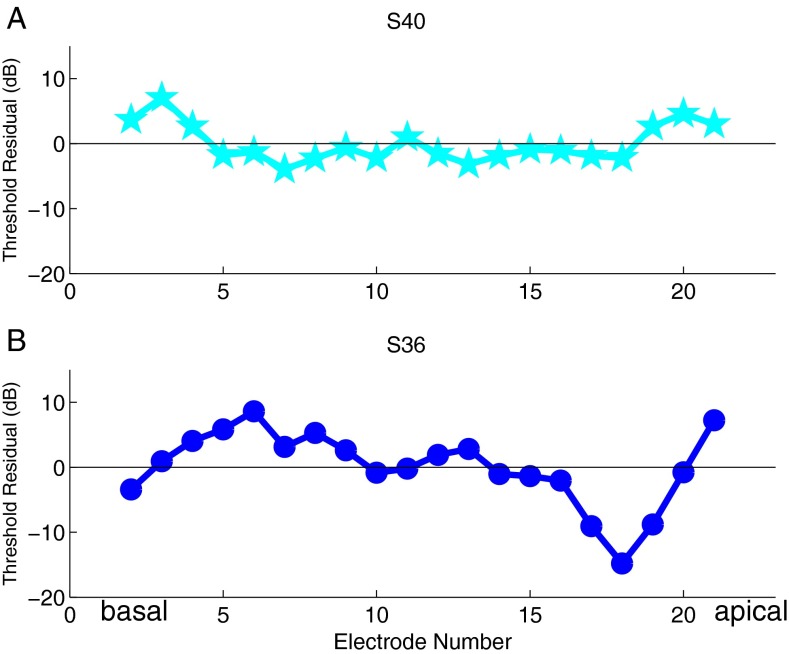
One way to characterize the relationship between EMML distance and threshold is in terms of the error of each linear fit of threshold versus distance. Figure 5A shows the error of the distance model (i.e., the residuals) versus electrode number for subject S40. Taking the root mean square error (RMSE) of these gives a metric of how much error there is in a linear distance model for each subject. For subject S40, the RMSE is 3 dB, and the error is small from electrodes 5 to 18. At the two extremes, the error is larger, which we speculate might be due to the limitations of PA focusing with fewer flanking electrodes to focus the current on one side of the channel center. Figure 5B shows the errors for subject S36. For S36 the RMSE is 6 dB indicating that distance is a less successful predictor of threshold for this subject.
FIG. 5.
Residual of the threshold–distance model versus electrode number for two subjects. A S40’s residuals are near zero across the array and the root mean square error of the residual of the distance model is 3 dB. B S36’s residuals are farther from zero and the root mean square error of the residual of the distance model for this subject is 6 dB.
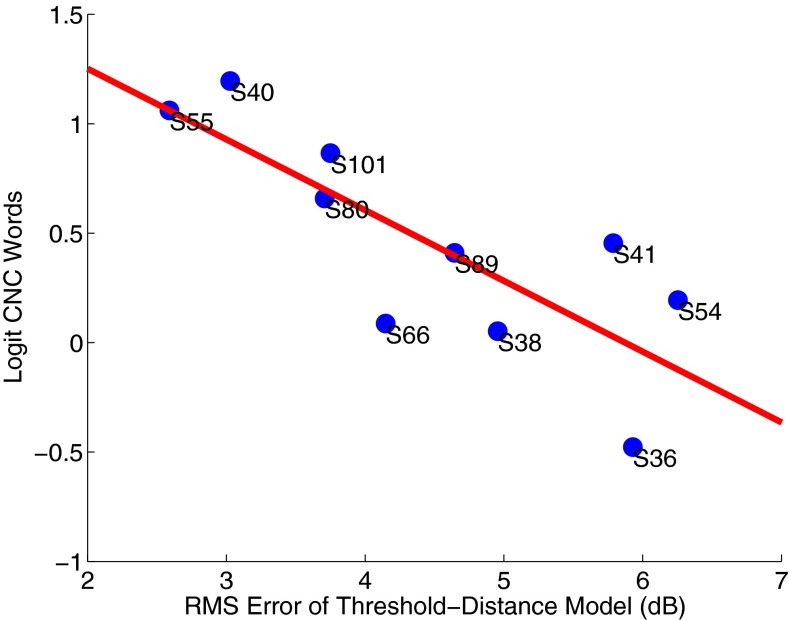
We examined the relationship between the RMSE of the distance model and the speech understanding score (expressed as the logit) for each subject and there was a negative correlation between them. The example subject whose thresholds were better predicted by the distance model (S40) had the best speech scores and the other example subject with higher RMSE (S36) had the poorest speech scores. Overall, the larger the error, the worse the speech understanding (r2 = 0.63; F(1,8) = 13.34; p = 0.0065; n = 10), as shown in Figure 6. In other words, the better the distance model predicts threshold, the better the speech understanding. If we only include those seven subjects whose thresholds were significantly predicted by distance, the negative correlation is stronger and is still significant (r2 = 0.88; F(1,5) = 35.04; p = 0.0020; n = 7).
FIG. 6.
Logit-transformed CNC word score versus RMS error of the threshold–distance model. There is a significant correlation between RMSE and speech understanding (r 2 = 0.63; F(1,8) = 13.34; p = 0.0065; n = 10).
Note, mean threshold alone was not a significant predictor of speech understanding (r2 = 0.18; F(1,8) = 1.80; p = 0.216; n = 10) indicating that simple elevation of thresholds was not problematic. EMML distance alone was not significantly predictive of speech understanding (r2 = 0.07; F(1,8) = 0.61; p = 0.456; n = 10), although the statistical power for this analysis was only 21 % given ten subjects and the effect of distance (wrapping factor) reported by Holden et al. (2013), so the absence of an effect does not contradict their results. The data also do not support an alternative hypothesis that predicted-threshold at the mean distance (0.89 mm) would be a significant predictor of speech understanding (r2 = 0.08; F(1,8) = 0.67; p = 0.438; n = 10).
In the study of Holden et al. (2013), the subjects with electrodes crossing into scala vestibuli (SV) from scala tympani (ST) had poorer speech understanding on average. In our study, the two subjects with electrodes in SV (S36 and S66) had CNC word scores of 36.5 % on average, while the eight subjects who had no electrodes in SV had CNC word scores of 72.5 % on average — a significant difference on a two tailed t-test (for logit percent correct: t(8) = −2.51; p = 0.036; n = 10; for percent correct t-test t(8) = −2.9779; p = 0.0176; n = 10).
DISCUSSION
Medial–Lateral Electrode Position Influences Perceptual Thresholds
Focused (PA) thresholds increased significantly with electrode distance at 11 dB/mm on average (std = 7 dB/mm). The magnitude of the effect of distance observed with TP (mean = 13 dB/mm and std = 11 dB/mm) and BP (mean = 10 dB/mm and std = 8 dB/mm) was similar to that of PA. In contrast, the magnitude of the effect of distance on MP thresholds (mean = 2 dB/mm and std = 2 dB/mm) was much smaller, yet still significant across subjects. Thus, electrode position should be accounted for in analyses of the electro-neural interface, studies that analyze the relationship between psychophysical thresholds and SGC counts, and in efficiency considerations in the design of cochlear implants.
A re-analysis of the previous literature shows results consistent with our findings. Cohen et al. (2001) plotted threshold as a function of distance with three listeners using BP + 1 mode in their Figure 3, which shows an average of 11 dB/mm (22, 6, and 5 dB/mm for the three subjects). Kawano et al. (1998) reported a significant, positive correlation of distance (measured in cadavers) of the electrode to Rosenthal's canal center in four of five subjects with BP threshold at p ≤ 0.05 using non-parametric statistics. Our re-examination of their data using a multi-level linear model shows a significant slope of bipolar threshold versus electrode distance across the five subjects of 11 dB/mm (p = 0.0326) with individual slopes of −1, 10, 14, 15, 21 dB/mm. The −1 dB/mm was obtained with less focused, BP + 2, thresholds as opposed to the others with BP + 1 thresholds. The present data expands the threshold–distance correlation to new stimulation paradigms and to more subjects with measures based on CT scans available in life.
Monopolar Speech Understanding Is Correlated with Threshold Variance and How Well Electrode Distances Predict Focused Thresholds
Neither mean threshold alone nor mean distance alone significantly predicted monopolar speech understanding in these recipients. The variance of the PA thresholds was significantly correlated with the logit of the speech understanding score (r2 = 0.82; F(1,8) = 36.7; p = 0.0003; n = 10) consistent with one of the metrics proposed by Pfingst et al. 2004. For their BP data, the correlation of consonant understanding and the variance of threshold was significant (r2 = 0.31; p < 0.05; n = 21). In our re-analysis of the data of Bierer (2007), the variance metric was also significantly correlated with speech understanding for BP thresholds (r2 = 0.97; F(1,4) = 91.9; p = 0.002; n = 5) and TP thresholds (r2 = 0.83; F(1,4) = 19.8; p = 0.011; n = 6).
In contrast, the variability of the distance was not significantly correlated with speech understanding in this study (r2 = 0.03; F(1,8) = 0.24; p = 0.686; n = 10). Speech understanding was, however, well predicted by how well a model of electrode distance predicted focused thresholds. This builds on previous work showing that location related, site-to-site differences in focused threshold negatively correlate with speech understanding. Pfingst and Xu (2004) used the mean of the absolute value of neighbor-to-neighbor differences of focused thresholds as a negative predictor of speech understanding. We examined this metric’s correlation with speech understanding in our data set and found only a marginally significant correlation (r2 = 0.32; F(1,8) = 3.71; p = 0.09; n = 10). Bierer (2007) used the standard deviation of the absolute value of the neighbor-to-neighbor differences of focused threshold. We examined this metric’s correlation with speech understanding and observed a marginally significant effect (r2 = 0.33; F(1,8) = 3.99; p = 0.081; n = 10).
Since EMML distance should be a smoothly varying function, as constrained by the mechanical properties of the electrode array and the smooth spiral of the cochlear canal, site-to-site variation in focused thresholds would be expected to be low for a subject whose thresholds were well predicted by electrode distance. Our work using the RMSE of the threshold–distance model extends the previous work by adding information about the position of the electrode array. For our data set, including electrode position information explained more of the variance of speech understanding (r2 = 0.63; F(1,8) = 13.34; p = 0.0065; n = 10) than either of the previously proposed site-to-site metrics.
The finding that speech understanding correlates with the variance of the focused thresholds is consistent with previous studies. Our work extends this concept by showing that, although threshold significantly depends on distance, speech understanding does not correlate with the within-subject variability of the distance. Rather, speech understanding correlates with the variability of the residual of the relationship between threshold and distance.
Effect of Electrodes in Scala Vestibuli
There are two main hypotheses regarding the mechanism by which having electrodes in SV causes a detriment to speech understanding: the incorrect position of the electrodes causes altered current pathways, perhaps from across-turn stimulation (Holden et al. 2013), or tissue damage where the electrode crosses from ST to SV reduces SGC counts (Nadol et al. 2001).
In our data, thresholds were significantly higher for the electrodes in the transition zone from ST to SV based on the two subjects with electrodes traversing the scalae (S36: electrodes 7 to 12; S66 electrodes 9 to 11), but this threshold elevation was explainable by distance (with no significant contribution of scalar position when controlling for distance from the modiolar wall). That is, the medial–lateral distances were also significantly higher for those electrodes traversing the scalae than for other electrodes. Thresholds in ST and SV were not significantly different from each other (nor were distances). Also, including vertical (tympano-vestibuli) distance as an additional factor, or as a measure to allow derivation of the hypotenuse of a triangle of distance from the putative site of SGCs, did not improve the threshold predictions. The EMML distance appears to have the greatest explanatory power for threshold.
In addition, controlling for RMSE of the threshold to distance model, the presence of electrodes in SV was not a significant predictor of speech understanding (F(2,7) = 2.88; p = 0.133; n = 10). While controlling for presence of electrodes in SV, RMSE remained a significant predictor of speech understanding (F(2,7) = 8.29; p = 0.024; n = 10). The variance of threshold and RMSE have the greatest explanatory power for speech understanding for our data set.
Inferences About Neural Survival
Subjects with focused thresholds that were not well explained by EMML distance understood speech less well than those whose thresholds were well described by distance. The simplest explanation for changes to thresholds not due to EMML distance is that changes in effective distance caused by gaps in the neural substrate changed thresholds. There clearly are cochlear implant users with extensive loss of SGCs (Fayad and Linthicum 2006; Khan et al. 2005). Variability in SGC count along the cochlea would introduce variability in the effective distance from the electrodes to the neural tissue and potentially manifest as increased variability in thresholds and particularly in increased variability in thresholds controlling for EMML distance. Thus, variability in threshold not explainable by distance likely indicates variability in the pattern of neural survival.
Alternative Hypotheses
This study makes inferences about neural survival based on thresholds, CT scans, and speech understanding. An alternative hypothesis to explain threshold variation within subjects and speech understanding across subjects would involve bone or fibrous tissue blocking the current flow to the neural tissue. There are a number of factors which argue against this interpretation. We did not observe signs indicative of bone growth in the CT scans and our subject population did not have etiologies associated with rapid bone formation (e.g., meningitis and otosclerosis). Only one of the ten subjects (S40) had any electrodes which showed a significant increase in threshold over time (at a significance level of 0.05 with Bonferroni correction for the number of electrodes per subject), but this subject had very low variability of thresholds across electrodes and one of the best speech understanding scores. Also, other researchers have reported that neither new bone growth nor fibrous tissue growth correlated with speech understanding (Li et al. 2007), so such factors would not explain our speech understanding results. The above factors argue against bone or fibrous tissue having a significant effect on the results.
Implications for Interventions to Improve Speech Understanding
This study has focused on making inferences about neural survival in cochlear implant users, information that may be useful for optimizing clinical outcomes. Several researchers have proposed clinical parameter modifications or removal of electrodes based on psychophysical or objectives measures (e.g., Garadat et al. 2012; Noble et al. 2013; Zwolan et al. 1997), but there is at present no clinical recommendation that has been widely adopted for such modifications. Our work confirms that the focused threshold variation across the cochlea — and in particular threshold variation not due to EMML distance — is a strong predictor of speech understanding. Deactivation of channels based on high focused thresholds, with or without controlling for EMML distance, might potentially provide benefits to speech understanding.
To explore whether useful predictions can be made without a CT scan, we examined whether the mean distances per electrode across subjects have similar predictive value. The mean distance across all subjects was used to determine a set of residuals of threshold versus this mean distance. This metric was significantly correlated with speech understanding (r2 = 0.50; F(1,8) = 7.93; p = 0.023; n = 10). Thus, a generic threshold-controlling-for-distance metric is possible, to the degree that insertion results from two surgeons and ten subjects can be generalized to all users of the Contour Advance. Studies of insertions of the Contour Advance electrode array in cadaveric temporal bones (Roland 2005; Roland and Wright 2006) have suggested that mid-array electrodes tend to be less perimodiolar than more apical or basal electrodes; and a modified insertion technique that attempts to pull the middle electrodes of the array closer to the modiolus resulted in reduced spread of excitation on mid-array electrodes (Todt et al. 2005). In patients, ECAP and behavioral thresholds were found to be lowest at the apex and highest at mid-array for Contour electrodes (Gordon and Papsin 2013). Thus, the distance pattern observed in this study is likely to be representative of many implanted Contour Advance devices so a generic threshold-controlling-for-distance metric may be generalizable.
Another motivation for understanding the status of neural survival and its effect on speech understanding is the growing focus on neurotrophic factors (e.g., Landry et al. 2013; Leake et al. 2013). If neural loss is a key factor, then neural preservation and regeneration methods may provide an important therapeutic option.
Our approach has been to examine the possible mechanisms underlying variability of focused thresholds and speech understanding. These results have been obtained in a special population of cochlear implant subjects with a research interface. One of our ongoing goals is to apply information about the underlying mechanisms to guide deactivation of channels by techniques that might not require a CT scan, special devices, or laborious psychophysical measures.
CONCLUSIONS
Our results point to SGC loss being an important contributor to reduced speech understanding with a cochlear implant and may explain much of the variability in clinical outcomes across patients. This validates the importance of atraumatic surgery, of research into preservative or regenerative interventions, and the potential for clinical parameter modifications to optimize cochlear implant outcomes.
Acknowledgments
CJL, ZMS, WSP are employed by Cochlear Ltd. We thank the patients for their hard work, the audiologists at the clinical study sites for programming and data collection, Chris van den Honert for numerous contributions, and two anonymous reviewers for their useful suggestions. TAH acknowledges the support of the National Institutes of Health, National Institute on Deafness and Communication Disorders R01 DC00581 and R01 DC009010.
References
- Bierer JA. Threshold and channel interaction in cochlear implant users: evaluation of the tripolar electrode configuration. J Acoust Soc Am. 2007;121:1642. doi: 10.1121/1.2436712. [DOI] [PubMed] [Google Scholar]
- Bierer JA. Probing the electrode–neuron interface with focused cochlear implant stimulation. Trends Amplif. 2010;14:84–95. doi: 10.1177/1084713810375249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blamey P, Arndt P, Bergeron F, Bredberg G, Brimacombe J, Facer G, Larky J, Lindström B, Nedzelski J, Peterson A, Shipp D, Staller S, Whitford L. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants. Audiol Neurootol. 1996;1:293–306. doi: 10.1159/000259212. [DOI] [PubMed] [Google Scholar]
- Blamey PJ, Pyman BC, Gordon M, Clark GM, Brown AM, Dowell RC, Hollow RD. Factors predicting postoperative sentence scores in postlinguistically deaf adult cochlear implant patients. Ann Otol Rhinol Laryngol. 1992;101:342–8. doi: 10.1177/000348949210100410. [DOI] [PubMed] [Google Scholar]
- Cohen LT, Saunders E, Clark GM. Psychophysics of a prototype peri-modiolar cochlear implant electrode array. Hear Res. 2001;155:63–81. doi: 10.1016/S0378-5955(01)00248-9. [DOI] [PubMed] [Google Scholar]
- Fayad JN, Linthicum FH. Multichannel cochlear implants: relation of histopathology to performance. Laryngoscope. 2006;116:1310–20. doi: 10.1097/01.mlg.0000227176.09500.28. [DOI] [PubMed] [Google Scholar]
- Garadat SN, Zwolan TA, Pfingst BE. Across-site patterns of modulation detection: relation to speech recognition. J Acoust Soc Am. 2012;131:4030–41. doi: 10.1121/1.3701879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon KA, Papsin BC. From nucleus 24 to 513: changing cochlear implant design affects auditory response thresholds. Otol Neurotol. 2013;34:436–42. doi: 10.1097/MAO.0b013e3182804784. [DOI] [PubMed] [Google Scholar]
- Holden LK, Finley CC, Firszt JB, Holden TA, Brenner C, Potts LG, Gotter BD, Vanderhoof SS, Mispagel K, Heydebrand G, Skinner MW. Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear. 2013;34:342–60. doi: 10.1097/AUD.0b013e3182741aa7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Honert C, Carlyon R, Long C, Smith Z, Shelton C, Kelsall D. Perceptual evidence of spatially-restricted excitation with focused stimulation. Conf Implantable Audit Prostheses. 2007;2:58. [Google Scholar]
- Van den Honert C, Kelsall DC. Focused intracochlear electric stimulation with phased array channels. J Acoust Soc Am. 2007;121:3703–16. doi: 10.1121/1.2722047. [DOI] [PubMed] [Google Scholar]
- Judd CM, McClelland GH, Ryan CS (2009) Data analysis: a model comparison approach, 2nd ed. Routledge, New York, p 328
- Kawano A, Seldon HL, Clark GM, Ramsden RT, Raine CH. Intracochlear factors contributing to psychophysical percepts following cochlear implantation. Acta Otolaryngol (Stockh) 1998;118:313–326. doi: 10.1080/00016489850183386. [DOI] [PubMed] [Google Scholar]
- Khan AM, Handzel O, Burgess BJ, Damian D, Eddington DK, Nadol JB. Is word recognition correlated with the number of surviving spiral ganglion cells and electrode insertion depth in human subjects with cochlear implants? Laryngoscope. 2005;115:672–7. doi: 10.1097/01.mlg.0000161335.62139.80. [DOI] [PubMed] [Google Scholar]
- Landry TG, Fallon JB, Wise AK, Shepherd RK. Chronic neurotrophin delivery promotes ectopic neurite growth from the spiral ganglion of deafened cochleae without compromising the spatial selectivity ofcochlear implants. J Comp Neurol. 2013 doi: 10.1002/cne.23318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake PA, Stakhovskaya O, Hetherington A, Rebscher SJ, Bonham B. Effects of brain-derived neurotrophic factor (BDNF) and electrical stimulation on survival and function of cochlear spiral ganglion neurons in deafened, developing cats. J Assoc Res Otolaryngol. 2013;14:187–211. doi: 10.1007/s10162-013-0372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychophysics. J Acoust Soc Am. 1971;49:467–477. doi: 10.1121/1.1912375. [DOI] [PubMed] [Google Scholar]
- Li PM, Somdas MA, Eddington DK, Nadol JB. Analysis of intracochlear new bone and fibrous tissue formation in human subjects with cochlear implants. Ann Otol Rhinol Laryngol. 2007;116:731–8. doi: 10.1177/000348940711601004. [DOI] [PubMed] [Google Scholar]
- Makary CA, Shin J, Kujawa SG, Liberman MC, Merchant SN. Age-related primary cochlear neuronal degeneration in human temporal bones. J Assoc Res Otolaryngol. 2011;12:711–7. doi: 10.1007/s10162-011-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadol JB, Young YS, Glynn RJ. Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1989;98:411–6. doi: 10.1177/000348948909800602. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Shiao J, Burgess B, Ketten D, Eddington D, Gantz B, Kos I, Montandon P, Coker N, Roland JJ, Shallop J (2001) Histopathology of cochlear implants in humans. Ann Otol Rhinol Laryngol 110:883–891 [DOI] [PubMed]
- Noble JH, Labadie RF, Gifford R, Dawant B. Image-guidance enables new methods for customizing cochlear implant stimulation strategies. IEEE Trans Neural Syst Rehabil Eng. 2013 doi: 10.1109/TNSRE.2013.2253333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson GE, Lehiste I. Revised CNC lists for auditory tests. J Speech Hear Dis. 1962;27:62–70. doi: 10.1044/jshd.2701.62. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Xu L. Across-site variation in detection thresholds and maximum comfortable loudness levels for cochlear implants. J Assoc Res Otolaryngol. 2004;5:11–24. doi: 10.1007/s10162-003-3051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Xu L, Thompson CS. Across-site threshold variation in cochlear implants: relation to speech recognition. Audiol Neurootol. 2004;9:341–52. doi: 10.1159/000081283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb RA. The biomedical imaging resource at Mayo Clinic. IEEE Trans Med Imaging. 2001;20:854–67. doi: 10.1109/42.952724. [DOI] [PubMed] [Google Scholar]
- Roland JT. A model for cochlear implant electrode insertion and force evaluation: results with a new electrode design and insertion technique. Laryngoscope. 2005;115:1325–39. doi: 10.1097/01.mlg.0000167993.05007.35. [DOI] [PubMed] [Google Scholar]
- Roland P, Wright C. Surgical aspects of cochlear implantation: mechanisms of insertional trauma. Adv Otorhinolaryngol. 2006;64:11–30. doi: 10.1159/000094642. [DOI] [PubMed] [Google Scholar]
- Skinner MW, Holden TA, Whiting BR, Voie AH, Brunsden B, Neely JG, Saxon EA, Hullar TE, Finley CC. In vivo estimates of the position of advanced bionics electrode arrays in the human cochlea. Ann Otol Rhinol Laryngol Suppl. 2007;197:2–24. [PubMed] [Google Scholar]
- Smith ZM, Parkinson WS, Long CJ (2013) Multipolar current focusing increases spectral resolution in cochlear implants. Conf Proc IEEE Eng Med Biol Soc Jul 2013:2796–2799. [DOI] [PubMed]
- Stakhovskaya O, Sridhar D, Bonham BH, Leake PA. Frequency map for the human cochlear spiral ganglion: implications for cochlear implants. J Assoc Res Otolaryngol. 2007;8:220–33. doi: 10.1007/s10162-007-0076-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teymouri J, Hullar TE, Holden TA, Chole RA. Verification of computed tomographic estimates of cochlear implant array position: a micro-CT and histologic analysis. Otol Neurotol. 2011;32:980–6. doi: 10.1097/MAO.0b013e3182255915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todt I, Basta D, Eisenschenk A, Ernst A. The “pull-back” technique for Nucleus 24 perimodiolar electrode insertion. Otolaryngol Head Neck Surg. 2005;132:751–4. doi: 10.1016/j.otohns.2005.01.046. [DOI] [PubMed] [Google Scholar]
- Vanpoucke F, Boermans P, Frijns J. Assessing the placement of a cochlear electrode array by multidimensional scaling. IEEE Trans Biomed Eng. 2012;59:307–310. doi: 10.1109/TBME.2011.2173198. [DOI] [PubMed] [Google Scholar]
- Voie AH. Imaging the intact guinea pig tympanic bulla by orthogonal-plane fluorescence optical sectioning microscopy. Hear Res. 2002;171:119–128. doi: 10.1016/S0378-5955(02)00493-8. [DOI] [PubMed] [Google Scholar]
- Zwolan TA, Collins LM, Wakefield GH. Electrode discrimination and speech recognition in postlingually deafened adult cochlear implant subjects. J Acoust Soc Am. 1997;102:3673–85. doi: 10.1121/1.420401. [DOI] [PubMed] [Google Scholar]