Abstract
Hippocampal sclerosis of aging (HS-Aging) neuropathology was observed in more than 15% of aged individuals in prior studies. However, much remains unknown about the clinical correlates of HS-Aging pathology or the association(s) between HS-Aging, Alzheimer's disease (AD), and frontotemporal lobar degeneration (FTLD) pathology. Clinical and comorbid pathological features linked to HS-Aging pathology were analyzed using National Alzheimer's Coordinating Center (NACC) data. From autopsy data extending back to 1990 (N=9,817 participants), the neuropathologic diagnoses were evaluated from American AD Centers (ADCs). Among participants who died between 2005-2012 (N=1,422), additional analyses identified clinical and pathological features associated with HS-Aging pathology. We also compared cognitive testing and longevity outcomes between HS-Aging cases and a subsample with non-tauopathy FTLD (N=210). Reporting of HS pathology increased dramatically among ADCs in recent years, to nearly 20% of autopsies in 2012. Participants with relatively “pure” HS-Aging pathology were often diagnosed clinically as having probable (68%) or possible (15%) AD. However, the co-occurrence of HS-Aging pathology and AD neuropathology (AD-NP) did not indicate any pattern of correlation between the two pathologies. Compared other pathologies, participants with HS-Aging pathology had higher overall cognitive/functional ability (versus AD-NP) and verbal fluency (versus both AD-NP and FTLD) but similar episodic memory impairment at one clinic visit 2 -5 years prior to death. Patients with HS-Aging live considerably longer than patients with non-tauopathy FTLD. We conclude that the manifestations of HS-Aging, increasingly recognized in recent years, probably indicate a separate disease process of direct relevance to patient care, dementia research, and clinical trials.
Keywords: TDP-43, oldest-old, hippocampus, human, APOE
Introduction
Hippocampal sclerosis of aging (HS-Aging) is a common neuropathological finding characterized by cell loss, gliosis, and atrophy in the hippocampal formation that is out of proportion to Alzheimer's disease (AD) neuropathologic change in the same structures [1–4]. The clinical presentation of participants with HS-Aging pathology is generally confused with AD because of overlapping neurocognitive and radiographic features [5]. The presence of HS-aging pathology is associated with substantial cognitive impairment, which is disease-specific whether or not other comorbid pathologies are present [6]. HS-Aging pathology is observed in up to 20% of individuals over age 85 in autopsy series,[2,3,7] and thus, HS-Aging pathology rivals AD pathology as a cause of cognitive impairment in the elderly [2,3].
Despite recent advances, there is still a generally poor understanding of HS-Aging.[4] Key knowledge gaps include clinical correlates of the disease and understanding the correlations between HS-Aging pathology, AD pathology, and frontotemporal lobar degeneration (FTLD) in individual patients. There are currently no clinical biomarkers or animal models specific to HS-Aging to aid clinical differential diagnosis or therapeutic trials. HS -Aging differs fundamentally from other conditions linked to hippocampal neuron loss (AD, epilepsy, and hypoperfusion/reperfusion of the brain) including other diseases referred to as “hippocampal sclerosis”, because HS-Aging has a pathologic marker -- aberrant TDP-43 immunohistochemistry [2,8]. There are important differences between FTLD-TDP and HS-Aging (clinically and pathologically) although the diseases both manifest with TDP-43 pathology [4]; more work is required to delineate the “border zones” of these diseases. Genetic polymorphisms have been linked to pathology similar to HS-Aging [9,10] but their direct relevance to HS-Aging is yet to be confirmed. There is a need for new research using large autopsy cohorts to provide insight into clinical measures that may be used to identify individuals with eventual HS-Aging pathology.
To learn more about individuals who died with HS-Aging pathology, we used National Alzheimer's Coordinating Center (NACC) data from U.S. Alzheimer's Disease Centers (ADCs). Specifically, data from participants who had been examined clinically with subsequent neuropathological evaluation were analyzed to gain the following new information: (1) historical trends in NACC/ADC research participants' pathologic diagnoses related to HS-Aging (2) demographics, antemortem clinical diagnoses, and APOE ε4 allele of individuals with eventual HS-Aging pathology (3) evidence for correlation or independence of HS-Aging pathology with AD pathology in the same cases, and (4) clinical (neurocognitive) measures of individuals with HS-Aging pathology.
Methods
Data Source and Study Sample
Research volunteers evaluated in one of 34 past and present ADCs throughout the United States with both reported clinical and autopsy data, beginning in 1990, were the initial data source for these analyses. For analyses of HS-Aging, participants were excluded if they were younger than 70 at death to best match all participants to HS-Aging age range, had autopsy determined prion disease, triplet repeat diseases, brain cancer, any subtype of FTLD, genetic abnormalities, and other rare neurological diseases. Participants with missing CERAD stages of neuritic plaque densities [11], Braak stages for neurofibrillary tangles [12], or assessment of vascular pathology were also excluded. A separate analysis included participants of any age at death with non-tauopathy subtypes of FTLD (e.g. ubiquitin positive, TDP-43 positive, no distinctive histopathology) in order to make comparisons with HS-Aging. Data were obtained from the NACC Minimum Data Set (MDS), Uniform Data Set (UDS), and Neuropathology (NP) Data Set [13–15]. The MDS includes standardized diagnostic data from participants evaluated from 1985 and the UDS includes standardized clinical, neuropsychological, and diagnostic data from participants that were evaluated between September 1, 2005 and December 31, 2012. Methods and rationale for the UDS clinical examination has been previously published [14,15], but briefly, data were collected by trained clinicians and interviewers. All participants received an initial in-person clinical evaluation and up to seven follow-up evaluations; data were collected on an approximately annual basis [14,15]. Research using NACC data was approved by the University of Washington Human Subjects Division.
Pathological Features
Neuropathologic evaluations, including autopsy, histopathology, and diagnostic assessment, were performed at individual ADCs, according to their own protocols, and entered into a standardized format for NACC purposes. In this analysis, HS-Aging pathology was defined as present if the neuropathologist recorded a “primary or contributing pathologic diagnosis of hippocampal sclerosis” among participants who died at age 70 years or older, and who did not have any subtype of FTLD pathology (HS is seen in many FTLD cases but we hypothesize this indicates a different disease process for reasons described previously[4]). AD neuropathology (referred to hereafter as “AD-NP”) was operationalized using CERAD stages of neuritic plaque densities (frequent, moderate, sparse, or none) [11] and Braak stages for neurofibrillary pathology (Stages 0-VI) [12]. Without recourse to more quantitative metrics of AD-NP, no operationalization of pathology is perfect. For the dichotomous operationalization of AD-NP, we applied two different criteria, one more specific and one more sensitive, to minimize the likelihood of a spurious result linked to our chosen method. For the more stringent criteria, AD-NP was indicated by Braak Stages V or VI with CERAD neuritic plaque densities of “moderate” or “frequent”; this analogous to “high severity” AD neuropathologic changes [16]. Because this stringent criteria could be insufficiently sensitive to include lower-severity AD cases, we also applied a less stringent (more sensitive) criteria: Braak Stages III, IV, V, or VI with CERAD “moderate or frequent”, which is analogous to “intermediate severity” of AD neuropathologic changes [16] and is referred to as “AD-NP(I)”. Lewy body pathology was characterized according to established guidelines [17]. Presence of FTLD subtypes were documented and defined for this analysis according to tau- positive subtypes (e.g. Pick's disease, corticobasal degeneration, progressive supranuclear palsy, and other tauopathies) and non-tauopathy subtypes (e.g. ubiquitin-positive/tau-negative inclusions, no distinctive histology, or not specified but not a tauopathy). Assessment of TDP-43 was not questioned on the NACC NP form, but allowed as a write-in.
Clinical Characteristics
Demographic characteristics were obtained via structured interviews of the subject and/or study partner during clinic visits age and year of death were recorded at autopsy. The total number of UDS visits was recorded and the duration of cognitive symptoms was calculated as age at death minus age at onset of cognitive symptoms. At each visit, severity of cognitive symptoms was measured using the Clinical Dementia Rating “sum of boxes” (CDR-SB) score [18], which ranges from 0-18, with higher scores corresponding to increasing impairment. A neuropsychological battery was administered to able participants and included verbal fluency using category fluency, animals generation test [19] and episodic memory using Delayed Logical Memory Recall [20]. Using all available information, clinicians recorded cognitive status (normal, impaired not MCI, MCI, or dementia) at each visit and determined a clinical diagnosis indicating the suspected etiology of cognitive impairment for those with impaired cognition. Diagnosis of Probable or Possible AD was based on NINCDS/ADRDA criteria [21]. APOE genotype was assessed for subset of participants; number of APOE ε4 alleles was categorized as none, 1, or 2.
Statistical Analyses
HS-Aging as a proportion of pathological diagnoses was described by calendar year of death for participants who died from 1990-2012 (N=9,817). Additional studies addressed data on UDS participants among research participants who died between 2005 and 2012 (N=1,422). Descriptive statistics characterized demographic and clinical features at last visit for participants with at least one UDS visit. Age-related trends in prevalence of any HS-Aging, AD, and Vascular neuropathological diagnoses as well as prevalence of clinical primary Probable AD were also examined (due to small numbers in this convenience sample, non-demented participants were excluded). Participants could have multiple pathologies (primary and contributing). Proportions of diagnoses across age were displayed using fitted polynomial curves. Since HS may share common pathways with, or considered a part of FTLD (especially FTLD with TDP-43 inclusions), we also examined age-related trends in prevalence of any HS and non-tauopathy FTLD by plotting Kaplan-Meier survival curves with age as the time scale of deaths in all UDS participants with HS and/or non-tauopathy FTLD. Equality of survival functions was tested using the logrank test.
In order to evaluate whether HS-Aging pathology occurs independently of AD-NP, we described the co-occurrence of HS-Aging pathology with each combination of CERAD neuritic plaque score and Braak stage. We also described the co-occurrence of Diffuse or Limbic Lewy body pathology, with each combination of CERAD neuritic plaque score and Braak stage in order to compare AD pathology with a pathological feature that tends to be associated with at least moderate AD severity as shown previously [22–24].
To understand how clinical manifestations of HS-Aging pathology and AD-NP differ, we compared neuropsychological tests previously found to be predictive of HS-Aging (episodic memory and verbal fluency) [2] among cognitively impaired participants with and without HS-Aging and AD-NP. This analysis focused on participants with longitudinal data and mild-to-moderate cognitive impairment (CDR-SB 0.5-15.5) [25] at a visit 2-5 years prior to death in order to identify cognitive patterns evident in participants likely to receive extensive cognitive testing in a clinical settings. Participants with severe dementia were excluded since they often could not complete extensive testing; participants missing education and participants who did not test in English were also excluded. Cognitive test scores were recorded as missing for participants unable or unwilling to complete testing. Multivariable linear regression using generalized estimating equations (GEE) was performed to compare test scores from a visit 2-5 years prior to death, separately, for participants with and without HS-Aging and AD pathologies after adjustment for education, and years between visit and death. Models were additionally adjusted for sex, however, results were similar. Primary outcomes were CDR-SB, animals generation (verbal fluency) and Logical Memory Delayed story recall (episodic memory) test scores. The primary exposure was the 4-level HS-Aging (yes, no) and AD-NP (yes, no) categorical variable. In order to make comparisons in test scores in relation to relatively “pure” HS-Aging pathology, participants with HS-Aging pathology and no AD-NP were chosen as the reference group. Predicted test score values were calculated for each participant based on fitted models for verbal fluency and episodic memory and displayed using box plots. We also performed a similar analysis to compare test scores between UDS participants (any ages at death) with non-tauopathy FTLDs, HS-Aging, and neither. This analysis was restricted to those without AD-NP, since AD could impact clinical symptoms of HS-Aging or FTLD. Statistical analyses were performed using STATA 12.0. All tests were two-tailed with α -levels set to 0.05.
Results
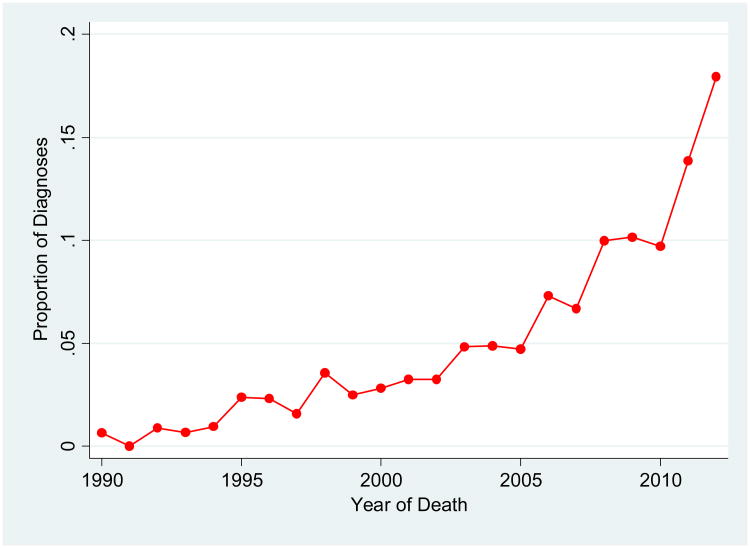
We first assessed diagnostic trends among ADC neuropathologists to highlight changes in the recognition of HS-Aging pathology over time. HS-Aging pathologic diagnoses between 1990 and December 2012 increased from approximately 0% to over 18% of diagnoses in autopsied NACC participants (Figure 1).
Figure 1.
Proportion of Hippocampal Sclerosis of Aging pathological diagnoses (primary and contributing) among autopsied participants in the NACC Neuropathology Data Set, by year of death, 1990-2012 (N=9,187).
Subsequent analyses were performed on individuals who died after the UDS collection began; an interval (2005 through 2012) of steady increase in the recognition of HS-Aging pathology by neuropathologists at ADCs. According to our operationalization among UDS cases, 118 were cases of HS-Aging (8.2% of autopsies), of which 71 also had AD-NP. Among the 675 participants who did not have HS-Aging or AD-NP the majority had one or multiple pathologies: 573 (84.9%) had any severity of vascular pathology (only 39% with primary or contributing pathological diagnosis of vascular disease), 250 (37.0%) had intermediate severity of AD (Braak Stage III/IV and frequent or moderate neuritic plaques), 164 (24.0%) had diffuse or limbic Lew body pathology (n=162, 24%), and 89 (13.3%) had a diagnosis of normal brain. Presence of vascular pathology, Lewy body, or other major pathologies did not differ significantly between groupings of HS-Aging and AD-NP (p<0.05 for all). The mean number of participants contributed by each ADC was 47.4 (range 2-160 participants).
Subject demographic and clinical characteristics stratified by HS-Aging and AD-NP categories are described in Table 1. Compared to participants with no HS-Aging pathology, a higher proportion of participants diagnosed with HS-Aging pathology died in advanced age (90 years or older) (Table 1). Participants with AD-NP, regardless of HS-Aging pathology status, however, tended to have died at a younger age, have a family history of dementia, have one or more APOE ε4 alleles, have an earlier age of onset, and a longer duration of disease (Table 1). On average, participants with HS-Aging pathology and the same AD-NP status also had worse cognitive and functional impairment at last visit compared to those without HS-Aging pathology, but age of onset was similar (Table 1). There was no significant difference in APOE ε4 allele status between participants with HS-Aging and without (p=0.23). Results were substantially similar when less stringent criteria (including cases with less severe AD pathology) for AD neuropathologic changes were applied (Supplementary Tables 1, 3).
Table 1. Participant characteristics at last visit by Hippocampal Sclerosis of aging and Alzheimer's disease neuropathology (N=1,422).
| Characteristic | No HS-Aging Pathology | HS-Aging Pathology Present | |||
|---|---|---|---|---|---|
|
|
|
||||
| No AD-NP (n=675) | AD-NP (n=629) | No AD-NP (n=47) | AD-NP (n=71) | ||
|
| |||||
| N (%) | N(%) | ||||
| Age at death (years) | |||||
| 70-79 | 126 (18.7) | 219 (34.8) | 6 (12.8) | 12 (16.9) | |
| 80-89 | 286 (42.4) | 297 (47.2) | 20 (42.6) | 38 (53.5) | |
| 90+ | 263 (39.0) | 113 (18.0) | 21 (44.7) | 21 (29.6) | |
| Sex | |||||
| Female | 323 (47.9) | 279 (44.4) | 24 (51.1) | 32 (45.1) | |
| Race* | |||||
| White | 649 (96.3) | 597 (94.9) | 42 (89.4) | 65 (91.6) | |
| Black | 14 (2.1) | 23 (3.7) | 4 (8.5) | 4 (5.6) | |
| Other | 11 (1.6) | 9 (1.4) | 1 (2.1) | 2 (2.8) | |
| Hispanic Ethnicity* | 27 (4.0) | 21 (3.4) | 3 (6.4) | 1 (1.4) | |
| Education* | |||||
| College graduate | 376 (56.0) | 338 (54.3) | 28 (62.2) | 40 (57.1) | |
| Family History of Dementia* | |||||
| Yes | 208 (40.4) | 260 (55.2) | 15 (42.9) | 23 (48.9) | |
| APOE ε4 alleles* | |||||
| 0 | 336 (68.9) | 171 (36.5) | 22 (68.8) | 16 (33.3) | |
| 1 | 134 (27.5) | 226 (48.2) | 9 (28.1) | 24 (50.0) | |
| 2 | 18 (3.7) | 72 (15.4) | 1 (3.1) | 8 (16.7) | |
| Cognitive Status | |||||
| Demented | 363 (53.8) | 598 (95.1) | 37 (78.7) | 70 (98.6) | |
| Primary Clinical Diagnosis | |||||
| Probable AD | 209 (31.0) | 471 (74.9) | 32 (68.1) | 66 (93.0) | |
| Possible AD | 75 (11.1) | 59 (9.4) | 7 (14.9) | 2 (2.8) | |
| Normal | 204 (30.2) | 8 (1.3) | 2 (4.3) | 1 (1.4) | |
| Other | 187 (27.7) | 91 (14.5) | 6 (12.8) | 2 (2.8) | |
| Mean (SD) | Mean (SD) | ||||
| Number of Visits to ADC | 2.4 (1.4) | 2.2 (1.3) | 2.8 (1.7) | 2.5 (1.7) | |
| Age of Onset† (years)* | 79.2 (10.1) | 73.1 (8.5) | 80.6 (9.5) | 73.3 (7.6) | |
| Duration of cognitive symptoms† (years)* | 6.6 (4.3) | 9.4 (4.1) | 7.4 (3.8) | 11.6 (3.9) | |
| CDR-SB at Last Visit | 5.7 (6.0) | 13.2 (5.1) | 8.8 (6.3) | 14.7 (4.6) | |
Abbreviations: AD-NP, Alzheimer's disease neuropathology (Braak Stages V or VI and “moderate” or “frequent” CERAD neuritic plaque frequency); CDR-SB, Clinical Dementia Rating “sum of boxes”; HS-Aging, Hippocampal Sclerosis of Aging.
Missing data: race (n=1, <1%), ethnicity (n=4, <1%), education (n=14, <1%), family history (n=354, 25.2%), APOE ε4 (385, 27.1%), and age of onset/symptom duration (n=48, 2.6%).
Among participants with MCI or dementia (n=1,227).
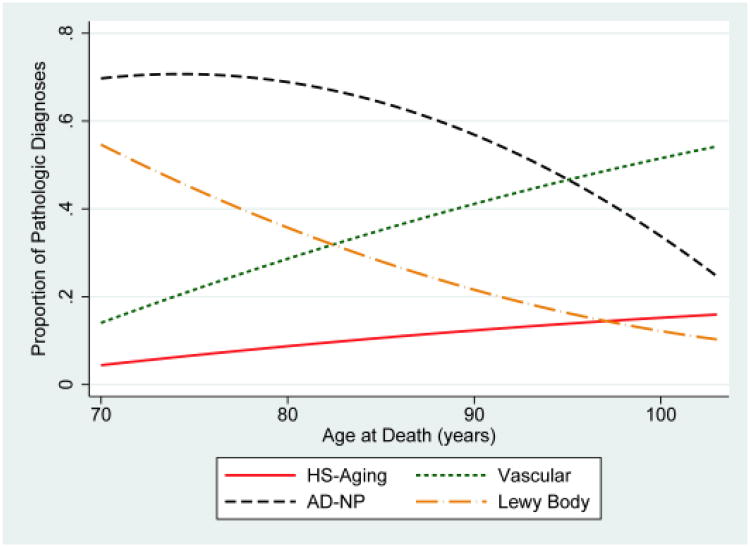
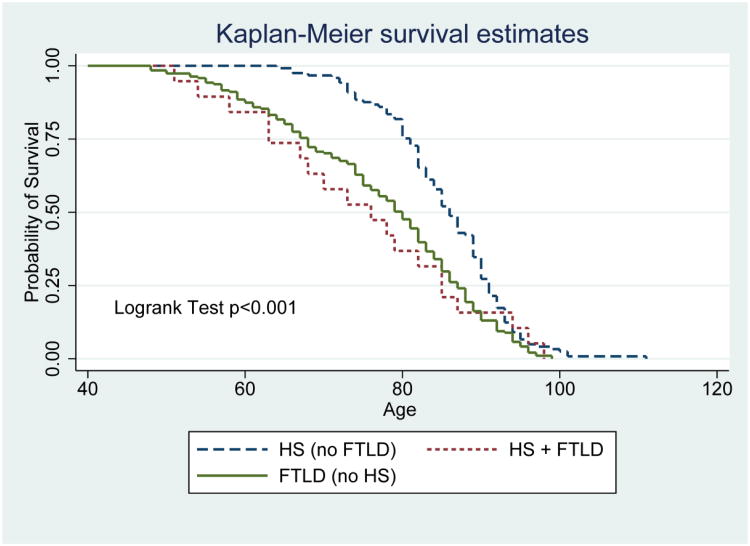
Participants with HS-Aging at autopsy were most often diagnosed clinically with AD (Probable or Possible) at last visit (Table 1). Over 90% of participants with HS-Aging pathology and AD-NP were diagnosed with Probable AD compared to only 74.9% of participants with only AD-NP. Even 68.1% of participants with HS-Aging and no AD-NP were diagnosed with Probable AD. Rates of pathological diagnoses, charted by age of death, are displayed in Figure 2 among demented participants. For data with a more sensitive operationalization of AD-NP to include earlier and less severe cases, see Supplementary Figure 2. Prevalence of HS-Aging pathological diagnosis was 10.0% overall among participants with dementia and increased by age at death, as did prevalence of diagnosis of vascular pathology, which was overall 33.1%. In contrast, AD-NP, with an overall prevalence of 62.9% among demented participants, decreased as a proportion of dementia diagnoses every year past age 85 (age at death). Notably, the proportion of primary Probable clinical AD diagnosed at the last clinical visit remained relatively constant at around 70% of all clinical dementia diagnoses, even in advanced age of death. Survival curves illustrate that participants with non-tauopathy FTLD (with and without HS) tended to die younger than those with HS and no FTLD (Figure 3).
Figure 2.
Trends by age at death for pathological diagnoses in individuals with dementia. Shown are the primary or contributing pathological diagnosis of Hippocampal Sclerosis of Aging (HS-Aging), Alzheimer's disease neuropathology (AD-NP), Vascular disease, and Lewy bodies charted as a proportion of all pathological diagnoses among participants (70-103 years old at death) with dementia at last visit using fitted curves. (N=1,061).
Figure 3.
Survival by age for pathological diagnoses for participants with non-tauopathy frontotemporal lobar degeneration (FTLD), any hippocampal sclerosis (HS), and both. Survival curves show proportion with HS (n=121), FTLD (n=191), or both (n=19) remaining alive according to age. Participants with non-tauopathy FTLD (with and without HS) tended to die younger than those with HS and no FTLD.
The relationship between HS-Aging pathology and the continuum of AD-NP was further evaluated by describing the prevalence of HS-Aging pathology, by each combination of CERAD neuritic plaques and Braak Stages (Table 2). HS-Aging pathology did not indicate a specific correlation with AD-NP; however, this neuropathologic manifestation clearly can be seen both in participants whose brains had high or low levels of AD-NP. For comparison, we also assessed Diffuse (neocortical) or Limbic Lewy body type pathology which tended to occur in the highest proportion among those with moderately severe scoring of neuritic plaques and tangles.
Table 2.
Distribution of Hippocampal Sclerosis of Aging pathology and Diffuse or Limbic Lewy Body pathology by severity of Alzheimer's disease pathological features.
| All participants (n=1,422) | ||||||||
| Braak Stage (Neurofibrillary tangles) | ||||||||
| 0 | I | II | III | IV | V | VI | ||
| CERAD Neuritic amyloid plaque densities | None | 21 | 49 | 68 | 32 | 33 | 1 | 3 |
| Sparse | 4 | 21 | 33 | 41 | 54 | 8 | 9 | |
| Moderate | 4 | 14 | 38 | 64 | 96 | 61 | 51 | |
| Severe | 2 | 6 | 14 | 41 | 66 | 299 | 359 | |
| Percent of participants with HS-Aging pathology* | ||||||||
| Braak Stage (Neurofibrillary tangles) | ||||||||
| 0 | I | II | III | IV | V | VI | ||
| CERAD Neuritic amyloid plaque densities | None | 9.5 | 8.2 | 1.5 | 12.5 | 3 | ||
| Sparse | 14.3 | 6.1 | 4.9 | 7.4 | ||||
| Moderate | 14.3 | 2.6 | 1.6 | 6.3 | 11.5 | 9.8 | ||
| Severe | 7.1 | 7.3 | 10.6 | 10.4 | 7.8 | |||
| Percent of participants with Diffuse or Limbic Lewy body-type pathology*,† | ||||||||
| Braak Stage (Neurofibrillary tangles) | ||||||||
| 0 | I | II | III | IV | V | VI | ||
| CERAD Neuritic amyloid plaque densities | None | 15 | 12.2 | 20.9 | 31.3 | 15.2 | ||
| Sparse | 5 | 30.3 | 22 | 27.8 | ||||
| Moderate | 7.1 | 8.1 | 28.6 | 25 | 27.9 | 37.3 | ||
| Severe | 21.4 | 39 | 36.4 | 25 | 20.4 | |||
Notes: Numbers of all participants in each cell are presented in top grid, followed by percentages of participants with HS-Aging as well as Diffuse or Limbic Lewy body pathology
Coloring displays the range of observed percentages from low percentages (dark blue) to high percentages (dark red) per pathology. Boxes containing <10 cases are black.
Assessed in 1,415 participants with complete Lewy body pathology scoring.
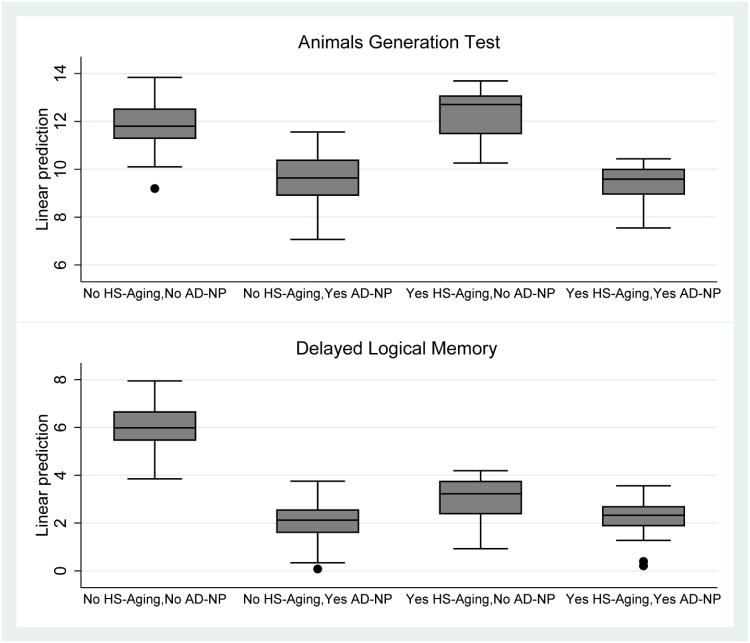
In secondary analyses, we examined potential cognitive predictors of HS-Aging pathology among 689 participants who had mild-to-moderate cognitive impairment (CDR-SB 0.5-15) at a visit 2-5 years prior to death (Table 3). This visit corresponded to the initial UDS visit for approximately 90% of participants. Verbal fluency was missing for 80 participants (11.6%) and episodic memory was missing for 107 participants (15.5%). Missingness for each test occurred across the range of CDR-SB scores, but was highest among participants with AD-NP regardless of HS-Aging (range 13.3%-20.0 %) lower among those without either HS-Aging or AD-NP (9.5 -11.7%) and lowest among those with HS-Aging and no AD-NP (3.8% for both tests). Supplementary Table 2 shows the results of the same analysis but using those without either HS-Aging pathology or AD-NP as the reference group instead of those with HS-Aging pathology and no AD-NP. Participants with HS-Aging pathology had higher functioning in verbal fluency and overall cognitive/functional ability 2-5 years prior to death compared to participants with AD-NP, in multivariable regression analyses (Table 3). Participants with HS-Aging pathology had lower functioning in episodic memory 2-5 years prior to death compared to participants without either HS-Aging pathology or AD-NP. Episodic memory in participants with HS-Aging pathology was not significantly different from participants with AD-NP. Results for verbal fluency and episodic memory are illustrated using box plots of the predicted values based on the fitted models (Figure 4). Results were similar when criteria for AD neuropathologic changes were applied that included less severe pathology (Supplementary Table 3).
Table 3. Comparison of Test Scores between Participants with or without HS-Aging and AD-NP.*.
| Neuropsychological or Clinical Test | β† | 95% CI | p |
|---|---|---|---|
| CDR-SB‡ (N= 689) | |||
| Yes HS-Aging, no AD-NP | --- | --- | --- |
| No HS-Aging, no AD-NP | 0.42 | -1.04, 1.88 | 0.57 |
| No HS-Aging, yes AD-NP | -2.08 | -3.53, -0.64 | 0.01 |
| Yes HS-Aging, yes AD-NP | -3.43 | -5.27, -1.60 | <0.001 |
| Animals Generation Test (N=609) | |||
| Yes HS-Aging, no AD-NP | --- | --- | --- |
| No HS-Aging, no AD-NP | 0.04 | -2.04, 2.12 | 0.97 |
| No HS-Aging, yes AD-NP | -2.48 | -4.54, -0.42 | 0.02 |
| Yes HS-Aging, yes AD-NP | -3.19 | -5.88, -0.49 | 0.02 |
| Delayed Logical Memory (N=582) | |||
| Yes HS-Aging, no AD-NP | --- | --- | --- |
| No HS-Aging, no AD-NP | 3.32 | 1.60, 5.05 | <0.001 |
| No HS-Aging, yes AD-NP | -0.54 | -2.25, 1.17 | 0.54 |
| Yes HS-Aging, yes AD-NP | -0.66 | -2.92, 1.58 | 0.56 |
Abbreviations: CDR-SB, Clinical Dementia Rating “sum of boxes”; HS-Aging, Hippocampal Sclerosis of Aging; AD-NP, Alzheimer's disease neuropathology (Braak Stages V or VI and “moderate” or “frequent” CERAD neuritic plaque frequency).
Based on linear regression of each test score from a visit 2-5 years prior to death among participants with mild to moderate cognitive impairment, and adjusted for age at death, education and years between visit and death.
A positive β represents a higher functioning compared to participants with HS-Aging pathology, no AD-NP (chosen as the reference group so comparisons would be made to those with relatively “pure” HS-Aging pathology);
CDR-SB scores were inverted so that an increase in score =higher functioning
Figure 4.
Box plots showing distribution of predicted scores for animals generation test (verbal fluency) and Delayed Logical Memory (episodic memory). Predicted values are based on separate fitted models that included HS-Aging and AD-NP grouping, age at death, education, and years between clinical visit and death as predictors of each test score.
Next we examined potential cognitive predictors of HS-Aging pathology compared to non-tauopathy FTLD pathology among 380 participants without AD-NP who had mild-to-moderate cognitive impairment (CDR-SB 0.5-15) at a visit 2-5 years prior to death (Table 4). Similar to the comparisons above, participants with HS-Aging pathology had higher functioning in verbal fluency but similar overall cognitive/functional ability and episodic memory 2-5 years prior to death compared to participants with non-tauopathy FTLD pathology, in multivariable regression analyses (Table 4).
Table 4.
Comparison of Test Scores between HS-Aging and Non-tauopathy FTLD.*
| Neuropsychological or Clinical Test | β† | 95% CI | p |
|---|---|---|---|
| CDR-SB‡ (N= 380) | |||
| Yes HS-Aging, no FTLD | --- | --- | --- |
| No HS-Aging, no FTLD | 0.33 | -1.08, 1.74 | 0.65 |
| No HS-Aging, yes FTLD | -1.03 | -2.73, 0.65 | 0.23 |
| Animals Generation Test(N=342) | |||
| Yes HS-Aging, no FTLD | --- | --- | --- |
| No HS-Aging, no FTLD | -0.46 | -2.58, 1.66 | 0.67 |
| No HS-Aging, yes FTLD | -3.64 | -6.26, -1.01 | 0.007 |
| Delayed Logical Memory (N=333) | |||
| Yes HS-Aging, no FTLD | --- | --- | --- |
| No HS-Aging, no FTLD | 3.31 | 1.21, 5.42 | 0.002 |
| No HS-Aging, yes FTLD | 0.99 | -1.61, 3.58 | 0.45 |
Abbreviations: CDR-SB, Clinical Dementia Rating “sum of boxes”; HS-Aging, Hippocampal Sclerosis of Aging; FTLD; Frontotemporal lobar degeneration.
Based on linear regression results of test scores from a visit 2-5 years prior to death among participants with mild to moderate cognitive impairment adjusted for age at death, education and years between visit and death. Participants with Braak Stages V or VI and “moderate” or “frequent” CERAD neuritic plaque frequency were excluded.
A positive β represents better functioning compared to participants with HS-Aging, no FTLD pathology
CDR-SB scores were inverted so that an increase in score =higher functioning
Discussion
In the largest sample to date of autopsy-verified HS-Aging pathology in terms of both cases and controls, we find that HS-Aging pathology is being recognized at autopsy with increased frequency at the sampled American research centers, particularly in the last several years. The large majority of autopsy-confirmed HS-Aging cases reported to NACC were conflated clinically with AD, yet HS-Aging pathology is not linked to APOE ε4 alleles and prevalence increased (as opposed to AD-NP which decreased) with increasing age of death beyond age 85. HS-Aging pathology was found in participants with both low severity of AD and high severity of AD pathology. These findings suggest that HS-Aging and AD pathologies may occur independently of each other. Among a subset of participants showing signs of mild to moderate cognitive impairment 2-5 years prior to death, we confirm the presence of an HS-Aging neurocognitive profile (relatively low or impaired Logical Memory Delayed Recall, with relatively high or preserved verbal fluency). HS-Aging participants were also less impaired overall (based on CDR-SB), so it could be that they have disproportionate episodic memory impairment rather than less fluency impairment, however longitudinal analyses would be needed to investigate this hypothesis. Nevertheless, this cognitive profile provides an indicator of increased risk of HS-Aging pathology.
We performed the current study mindful of caveats linked to retrospective analyses of large autopsy data sets. There are important sources of bias that encourage caution in interpreting these data. No autopsy series has true epidemiologic scope because each involves a limited fraction of the population, <100% autopsy rate, and imperfect clinical and pathologic diagnostic rubrics. “Dementia clinics”, as represented by most U.S. ADCs, follow cohorts that differ from a broader population. For example, FTLD prevalence tends to be 5% or higher at dementia clinics, whereas in epidemiological samples FTLD prevalence is usually <1% of demented participants [7,26]. Yet, it is difficult to understand how the biases that lead to over-representation of FTLD, a disease that is clinically devastating, familial in inheritance, rare, affects younger individuals, attracts arguably disproportionate academic interest, and is usually accurately diagnosed clinically, would also lead to an over-representation of HS-Aging, a disease that, by contrast, has a less severe clinical manifestation, is highly prevalent, affects very old individuals, is almost never accurately diagnosed clinically, and thus, is largely overlooked by clinicians. Since most individuals with HS-Aging pathology were diagnosed clinically with AD, the observed differences are probably not related to recruitment biases even if the prevalence of HS-Aging pathology in the NACC cohort is not an accurate epidemiologic representation.
The NACC database represents one of the world's largest and highest-quality multicenter databases, with both detailed clinical and pathological information. The database has been extensively audited and, indeed, the multicenter derivation of these data provides opportunities as well as potential pitfalls. This is particularly true for HS-Aging, a disease that currently lacks comprehensive, consensus-based diagnostic rubrics (clinical or pathological) and is relatively unknown even at some academic research centers. Thus HS-Aging may be assessed differently at each ADC, including whether neuropathologists studied one or both hippocampi. However, any analysis that focuses only on a single research center cannot indicate the “state of the field” in terms of diagnostic tendencies, as can be readily achieved with the NACC database (after all, our data do not indicate that HS-Aging pathology is becoming more prevalent, just that the diagnosis is becoming more prevalent across multiple centers). Our finding of dramatically increased rate of pathologic diagnosis -- presumably in part due to the advent of a more specific pathologic marker, TDP-43 -- for HS-Aging during recent years is certainly more meaningful for having used a database that draws on dozens of different academic research centers. This finding also indicates that HS-Aging was almost certainly underdiagnosed in our sample, and some participants without HS-Aging may have been misclassified; this would have the result of attenuating true associations.
Although HS-Aging pathology may be associated with heterogeneous underlying cause[s] – linked previously to vascular factors, AD, and FTLD (see Ref. 4) – there is a need to better understand the implications of the pathologic observation. We do not find data to indicate that HS-Aging pathology is either strongly positively or negatively associated with AD-NP. There is no association between HS-Aging risk and APOE gene alleles (as shown previously [27,28]) which is a salient observation because APOE ε4 strongly drives AD-NP. There also is no systematic pattern of overlap between HS-Aging pathologic presence and increasing severity of AD-NP. Some cases with high AD-NP levels have comorbid HS-Aging pathology, and our data do not rule out some sort of pathologic synergy in a subset of cases. However, there clearly are many cases with low AD-NP, and also with HS-Aging pathology, which indicates conclusively that, at the very least, HS-Aging pathology does not require high AD-NP to occur.
These data confirm the importance of differentiating the clinical condition of “dementia” from AD, according to the underlying pathologies [29]. The natural history of different brain diseases of advanced age also may differ, although the current study was not designed to characterize the longitudinal courses of the diseases in detail. In this study sample we found that participants with relatively “pure” HS-Aging had shorter duration of symptoms, later age of onset and less severe overall cognitive/functional impairment compared to those with AD-NP (regardless of our operationalization of AD-NP). This phenomenon may be due to the relative lack of involvement of neocortical and subcortical structures in HS-Aging.
The presence of HS-Aging pathology in cases with minimal AD pathology is one reason for the perceived “disassociation” between cognitive status and AD pathology in the “oldest-old.”[30] Perhaps even more important conceptually, HS-Aging appears to be a true “age-linked” disease because the peak prevalence correlates with the final stage of human life (age >90) and each added year of life is associated with an increased risk for demonstrating HS-Aging pathology at autopsy. Unlike for HS-Aging, our data, in addition to prior work [31], suggest that the statement “Age is the greatest risk factor for AD” is not accurate because surviving each additional year above age 90 does not correlate with increased risk for developing AD pathology.
We conclude that clinicians' abilities to diagnose HS-Aging currently lags far behind that of pathologists. Over eighty percent of participants with relatively “pure” HS-Aging pathology in this study were diagnosed clinically with AD. Thus, we need new methods to help discriminate HS-Aging from AD in clinical participants in order to ensure good clinical trials for either of these as-yet incurable conditions. Here we provide confirmation that our earlier-identified neurocognitive profile of HS-Aging [2] (with relatively preserved verbal fluency despite impaired word list delayed recall performance) indeed points to individuals at increased risk for HS-pathology. Hopefully, more sensitive and specific methods, i.e. biomarkers, for identifying HS-Aging cases clinically will be developed. In the meantime, HS-Aging remains an under-appreciated brain disease of strong relevance to the field of dementia research.
Supplementary Material
Supplementary Figure 1. Study sample flow chart. Abbreviations: AD-NP, Alzheimer's disease neuropathology (Braak Stages V or VI and “moderate” or “frequent” CERAD neuritic plaque frequency); CDR-SB, Clinical Dementia Rating “sum of boxes”; FTLD, Frontoemporal lobar degeneration; HS-Aging, Hippocampal Sclerosis of Aging; NP; neuropathology; UDS, Uniform Data Set.
Supplementary Figure 2. Trends by age at death for pathological diagnoses in individuals with dementia. Shown are the primary or contributing pathological diagnosis of Hippocampal Sclerosis of Aging (HS-Aging), “intermediate/moderate” Alzheimer's disease neuropathology (AD-NP(I)), vascular disease, and Lewy bodies charted as a proportion of all pathological diagnoses among participants (70-103 years old at death) with dementia at last visit using fitted curves. (N=1,061).
Supplementary Table 1. Participant characteristics at last visit by Hippocampal Sclerosis of aging and Alzheimer's disease neuropathology (N=1,422), which is operationalized using a relative sensitive method AD-NP(I) which includes Braak stages III and IV.
Abbreviations: AD-NP (I), “intermediate to moderate” Alzheimer's disease neuropathology (Braak Stages III- VI and “moderate” or “frequent” CERAD neuritic plaque frequency); CDR-SB, Clinical Dementia Rating “sum of boxes”; HS-Aging, Hippocampal Sclerosis of Aging. *Missing data: race (n=1, <1%), ethnicity (n=4, <1%), education (n=14, <1%), family history (n=354, 25.2%), APOE ε4 (385, 27.1%), and symptom duration (n=46, 3.7%). †Among participants with MCI or dementia (n=1,227)
Supplementary Table 2. Comparison of Test Scores between Participants with or without HS-Aging and AD-NP (no HS-Aging pathology, no AD-NP chosen as the reference group).*
Abbreviations: CDR-SB, Clinical Dementia Rating “sum of boxes”; HS-Aging, Hippocampal Sclerosis of Aging; AD-NP, Alzheimer's disease neuropathology (Braak Stages V or VI and “moderate” or “frequent” CERAD neuritic plaque frequency). *Based on linear regression of each test score from a visit 2-5 years prior to death among participants with mild to moderate cognitive impairment, and adjusted for age at death, education and years between visit and death. †A positive β represents a higher functioning compared to participants with no HS-Aging pathology, no AD-NP (reference) ‡CDR-SB scores were inverted so that an increase in score =higher functioning
Supplementary Table 3. Comparison of Test Scores between Participants with or without HS-Aging and AD-NP(I). Abbreviations: CDR-SB, Clinical Dementia Rating “sum of boxes”; HS-Aging, Hippocampal Sclerosis of Aging; AD-NP (I), “intermediate to moderate” Alzheimer's disease neuropathology (Braak Stages III- VI and “moderate” or “frequent” CERAD neuritic plaque frequency).
*Based on linear regression of each test score from a visit 2-5 years prior to death among participants with mild to moderate cognitive impairment, and adjusted for age at death, education and years between visit and death.
†A positive β represents a higher functioning compared to participants with HS-Aging pathology, no AD-NP (reference)
‡CDR-SB scores were inverted so that an increase in score =higher functioning
Acknowledgments
We are deeply grateful to all of the study participants, clinicians, and other workers at ADCs that make this research possible. We also thank NACC staff for help with NACC data.
Corresponding Author Dr. Nelson had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study funding: Supported by NIH (R01 NS061933, P30 AG028383, and U01 AG016976)
Footnotes
Statistical analyses: Conducted by Willa Brenowitz
Authors' contributions and conflict of interest disclosures; and: Willa D. Brenowitz, MPH (contributions: data analyses, writing, and editing) reports no disclosures
Sarah E. Monsell, MS (contributions: data analyses, writing, and editing) reports no disclosures
Walter A. Kukull, PhD (contributions: data analyses, writing, and editing) reports no disclosures
Frederick A. Schmitt PhD (contributions: data analyses, and editing) reports no disclosures
Peter T. Nelson MD PhD (contributions: data analyses, writing, and editing) reports no disclosures
References
- 1.Amador-Ortiz C, Ahmed Z, Zehr C, Dickson DW. Hippocampal sclerosis dementia differs from hippocampal sclerosis in frontal lobe degeneration. Acta Neuropathol. 2007;113:245–252. doi: 10.1007/s00401-006-0183-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson PT, Schmitt FA, Lin Y, Abner EL, Jicha GA, Patel E, Thomason PC, Neltner JH, Smith CD, Santacruz KS, Sonnen JA, Poon LW, Gearing M, Green RC, Woodard JL, Van Eldik LJ, Kryscio RJ. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain. 2011;134:1506–1518. doi: 10.1093/brain/awr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarow C, Weiner MW, Ellis WG, Chui HC. Prevalence, laterality, and comorbidity of hippocampal sclerosis in an autopsy sample. Brain Behav. 2012;2:435–442. doi: 10.1002/brb3.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson PT, Smith CD, Abner EL, Wilfred BJ, Wang W-X, Neltner JH, Baker M, Fardo DW, Kryscio RJ, Scheff SW, Jicha GA, Jellinger KA, Van Eldik LJ, Schmitt FA. Hippocampal sclerosis of aging, a prevalent and high-morbidity brain disease. Acta Neuropathol. 2013;126:161–177. doi: 10.1007/s00401-013-1154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pao WC, Dickson DW, Crook JE, Finch NA, Rademakers R, Graff-Radford NR. Hippocampal sclerosis in the elderly: genetic and pathologic findings, some mimicking Alzheimer disease clinically. Alzheimer Dis Assoc Disord. 2011;25:364–368. doi: 10.1097/WAD.0b013e31820f8f50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, Davis DG, Poduska JW, Patel E, Mendiondo MS, Markesbery WR. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol. 2010;20:66–79. doi: 10.1111/j.1750-3639.2008.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA. The Neuropathology of Older Persons with and Without Dementia from Community versus Clinic Cohorts. J Alzheimers Dis. 2009;18:691–701. doi: 10.3233/JAD-2009-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickson DW, Baker M, Rademakers R. Common variant in GRN is a genetic risk factor for hippocampal sclerosis in the elderly. Neurodegener Dis. 2010;7:170–174. doi: 10.1159/000289231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rutherford NJ, Carrasquillo MM, Li M, Bisceglio G, Menke J, Josephs KA, Parisi JE, Petersen RC, Graff-Radford NR, Younkin SG, Dickson DW, Rademakers R. TMEM106B risk variant is implicated in the pathologic presentation of Alzheimer disease. Neurology. 2012;79:717–718. doi: 10.1212/WNL.0b013e318264e3ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 12.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beekly DL, Ramos EM, van Belle G, Deitrich W, Clark AD, Jacka ME, Kukull WA. The National Alzheimer's Coordinating Center (NACC) Database: an Alzheimer disease database. Alzheimer Dis Assoc Disord. 2004;18:270–277. [PubMed] [Google Scholar]
- 14.Morris JC, Weintraub S, Chui HC, Cummings J, DeCarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA. The Uniform Data Set (UDS): Clinical and Cognitive Variables and Descriptive Data From Alzheimer Disease Centers. Alzheimer Disease & Associated Disorders. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 15.Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J, Hubbard JL, Koepsell TD, Morris JC, Kukull WA. The National Alzheimer's Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21:249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 16.Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Trojanowski JQ, Vinters HV, Hyman BT. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VMY, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 18.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 19.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 20.Wechsler D. Wechsler Memory Scale-Revised Manual. The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- 21.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 22.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 23.Jellinger KA, Attems J. Prevalence and impact of vascular and Alzheimer pathologies in Lewy body disease. Acta Neuropathol. 2008;115:427–436. doi: 10.1007/s00401-008-0347-5. [DOI] [PubMed] [Google Scholar]
- 24.Nelson PT, Kryscio RJ, Jicha GA, Abner EL, Schmitt FA, Xu LO, Cooper G, Smith CD, Markesbery WR. Relative preservation of MMSE scores in autopsy-proven dementia with Lewy bodies. Neurology. 2009;73:1127–1133. doi: 10.1212/WNL.0b013e3181bacf9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Bryant SE, Waring SC, Cullum CM, Hall J, Lacritz L, Massman PJ, Lupo PJ, Reisch JS, Doody R. Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer's research consortium study. Arch Neurol. 2008;65:1091–1095. doi: 10.1001/archneur.65.8.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, Craft S, Leverenz JB, Montine TJ. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62:406–413. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- 27.Leverenz JB, Lipton AM. Clinical aspects of hippocampal sclerosis. Handb Clin Neurol. 2008;89:565–567. doi: 10.1016/S0072-9752(07)01252-3. [DOI] [PubMed] [Google Scholar]
- 28.Troncoso JC, Kawas CH, Chang CK, Folstein MF, Hedreen JC. Lack of association of the apoE4 allele with hippocampal sclerosis dementia. Neurosci Lett. 1996;204:138–140. doi: 10.1016/0304-3940(96)12331-4. [DOI] [PubMed] [Google Scholar]
- 29.Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del Tredici K, Duyckaerts C, Frosch MP, Haroutunian V, Hof PR, Hulette CM, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kövari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee AC, Montine TJ, Morris JC, Schneider JA, Sonnen JA, Thal DR, Trojanowski JQ, Troncoso JC, Wisniewski T, Woltjer RL, Beach TG. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giannakopoulos P, Bouras C, Hof PR. Clinicopathologic correlates in the oldest-old: Commentary on “No disease in the brain of a 115-year-old woman.”. Neurobiol Aging. 2008;29:1137–1139. doi: 10.1016/j.neurobiolaging.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson PT, Head E, Schmitt FA, Davis PR, Neltner JH, Jicha GA, Abner EL, Smith CD, Van Eldik LJ, Kryscio RJ, Scheff SW. Alzheimer's disease is not “brain aging”: neuropathological, genetic, and epidemiological human studies. Acta Neuropathol. 2011;121:571–587. doi: 10.1007/s00401-011-0826-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Study sample flow chart. Abbreviations: AD-NP, Alzheimer's disease neuropathology (Braak Stages V or VI and “moderate” or “frequent” CERAD neuritic plaque frequency); CDR-SB, Clinical Dementia Rating “sum of boxes”; FTLD, Frontoemporal lobar degeneration; HS-Aging, Hippocampal Sclerosis of Aging; NP; neuropathology; UDS, Uniform Data Set.
Supplementary Figure 2. Trends by age at death for pathological diagnoses in individuals with dementia. Shown are the primary or contributing pathological diagnosis of Hippocampal Sclerosis of Aging (HS-Aging), “intermediate/moderate” Alzheimer's disease neuropathology (AD-NP(I)), vascular disease, and Lewy bodies charted as a proportion of all pathological diagnoses among participants (70-103 years old at death) with dementia at last visit using fitted curves. (N=1,061).
Supplementary Table 1. Participant characteristics at last visit by Hippocampal Sclerosis of aging and Alzheimer's disease neuropathology (N=1,422), which is operationalized using a relative sensitive method AD-NP(I) which includes Braak stages III and IV.
Abbreviations: AD-NP (I), “intermediate to moderate” Alzheimer's disease neuropathology (Braak Stages III- VI and “moderate” or “frequent” CERAD neuritic plaque frequency); CDR-SB, Clinical Dementia Rating “sum of boxes”; HS-Aging, Hippocampal Sclerosis of Aging. *Missing data: race (n=1, <1%), ethnicity (n=4, <1%), education (n=14, <1%), family history (n=354, 25.2%), APOE ε4 (385, 27.1%), and symptom duration (n=46, 3.7%). †Among participants with MCI or dementia (n=1,227)
Supplementary Table 2. Comparison of Test Scores between Participants with or without HS-Aging and AD-NP (no HS-Aging pathology, no AD-NP chosen as the reference group).*
Abbreviations: CDR-SB, Clinical Dementia Rating “sum of boxes”; HS-Aging, Hippocampal Sclerosis of Aging; AD-NP, Alzheimer's disease neuropathology (Braak Stages V or VI and “moderate” or “frequent” CERAD neuritic plaque frequency). *Based on linear regression of each test score from a visit 2-5 years prior to death among participants with mild to moderate cognitive impairment, and adjusted for age at death, education and years between visit and death. †A positive β represents a higher functioning compared to participants with no HS-Aging pathology, no AD-NP (reference) ‡CDR-SB scores were inverted so that an increase in score =higher functioning
Supplementary Table 3. Comparison of Test Scores between Participants with or without HS-Aging and AD-NP(I). Abbreviations: CDR-SB, Clinical Dementia Rating “sum of boxes”; HS-Aging, Hippocampal Sclerosis of Aging; AD-NP (I), “intermediate to moderate” Alzheimer's disease neuropathology (Braak Stages III- VI and “moderate” or “frequent” CERAD neuritic plaque frequency).
*Based on linear regression of each test score from a visit 2-5 years prior to death among participants with mild to moderate cognitive impairment, and adjusted for age at death, education and years between visit and death.
†A positive β represents a higher functioning compared to participants with HS-Aging pathology, no AD-NP (reference)
‡CDR-SB scores were inverted so that an increase in score =higher functioning