Abstract
Prenatal ethanol exposure causes persistent neurodevelopmental deficits by inducing apoptosis within neuronal progenitors including the neural crest. The cellular signaling events underlying this apoptosis are unclear. Using an established chick embryo model, we previously identified ethanol’s activation of CaMKII as a crucial early step in this pathway. Here we report that CaMKII is pro-apoptotic because it mediates the loss of transcriptionally active β-catenin, which normally provides trophic support to these cells. β-catenin overexpression normalized cell survival in ethanol’s presence. CaMKII inhibition similarly restored β-catenin content and transcriptional activity within ethanol-treated cells and prevented their cell death. In contrast, inhibition of alternative effectors known to destabilize β-catenin, including GSK3β, Protein Kinase C, JNK, and calpain, failed to normalize cell survival and β-catenin activity in ethanol’s presence. Importantly, we found that purified CaMKII can directly phosphorylate β-catenin. Using targeted mutagenesis we identified CaMKII phosphorylation sites within human β-catenin at T332, T472, and S552. This is the first demonstration that β-catenin is a phosphorylation target of CaMKII and represents a novel mechanism by which calcium signals could regulate β-catenin-dependent transcription. These results inform ethanol’s neurotoxicity and offer unexpected insights into other neurodevelopmental and neurodegenerative disorders having dysregulated calcium or β-catenin signaling.
Keywords: ethanol, neural crest, β-catenin, CaMKII, apoptosis, intracellular calcium, fetal alcohol spectrum disorders, birth defect, teratology, Wnt
Introduction
Fetal Alcohol Spectrum Disorders (FASD) are a leading cause of neurodevelopmental disability in most western societies. The most severe form, Fetal Alcohol Syndrome, affects 0.33 to 2.2 per 1000 live births, with substantially higher rates (8 to 89.2 per 1000) in populations with appreciable alcohol use (May et al. 2009). Prenatal alcohol exposure (PAE) causes significant apoptosis within developing neuronal populations, and these losses contribute to the pathologies underlying FASD (Cartwright et al. 1998; Dunty et al. 2001; Ikonomidou et al. 2000). An early neural progenitor population whose development is disrupted by ethanol is the neural crest, a unique stem cell population that originates within the dorsal neuroectoderm (LeDouarin and Kalcheim, 1999). As the elevated neural folds fuse, neural crest cells emigrate into peripheral tissues and contribute to diverse structures, including the sympathetic and parasympathetic nervous system, elements of the cranial ganglia, and craniofacial bone and cartilage. Clinically relevant ethanol concentrations (0.05% – 0.3%, or 8.6 – 52 mM) cause substantial apoptosis within neural crest progenitors as revealed by the cells’ pyknotic appearance, their detection using TUNEL, and dependence of this cell death upon caspase activity (Cartwright et al. 1998; Dunty et al. 2001). These neural crest losses contribute to the deficits in cranial nerves, facial structure, and other neurocristopathies that partly typify those with FASD.
To elucidate the mechanism that underlies this ethanol-mediated apoptosis, we investigated the early cellular signaling events that are altered by acute ethanol exposure. Using a chick embryo FASD model, we found that clinically relevant ethanol concentrations (EC50 = 52 mM) cause a rapid (1–2 sec), 3-fold elevation of intracellular calcium (Cai+2) stores within the premigratory neural crest progenitors that reside in the dorsal neural folds (Debelak-Kragtorp et al. 2003; Garic-Stankovic et al. 2006). We showed that this Cai+2 transient originates from ethanol’s stimulation of G protein-coupled signaling via Gαi2/3 and Gβγ, the latter of which activates phospholipase Cβ and phosphoinositide-mediated release of Cai+2 stores (Garic-Stankovic et al. 2005, 2006). Pretreatment with Cai+2 chelators prevents the ethanol-induced Cai+2 rise and subsequent cell death, thus normalizing neural crest survival (Debelak-Kragtorp et al. 2003; Garic-Stankovic et al. 2005). How this ethanol-induced Cai+2 transient initiates neural crest apoptosis is unclear. However, we recently identified two signals downstream of the Cai+2 transient that are essential participants in ethanol’s pro-death signal. The first is CaMKII, whose activity in premigratory neural crest increases 3-fold within 1 min of ethanol addition (Garic et al., 2011). We found that forced expression of CaMKII is sufficient to cause neural crest apoptosis in ethanol’s absence, whereas inhibition of CaMKII activity prevents this cell death and rescues neural crest survival in ethanol’s presence (Garic et al., 2011). The second key signal is the transcriptional effector β-catenin, which is essential for neural crest induction, migration, and survival (Stuhlmiller and Garcia-Castro, 2012). We found that nuclear β-catenin is selectively depleted from neural crest within 2hr of ethanol addition, and this loss is mediated by ethanol’s Cai+2 transient (Flentke et al., 2011). Forced expression of β-catenin similarly protects neural crest from ethanol-induced cell death and rescues their survival (Flentke et al., 2011). This loss of β-catenin protein is post-transcriptional, as its transcript levels are unaffected by ethanol.
It is unclear whether ethanol’s effects upon CaMKII and β-catenin are mechanistically linked. However, studies in other models suggest a potential interaction between them. Two intersecting pathways act posttranslationally to regulate β-catenin stability and thereby its transcriptional activity. In the Canonical Wnt pathway, secreted Wnt glycoproteins interact with their cell-surface Frizzled receptors to inhibit the protein kinase GSK3β (reviewed in MacDonald et al. 2009). In the presence of canonical Wnt/Frizzled signals, β-catenin interacts with T cell transcription factor/lymphoid enhancer factor (TCF/LEF) proteins to initiate transcription. When Wnt signals are absent, GSK3β is no longer silenced and it phosphorylates β-catenin, targeting β-catenin for ubiquitination and proteosomal destruction. In the second pathway, the Noncanonical Ca/Wnt pathway, Wnt interactions with G proteins elevate Cai+2 and repress β-catenin’s transcriptional activity through both transcriptional and post-transcriptional means (reviewed in Kohn and Moon, 2005). This pathway is GSK3β-independent and utilizes several calcium effectors in a cell-dependent manner. CaMKII can indirectly silence β-catenin-mediated transcription through its phosphorylation of both LEF-1 and the TAK1/NLK kinases that negatively modulate TCF/LEF activity (Ishitani et al. 2003). Protein Kinase C can antagonize canonical Wnt signaling through its phosphorylation of Wnt pathway effectors, including dishevelled and β-catenin itself (Gwak et al. 2006). Calcineurin negatively regulates β-catenin via its nuclear NFAT targets to decrease β-catenin transcription (Saneyoshi et al. 2002). Finally, the calcium-activated proteases, calpains, can directly cleave β-catenin and inactivate the protein (Li and Iyengar 2002). We also note that ethanol itself enhances GSKβ3 activity in selected neuronal populations (French and Heberlein 2009; Liu et al. 2009), although the consequences of this to β-catenin have yet to be examined.
Here we evaluate how these calcium-dependent and -independent effectors of β-catenin contribute to ethanol’s neurotoxicity in the neural crest. We show that activated CaMKII, and not other Wnt effectors, selectively mediates both the loss of transcriptionally active β-catenin from these cells and their subsequent death. β-catenin gain-of-function and CaMKII inhibition normalizes canonical Wnt signaling within these cells and rescues them from ethanol’s neurotoxicity. Importantly, we show that purified CaMKII can directly phosphorylate β-catenin in a cell-free context, and we identify those target sites within the protein. This work uncovers a novel mechanism by which calcium signals could regulate the transcriptional activity of the β-catenin/TCF-LEF complex.
Methods
Embryos and Ethanol Treatment
Fertile White Leghorn chick eggs (W98, Hyline, Spencer, IA; Special Black, Sunnyside Farms, Beaver Dam, WI) were incubated to the desired developmental stage (Hamburger and Hamilton, 1951). For in ovo studies, control saline or 0.43 mmol ethanol in isotonic saline was injected into the egg yolk center at stage 8/9 (3–7 somites); this produces a peak embryonic ethanol concentration of 50–60 mM for 1–1.5 hr post-injection (Debelak and Smith, 2000). Ex vivo studies incubated stage 8/9 embryos 2hr in Tyrode’s buffer ± 52 mM ethanol; this ethanol concentration causes half-maximal calcium release in these embryos (Garic-Stankovic et al. 2006). All embryos were precisely matched in somite number.
Pharmacological Intervention
Prior to ethanol challenge some embryos were pretreated with selective pharmacological compounds to investigate signaling pathway contributions. Membrane-permeable forms were used and included ALLN (20 μM), Bapta-AM (1 mM), 1-methyl-BIO (1 μM), calmidazolium (10 μM), Calpain Inhibitor V (2 mM), Gö-6983 (50 μM), JNK Inhibitor II (1 μM), Ro-32-0432 (10 μM; all from Calbiochem), myristylated-CaMKII Autoinhibitory Peptide (myr-AIP, 2.5 μM, BioMol Research Laboratories), ionomycin (50 μM, Sigma), and SB-415,286 (1 μM, Sigma). For in ovo treatment, compounds were transiently delivered using hydrophobic (SM2, 100 μm diameter) or anion-exchange beads (AG-50W, 75 μm, both from Bio-Rad, Hercules CA) that were presorbed with the agent and washed prior to implant. Pilot studies ascertained the appropriate concentration to be tested; because bead-mediated delivery is diffusion dependent, the bead soaking concentrations were generally 100- to 1000-fold greater than levels administered by direct delivery (Garic-Stankovic et al. 2005; Garic et al. 2011). Beads were placed adjacent the HH8 presumptive hindbrain. 3hr later embryos were treated with saline or ethanol, and beads were removed by gentle aspiration 3hr thereafter. Controls received beads treated with the DMSO carrier solvent at final concentrations ≤0.1%. Cell death was evaluated 20hr later, at HH12/13- (16–18 somites), as described below. For ex vivo treatment, HH8 embryos were incubated in the compound for 15 min prior to saline or ethanol challenge, and their β-catenin protein levels were evaluated 2hr later by immunostain or western blot as described below.
Cell Death Analysis
Cell death was visualized at HH12/13- using LysoTracker Red (0.5 μM, Invitrogen), which we and others have shown detects apoptotic death in the early embryo FAS model (Cartwright et al. 1998; Dunty et al. 2006). To quantify neural crest cell death, the LTR-stained hindbrain was sectioned and immunostained for the neural crest markers Slug (aka Snail2, #27568, 1/1000, AbCam, Cambridge MA) or Sox9 (1/1000, Abcam) followed by Alexa488-conjugated secondary antibody. We counted the total number of slug+ (Sox9+) and LTR+slug+ (Sox9+) cells residing in the dorsal neural roof and adjacent lateral quadrant of hindbrain rhomobomeres 4 (r4) and r6, which normally do not experience significant cell death. We enumerated 6–10 sections per embryo and 3–4 embryos per treatment, counting >100 slug+(Sox9+) cells per embryo and normalized to the number of sections counted. In the small molecule screen, we counted the number of LTR+ cells in r4. The screens evaluated 15–20 embryos per treatment and were performed in at least triplicate.
β-Catenin Immunostain
HH8 embryos were incubated 15 min with the indicated compound, washed, treated 2hr with saline or 52 mM ethanol and then were fixed in Dent’s solution, sectioned, and immunostained for β-catenin with antibody #15B8 (1:1000, Novus Biologicals, Littleton CO) and Alexa 488-conjugated secondary antibody. DAPI counterstain visualized nuclei. Photographic exposure time was uniform between images.
Western Analysis
This was performed as described (Flentke et al. 2011). Ex vivo somite-matched HH8/9 embryos were pretreated as above with the indicated compound and then challenged 2hr with saline or 52mM ethanol. Proteins extracted from dissected crania were identified using antibodies against β-catenin (15B8, 1:50,000) and GAPDH (#G8795, 1:50,000, Sigma). Experiments were performed in triplicate and analyzed one dissected head per lane and 3–5 embryos per treatment.
Electroporation and Cell Death Assays
This was performed as described (Flentke et al. 2011). cDNA encoding eGFP and the parent expression vector pCAGGS (Niwa et al. 2001) were kind gifts of T. Suzuki. The β-catenin construct was obtained from Addgene (#13435, Cambridge MA; Lee et al. 2001). The ΔTCF construct (Addgene #14019; Kardon et al. 2003) lacks the β-catenin binding domain (amino acids 2–50) and thus has dominant-negative activity. Constructs were used at a 3:1 ratio (1.2:0.4 μg/μl) of experimental:eGFP cDNA. DNA (2 μl) was injected into the lumen of the HH9 posterior hindbrain, and the in ovo embryos were immediately electroporated as described (Flentke et al. 2011). Eggs were injected 3hr later with saline or 0.43 mmol ethanol. At 20 hr thereafter (HH12/13−), cell death and neural crest numbers were enumerated as above using LysoTracker Red and Sox9 immunostain. Embryos lacking hindbrain expression of the parent eGFP vector were discarded from analysis.
Quantitation of Endogenous β-Catenin/TCF Transcriptional Activity
This was performed as described (Flentke et al. 2011). In ovo embryos were electroporated with the β-catenin/TCF luciferase reporter construct pBARL or the parallel, inactive construct pfuBARL (5 μg/μl; kind gift of R. Moon; Biechele and Moon, 2008) + CMV-Renilla luciferase vector (0.06 μg/μl; Promega). Prior to electroporation some embryos were treated 3hr with Bapta-AM or calmidazolium as described above. Luciferase activity of individual crania was quantified 20 hr thereafter using a modification of the Dual Luciferase Reporter Assay System (Promega) as described (Flentke et al. 2011). Luciferase values were normalized to Renilla activity. Extracts were assayed in duplicate using 6–8 embryos per treatment; experiments were repeated in at least duplicate.
In Vitro Phosphorylation Studies
cDNA encoding human recombinant β-catenin in pET28a (#17198, Addgene, Cambridge MA; Xing et al., 2008) was expressed and purified using a cobalt affinity column (Clontech, Mountain View, CA). The eluate was treated with tobacco etch virus protease (Promega) to cleave the His-tag and repurified as above before use. Site-directed mutagenesis to substitute Alanine at Threonine-332, Serine-389, Threonine-472, and/or Serine-552 was performed using the Quick Change Lightning mutagenesis kit (Stratagene) according to manufacturer’s directions. Truncated rat recombinant CaMKII (AA 1-325) was prepared in Sf9 baculovirus system and obtained from New England Biolabs (Beverly, MA); it was activated by phosphorylation following manufacturer’s guidelines. Unactivated CaMKII controls were run in parallel with omission of calcium and calmodulin. The protein D52, a known CaMKII substrate, was a generous gift of G. Groblewski (Chew et al. 2008). For the reaction, recombinant β-catenin (0.96 μg) or D52 (0.1 μg) were incubated (3hr, 30°C) with either activated (11 units) or unactivated (11 unit equivalents) recombinant CaMKII, in a CaMKII reaction mixture (New England Biolabs) containing 50 mM Tris-HCl, 10 mM MgCl2, 0.1 mM EDTA, 0.01% Brij-35, 0.3 mM ATP, 2.5 μCi [γ32P]-ATP (Perkin-Elmer). This represented three molar equivalents of β-catenin (89.9 kD) over D52 (28 kD). Samples were separated using 10% SDS-PAGE, transferred to Immobilon-P membrane, and autoradioimages were acquired using a Typhoon FLA-7000 (GE BioSciences, Piscataway, NJ).
Statistics
Normally distributed data were subjected either to unpaired t-test or one-way ANOVA and the appropriate post-hoc analysis as indicated, using SigmaStat v.3.5 (Systat Software, Point Richmond, CA). Data not normally distributed were analyzed using Kruskal-Wallis one-way ANOVA on ranks and the nonparametric version of Dunn’s post hoc analysis, again using SigmaStat. P<0.05 was the criterion for significance. Results are presented as mean ± SEM, unless otherwise indicated.
Results
Cai+2 Mediated Loss of β-Catenin and Neural Crest
As we showed previously (Cartwright et al. 1998), exposure of the neurulation-stage chick embryo (3–5 somites) to clinically relevant ethanol concentrations (50–60 mM) initiates substantial cell death within the early neural tube and hindbrain. Many of the affected cells are of neural crest origin, revealed by immunostain for the neural crest marker Sox9+ and their location within the dorsal hindbrain roof (Fig. 1A, B). The level of neural crest death rises from 8.7 ± 4.5% to 35.5 ± 7.6% (p<0.001) following ethanol exposure (Fig. 1E), and neural crest numbers drop to 60% of normal (p<0.001, Fig. 1F). As we reported previously (Debelak-Kragtorp et al. 2003; Garic-Stankovic et al. 2005), a well-documented ethanol-invoked Cai+2 transient initiates this cell death. Treatment with the intracellular calcium chelator Bapta-AM immediately prior to ethanol exposure normalized neural crest numbers (35.1 ± 2.5, Fig. 1F) and prevented the ethanol-induced cell death (4.8 ± 6.2%, both p<0.001 vs. ethanol; Fig. 1C, E). In the absence of ethanol, treatment with the calcium ionophore Ionomycin initiated significant neural crest death (41.3 ± 10.7%, p<0.001; Fig. 1D, E) to levels indistinguishable from those caused by ethanol, showing that a transient elevation in Cai+2 is sufficient to cause neural crest death.
Figure 1. Calcium signals mediate cell death in ethanol-treated neural crest.
(A–D) Transverse sections of embryos treated as indicated at neural fold-stage (HH8), and evaluated at HH12/13 for neural crest (Sox9, green), cell death (LTR, red) and nuclei (DAPI, blue). Arrows indicate Sox9+ neural crest cells within the dorsal roof of hindbrain rhombomere 4 (r4); these cells normally exhibit little cell death. (A) Saline-treated control has little cell death in hindbrain r4. (B) Significant cell death occurs in hindbrain r4 including Sox9+ neural crest following exposure to 52mM ethanol. (C) Treatment with BAPTA-AM prior to 52mM ethanol exposure prevents neural crest death in r4; BAPTA also caused minor neural tube dilatation. (D) Hindbrain treated with ionomycin (iono) and not ethanol has appreciable cell death and Sox9 losses. Scale bars represent 50 μm. (E–F) Quantitation of neural crest survival in rhombomere 4. (E) Frequency of neural crest cell death (LTR+Sox9+ cells) in rhombomere 4. (F) Number of Sox9+ neural crest cells in rhombomere 4. Mean ± SD of 6 embryos/treatment, * p<0.001 vs. saline; ^ p<0.001 vs. ethanol-only by one-way ANOVA and Holm-Sidak comparison.
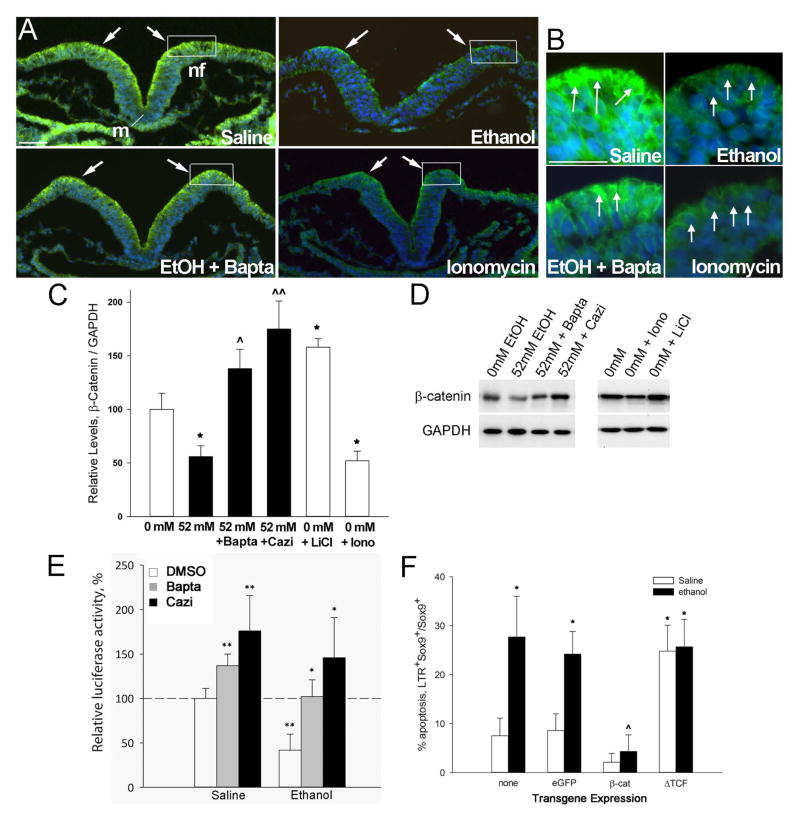
At these stages (HH8, 3–5 somites), the dorsal neuroepithelium including neural crest progenitors was selectively enriched in β-catenin protein (Fig. 2A, B), as we and others reported previously (Flentke et al. 2011; Brault et al., 2001). A 2hr exposure to 52 mM ethanol substantially down-regulated β-catenin protein levels within these dorsal neuroepithelial populations, including neural crest progenitors within the dorsal lip of the neural folds (boxed region, Fig. 2A, B). Western blot analysis of ethanol-treated hindbrain confirmed these observations and showed that β-catenin protein content decreased to 56 ± 10% of control values (Fig. 2C, D; p<0.005). The ethanol-mediated loss of β-catenin was calcium-dependent. Pretreatment with Bapta-AM prevented the β-catenin loss and normalized its expression in the presence of ethanol (Figs. 2A–D; p=0.037 vs. ethanol). BAPTA pretreatment also exaggerated neural fold elevation in 6 out of 7 embryos, an action consistent with calcium’s known role within the developing neural plate (Ferreira and Hilfer, 1993). In contrast, treatment of otherwise-normal embryos with ionomycin caused a significant loss of β-catenin protein as evaluated using immunostain (Fig. 2A, B) and western blot (Fig. 2C, D). The level of loss was similar to that caused by ethanol (52 ± 9%, p=0.003 vs. saline; p=0.74 vs. ethanol). This suggested that the β-catenin protein in neural crest progenitors was sensitive to Cai+2 –mediated destabilization and degradation. To further investigate the calcium mechanism, we pretreated neural folds with the calmodulin inhibitor calmidazolium (Gietzen et al. 1982); calmodulin is a cytoskeletal calcium sensor protein that converts the calcium transient into a lasting effector of cell fate through the calcium-dependent interactions with protein kinases and phosphatases. Calmidazolium pretreatment prevented the β-catenin loss in ethanol-treated neural crest (p=0.003 vs. ethanol; Fig. 2C, D) indicating that the calcium transient might act upstream through activation of calmodulin-dependent signaling pathways. The β-catenin levels following ethanol + calmidazolium treatment were significantly greater than that of saline controls (p=0.01 vs. saline), suggesting that calcium may also have a role in controlling endogenous β-catenin levels within this population.
Figure 2. Ethanol depletes β-catenin from early neural progenitors.
(A, B) Calcium signals rapidly deplete β-catenin from ethanol-treated neural folds. Transverse sections through presumptive hindbrain of HH8 embryos treated as indicated for 2hr and stained for β-catenin protein (green) and nuclei (blue). Dorsal is at the top. Ethanol (52 mM) substantially reduces β-catenin content from dorsal neuroectoderm including neural crest-enriched populations (boxed region, arrows), as compared with saline-treated controls. The calcium chelator BAPTA-AM prior to ethanol exposure mitigates the β-catenin losses, whereas the calcium ionophore ionomycin similarly depletes β-catenin. (C, D) Quantitation of β-catenin protein by western blot analysis. (C) β-Catenin content, normalized to GAPDH, for neuroepithelia treated as indicated. Shown is the mean ± SEM of three independent experiments as detailed in Methods. * p <0.005 vs. saline, ^ p = 0.037 and ^^ p < 0.005 vs. ethanol-only by one-way ANOVA and Holm-Sidak comparison. (D) Representative western blots with treatments indicated, each lane evaluating a single crania. (E) Endogenous transcriptional activity of β-catenin in ethanol-treated hindbrain, measured using the pBARL luciferase reporter relative to Renilla luciferase, and normalized to the activity in saline controls. Mean ± SEM of four experiments having 6–8 embryos per treatment. * p<0.05, ** p<0.003 by ANOVA and Holm-Sidak comparison. (F) Quantitation of neural crest (Sox9+) apoptosis in r4 of ethanol-treated hindbrains transfected with β-catenin, dominant-negative TCF (ΔTCF) or eGFP control vector; “none” is non-transfected hindbrain. Mean ± SD of 3–4 embryos per treatment. * p < 0.05 vs. Saline-only, ^ p < 0.05 vs. Ethanol-only, by Kruskal-Wallis statistic. Cazi, calmidizolium.
In the nucleus, β-catenin interaction with the transcriptional effectors TCF/LEF1 converts the latter from transcriptional repressors to activators (MacDonald et al. 2009). To ascertain if this β-catenin loss affected its transcriptional function, we transfected hindbrain neural crest with a luciferase-based transcriptional reporter construct and quantified its activity in the presence or absence of ethanol treatment. The pBARL reporter contains twelve TCF response elements that drive luciferase expression (Biechele and Moon, 2008). Ethanol challenge significantly reduced the luciferase expression in pBARL-transfected cells as compared with saline-treated controls (41.8 ± 18.0%, p<0.003, Fig. 2E), indicating that ethanol treatment reduced the transcriptional activity of the β-catenin/TCF complex. Ethanol does not suppress luciferase or Renilla activity per se (Flentke et al., 2011).
The loss of β-catenin/TCF transcriptional activity was mediated by ethanol’s calcium transient. Bapta-AM treatment prior to ethanol challenge rescued β-catenin/TCF transcriptional activity and prevented the ethanol-mediated transcriptional loss (102 ± 19%, p<0.05 vs. ethanol-only, Fig. 2E). The calmodulin inhibitor calmidizolium similarly prevented the loss of β-catenin/TCF transcription following ethanol treatment (146 ± 45%, p<0.05 vs. ethanol-only, Fig. 2E). Commensurate with their effects on β-catenin content, Bapta-AM and calmidizolium also enhanced β-catenin/TCF transcriptional activity in the saline controls (p<0.001), suggesting that calcium signals might normally govern β-catenin stability in neural crest progenitors, in addition to mediating the effects of ethanol.
This loss of β-catenin/TCF transcriptional activity was responsible for the neural crest cell death following ethanol exposure. In ethanol-treated embryos, transient transfection of neural crest with ectopic β-catenin substantially reduced the numbers of apoptotic neural crest, identified by their coexpression of LTR+Sox9+, as compared to ethanol-treated controls that were untransfected or were transfected with the eGFP parent construct (4.3 ± 3.4% vs. 24.2 ± 8.3%, p < 0.05 vs. ethanol-only, Fig. 2F). Conversely, overexpression of the TCF construct ΔTCF, which lacks the β-catenin binding site and thus has dominant-negative activity against the β-catenin/TCF transcriptional complex, caused substantial neural crest losses following both ethanol (25.7 ± 5.6%) and saline treatment (24.8 ± 5.3%, for both p<0.05 vs. saline-only). The effects of ΔTCF and ethanol were not additive, suggesting their actions were convergent and not separate. Transfection with the parent eGFP construct did not alter the effects of ethanol or saline on neural crest survival.
CaMKII Destabilizes β-Catenin in Ethanol-Treated Neural Crest
In other model systems, several calcium-dependent signals have been shown to alter β-catenin expression, protein stability, and transcriptional activity, including CaMKII, Protein Kinase C, calpains, and JNK. GSK3β is also a potent negative effector, although its activity is not necessarily linked to calcium signaling. Several of these (GSK3β, CaMKII, PKC) are ethanol responsive (Garic et al. 2011; French and Heberlein 2009; Lee and Messing 2008; Liu et al. 2009). We investigated which of these effectors might contribute to the calcium-mediated reductions in β-catenin protein and cell survival in these ethanol-treated cells. To narrow the list of candidates, we performed a screen of small molecule inhibitors that were selective for these effectors and investigated their ability to prevent the ethanol-induced neural crest death.
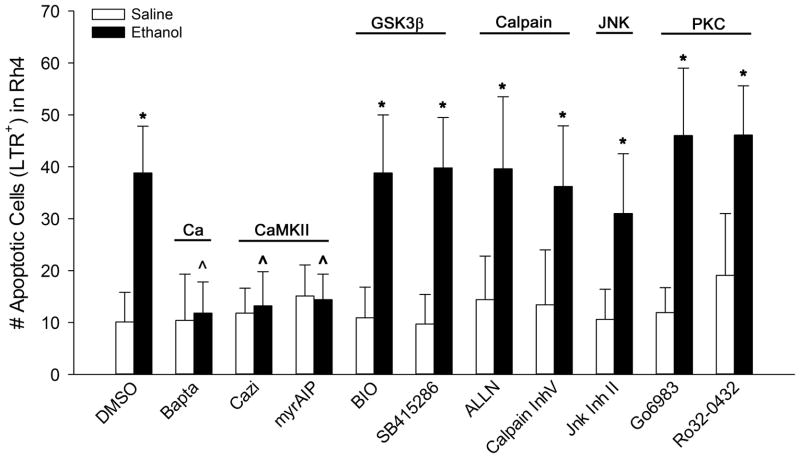
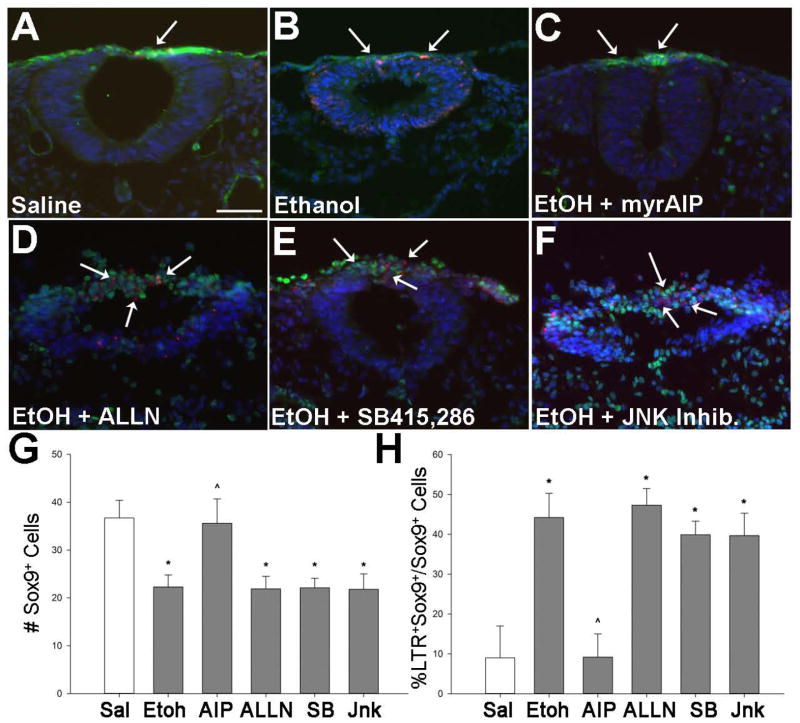
We first explored the potential involvement of CaMKII because, in our previous studies, we showed that it is a downstream target of ethanol’s calcium transient, and that inhibition of CaMKII activity prevents the ethanol-induced neural crest death studied here (Garic et al. 2011). Consistent with that work, we found that inhibitors selective against CaMKII (myrAIP; Ishida and Fujisawa 1995), or its upstream activator calmodulin (calmidazolium), both prevented the ethanol-induced cell death as shown by the significant reductions in the number of LTR+ cells within rhombomere 4, which normally does not experience significant cell death (p<0.001 vs. ethanol-only; Fig. 3). This represented an effect upon neural crest because myrAIP significantly enhanced the survival of Sox9+ neural crest following ethanol treatment (Ethanol + myrAIP: 35.6 ± 5.1 Sox9+ cells, Ethanol-only: 22.3 ± 2.6 Sox9+ cells, p< 0.001; Fig. 4A–C, G) and reduced the percentage of Sox9+LTR+ cells within rhombomere 4 (Ethanol + myrAIP: 9.2 ± 5.8%, Ethanol-only: 44.2 ± 6.1%, p<0.001; Fig. 4H). In contrast, inhibitors directed against PKC (Go-6983, Ro-32-0432), Calpain (ALLN, Inhibitor V), JNK (JNK Inhibitor II), and GSK3β (BIO, SB-415,286) all failed to prevent the pro-apoptotic effects of ethanol. For those inhibitors, both the number of LTR+ cells in rhombomere 4 (Fig. 4) and the absolute number of Sox9+LTR+ neural crest did not differ from ethanol-only treatment (Fig. 5, Fig. 6D–F). These data were consistent with our previous findings that CaMKII activity is an essential component of the ethanol-induced apoptosis in neural crest (Flentke et al. 2011). It also suggested that other effectors of calcium signaling and/or β-catenin stability, including GSK3β, calpain, PKC, and JNK, were unlikely participants in this model of ethanol’s neurotoxicity.
Figure 3. Small molecule screen implicates CaMKII in ethanol-mediated neural crest death.
HH8 embryos were pretreated with the indicated effector of calcium signaling prior to 52 mM ethanol challenge. The number of LTR+ cells in r4 was quantified at HH12/13. Inhibitors selective for intracellular calcium (BAPTA-AM), calmodulin (calmidizolium, cazi), and CaMKII (myrAIP) prevented ethanol-mediated cell death, whereas inhibitors directed against GSK3β, calpain, JNK and Protein Kinase C did not affect cell death. Mean ± SD of 11–22 embryos per treatment group. * p < 0.001 vs. saline-DMSO control, ^ p < 0.001 vs. ethanol-DMSO using one-way ANOVA and Student-Newman-Kuels pairwise multiple comparisons.
Figure 4. CaMKII but not other β-catenin effectors contribute to ethanol-induced neural crest death.
(A–F) Representative cross-sections through r4 of embryos exposed to 52mM ethanol following treatment with the indicated inhibitor of calcium signaling. Neural crest is visualized using Sox9+ immunostain (green) and cell death using LTR (red); nuclei are blue. Saline control (A) has little neural crest death (arrow). Significant cell death occurs in ethanol-treated hindbrain (B) including neural crest (arrows), and. the CaMKII-selective inhibitor Myr-AIP (C) significantly reduces that cell death. In contrast, pretreatment with inhibitors directed against calpain (ALLN, D), GSK-3β (SB-415,286, E) or JNK (JNK Inhibitor-2, F) did not prevent ethanol-induced cell death. Calpain and JNK inhibition also substantially altered neural tube morphology. Scale bar represents 50 μm. (G) Enumeration of Sox9+ neural crest cells per section of r4 following the indicated treatments. MyrAIP, but not other inihibitors, normalized neural crest numbers in ethanol-treated hindbrain. (H) Percentage of LTR+ neural crest (Sox9+) in r4 following the indicated treatments. Only myrAIP prevented neural crest death in ethanol-treated hindbrain. * p < 0.001 vs. saline, ^ p < 0.001 vs. ethanol using one-way ANOVA and Holm-Sidak comparison.
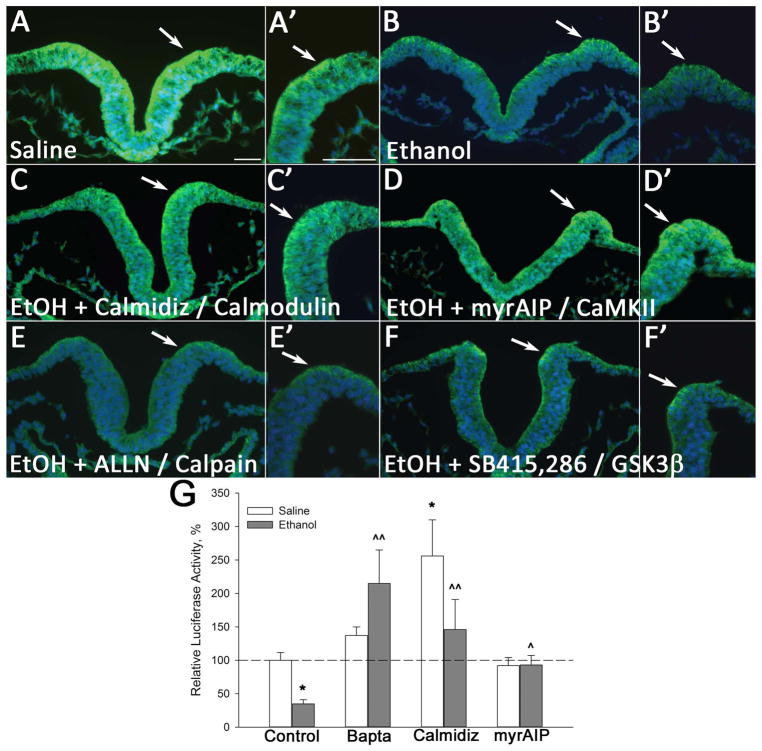
Figure 5. CaMKII inhibition but not other β-Catenin effectors stabilize β-catenin protein.
(A–F) Neural folds of 3-somite embryos were treated with the indicated inhibitor prior to 52mM ethanol exposure; β-catenin was visualized 2hr thereafter in presumptive hindbrain. Arrows indicate the neural crest-enriched dorsal neural fold; scale bar represents 50 μm. Saline control (A) has robust β-catenin distribution within the dorsal neuroepithelium (arrow), and this is substantially reduced by ethanol (B). The calmodulin-selective inhibitor calmidizolium (C) (20 μM) and the CaMKII-selective inhibitor myrAIP (D) (5 μM) both prevent β-catenin loss within the ethanol-treated dorsal neural folds. In contrast, inhibitors directed against calpain (E, ALLN, 5 μM) and GSK-3β (F, SB-415,286, 5 μM) do not prevent ethanol-mediated β-catenin loss. (G) CaMKII and Cai2+ inhibition restore β-catenin transcriptional activity in ethanol-treated hindbrain. Hindbrain expression of TopFlash luciferase reporter was quantified and normalized against Renilla luciferase co-expression in hindbrains pretreated as indicated prior to saline or ethanol challenge. Mean ± SEM of three experiments having 6–8 embryos/treatment, normalized to saline control. * p < 0.001 vs. saline-only; ^ p < 0.05 and ^^ p < 0.001 vs. ethanol-only, analyzed using one-way ANOVA with Holm-Sidak method.
Figure 6. CaMKII can directly phosphorylate β-catenin.
(A) Amino acid sequence comparison of Gallus gallus and human β-catenin. Dashes indicate conserved residues. Boldface / underline indicates putative CaMKII binding sites (LXXLL) and CaMKII phosphorylation sites (RXXT/S) identified in silico. (B–D) SDS-PAGE and autoradiography of purified phosphoproteins labeled with [γ32P]-ATP by CaMKII. Reactions contained the respective purified proteins as indicated. (B) CaMKII can phosphorylate the known target protein thrombin-cleaved D52 and purified human β-catenin. (C) CaMKII phosphorylation of wild-type β-catenin or β-catenin constructs harboring one of four mutations in putative phosphorylation sites, as indicated. (D) CaMKII phosphorylation of wild-type β-catenin and a β-catenin construct harboring a triple mutation of the CaMKII phosphorylation sites as indicated. For all three experiments, reactions that lack activated CaMKII instead contain unactivated CaMKII prepared in the absence of calcium and calmodulin. The β-catenin doublet in (C) and (D) represents β-catenin with an intact (upper band) or cleaved 4.4kDa His-tag sequence (lower band). For all experiments, lanes contained equimolar levels of β-catenin.
We investigated which of these calcium effectors contributed to the β-catenin loss in ethanol-exposed neural crest. Pretreatment with either calmidazolium or myr-AIP prior to ethanol challenge counteracted ethanol’s effects and normalized the β-catenin protein expression within the ethanol-treated neural folds (Fig. 5A–D). The CaMKII and calmodulin inhibitors also restored the transcriptional activity of β-catenin/TCF within ethanol-treated cells to the level observed in controls, as evaluated using the pBARL TopFlash reporter construct (calmidazolium + ethanol: 184 ± 51% p<0.05, myr-AIP + ethanol: 98 ± 23% p<0.001 vs. ethanol-only: 43±18%, Figure 5G). In contrast, inhibitors selective for GSK3β (SB415,286; Fig. 5F) or calpain (ALLN; Fig. 5E), although known to modulate β-catenin protein stability, failed to override ethanol’s effects upon β-catenin and the protein levels remained low within the ethanol-treated neural fold. These findings, combined with our previous demonstration that β-catenin loss was responsible for the ethanol-mediated neural crest death, indicated that ethanol’s activation of CaMKII was responsible for the β-catenin loss within this cell population.
CaMKII Can Phosphorylate β-Catenin
Little is known about how CaMKII might destabilize β-catenin. Ethanol treatment does not alter β-catenin transcript levels in these cells (Flentke et al. 2011). Moreover, β-catenin protein disappears from these cells within 2hr of ethanol addition (Flentke et al. 2011), implicating a post-translational effect. Direct phosphorylation of β-catenin by the protein kinase GSK3β targets β-catenin for proteosomal transport and degradation. We considered whether CaMKII might possess similar kinase activity against β-catenin; such activity has not been reported previously. In silico analysis of the amino acid sequences of chick and human β-catenin revealed four conserved sites that contain the CaMKII phosphorylation motif RXX(T/S). These were located at Threonine 332, Serine 389, Threonine 472, and Serine 552 (Fig. 6A). An additional fifth site at Serine 96 of chick β-catenin was not present in human. β-catenin from both species also shared four conserved sites containing the putative CaMKII binding motif LXXLL, and these were located starting at Leucines 156, 405, 536, and 640. Incubation of purified human β-catenin with activated CaMKII and [γ32P]-labeled ATP, followed by SDS-PAGE, revealed the presence of a 85kDa phosphorylated protein that was absent from samples incubated with inactive CaMKII (Fig. 6B). As a positive control, the protein D52, a thrombin-cleaved, known CaMKII target, produced a 32P-labeled protein when incubated with activated CaMKII but not with unactivated CaMKII. This result indicated that CaMKII was capable of directly phosphorylating β-catenin.
To establish which of the putative phosphorylation sites in β-catenin could be phosphorylated by CaMKII, we individually mutated the four target sites (T332A, S389A, T472A, S552A) in human β-catenin and tested the ability of activated CaMKII to phosphorylate each mutant protein. We found that β-catenin proteins that harbored the individually mutated sites could still be phosphorylated by CaMKII. Importantly, sensitivity to phosphorylation was not equivalent among the four mutants. The lowest phosphorylation with 32P was observed with the β-catenin S552A mutant, and moderate 32P labeling was observed with the T332A and T472A β-catenin constructs (Fig. 6C). The S389A mutant had robust phosphorylation labeling that did not appreciably differ from the results obtained with equimolar wild-type β-catenin. These results suggested that CaMKII may have targeted more than one site within β-catenin, and phosphorylation at T332 and T472 might depend on the phosphorylation status at S552. It additionally suggested that S389 was not a phosphorylation target of CaMKII. We then tested the ability of activated CaMKII to phosphorylate the β-catenin triple mutant T332A/T472A/S552A. As compared with equimolar wild-type β-catenin, mutation at T332A/T472A/S552A substantially abolished the ability of CaMKII to phosphorylate β-catenin (Fig. 6D), demonstrating that these sites represent bona fide targets of CaMKII’s protein kinase activity. Because a faint band was still observed in the triple mutant protein, it further suggested there might be an additional, cryptic phosphorylation site within the protein.
Discussion
β-Catenin is a central regulator of proliferation, differentiation and migration in many cell populations, including those of neural origin. A major finding of this study is the demonstration that CaMKII can directly phosphorylate β-catenin, and this represents a novel mechanism by which calcium and its downstream kinase effectors could govern canonical Wnt signaling and thus control cellular fate. Ethanol’s dysregulation of β-catenin via CaMKII also represents a novel mechanism by which ethanol affects cellular activity and survival. Additionally, these findings highlight β-catenin and CaMKII as central mediators of ethanol’s neurotoxicity within early neural progenitors. We and others showed previously that ethanol causes significant apoptosis within neural crest progenitors and these cellular losses contribute to the neurochristopathies that partly typify individuals who experienced prenatal alcohol exposure during early development. Our past work has separately shown that two upstream events that are necessary for this apoptosis are the calcium-mediated loss of transcriptionally active β-catenin (Flentke et al. 2011) and the activation of CaMKII (Garic et al. 2011). Here, we show that these events are mechanistically linked and that CaMKII is responsible for the loss of transcriptionally active β-catenin and subsequent cell death. The ability of CaMKII to directly phosphorylate and thereby modulate β-catenin’s transcriptional activity, which is essential for neural crest survival, suggests a mechanism by which these cells are sensitive to ethanol during this developmental period.
The Wnt/β-catenin signaling cascade is a crucial regulator of cellular activities within neural progenitors, including brain patterning, neurogenesis, plasticity, and stem cell production, and disruptions in those signals contribute to disorders also seen in FASD including microcephaly, learning impairments, and autism (De Ferrari and Moon 2006; MacDonald et al. 2009; Toledo et al 2008). Growing evidence indicates that altered canonical Wnt/β-catenin signaling contributes to ethanol’s damage as revealed by expression screens of ethanol-treated cell populations (Chen et al. 2010; Himes et al. 2008; Serrano et al. 2010). This broad dysregulation within disparate tissues impairs processes including bone healing (Chen et al. 2010; Lauing et al. 2012), neural differentiation (Vangipuram and Lyman, 2012), and cell survival (Flentke et al. 2011; Yeh et al. 2008), and suggests that β-catenin/Wnt dysregulation may be a fundamental mechanism underlying ethanol’s pathogenicity. Further supporting this are recent demonstrations that Wnt/β-catenin protects hepatocytes against apoptosis and pathogenesis in alcoholic liver disease (Liu et al. 2012; Tao et al. 2013). While the β-catenin losses in this acute exposure model are clearly mechanistic, reports of increased β-catenin in chronic exposures could reflect compensatory responses to these earlier β-catenin losses. Further investigation into this understudied ethanol target will provide additional insights into how its dysregulation contributes to ethanol’s pathogenicity.
This work is also the first demonstration that CaMKII is capable of directly phosphorylating β-catenin. Given that β-catenin contains multiple, conserved CaMKII recognition sequences, and that small molecule CaMKII inhibitors selectively stabilize cellular β-catenin protein and its transcriptional activity, these findings suggest their interaction is functionally relevant. In the non-canonical Ca/Wnt pathway, ligands such as Wnt5a interact with Frizzled receptors, which act, in this context, as G protein-coupled receptors to elevate intracellular calcium and antagonize canonical Wnt/β-catenin signaling (Kohn and Moon, 2005). The CaMKII/β-catenin interaction may represent a novel mechanism by which calcium signals, perhaps including those originating from non-canonical Ca/Wnt signaling, control cellular function. CaMKII negatively regulates β-catenin indirectly, through its phosphorylation and stimulation of the TAK1-NLK mitogen-activated protein kinases, which in turn phosphorylate TCFs to inhibit β-catenin/TCF-mediated transcription (Ishitani et al. 2003). β-Catenin contains four CaMKII binding motifs (LXXLL) and four putative phosphorylation sites (RXXT/S) that are 100% conserved between chick and human, despite having diverged ~300 million years ago. All four predicted sites reside within the Arm repeat structure and three of these (T332, T472, S552) could be phosphorylated by CaMKII, suggesting they are functional. The S552 site was previously identified as a phosphorylation target for Akt and PKA (the latter, weakly); paradoxically, pS552 enhanced nuclear β-catenin accumulation and β-catenin/TCF/LEF transcriptional activity (Fang et al. 2007; Taurin et al. 2006). Given that CaMKII destabilized nuclear β-catenin and reduced its transcriptional activity, it is possible that pS552 may function to integrate the competing activities of CaMKII and Akt and thereby control canonical Wnt sensitivity.
It is unclear why the direct phosphorylation of β-catenin by CaMKII has not been previously identified. One explanation is technical. The β-catenin form often used for protein expression studies is a GST-fusion protein. In pilot studies (Flentke and Smith, unpublished) we found that CaMKII, at best, only weakly phosphorylated the β-catenin-GST fusion construct, perhaps due to steric bulk from the GST, which comprises approximately 30% of the protein’s mass. In support of this interpretation, CaMKII readily phosphorylated the β-catenin-HisTag construct that had its 4.4 kDa HisTag removed. The details of how the CaMKII phosphorylation sites regulate β-catenin stability, both individually and through potential interactions with each other, are the focus of a separate investigation.
We found no evidence that two additional effectors of β-catenin stability, PKC and GSK3β, contributed to the β-catenin losses and cell death caused by ethanol here. PKC negatively regulates canonical Wnt signaling through phosphorylation of β-catenin at S33, S37 and S45 (Gwak et al. 2006). PKC is also stimulated by ethanol in some neuronal cell lines (Lee and Messing 2008). However, ethanol treatment does not increase PKC activity in this neural crest population, nor does PKC inhibition prevent their apoptosis (Garic et al. 2011), and that PKC inhibition could not stabilize β-catenin here is consistent with that work. Another significant, Wnt effector, GSK3β, is not known to be calcium-dependent, but in certain neuronal populations acute ethanol exposure significantly enhances its activity to mediate neuronal apoptosis (French and Heberlein 2009; Liu et al. 2009). Thus it was unexpected that it did not contribute to the cell death studied here, nor did its inhibition stabilize β-catenin. However, a similar lack of GSK3β participation occurs in ethanol-exposed neonatal mouse brain, in which lithium but not SB-415,286 blocks neuronal apoptosis independent of GSK3β activation (Young et al. 2008; Zhong et al. 2006). Thus ethanol’s stimulation of neuronal GSK3β may be lineage-dependent. Overall we conclude that ethanol’s dysregulation of β-catenin did not involve signaling through the canonical Wnt pathway, an interpretation that is consistent with our prior demonstration of no expression differences in the canonical Wnt pathway (Flentke et al. 2011).
How does the calcium/CaMKII-mediated loss of transcriptional β-catenin result in cell death? β-Catenin is indispensible for neural crest survival and normal craniofacial development through its induction of genes essential for neural crest development including Wnt6 and FoxD3 (Stuhlmiller and Garcia-Castro, 2012). We showed previously that ethanol sharply down-regulates these inductive signals (Flentke et al. 2011), and their repression could reduce neural crest progenitor pools and contribute to neural crest deficits in FASD. Ethanol also significantly reduces slug/snail2 (Flentke et al. 2011), an anti-apoptotic transcriptional repressor that controls the expression of caspase and Bcl-2 family members (Tribulo et al. 2004). Later in development, β-catenin participates in neural crest migration (De Calisto et al. 2005) and differentiation into sensory neurons (Lee et al. 2004), suggesting this dysregulation may also contribute to additional neurochristopathies associated with prenatal alcohol exposure.
In summary, transcriptional β-catenin is an immediate and important target of prenatal alcohol exposure. The loss of β-catenin/TCF/LEF signaling mediates the apoptosis of ethanol-exposed neural crest, and, given the importance of canonical Wnt signaling to neural crest induction and differentiation, this repression may also play a broader role in FASD neurochristopathies. Because β-catenin also has critical roles in neuronal development, its disruption by ethanol may underlie additional FASD pathologies and, as suggested by recently published studies of liver, in adult alcoholics. It is unlikely here that ethanol targeted canonical Wnt pathways. Rather, ethanol is teratogenic because it activates pathways that mimic or converge upon canonical Wnt and Ca/Wnt pathways within neural crest. These pathways, and how ethanol interacts with them, are shown in Figure 7. Ethanol’s activation of CaMKII, via stimulation of PLC/PIP2-mediated calcium release, mediates the loss of transcriptionally active β-catenin and its trophic support. Given that both acute and chronic ethanol exposures modulate intracellular calcium in diverse cell types, the calcium-mediated destabilization of β-catenin described here might also underlie the seemingly diverse alcoholic pathologies in other fetal and adult tissues.
Figure 7. Diagram of ethanol signaling pathway that initiates cell death in neural crest.
Shown is a summary of published studies (Debelak-Kragtorp et al., 2003; Garic-Stankovic et al., 2005, 2006; Garic et al. 2011; Flentke et al. 2011) and results herein. Ethanol’s interaction with a G protein-coupled receptor (GPCR) of unknown identity activates Gαi2/3 and Gβγ. Within seconds, the latter stimulates the phospholipase Cβ (PLCβ)-mediated synthesis of inositol-1,4,6-trisphosphate (Ins[1,4,5]P3) and calcium release predominantly from intracellular stores. The calcium transient activates CaMKII, which may directly phosphorylate and destabilize β-catenin. CaMKII might additionally act indirectly via NLK/TCF. This loss terminates β-catenin’s transcriptional activity and its protective effects on neural crest survival, and instead promotes cell death within these cells.
Acknowledgments
Supported by NIH MERIT Award R37 AA11085 to SMS. The authors have no conflict of interest to declare.
References
- Biechele TL, Moon RT. Assaying β-catenin/TCF transcription with β-catenin/TCF transcriptional-based reporter constructs. Methods Mol Biol. 2008;468:99–110. doi: 10.1007/978-1-59745-249-6_8. [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Cartwright MM, Tessmer LL, Smith SM. Ethanol-induced neural crest apoptosis is coincident with their endogenous death, but is mechanistically distinct. Alcohol Clin Exp Res. 1998;22:142–149. [PubMed] [Google Scholar]
- Chen JR, Lazarenko OP, Shankar K, Blackburn ML, Badger TM, Ronis MJ. A role for ethanol-induced oxidative stress in controlling lineage commitment of mesenchymal stromal cells through inhibition of Wnt/beta-catenin signaling. J Bone Miner Res. 2010;25:1117–27. doi: 10.1002/jbmr.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew CS, Chen X, Zhang H, Berg EA, Zhang H. Calcium/calmodulin-dependent phosphorylation of tumor protein D52 on serine residue 136 may be mediated by CAMK2delta6. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1159–1172. doi: 10.1152/ajpgi.90345.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debelak KA, Smith SM. Avian genetic background modulates the neural crest apoptosis induced by ethanol exposure. Alcohol Clin Exp Res. 2000;24:307–314. [PubMed] [Google Scholar]
- Debelak-Kragtorp KA, Armant DR, Smith SM. Ethanol-induced cephalic apoptosis requires phopholipase C-dependent intracellular calcium signaling. Alcohol Clin Exp Res. 2003;27:515–523. doi: 10.1097/01.ALC.0000056615.34253.A8. [DOI] [PubMed] [Google Scholar]
- De Calisto J, Araya C, Marchant L, Riaz CF, Mayor R. Essential role of non-canonical Wnt signaling in neural crest migration. Development. 2005;132:2587–2597. doi: 10.1242/dev.01857. [DOI] [PubMed] [Google Scholar]
- De Ferrari GV, Moon RT. The ups and downs of Wnt signaling in prevalent neurological disorders. Oncogene. 2006;25:7545–7553. doi: 10.1038/sj.onc.1210064. [DOI] [PubMed] [Google Scholar]
- Dunty WC, Chen SY, Zucker RM, Dehart DB, Sulik KK. Selective vulnerability of embryonic cell populations to ethanol-induced apoptosis: implications for alcohol-related birth defects and neurodevelopmental disorder. Alcohol Clin Exp Res. 2001;25:1523–1535. [PubMed] [Google Scholar]
- Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z. Phosphorylation of β-catenin by AKT promotes β-catenin transcriptional activity. J Biol Chem. 2007;282:11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira MC, Hilfer SR. Calcium regulation of neural fold formation: visualization of the actin cytoskeleton in living chick embryos. Dev Biol. 1993;159:427–440. doi: 10.1006/dbio.1993.1253. [DOI] [PubMed] [Google Scholar]
- Flentke GR, Garic A, Amberger E, Hernandez M, Smith SM. The calcium-mediated repression of β-catenin and Its transcriptional signaling mediates neural crest cell death in an avian model of Fetal Alcohol Syndrome. Birth Defects Res A. 2011;91:591–602. doi: 10.1002/bdra.20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French RL, Heberlein U. Glycogen synthase kinase-3/Shaggy mediates ethanol-induced excitotoxic cell death of Drosophila olfactory neurons. Proc Natl Acad Sci USA. 2009;106:20924–20929. doi: 10.1073/pnas.0910813106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garic A, Flentke GR, Amberger E, Hernandez M, Smith SM. CaMKII activation is a novel effector of alcohol’s neurotoxicity in neural crest stem/progenitor cells. J Neurochem. 2011;118:646–657. doi: 10.1111/j.1471-4159.2011.07273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garic-Stankovic A, Hernandez M, Chiang PJ, Armant DR, Debelak-Kragtorp KA, Smith SM. Ethanol selectively triggers neural crest apoptosis thru its activation of a pertussis toxin-sensitive G-protein and a phospholipase Cβ-dependent Ca2+ transient. Alcohol Clin Exp Res. 2005;29:1237–1246. doi: 10.1097/01.alc.0000172460.05756.d9. [DOI] [PubMed] [Google Scholar]
- Garic-Stankovic A, Hernandez M, Flentke GR, Smith SM. Structural constraints for alcohol-stimulated Ca2+ release in neural crest, and dual agonist/antagonist properties of n-octanol. Alcohol Clin Exp Res. 2006;30:552–559. doi: 10.1111/j.1530-0277.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- Gietzen K, Sadorf I, Bader H. A model for the regulation of the calmodulin-dependent enzymes erythrocyte Ca2+-transport ATPase and brain phosphodiesterase by activators and inhibitors. Biochem J. 1982;207:541–548. doi: 10.1042/bj2070541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak J, Cho M, Gong S-J, Won J, Kim D-E, Kim E-Y, Lee SS, Kim M, Kim TK, Shin J-G, Oh S. Protein kinase C-mediated β-catenin phosphorylation negatively regulates the Wnt/β-catenin pathway. J Cell Sci. 2006;119:4702–4709. doi: 10.1242/jcs.03256. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Himes R, Wezeman FH, Callaci JJ. Identification of novel bone-specific molecular targets of binge alcohol and ibandronate by transcriptome analysis. Alcohol Clin Exp Res. 2008;32:1167–80. doi: 10.1111/j.1530-0277.2008.00736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Hörster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Ishida A, Fujisawa H. Stabilization of calmodulin-dependent protein kinase II through the autoinhibitory domain. J Biol Chem. 1995;270:2163–2170. doi: 10.1074/jbc.270.5.2163. [DOI] [PubMed] [Google Scholar]
- Ishitani T, Kishida S, Hyodo-Miura J, Ueno N, Yasuda J, Waterman M, Shibuya H, Moon RT, Ninomiya-Tsuji J, Matsumoto K. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol Cell Biol. 2003;23:131–139. doi: 10.1128/MCB.23.1.131-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardon G, Harfe BD, Tabin CJ. A Tcf-4-positive mesodermal population provides a prepattern for vertebrate limb muscle patterning. Dev Cell. 2003;5:937–944. doi: 10.1016/s1534-5807(03)00360-5. [DOI] [PubMed] [Google Scholar]
- Kohn AD, Moon RT. Wnt and calcium signaling: β-catenin-independent pathways. Cell Calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Lauing KL, Roper PM, Nauer RK, Callaci JJ. Acute alcohol exposure impairs fracture healing and deregulates β-catenin signaling in the fracture callus. Alcohol Clin Exp Res. 2012 doi: 10.1111/j.1530-0277.2012.01830.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin NM, Kalcheim C. The Neural Crest. 2. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Lee AM, Messing RO. Protein kinases and addiction. Ann NY Acad Sci. 2008;1141:22–57. doi: 10.1196/annals.1441.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Kleber M, Hari L, Brault V, Suter U, Taketo MM, Kemler R, Sommer L. Instructive role of Wnt/β-catenin in sensory fate specification in neural crest stem cells. Science. 2004;303:1020–1023. doi: 10.1126/science.1091611. [DOI] [PubMed] [Google Scholar]
- Lee E, Salic A, Kirschner MW. Physiological regulation of β-catenin stability by Tcf3 and CK1ε. J Cell Biol. 2001;154:983–993. doi: 10.1083/jcb.200102074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Iyengar R. Calpain as an effector of the Gq signaling pathway for inhibition of Wnt/β-catenin-regulated cell proliferation. Proc Natl Acad Sci USA. 2002;99:13254–13259. doi: 10.1073/pnas.202355799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chen G, Ma C, Bower KA, Xu M, Fan Z, Shi X, Ke ZJ, Luo J. Overexpression of glycogen synthase kinase 3β sensitizes neuronal cells to ethanol toxicity. J Neurosci Res. 2009;87:2793–2802. doi: 10.1002/jnr.22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Yeh TH, Singh VP, Shiva S, Krauland L, Li H, Zhang P, Kharbanda K, Ritov V, Monga SP, Scott DK, Eagon PK, Behari J. β-catenin is essential for ethanol metabolism and protection against alcohol-mediated liver steatosis in mice. Hepatology. 2012;55:931–940. doi: 10.1002/hep.24766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–200. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Saneyoshi T, Kume S, Amasaki Y, Mikoshiba K. The Wnt/calcium pathway activates NF-AT and promotes ventral cell fate in Xenopus embryos. Nature. 2002;417:295–299. doi: 10.1038/417295a. [DOI] [PubMed] [Google Scholar]
- Serrano M, Han M, Brinez P, Linask KK. Fetal alcohol syndrome: cardiac birth defects in mice and prevention with folate. Am J Obstet Gynecol. 2010;203:75.e7–75.e15. doi: 10.1016/j.ajog.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Stuhlmiller TJ, Garcia-Castro MI. Current perspectives of the signaling pathways directing neural crest induction. Cell Mol Life Sci. 2012;69:3715–3737. doi: 10.1007/s00018-012-0991-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao GZ, Lehwald N, Jang KY, Baek J, Xu B, Omary MB, Sylvester KG. Wnt/β-Catenin Signaling Protects Mouse Liver against Oxidative Stress-induced Apoptosis through the Inhibition of Forkhead Transcription Factor FoxO3. J Biol Chem. 2013;288:17214–17224. doi: 10.1074/jbc.M112.445965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo EM, Colombres M, Inestroso NC. Wnt signaling in neuroprotection and stem cell differentiation. Prog Neurobiol. 2008;86:281–296. doi: 10.1016/j.pneurobio.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Tribulo C, Aybar MJ, Sanchez SS, Mayor R. A balance between the anti-apoptotic activity of Slug and the apoptotic activity of msx1 is required for the proper development of neural crest. Dev Biol. 2004;275:325–342. doi: 10.1016/j.ydbio.2004.07.041. [DOI] [PubMed] [Google Scholar]
- Vangipuram SD, Lyman WD. Ethanol affects differentiation-related pathways and suppresses Wnt signaling protein expression in human neural stem cells. Alcohol Clin Exp Res. 2012;36:788–797. doi: 10.1111/j.1530-0277.2011.01682.x. [DOI] [PubMed] [Google Scholar]
- Xing Y, Takemaru KI, Liu J, Berndt JD, Zheng JJ, Moon RT, Xu W. Crystal structure of a full-length β-catenin. Structure. 2008;16:478–487. doi: 10.1016/j.str.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CH, Chang JK, Wang YH, Ho ML, Wang GJ. Ethanol may suppress Wnt/beta-catenin signaling on human bone marrow stroma cells: a preliminary study. Clin Orthop Relat Res. 2008;466:1047–53. doi: 10.1007/s11999-008-0171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C, Straiko MMW, Johnson SA, Creeley C, Olney JW. Ethanol causes and lithium prevents neuroapoptosis and suppression of pERK in the infant mouse brain. Neurobiol Dis. 2008;31:355–360. doi: 10.1016/j.nbd.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Yang X, Yao W, Lee W. Lithium protects against ethanol-induced neuronal apoptosis. Biochem Biophys Res Commun. 2006;350:905–910. doi: 10.1016/j.bbrc.2006.09.138. [DOI] [PubMed] [Google Scholar]