Abstract
The N-acylethanolamines (NAEs) exert important behavioral, physiological and immunological effects through actions at cannabinoid and other receptors. We measured concentrations of three NAEs, the Km and Vmax for fatty acid amide (FAAH) hydrolysis, FAAH protein and FAAH mRNA in prefrontal cortex, hippocampus, hypothalamus, amygdala, striatum and cerebellum at 4 hour intervals, starting at 0300. Significant differences in N-arachidonylethanolamine (AEA) contents among the times examined occur in the prefrontal cortex (PFC), hippocampus, hypothalamus and striatum. N-Oleoylethanolamine (OEA) concentrations exhibit large fluctuations over the day in the cerebellum, including a three-fold decrease between 1900 and 2300. N-Palmitoylethanolamine (PEA) and OEA were significantly, positively correlated in all regions examined except the hypothalamus. Although FAAH Km values are significantly affected by time of day in PFC, hippocampus and amygdala and FAAH Vmax values are significantly affected in PFC, hippocampus and cerebellum none of the data support a primary role for FAAH in the circadian regulation of the NAE concentrations. FAAH protein expression is not significantly different among the harvest times in any brain region examined. Concentrations of 2-arachidonoylglycerol are significantly affected by time of harvest in the striatum and cerebellum, but not in other brain regions. Together, these data indicate that the NAEs exhibit diverse patterns of change with time of day that are likely the result of alterations in biosynthesis; and support the hypothesis that AEA is a tonic activator of cannabinoid receptor signaling.
Keywords: N-arachidonylethanolamine, palmitoylethanolamide, N-oleoylethanolamine, fatty acid amide hydrolase, circadian, 2-arachidonoylglycerol
Introduction
The brain endocannabinoid signaling (ECS) system consists of the CB1 cannabinoid receptor (CB1R) and two endocannabinoids (eCBs), 2-arachidonoylglycerol (2-AG) and N-arachidonylethanolamine (AEA). ECS occurs widely throughout the brain and subserves activity-dependent, retrograde regulation of synaptic plasticity (Freund et al. 2003). In particular, CB1Rs are present at high density on glutamatergic and GABAergic terminals and their activation results in inhibition of neurotransmitter release, thereby significantly affecting the balance of excitation and inhibition in a regional circuit. Activity-dependent regulation of synaptic activity is primarily mediated by increased synthesis of 2-AG, which begs the question of the role of AEA in CB1R activation. One possibility is that AEA is a tonic activator while 2-AG is phasic (Vaughn et al. 2010). In this scenario, the concentration of AEA regulates the CB1R set point while mobilization of 2-AG, which has greater efficacy than AEA at G protein signaling (Hillard 2000), can increase CB1R activation further. A consequence of this scheme is that endogenous CB1R activation has a large dynamic range and can be altered by increases and decreases in AEA concentrations. Support for this hypothesis comes from findings that stress alters brain function through a reduction in AEA concentration as a result of an increase in the activity of its catabolic enzyme, fatty acid amide hydrolase (FAAH) (Patel et al. 2004, Hill et al. 2007, Hill et al. 2008, Hill et al. 2009).
ECS occurs widely throughout the brain and alters many fundamental processes, including sleep-wake cycles (Murillo-Rodriguez 2008); temperature regulation (Maccarrone & Wenger 2005); food consumption and fat storage (de Kloet & Woods 2009); autonomic (Pacher et al. 2005) and endocrine function (Maccarrone & Wenger 2005), reward-driven behavior (Solinas et al. 2008), mood (Hill & Gorzalka 2009) and sensory perception (Biro et al. 2009). Not only do these functions involve different brain regions, but they are also engaged to a greater or lesser degree at different times of the day. We hypothesize that AEA concentrations are “tuned” to maintain tonic CB1R activity at a state optimal for the brain region and time of day and, therefore, we predict that brain regional AEA concentrations will exhibit individualized time patterns in different brain regions. Earlier studies have provided support for this hypothesis. AEA concentrations in the pons (Valenti et al. 2004, Murillo-Rodriguez et al. 2006), nucleus accumbens, prefrontal cortex, hippocampus and striatum (Valenti et al. 2004) of rats are higher in tissues harvested during the night than during the day. On the other hand, AEA content in hypothalamus exhibits the opposite pattern (Murillo-Rodriguez et al. 2006).
AEA and other N-acylethanolamines (NAEs) are substrates for fatty acid amide hydrolase (FAAH) which hydrolyzes the amide bond of this family of lipids (Cravatt et al. 1996). Although there are other enzymes that hydrolyze the NAEs in the periphery (Ueda et al. 2010b), brain tissue from FAAH null mice does not exhibit appreciable hydrolysis of AEA (Patel et al. 2005) indicating that it is the primary, if not sole, mechanism of AEA hydrolysis in the brain. However, both AEA and 2-AG are substrates for enzymes that oxygenate arachidonic acid, including cyclooxygenase type 2 (COX-2) (Kozak et al. 2002) and COX-2 in particular has been shown to regulate synaptic endocannabinoid signaling in the hippocampus (Kim & Alger 2004) and raphe (Wang et al. 2012). The mechanisms of synthesis of the NAEs are not completely understood; however, data from Cravatt and colleagues indicate that members of the NAE family are synthesized via different pathways (Simon & Cravatt 2010).
We have tested two hypotheses with the studies in this report: first, AEA concentrations change with time in a brain region-specific manner and second, changes in AEA are accompanied by changes in other NAEs and are regulated by FAAH activity. To test these hypotheses, brain regional concentrations of 2-AG, AEA and two additional NAEs: N-oleoylethanolamine (OEA) and N-palmitoylethanolamine (PEA) and FAAH activity were measured at 4 hour intervals throughout the day. We find significant and diverse differences in AEA, PEA and OEA that indicate the basal concentrations of these three NAEs are largely independent of one another over the times sampled. In addition, our data indicate that FAAH activity also changes with time of day, but FAAH activity is a minor contributor to the regulation of “basal” NAE concentrations in most brain regions. These results support the hypothesis that AEA is a tonic regulator of CB1R function that is tuned to the time of day and brain region. These data suggest further that multiple and diverse mechanisms regulate concentrations of individual members of the NAE family of lipids.
Materials and Methods
Animals
Male, ICR mice were used in these studies (Harlan, Indianapolis, IN). The mice were 9 to 10 weeks of age at the time of brain removal and were otherwise untreated. Colony rooms were maintained at 21°C on a 12 h light/dark cycle, with lights on at 6AM. All mice were conditioned to this light cycle for at least two weeks prior to use, during which time they were given free access to food and water. All procedures were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin and were in accordance with the guidelines of the National Institutes of Health and with the ARRIVE guidelines.
Brain removal and dissection
Mice were euthanized by cervical dislocation followed by rapid decapitation at 6 equally-spaced time points across the light/dark cycle: 0300 (3AM), 0700 (7AM), 1100 (11AM), 1500 (3PM), 1900 (7PM), and 2300 (11PM). Euthanasia was carried out under red light conditions at 1900, 2300 and 0300. Brains were removed and rapidly frozen in liquid nitrogen and stored at −80°C until they were dissected into prefrontal cortex (PFC), hippocampus, amygdala, ventral striatum, hypothalamus and cerebellum. Separate cohorts of mice were used for endocannabinoid measurement and FAAH activity measurement.
Lipid extraction and NAE and 2-AG quantification
Brain regions were subjected to lipid extraction and the contents of 2-AG, AEA, PEA, and OEA were determined simultaneously using isotope-dilution, liquid chromatography-mass spectrometry (LC-MS) as previously described (Hill et al. 2009).
FAAH activity assays
Membranes were prepared from dissected brain regions as previously described (Hill et al. 2009). Protein concentrations were determined using the Bradford method (Bio-Rad Laboratories, Hercules, CA). FAAH activity was measured as the conversion of A[3H]EA (American Radiolabeled Chemicals, Inc., St. Louis, MO) to [3H]ethanolamine using liquid extraction to separate the radiolabelled species as described previously (Hillard et al. 1995). Saturation isotherms were constructed using eight concentrations of AEA ranging from 0.05 μM to 1.5 μM; the Kd and Vmax values for the hydrolysis were calculated using the nonlinear regression equation of GraphPad Prism (San Diego, CA).
Quantification of FAAH by Immunoblot
Expression levels of FAAH proteins were measured in membranes by Western blotting. Laemmli loading buffer (5X) was added to each sample, and the samples were denatured at 95°C for 5 minutes. Protein samples were loaded onto a 10% SDS-PAGE gel, and separated by electrophoresis at 200 volts for one hour. Transfer onto nitrocellulose membranes took place at 100 volts for one hour. Membranes were blocked with a one hour incubation at room temperature in Tris-buffered saline containing 0.2% Tween-20 (TBST) and 5% nonfat dry milk. Primary antibodies to FAAH (1:300) were diluted in TBST/2% milk, and incubated with the nitrocellulose membranes overnight at 4°C. A primary antibody to GAPDH (1:10000) was obtained from Cell Signaling Technology (Beverly, MA). The FAAH antibody was raised in rabbits by our laboratory (Tsou et al, 1998). After washing, the membranes were incubated with HRP-conjugated goat anti-rabbit secondary antibody (diluted in TBST/2% milk) for one hour at room temperature. Following washing, each membrane was incubated with SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL) for 3 minutes at room temperature and developed on X-ray film. GAPDH served as a loading control to ensure that the same amount of protein was added into each well. Densitometry was performed using ImageJ computer software to normalize the amount of FAAH protein to GAPDH.
Quantification of FAAH mRNA using qPCR
RNA was isolated from dissected brain regions using Trizol following the manufacturer’s instructions (Invitrogen, Carlsbad, CA). cDNA was synthesized using the iScript cDNA synthesis kit from Bio-Rad (Hercules, CA). Quantification of mRNA was carried out using SYBR-Green as the detection agent. Amplifications were performed using the iCycler iQ (Bio-Rad) in 20 μL reaction volumes. Each reaction contained 10 μL of SYBR-Green, 7.6 μL of PCR water, 1.6 μL of RNA, and 0.4 μL of each primer (final concentration 100 nM). All reactions were performed in duplicate. Thermal cycling proceeded with one amplification cycle of denaturation at 95°C for 5 min, followed by 40 cycles of 95°C for 10 sec and 65°C for 1 min. The primers used were: FAAH forward: 5′-CTTGTGGTGAGTGAGTGAGAC-3′ and reverse: 5′-TGGATGGCATAGGAAGTAATCG-3′; GAPDH forward: 5′-TTCACCACCATGGAGAAGGGC-3′ and reverse: 5′-GGCATGGACTGTGGTCATGA-3′. Relative mRNA expression was calculated using the formula 2^(CT GAPDH – CT FAAH).
Data Presentation and Statistics
To facilitate visualization of the temporal relationships of the lipids and FAAH parameters, two cycles of the same data are displayed. Analyses of the temporal differences in NAE content and FAAH activity were performed using a one-way analysis of variance (ANOVA). Post hoc Bonferroni’s t-tests were used to compare data obtained from tissues harvested at consecutive times, when the p value for the ANOVA was less than 0.05. Differences were considered significant if p < 0.05. To examine correlations among the lipids, data from each animal were compared in a matched sample set. To examine the correlations between the Km and Vmax values for FAAH and NAE concentrations, unmatched samples were compared at each of the time points. In both cases, Pearson’s r values were determined and a correlation was considered significant if the p value was less than 0.05.
Results
Prefrontal Cortex (PFC)
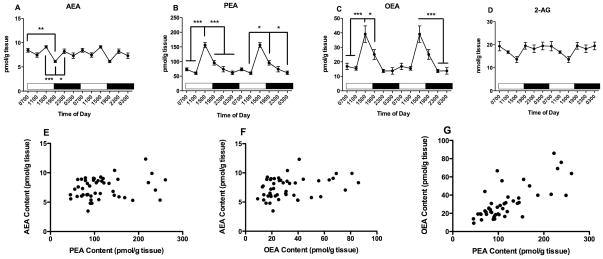
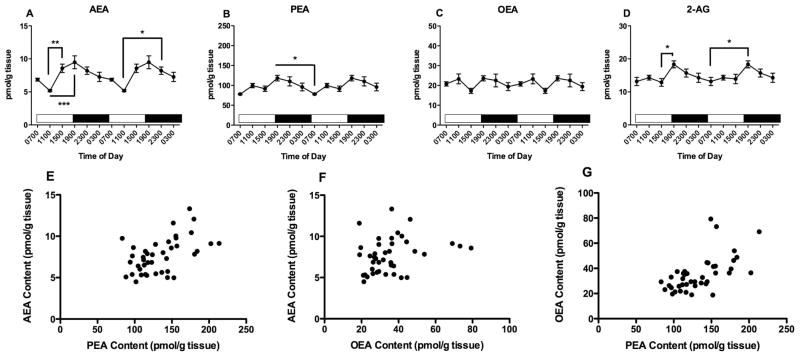
PFC tissue contents of all three NAEs exhibit significant differences as a function of time of tissue harvest (Fig 1). One way ANOVA reveals that AEA content in the PFC (Fig 1A) varies significantly with time of day (F5,47=5.8, p<0.001). AEA content peaks at 1500, the last time point examined in the light period and is lowest at 1900, the first time point examined after the light to dark transition which occurred at 1800. Bonferroni’s post hoc analyses indicate that AEA content at 1900 is significantly lower than AEA content in PFC harvested at 0700, 1500, and 2300.
Figure 1.
Effects of time of day on PFC contents of AEA (A), PEA (B) OEA (C) and 2-AG (D) were determined using isotope dilution, LC/MS. Each point is the mean of 6–9 determinations; vertical lines represent SEM. Two cycles of the same data are displayed. For AEA, PEA and OEA, Bonferroni’s t-tests were used to determine differences among harvest times; significant differences are noted; * p<0.05; ** p<0.01 and *** p<0.005. Correlations between AEA and PEA (E); AEA and OEA (F) and PEA and OEA (G) were examined using Pearson’s correlational analyses. The results of these analyses are reported in the text.
PFC contents of PEA (Fig 1B) and OEA (Fig 1C) are also significantly affected by harvest time, with patterns of change that are similar to AEA. ANOVA demonstrated that the contents of both PEA and OEA are significantly affected by time of harvest (PEA: F5,47=22, p<0.0001, OEA: F5,47=12, p<0.0001). Like AEA, the highest amounts of PEA and OEA occur in PFC harvested at 1500. The results of the Bonferroni’s post hoc analyses reveal that the concentrations of PEA and OEA are significantly higher in tissues harvested at 1500 than at all other times in the study. In addition, PEA concentrations in PFC harvested at 1900 are higher than PEA concentrations in PFC harvested at 1100 and 2300.
The contents of 2-AG in the PFC are not significantly affected by time of harvest (F5,47 = 1.93) (Fig 1D).
Since the NAEs have overlapping mechanisms of synthesis and degradation and were measured in the same samples, we calculated correlations among these lipids (Fig 1E, F, G). NAE concentrations determined in 47 different PFC samples are significantly correlated with each other. The strongest correlation is between contents of PEA and OEA (r2 = 0.65, p<0.0001, Fig 1G). The content of AEA is also significantly correlated with both PEA (r2 = 0.33, p<0.05, Fig 1E) and OEA (r2 = 0.47, p<0.001, Fig 1F). The content of 2-AG is not correlated with any of the NAEs (data not shown).
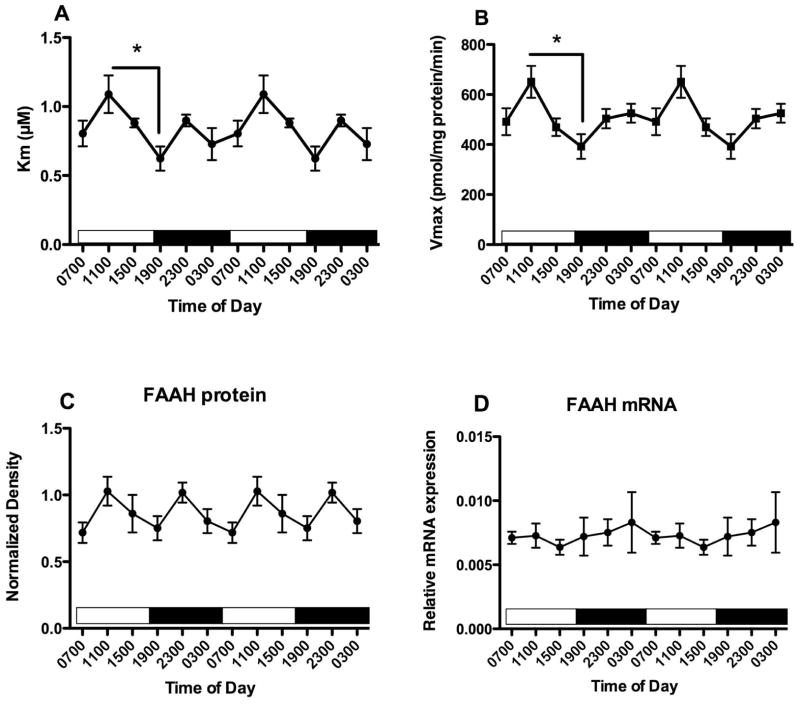
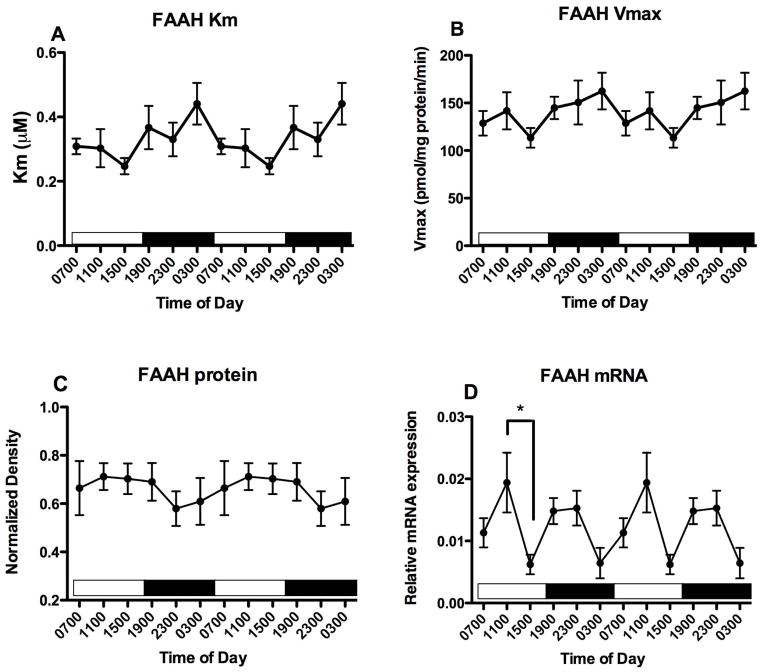
Membranes were obtained from PFC harvested from another set of mice at the same time points. Km and Vmax parameters for FAAH activity were determined (Fig 2A, B). There is a significant effect of time of harvest on both Km (F5,25 = 2.95, p<0.05) and Vmax (F5,25 = 2.95, p<0.05) values. Bonferroni’s multiple comparisons demonstrated that both Km and Vmax differ significantly between PFC harvested at 1100 and at 1900. There is no significant effect of harvest time on FAAH protein content estimated using Western blots (F5,25 = 1.75, n.s.). In a third set of mice, mRNA was isolated and the relative expression of FAAH mRNA was quantified, using GAPDH as an internal control. FAAH mRNA concentrations do not vary significantly with time of tissue harvest (Fig 2D). There were no significant correlations over the 6 harvest times between mean concentrations of the NAEs and either the Km (Table 1) or Vmax (Table 2) for FAAH activity.
Figure 2.
Effects of time of day on FAAH activity and expression in the PFC were determined. FAAH activity was measured as the conversion of AEA to ethanolamine in membranes harvested at the times indicated; Km and Vmax values were determined from isotherms created from 8 concentrations of AEA. Mean values of Km (A) and Vmax (B) are shown (n=4–5); vertical lines indicate SEM. Bonferroni’s t-tests were used to compare all values with each other; significantly different values are indicated; * p<0.05. (C) Western blot analyses were used to examine FAAH expression following separation of membrane proteins using gel electrophoresis. FAAH density was normalized to the density of GAPDH determined in the same sample. Each point is the mean of 4–6 separate tissues; vertical lines represent SEM. (D) mRNA for FAAH was determined in PFC harvested at the times shown using qPCR. Relative expression was calculated using GAPDH as a calibrator. Each point is the mean of 5–6 replicates, vertical lines represent SEM. For each graph, two cycles of the same data are displayed.
Table 1.
Correlations between NAE concentrations and Km for FAAH over time of day.
| Brain Region | AEA | PEA | OEA | |||
|---|---|---|---|---|---|---|
| Pearson r | p | Pearson r | p | Pearson r | p | |
| PFC | 0.46 | 0.35 | −0.11 | 0.84 | −0.12 | 0.81 |
| Hippocampus | 0.07 | 0.89 | −0.11 | 0.83 | 0.74 | 0.09 |
| Hypothalamus | 0.31 | 0.56 | 0.81 | 0.051 | 0.47 | 0.34 |
| Striatum | 0.07 | 0.90 | 0.31 | 0.56 | 0.20 | 0.70 |
| Amygdala | 0.07 | 0.90 | 0.69 | 0.13 | 0.63 | 0.18 |
| Cerebellum | 0.70 | 0.12 | 0.20 | 0.71 | 0.67 | 0.15 |
Correlations of the mean concentrations of each NAE were compared in a pair wise fashion to the mean Km for FAAH determined in tissue harvested at the same time of day.
Table 2.
Correlations between NAE concentrations and Vmax for FAAH over time of day.
| Brain Region | AEA | PEA | OEA | |||
|---|---|---|---|---|---|---|
| Pearson r | p | Pearson r | p | Pearson r | p | |
| PFC | 0.16 | 0.76 | −0.50 | 0.32 | −0.47 | 0.34 |
| Hippocampus | −0.07 | 0.90 | 0.10 | 0.85 | 0.56 | 0.25 |
| Hypothalamus | 0.13 | 0.81 | 0.68 | 0.13 | 0.52 | 0.29 |
| Striatum | −0.10 | 0.84 | 0.48 | 0.34 | 0.46 | 0.36 |
| Amygdala | 0.72 | 0.51 | 0.84 | <0.05* | 0.87 | <0.05* |
| Cerebellum | 0.54 | 0.27 | 0.33 | 0.52 | 0.68 | 0.14 |
Correlations of the mean concentrations of each NAE were compared in a pair wise fashion to the mean Vmax for FAAH determined in tissue harvested at the same time of day.
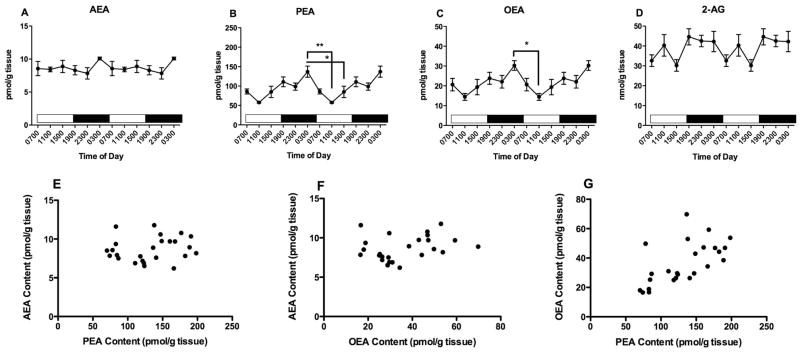
Hippocampus
Hippocampal tissue contents of all three NAEs exhibit significant differences as a function of time of tissue harvest (Fig 3). One way analysis of variance reveals that AEA content in the hippocampus varies significantly with time of day (Fig 3A; F5,46=3.1, p<0.05). Post hoc tests reveal that AEA content is significantly greater in hippocampal tissue harvested at 1900 than harvested at both 1100 and 1500. Hippocampal contents of PEA (Fig 3B) and OEA (3C) are also significantly affected by harvest time (PEA: F5,46=4.1, p<0.005, OEA: F5,46=3.9, p<0.05). PEA content in hippocampus is highest at 2300; post hoc tests reveal that PEA content is significantly greater in hippocampus harvested at 2300 than at 0700 and 1100. On the other hand, OEA content is highest in hippocampus harvested at 1500; post hoc tests reveal that OEA is significantly greater at 1500 than at 0300 and 0700. Thus, each of the NAEs reach their highest value in hippocampus at different times; OEA is highest at 1500, AEA is highest at 1900 and PEA is highest at 2300.
Figure 3.
Effects of time of day on hippocampal contents of AEA (A), PEA (B) OEA (C) and 2-AG (D) were determined using isotope dilution, LC/MS. Each point is the mean of 5–9 determinations; vertical lines represent SEM. Two cycles of the same data are displayed. For AEA, PEA and OEA, Bonferroni’s t-tests were used to determine differences among harvest times; significant differences are noted; * p<0.05; ** p<0.01. Correlations between AEA and PEA (E); AEA and OEA (F); PEA and OEA (G) and PEA and 2-AG (H) were examined using Pearson’s correlational analyses. The results of these analyses are reported in the text.
The content of 2-AG in hippocampus is not affected by time of tissue harvest (F5,46 = 0.98; n.s.).
Correlational analyses were carried out among the four lipids measured. The strongest correlation is between OEA and PEA (Fig 3G; r2 = 0.54, p<0.0001). PEA content also correlates significantly with AEA (Fig 3E; r2 = 0.34, p<0.05); however, OEA and AEA concentrations trend but do not reach significance (Fig 3F; r2 = 0.26, p=0.07). Interestingly, the hippocampal contents of PEA and 2-AG are significantly correlated (Fig 3H; r2 = 0.48, p<0.001) as are the contents of OEA and 2-AG (data not shown; r2 = 0.44, p<0.005). Contents of AEA and 2-AG in the hippocampus are not significantly correlated (data not shown).
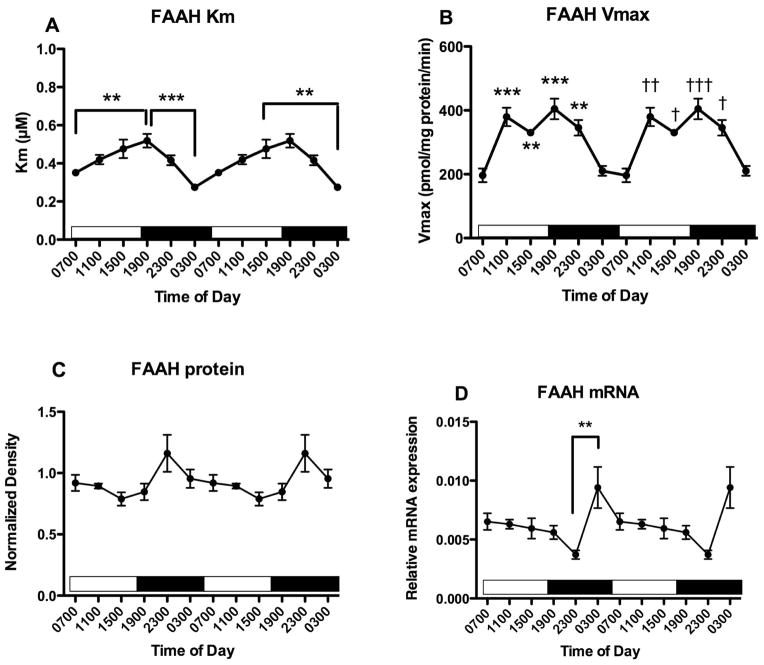
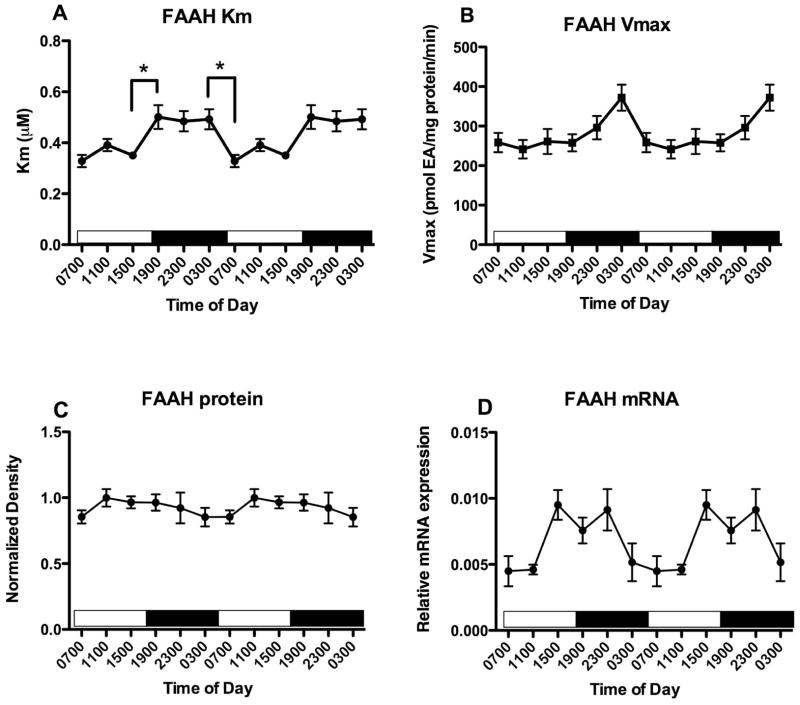
FAAH activity was measured in membranes harvested from hippocampus (Fig 4). There is a significant effect of time of harvest on both Km (Fig 4A; F5,28 = 7.8, p<0.0005) and Vmax (Fig 4B; F5,28 = 13.3, p<0.0001) values. Post hoc tests demonstrated that the Km value of AEA for FAAH is significantly greater in hippocampal tissue harvested at 1900 than at 0300 and 0700. In addition, FAAH Km is significantly higher in tissue harvested at 1500 than at 0300. The Vmax for FAAH exhibits a bi-modal distribution over harvest time; Vmax is low in tissues harvested at 0300 and 0700, and approximately 2-fold higher in tissues harvested at 1100, 1500, 1900 and 2300 (Fig 4B). Post hoc tests reveal that the Vmax for FAAH is significantly greater in tissues harvested at 1100, 1500, 1900 and 2300 than in tissues harvested at 0700; and is significantly greater in tissues harvested at 1100 and 1900 than at 0300. Neither mean Km (Table 1) nor mean Vmax (Table 2) values correlate with the concentrations of the NAEs determined at the same time of day.
Figure 4.
Effects of time of day on FAAH activity and expression in the hippocampus were determined. FAAH activity was measured as the conversion of AEA to ethanolamine in membranes harvested at the times indicated; Km and Vmax values were determined from isotherms created from 8 concentrations of AEA. Mean values of Km (A) and Vmax (B) are shown (n=4–5); vertical lines indicate SEM. Bonferroni’s t-tests were used to compare all values with each other; significantly different values are indicated; * p<0.05; ** p<0.01 and *** p<0.005. In panel B, * p<0.05; ** p<0.01 and *** p<0.005 refer to differences from Vmax in tissue harvested at 0700; † p<0.05; †† p<0.01 and ††† p<0.005 compared to Vmax in tissue harvested at 0300. (C) Western blot analyses were used to examine FAAH expression following separation of membrane proteins using gel electrophoresis. FAAH density was normalized to the density of GAPDH determined in the same sample. Each point is the mean of 4–6 separate tissues; vertical lines represent SEM. (D) mRNA for FAAH was determined in PFC harvested at the times shown using qPCR. Relative expression was calculated using GAPDH as a calibrator. Each point is the mean of 5–6 replicates, vertical lines represent SEM. For each graph, two cycles of the same data are displayed. ** p<0.01 by Bonferroni’s t-test.
FAAH protein concentrations, determined using Western blot, are not significantly affected by time of harvest (Fig 4C; F5,26 = 2.5, p=0.07). The mean Vmax values for FAAH activity and mean western blot relative densities do not correlate (Pearson r = −0.07, n.s.). The relative expression of mRNA for FAAH in hippocampus is significantly affected by harvest time (Fig 4D; F5,29 = 4.1. p<0.01). Post hoc tests reveal that FAAH mRNA content is higher at 0300 than at 2300.
Hypothalamus
The only NAE that exhibits a significant relationship to time of tissue harvest in the hypothalamus is AEA (Fig 5A; F5,26 = 6.9, p<0.001). Hypothalamic contents of PEA (Fig 5B) and OEA (Fig 5C) are not significantly affected by time of harvest (F5,26 = 1.4 and 0.8, respectively). AEA content is highest in the middle of the light period (i.e. 1100) and is low from 1500 until 0300, the last time investigated in the dark period. Post hoc tests reveal that hypothalamic AEA content is significantly higher at 1100 than at all other times examined except 0700. In addition, AEA content is significantly higher in the hypothalamus at 0700 than at 2300.
Figure 5.
Effects of time of day on hypothalamic contents of AEA (A), PEA (B) OEA (C) and 2-AG (D) were determined using isotope dilution, LC/MS. Each point is the mean of 5–9 determinations; vertical lines represent SEM. Two cycles of the same data are displayed. For AEA, Bonferroni’s t-tests were used to determine differences among harvest times; significant differences are noted; ** p<0.01. Correlations between AEA and PEA (E); AEA and OEA (F) and PEA and OEA (G) were examined using Pearson’s correlational analyses. The results of these analyses are reported in the text.
Hypothalamic content of 2-AG is not significantly affected by time of tissue harvest (Fig 5D; F5,34 = 0.6, n.s.).
Hypothalamic lipid contents do not correlate among the samples analyzed (Figs 5E, F, G). Correlation coefficients for each pair are: AEA/PEA r2 = 0.33; AEA/OEA r2 = 0.8; and OEA/PEA r2 = 0.26. The NAEs do not correlate with 2-AG concentrations (data not shown).
FAAH activity was measured in membranes harvested from hypothalamus. Neither Km (Fig 6A; F5,36 = 2.3, p=0.07) nor Vmax (Fig 6B; F5,36 = 2.0, p=0.1) values are significantly affected by time of harvest, although the influence of time of harvest on Km is nearly significant. There is a near significant effect of harvest time on FAAH protein measured using Western blot (Fig 6C; F5,26 = 2.3, p=0.07). The hypothalamic content of FAAH mRNA is significantly affected by time of harvest (Fig 6D; F5,22 = 3.9, p<0.05). Post hoc tests reveal that mRNA for FAAH is significantly greater in tissue harvested at 0300 than at 2300.
Figure 6.
Effects of time of day on FAAH activity and expression in the hypothalamus were determined. FAAH activity was measured as the conversion of AEA to ethanolamine in membranes harvested at the times indicated; Km and Vmax values were determined from isotherms created from 8 concentrations of AEA. Mean values of Km (A) and Vmax (B) are shown (n=4–5); vertical lines indicate SEM. (C) Western blot analyses were used to examine FAAH expression following separation of membrane proteins using gel electrophoresis. FAAH density was normalized to the density of GAPDH determined in the same sample. Each point is the mean of 4–6 separate tissues; vertical lines represent SEM. (D) mRNA for FAAH was determined in PFC harvested at the times shown using qPCR. Relative expression was calculated using GAPDH as a calibrator. Each point is the mean of 5–6 replicates, vertical lines represent SEM. For each graph, two cycles of the same data are displayed. * p<0.05 by Bonferroni’s t-test.
Striatum
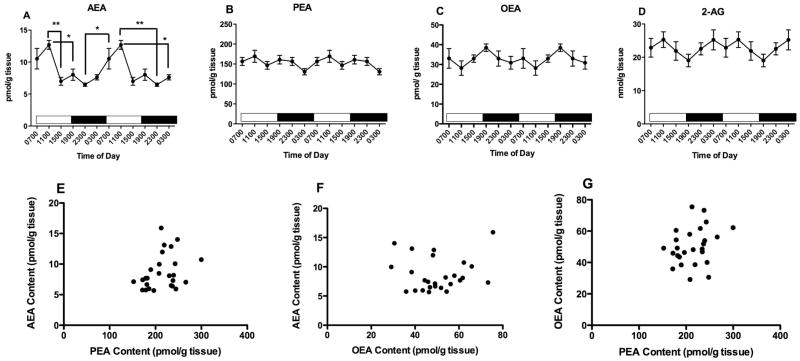
The striatal content of AEA is significantly affected by time of harvest (Fig 7A; F5,44 = 5.5, p<0.001). The striatal content of AEA is highest at 1900; falls steadily until it reaches a nadir at 1100; then increases again between 1100 and 1900. Post hoc analyses reveal that striatal AEA content is significantly lower in tissues harvested at 1100 than at 1500, 1900 and 2300.
Figure 7.
Effects of time of day on striatal contents of AEA (A), PEA (B) OEA (C) and 2-AG (D) were determined using isotope dilution, LC/MS. Each point is the mean of 5–9 determinations; vertical lines represent SEM. Two cycles of the same data are displayed. For AEA, PEA and 2-AG, Bonferroni’s t-tests were used to determine differences among harvest times; significant differences are noted; * p<0.05; ** p<0.01; ***p<0.005. Correlations between AEA and PEA (E); AEA and OEA (F) and PEA and OEA (G) were examined using Pearson’s correlational analyses. The results of these analyses are reported in the text.
Striatal PEA content is also significantly affected by time of harvest (Fig 7B; F5,44 = 3.2, p<0.05). Although the percent changes are smaller than the changes in AEA, striatal contents of PEA follow the same pattern across the harvest times as seen with AEA. Post hoc tests reveal that striatal PEA content is significantly higher at 1900 than at 0700. The striatal content of OEA (Fig 7C; F5,44 = 1.3) is not significantly affected by time of harvest.
Striatal 2-AG content is significantly altered by time of tissue harvest (Fig 7D; F5,49 = 2.8, p<0.05). Post hoc tests reveal that 2-AG content is highest at 1900 and is significantly greater than content in tissue harvested at both 1500 and 0700.
Correlational analyses demonstrate that striatal contents of AEA and PEA are significantly correlated (Fig 7E; r2 = 0.52, p<0.001). The contents of PEA and OEA are also highly correlated (Fig 7G; r2 = 0.61, p<0.0001), while contents of AEA and OEA trend to a significant correlation (Fig 7F; r2 = 0.27, p=0.09). There are no significant correlations among 2-AG and the NAEs (data not shown).
FAAH activity was measured in membranes harvested from striatum. Neither Km (Fig 8A; F5,26 = 1.7) nor Vmax (Fig 8B; F5,26 = 1.2) values are significantly affected by time of harvest. Similarly, FAAH protein content is not significantly affected by time of tissue harvest (Fig 8C; F5,23 = 0.5). However, striatal FAAH mRNA content is significantly affected (Fig 8D; F5,26 = 3.6, p<0.05); post hoc tests reveal that striatal FAAH mRNA content is significantly greater at 1100 than at 1500.
Figure 8.
Effects of time of day on FAAH activity and expression in the striatum were determined. FAAH activity was measured as the conversion of AEA to ethanolamine in membranes harvested at the times indicated; Km and Vmax values were determined from isotherms created from 8 concentrations of AEA. Mean values of Km (A) and Vmax (B) are shown (n=4–5); vertical lines indicate SEM. (C) Western blot analyses were used to examine FAAH expression following separation of membrane proteins using gel electrophoresis. FAAH density was normalized to the density of GAPDH determined in the same sample. Each point is the mean of 4–6 separate tissues; vertical lines represent SEM. (D) mRNA for FAAH was determined in PFC harvested at the times shown using qPCR. Relative expression was calculated using GAPDH as a calibrator. Each point is the mean of 5–6 replicates, vertical lines represent SEM. For each graph, two cycles of the same data are displayed. * p<0.05 by Bonferroni’s t-test.
Amygdala
Amygdalar contents of AEA are not affected by time of harvest (Fig 9A; F5,25 = 1.2); while PEA (Fig 9B; F5,28=5.4, p < 0.01) and OEA (Fig 9C; F5,26=3.0, p<0.05) are significantly affected. Amygdalar contents of both PEA and OEA are highest at 0300 and lowest at 1100. Post hoc tests reveal that amygdalar PEA content in tissue harvested at 0300 is significantly greater than in tissue harvested at 1100 and 1500. Post hoc tests reveal that OEA concentration is higher at 0300 than at 1100.
Figure 9.
Effects of time of day on amygdalar contents of AEA (A), PEA (B) OEA (C) and 2-AG (D) were determined using isotope dilution, LC/MS. Each point is the mean of 5–9 determinations; vertical lines represent SEM. Two cycles of the same data are displayed. For PEA and OEA, Bonferroni’s t-tests were used to determine differences among harvest times; significant differences are noted; * p<0.05; ** p<0.01. Correlations between AEA and PEA (E); AEA and OEA (F) and PEA and OEA (G) were examined using Pearson’s correlational analyses. The results of these analyses are reported in the text.
Amygdalar content of 2-AG is not significantly affected by time of harvest (Fig 9D; F5,26 = 1.9, n.s.).
Correlational analyses indicate that the amygdalar contents of PEA and OEA are significantly correlated (Fig 9G; r2 = 0.62, p<0.001). However, AEA content is not significantly correlated with either PEA (Fig 9E; r2 = 0.16) or OEA (Fig 9F; r2 = 0.29).
The activity of FAAH was measured in amygdalar membranes prepared from brains harvested at the same time points. The Km for FAAH is significantly affected by time of harvest (Fig 10A; F5,28 = 5.6, p<0.005); Km values are lower in tissues harvested in the light (0700, 1100 and 1500) compared to tissues harvested in the dark (1900, 2300 and 0300). Post hoc tests indicate that the Km value of FAAH for AEA is significantly lower at 1500 than 1900, and is significantly lower at 0700 than 0300. The Vmax for FAAH is also significantly affected by time of harvest (Fig 10B; F5,28 = 2.7, p<0.05); however, no individual times differ by post hoc tests. FAAH protein content in amygdala, measured using Western blot, is not affected by time of harvest (Fig 10C; F5,30 = 0.7, n.s.). The amygdalar content of FAAH mRNA is significantly affected by time of harvest (Fig 10D; F5, 28 = 3.6, p<0.05); however, no individual differences are indicated by post hoc tests.
Figure 10.
Effects of time of day on FAAH activity and expression in the amygdala were determined. FAAH activity was measured as the conversion of AEA to ethanolamine in membranes harvested at the times indicated; Km and Vmax values were determined from isotherms created from 8 concentrations of AEA. Mean values of Km (A) and Vmax (B) are shown (n=4–5); vertical lines indicate SEM. Bonferroni’s t-tests were used to compare all values with each other; significantly different values are indicated; * p<0.05. (C) Western blot analyses were used to examine FAAH expression following separation of membrane proteins using gel electrophoresis. FAAH density was normalized to the density of GAPDH determined in the same sample. Each point is the mean of 4–6 separate tissues; vertical lines represent SEM. (D) mRNA for FAAH was determined in PFC harvested at the times shown using qPCR. Relative expression was calculated using GAPDH as a calibrator. Each point is the mean of 5–6 replicates, vertical lines represent SEM. For each graph, two cycles of the same data are displayed.
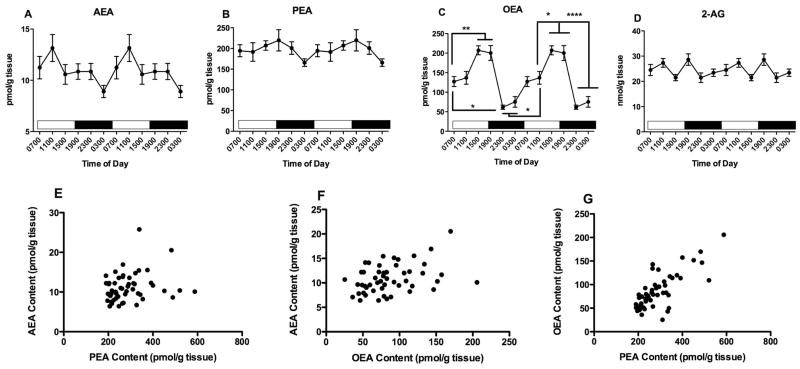
Cerebellum
The only NAE significantly affected by time of harvest in the cerebellum is OEA (Fig 11C; F5,50 = 20.4, p<0.0001); neither AEA (Fig 11A; F5,51 = 2.2, p=0.07) nor PEA (Fig 11 B; F5,51 = 0.75) contents are affected. OEA concentrations in the cerebellum are remarkably variable with time of harvest; the highest contents occurred in samples harvested at 1500 and 1900, while the lowest contents are at 2300 and 0300. Post hoc analyses of the OEA data reveal many differences among the time points (see Fig 11C).
Figure 11.
Effects of time of day on cerebellar contents of AEA (A), PEA (B) OEA (C) and 2-AG (D) were determined using isotope dilution, LC/MS. Each point is the mean of 5–9 determinations; vertical lines represent SEM. Two cycles of the same data are displayed. For OEA, Bonferroni’s t-tests were used to determine differences among harvest times; significant differences are noted; * p<0.05; ** p<0.01; **** p<0.0001. Correlations between AEA and PEA (E); AEA and OEA (F) and PEA and OEA (G) were examined using Pearson’s correlational analyses. The results of these analyses are reported in the text.
The cerebellar content of 2-AG is significantly affected by time of harvest (Fig 11D; F5,52 = 2.7, p<0.05). However, post hoc analyses reveal no significant differences between the individual times.
Correlational analyses indicate that the cerebellar contents of PEA and OEA are very significantly correlated (Fig 11G; r2 = 0.76, p<0.0001). However, AEA content is not significantly correlated with either PEA (Fig 11E; r2 = 0.22) or OEA (Fig 11F; r2 = 0.22).
FAAH activity was determined in membranes from cerebellum. The Vmax (Fig 12B; F5,27 = 4.2, p<0.01) but not Km (Fig 12A; F5,27 = 1.4) values are significantly affected by time of harvest. Post hoc tests revealed that there were no significant differences between individual Vmax values. Neither cerebellar FAAH protein content measured using Western blot (Fig 12C; F5,33 = 0.64) nor FAAH mRNA (Fig 12D; F5,27 = 0.4) are affected by time of tissue harvest.
Figure 12.
Effects of time of day on FAAH activity and expression in the cerebellum were determined. FAAH activity was measured as the conversion of AEA to ethanolamine in membranes harvested at the times indicated; Km and Vmax values were determined from isotherms created from 8 concentrations of AEA. Mean values of Km (A) and Vmax (B) are shown (n=4–5); vertical lines indicate SEM. (C) Western blot analyses were used to examine FAAH expression following separation of membrane proteins using gel electrophoresis. FAAH density was normalized to the density of GAPDH determined in the same sample. Each point is the mean of 4–6 separate tissues; vertical lines represent SEM. (D) mRNA for FAAH was determined in PFC harvested at the times shown using qPCR. Relative expression was calculated using GAPDH as a calibrator. Each point is the mean of 5–6 replicates, vertical lines represent SEM. For each graph, two cycles of the same data are displayed.
Discussion
Each of the brain regions examined in this study exhibit a significant effect of time of brain harvest on the concentrations of one or more of the NAEs. On the other hand, 2-AG concentrations are only related to time of harvest in the striatum. We conclude that the NAEs and 2-AG are not circadian per se, but rather exhibit concentration differences over the 24 hour day that are likely driven by biochemical processes that are specific to each brain region.
AEA concentrations are significantly affected by time of day in the PFC, hippocampus, hypothalamus and striatum, but not in the amygdala or cerebellum. In PFC, hippocampus and striatum, the significant changes in AEA content occur across the transition from the mid to late light period (inactive phase) to the early dark period (active phase). In the hippocampus and striatum, AEA contents are lowest at 1100 and increase in the early (striatum) or late (hippocampus) afternoon to a maximum at 1900, one hour after the lights are turned off. In the PFC, the opposite pattern occurs; AEA concentrations are high throughout the light phase and reach a nadir at 1900. In the hypothalamus, AEA concentrations are most affected by the dark to light transition; AEA concentrations are low from 1500 to 0300, then rise steadily through the first half of the light period to reach a peak at 1100. Although the data do not reach statistical significance, AEA concentrations in the cerebellum also rise from 0300 to 1100. We hypothesize that the changes in AEA concentrations have the purpose of setting the baseline activity of the CB1 receptors and thereby influence both network excitability (Lutz 2004) and the degree to which a phasic signal from 2-AG can modulate synaptic plasticity (Vaughn et al. 2010). Recent data from studies of stress-induced activation of the hypothalamic-pituitary-adrenal (HPA) axis suggest that AEA activation of the CB1 receptor also serves a gating function. In particular, high AEA concentrations in the amygdala are associated with dampened HPA axis activity and activation of the stress response requires FAAH-mediated reductions in AEA concentrations (Hill et al. 2009). Following this logic, we hypothesize that the pattern of AEA concentrations among brain regions and over time of day allow for tuning of CB1 receptor engagement which, in turn, contributes to the synaptic activity in that region.
A similar pattern was seen in an earlier study of rat brain NAE concentrations; in particular, hippocampal AEA was higher in the dark phase than the light while hypothalamic concentrations were higher in the light phase (Murillo-Rodriguez et al. 2006). In fact, the pattern of change in hypothalamic AEA concentrations over time in the earlier and current study is nearly identical. A second study compared AEA concentrations in rat brains harvested at 1000 and 2200 and found that AEA concentrations were significantly greater at 2200 in hippocampus and striatum (Valenti et al. 2004), which is in agreement with the current results obtained at 1100 and 2300. Valenti and colleagues also reported that AEA concentrations in PFC exhibited a similar pattern, which was not seen in the mouse brains examined in the current study at the closest times (AEA concentrations in PFC were 7.5 ± 0.4 and 8.2 ± 0.5 pmol/g tissue at 1100 and 2300, respectively).
The concentrations of PEA in the PFC and striatum are significantly correlated with the concentrations of AEA in those same regions and, not surprisingly, the pattern of change over the day is also similar. Like AEA, cerebellar concentrations of PEA are unrelated to time of day. Both AEA and PEA are significantly affected by time of day in the hippocampus and both reach a peak in the dark period and are lowest in the light period. The effect of time of day on PEA and AEA concentrations is dissimilar in the hypothalamus (AEA is significantly affected by time of day while PEA is not) and in the amygdala (PEA is significantly affected by time of day while AEA is not). OEA and PEA concentrations are very significantly correlated over the time of day in all of the brain regions examined except for the hypothalamus while OEA and AEA concentrations are only significantly correlated in the PFC. Interestingly, in spite of a high degree of correlation between hippocampal OEA and PEA concentrations across all data, the peak concentration of OEA is reached in the late light period (1500) while the peak concentration of PEA occurs at 2300, in the dark period. OEA concentrations are increased in the GI tract following consumption of food (Fu et al. 2007) and it is possible that the increase in OEA seen at 0300 is also the result of food consumption during the dark (active) phase. Protein targets for PEA and OEA include the nuclear transcription factor PPARα (Hansen 2010). OEA activation of PPARα in the small intestine results in enhanced memory consolidation via multi-synaptic inputs to the amygdala (Campolongo et al. 2009), the current data raise the possibility that OEA and PEA could also affect feeding, memory or other food-related behaviors through direct effects in the brain as well.
The most dramatic changes in the concentrations of OEA with time of brain harvest occurred in the cerebellum, where it was the only NAE that was altered in a significant manner and changed by 3 fold over the course of the day. OEA has been demonstrated to act as an endogenous agonist for an orphan receptor, GPR119 (Overton et al. 2006). In the periphery, the OEA/GPR119 signaling pair is proposed to suppress food intake and reduce body weight. Although there are no published studies of the potential role of GPR119 receptors in the CNS, the Allen Brain Atlas database reports high mRNA expression for this receptor in the cerebellum. Recent studies indicate that the cerebellum plays an important role in the regulation of feeding and satiety. For example, functional imaging studies in rodents and humans show feeding-induced changes in the activation of the cerebellum (Zhu & Wang 2008); and structural differences in the cerebellum between normal weight and obese individuals (Smucny et al. 2012). Thus, an intriguing hypothesis is that OEA activation of GPR119 in the cerebellum could participate in satiety signaling. As OEA exerts a satiety signal, low concentrations in the dark phase are consistent with increased feeding during this time of day in rodents.
The tissue concentrations of 2-AG were significantly affected by time of harvest in two of the tissues examined, striatum and cerebellum. In both brain regions, 2-AG concentrations increased across the transition from light to dark (i.e. between 1500 and 1900). 2-AG concentrations are also significantly greater at 1900 than 1500 in the PFC and amygdala when unpaired t-tests are used to compare only those values. Prevailing hypotheses indicate that 2-AG functions as an “on-demand” neurotransmitter for the CB1 receptor. If these data reflect a pool of signaling 2-AG, then it is possible that the processes involved in 2-AG synthesis are operating at a higher level in the early part of the active phase in the striatum, cerebellum, PFC and amygdala. A major mechanism for the synthesis of 2-AG at synapses is increased glutamatergic signaling (Freund et al. 2003). It is possible that the increased 2-AG detected at the beginning of the awake period is the result of increased glutamatergic signaling that accompanies the increased activity, search for food and other behaviors that occur upon wakening. However, these data need to be interpreted with caution as it is likely that a majority of 2-AG measured in lipid extracts from brain tissue does not serve a signaling purpose since 2-AG concentrations in microdialysates are orders of magnitude lower (Buczynski & Parsons 2010). Basal 2-AG concentrations available to signal could vary through day and not be detectable using bulk tissue measurements. In addition, these results conflict with the previous findings in male rats showing that 2-AG concentrations are significantly different between 1000 and 2200 in nucleus accumbens, prefrontal cortex, striatum and hippocampus (Valenti et al. 2004). The reasons for the difference between the two studies are not apparent.
A second hypothesis of this study was that FAAH activity plays an important role in diurnal regulation of NAE concentrations. The basis for this hypothesis is that AEA, PEA and OEA are all substrates of FAAH, although the catalytic efficiency of FAAH towards them differs in ex vivo preparations with AEA being the best substrate, followed by OEA and PEA (Desarnaud et al. 1995, Ueda et al. 1995). Furthermore, the concentrations of all three NAEs are significantly increased in FAAH null mice and after treatment with a selective FAAH inhibitor (Patel et al. 2005), suggesting that FAAH activity plays a vital role in maintaining the concentrations of the NAEs. To test this hypothesis, we measured FAAH activity, protein concentration, and quantified mRNA for FAAH in tissues harvested from each brain region at the same times that the lipids were measured. Because the tissue processing for lipid extraction, membrane preparation and mRNA isolations differ, the correlational analyses were limited to comparisons of the mean of the values obtained at each time.
We were surprised to find that AEA concentrations did not correlate with FAAH activity parameters in any of the brain regions examined. In fact, the only significant correlation among any of the NAEs occurred in the amygdala, where PEA and OEA concentrations and the Vmax for FAAH were significantly, positively correlated. This observation is counterintuitive, since one would expect a negative correlation if FAAH Vmax and the NAE concentrations were related in a causal manner. Closer inspection of the data indicates that it is driven by the fact that the highest Vmax for FAAH and highest concentrations of both OEA and PEA occur in tissue harvested at 0300. It is not likely that these parameters are casually related, pointing to the problems inherent in correlational analyses.
In spite of the lack of correlation between AEA concentrations and FAAH parameters, several brain regions exhibited significant effects of time of harvest on FAAH activity. Significant effects of time of harvest on both Km and Vmax values occurred in PFC, hippocampus, and amygdala while Vmax alone was significantly altered in the cerebellum. Two different patterns of change were seen. In the PFC, the Km and Vmax values are highly significantly correlated, suggesting that the same mechanism underlies changes in each parameter. Interestingly, two previous studies from our laboratory have found parallel reductions (Hill et al. 2007) and parallel increases (Hill et al. 2008) in the Vmax and Km for FAAH in rat brain. It is possible that a post-translational modification of FAAH itself or an accessory protein, as suggested previously (Grimaldi et al. 2009), could produce conformational changes that would alter both parameters. To address this issue, we have used Western blot as a method to gain semi-quantitative information about variation in the absolute amount of FAAH protein with time of day in the brain regions examined. Not only are there no significant effects of time of harvest on FAAH protein amounts, there are no significant correlations between the Vmax for FAAH and the amount of FAAH protein across the harvest times in any of the regions examined. A caveat to these results is that the FAAH activity and protein measurements were not carried out in the same group of tissues. In spite of the lack of effect of time of harvest on amounts of FAAH protein, the amount of FAAH mRNA was significantly affected by time in the hippocampus, hypothalamus, striatum and amygdala. Interestingly, only two pairs of consecutive harvest times accounted for all of the significant differences: 2300/0300 and 1100/1500. In both the hippocampus and hypothalamus, FAAH mRNA expression is significantly higher at 0300 than at 2300. In the striatum, FAAH mRNA is significantly lower at 1500 than 1100, while the opposite relationship occurs (although does not reach statistical significance) in the amygdala. Since the sensitivity to changes using quantitative PCR is greater than Western blot, the mRNA data suggest that there could be changes in the amount of FAAH protein that are not revealed by the Western blot analyses. In any case, the FAAH activity measures are likely the best method for examining the role of FAAH in the regulation of the NAE concentrations.
An earlier study found a small (10%) but significant decrease in cerebellar FAAH activity (most likely Vmax) in brains harvested at midnight compared to noon (Glaser & Kaczocha 2009). These findings are consistent with the current study as the Vmax for FAAH in the cerebellum was 40% lower in brains harvested at 2300 than those harvested at 1100 (327 ± 20 and 459 ± 47 pmol EA/min/mg protein, respectively). The earlier study found no differences in FAAH activity at the same time points in cortex, hippocampus, or striatum, findings that are also in agreement with the current results.
Although it is likely that the activity of FAAH contributes to the absolute amounts of the NAEs present at each time of day, our data indicate that other mechanisms are important contributors to the changes in basal NAE concentrations over the day. We suggest that NAE biosynthetic enzymatic activities and/or precursor concentrations likely exert significant effects on the variability in NAE concentrations observed in this study. Several biosynthetic mechanisms for the NAEs have been identified. More than 20 years ago, Schmid and colleagues outlined a mechanism for the synthesis of saturated and mono-unsaturated NAEs from a minor phospholipid, N-acyl phosphatidylethanolamine (NAPE) (Schmid et al. 1990). The enzyme responsible for this reaction is a phosphodiesterase of the phospholipid D type (NAPE-PLD) that hydrolyzes NAPE to NAE and phosphatidic acid. Studies showing that NAPE-PLD null mice have normal or slightly reduced concentrations of the NAEs suggest that alternative mechanisms for their synthesis are present in brain (Leung et al. 2006, Simon & Cravatt 2010). The observation that lyso-NAPE could also be converted to NAE by brain homogenates lead Simon and Cravatt to identify glycerophosphodiesterase 1 (GDE1) as an alternative mechanism for the biosynthesis of the NAEs, including AEA, OEA and PEA (Simon & Cravatt 2008). However, this group subsequently found that neurons from mice null for both NAPE-PLD and GDE1 exhibited normal conversion of NAPE to NAE (Simon & Cravatt 2010). They concluded that the primary enzyme or enzymes responsible for the synthesis of NAE from NAPE remain unidentified and are inoperative in brain homogenates (Simon & Cravatt 2010). A third synthetic pathway identified in macrophages involves formation of phospho-NAE through a phospholipase C mechanism, followed by conversion to NAE via phosphatases (Liu et al. 2006). This pathway has not been examined in brain nor has the N-acyl specificity been determined.
The precursor for all of these NAE synthetic pathways is NAPE; and it is generally accepted that the formation of NAPE through an N-acyltransferase mechanism is the rate limiting step in the formation of the NAEs (Ueda et al. 2010a). Thus, it is possible that the dependence of NAE concentrations on time of day could be due to changes in the concentrations of the NAPE precursors. In brain, biochemical data have established that a membrane-bound, calcium-dependent N-acyltransferase catalyzes the formation of NAPE by transferring an acyl chain from the sn-1 position of glycerophospholipid to the amino group of PE (Cadas et al. 1996, Cadas et al. 1997). However, this enzyme has not been purified or cloned so is difficult to study in brain samples. A second family of enzymes has been identified that can catalyze the same reaction, although in a calcium-independent manner and, importantly, can transfer sn-2 as well as sn-1 acyl chains (Jin et al. 2007). Since unsaturated acyl chains are essentially excluded from the sn-1 position, this second class of enzymes (named the phospholipase A/acyltransferase family) provides a mechanism for the formation of N-arachidonylPE and therefore, AEA (Jin et al. 2007). Both the calcium-dependent and independent transferase activities are detected in rat brain tissue (Jin et al. 2007), although no data regarding the brain regional expression of the biochemical activity or expression of the enzymes is available.
These results demonstrate significant variation in NAE content between brain regions and over the course of the day in male mice. We hypothesize that changes in AEA concentrations could result in changes in “basal” activation of CB1 receptors and, thus, regulate its ability to control activity-dependent synaptic plasticity. As a result, excitatory and/or inhibitory tone in a brain region could be “tuned” to amount of physical activity, feeding and sleep. These results also reveal that large and significant changes in PEA and OEA also occur throughout the day, which suggests that increased understanding of the targets of these lipids is an important goal.
Acknowledgments
These studies were supported by NIH grants DA09155 and DA026996 and by the Research and Education Component of the Advancing a Healthier Wisconsin Endowment of the Medical College of Wisconsin.
Abbreviations
- 2-AG
2-arachidonoylglycerol
- AEA
N-arachidonylethanolamine
- ANOVA
analysis of variance
- CB1R
type 1 cannabinoid receptor
- eCBs
endocannabinoids
- ECS
endocannabinoid signaling
- FAAH
fatty acid amide hydrolase
- LC-MS
liquid chromatography-mass spectrometry
- OEA
N-oleoylethanolamine
- PEA
palmitoylethanolamide
- PFC
prefrontal cortex
- PPAR
peroxisome proliferation-activator receptor
Footnotes
The authors have no conflicts of interest to disclose.
References
- Biro T, Toth BI, Hasko G, Paus R, Pacher P. The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol Sci. 2009;30:411–420. doi: 10.1016/j.tips.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczynski MW, Parsons LH. Quantification of brain endocannabinoid levels: methods, interpretations and pitfalls. Br J Pharmacol. 2010;160:423–442. doi: 10.1111/j.1476-5381.2010.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadas H, di Tomaso E, Piomelli D. Occurrence and biosynthesis of endogenous cannabinoid precursor, N-arachidonoyl phosphatidylethanolamine, in rat brain. J Neurosci. 1997;17:1226–1242. doi: 10.1523/JNEUROSCI.17-04-01226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadas H, Gaillet S, Beltramo M, Venance L, Piomelli D. Biosynthesis of an endogenous cannabinoid precursor in neurons and its control by calcium and cAMP. J Neurosci. 1996;16:3934–3942. doi: 10.1523/JNEUROSCI.16-12-03934.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolongo P, Roozendaal B, Trezza V, Cuomo V, Astarita G, Fu J, McGaugh JL, Piomelli D. Fat-induced satiety factor oleoylethanolamide enhances memory consolidation. Proc Natl Acad Sci U S A. 2009;106:8027–8031. doi: 10.1073/pnas.0903038106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- de Kloet AD, Woods SC. Minireview: Endocannabinoids and their receptors as targets for obesity therapy. Endocrinology. 2009;150:2531–2536. doi: 10.1210/en.2009-0046. [DOI] [PubMed] [Google Scholar]
- Desarnaud F, Cadas H, Piomelli D. Anandamide amidohydrolase activity in rat brain microsomes. J Biol Chem. 1995;270:6030–6035. doi: 10.1074/jbc.270.11.6030. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Fu J, Astarita G, Gaetani S, Kim J, Cravatt BF, Mackie K, Piomelli D. Food intake regulates oleoylethanolamide formation and degradation in the proximal small intestine. J Biol Chem. 2007;282:1518–1528. doi: 10.1074/jbc.M607809200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser ST, Kaczocha M. Temporal changes in mouse brain fatty acid amide hydrolase activity. Neuroscience. 2009;163:594–600. doi: 10.1016/j.neuroscience.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi P, Rossi G, Catanzaro G, Maccarrone M. Modulation of the endocannabinoid-degrading enzyme fatty acid amide hydrolase by follicle-stimulating hormone. Vitam Horm. 2009;81:231–261. doi: 10.1016/S0083-6729(09)81010-8. [DOI] [PubMed] [Google Scholar]
- Hansen HS. Palmitoylethanolamide and other anandamide congeners. Proposed role in the diseased brain. Exp Neurol. 2010;224:48–55. doi: 10.1016/j.expneurol.2010.03.022. [DOI] [PubMed] [Google Scholar]
- Hill MN, Barr AM, Ho WS, Carrier EJ, Gorzalka BB, Hillard CJ. Electroconvulsive shock treatment differentially modulates cortical and subcortical endocannabinoid activity. J Neurochem. 2007;103:47–56. doi: 10.1111/j.1471-4159.2007.04688.x. [DOI] [PubMed] [Google Scholar]
- Hill MN, Carrier EJ, McLaughlin RJ, Morrish AC, Meier SE, Hillard CJ, Gorzalka BB. Regional alterations in the endocannabinoid system in an animal model of depression: effects of concurrent antidepressant treatment. J Neurochem. 2008;106:2322–2336. doi: 10.1111/j.1471-4159.2008.05567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. The endocannabinoid system and the treatment of mood and anxiety disorders. CNS Neurol Disord Drug Targets. 2009;8:451–458. doi: 10.2174/187152709789824624. [DOI] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Morrish AC, Viau V, Floresco SB, Hillard CJ, Gorzalka BB. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology. 2009;34:2733–2745. doi: 10.1038/npp.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ. Biochemistry and pharmacology of the endocannabinoids arachidonylethanolamide and 2-arachidonylglycerol. Prostaglandins Other Lipid Mediat. 2000;61:3–18. doi: 10.1016/s0090-6980(00)00051-4. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Wilkison DM, Edgemond WS, Campbell WB. Characterization of the kinetics and distribution of N-arachidonylethanolamine (anandamide) hydrolysis by rat brain. Biochim Biophys Acta. 1995;1257:249–256. doi: 10.1016/0005-2760(95)00087-s. [DOI] [PubMed] [Google Scholar]
- Jin XH, Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Discovery and characterization of a Ca2+-independent phosphatidylethanolamine N-acyltransferase generating the anandamide precursor and its congeners. J Biol Chem. 2007;282:3614–3623. doi: 10.1074/jbc.M606369200. [DOI] [PubMed] [Google Scholar]
- Kim J, Alger BE. Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat Neurosci. 2004;7:697–698. doi: 10.1038/nn1262. [DOI] [PubMed] [Google Scholar]
- Kozak KR, Crews BC, Morrow JD, Wang LH, Ma YH, Weinander R, Jakobsson PJ, Marnett LJ. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J Biol Chem. 2002;277:44877–44885. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- Leung D, Saghatelian A, Simon GM, Cravatt BF. Inactivation of N-acyl phosphatidylethanolamine phospholipase D reveals multiple mechanisms for the biosynthesis of endocannabinoids. Biochemistry. 2006;45:4720–4726. doi: 10.1021/bi060163l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang L, Harvey-White J, et al. A biosynthetic pathway for anandamide. Proc Natl Acad Sci U S A. 2006;103:13345–13350. doi: 10.1073/pnas.0601832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz B. On-demand activation of the endocannabinoid system in the control of neuronal excitability and epileptiform seizures. Biochem Pharmacol. 2004;68:1691–1698. doi: 10.1016/j.bcp.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Wenger T. Effects of cannabinoids on hypothalamic and reproductive function. Handb Exp Pharmacol. 2005;168:555–571. doi: 10.1007/3-540-26573-2_18. [DOI] [PubMed] [Google Scholar]
- Murillo-Rodriguez E. The role of the CB(1) receptor in the regulation of sleep. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1420–1427. doi: 10.1016/j.pnpbp.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Murillo-Rodriguez E, Desarnaud F, Prospero-Garcia O. Diurnal variation of arachidonoylethanolamine, palmitoylethanolamide and oleoylethanolamide in the brain of the rat. Life Sci. 2006;79:30–37. doi: 10.1016/j.lfs.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Overton HA, Babbs AJ, Doel SM, et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006;3:167–175. doi: 10.1016/j.cmet.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. Blood pressure regulation by endocannabinoids and their receptors. Neuropharmacology. 2005;48:1130–1138. doi: 10.1016/j.neuropharm.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Carrier EJ, Ho WS, Rademacher DJ, Cunningham S, Reddy DS, Falck JR, Cravatt BF, Hillard CJ. The postmortal accumulation of brain N-arachidonylethanolamine (anandamide) is dependent upon fatty acid amide hydrolase activity. J Lipid Res. 2005;46:342–349. doi: 10.1194/jlr.M400377-JLR200. [DOI] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145:5431–5438. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- Schmid HHO, Schmid PC, Natarajan V. N-Acylated glycerophospholipids and their derivatives. Prog Lipid Res. 1990;29:1–43. doi: 10.1016/0163-7827(90)90004-5. [DOI] [PubMed] [Google Scholar]
- Simon GM, Cravatt BF. Anandamide biosynthesis catalyzed by the phosphodiesterase GDE1 and detection of glycerophospho-N-acyl ethanolamine precursors in mouse brain. J Biol Chem. 2008;283:9341–9349. doi: 10.1074/jbc.M707807200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GM, Cravatt BF. Characterization of mice lacking candidate N-acyl ethanolamine biosynthetic enzymes provides evidence for multiple pathways that contribute to endocannabinoid production in vivo. Mol Biosyst. 2010;6:1411–1418. doi: 10.1039/c000237b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny J, Cornier MA, Eichman LC, Thomas EA, Bechtell JL, Tregellas JR. Brain structure predicts risk for obesity. Appetite. 2012;59:859–865. doi: 10.1016/j.appet.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR, Piomelli D. The endocannabinoid system in brain reward processes. Br J Pharmacol. 2008;154:369–383. doi: 10.1038/bjp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda N, Kurahashi Y, Yamamoto S, Tokunaga T. Partial purification and characterization of the porcine brain enzyme hydrolyzing and synthesizing anandamide. J Biol Chem. 1995;270:23823–23827. doi: 10.1074/jbc.270.40.23823. [DOI] [PubMed] [Google Scholar]
- Ueda N, Tsuboi K, Uyama T. Enzymological studies on the biosynthesis of N-acylethanolamines. Biochim Biophys Acta. 2010a;1801:1274–1285. doi: 10.1016/j.bbalip.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Ueda N, Tsuboi K, Uyama T. N-acylethanolamine metabolism with special reference to N-acylethanolamine-hydrolyzing acid amidase (NAAA) Prog Lipid Res. 2010b;49:299–315. doi: 10.1016/j.plipres.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Valenti M, Vigano D, Casico MG, Rubino T, Steardo L, Parolaro D, Di Marzo V. Differential diurnal variations of anandamide and 2-arachidonoyl-glycerol levels in rat brain. Cell Mol Life Sci. 2004;61:945–950. doi: 10.1007/s00018-003-3453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn LK, Denning G, Stuhr KL, de Wit H, Hill MN, Hillard CJ. Endocannabinoid signalling: has it got rhythm? Br J Pharmacol. 2010;160:530–543. doi: 10.1111/j.1476-5381.2010.00790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Shen RY, Haj-Dahmane S. Endocannabinoids mediate the glucocorticoid-induced inhibition of excitatory synaptic transmission to dorsal raphe serotonin neurons. J Physiol. 2012;590:5795–5808. doi: 10.1113/jphysiol.2012.238659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JN, Wang JJ. The cerebellum in feeding control: possible function and mechanism. Cell Mol Neurobiol. 2008;28:469–478. doi: 10.1007/s10571-007-9236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]