Abstract
BACKGROUND
Acetabular dysplasia is a major predisposing factor for development of hip osteoarthritis, and may result from alterations to chondrolabral loading. Subject-specific finite element (FE) modeling can be used to evaluate chondrolabral mechanics in the dysplastic hip, thereby providing insight into mechanics that precede osteoarthritis.
OBJECTIVE
To evaluate chondrolabral contact mechanics and congruency in dysplastic hips and normal hips using a validated approach to subject-specific FE modeling.
METHODS
FE models of ten subjects with normal acetabula and ten subjects with dysplasia were constructed using a previously validated protocol. Labrum load support, and labrum and acetabular cartilage contact stress and contact area were compared between groups. Local congruency was determined at the articular surface for two simulated activities.
RESULTS
The labrum in dysplastic hips supported 2.8 to 4.0 times more of the load transferred across the joint than in normal hips. Dysplastic hips did not have significantly different congruency in the primary load-bearing regions than normal hips, but were less congruent in some unloaded regions. Normal hips had larger cartilage contact stress than dysplastic hips in the few regions that had significant differences.
CONCLUSIONS
The labrum in dysplastic hips has a far more significant role in hip mechanics than it does in normal hips. The dysplastic hip is neither less congruent than the normal hip, nor subjected to elevated cartilage contact stresses. This study supports the concept of an outside-in pathogenesis of osteoarthritis in dysplastic hips and that the labrum in dysplastic hips should be preserved during surgery.
Keywords: hip, cartilage mechanics, labrum, finite element, acetabular dysplasia, osteoarthritis
Introduction
Osteoarthritis (OA) of the hip is primarily caused by hip pathomorphologies, consisting of structural abnormalities of the acetabulum and/or femur [1, 2]. Acetabular dysplasia is characterized by a shallow acetabulum. Dysplasia is typically diagnosed radiographically by an anterior and/or lateral center edge angle (CEA) less than 20–25 degrees and an acetabular index greater than ten degrees, indicating a shallow acetabulum and an upwardly sloping sourcil, respectively [3]. An estimated 20% of all hip OA is secondary to mild to moderate acetabular dysplasia [4], which causes a 4.3-fold increased risk for radiographic hip OA [5]. The link between acetabular dysplasia and hip OA is thought to be altered cartilage mechanics [3, 6]. Clinical observation of labral hypertrophy and labral tears in the dysplastic hip suggests that the labrum also experiences altered mechanics [2, 7]. Chondrolabral mechanics cannot be measured in-vivo in the dysplastic hip as a means to understand the link between altered mechanics and OA, and similarly, it would be nearly impossible to assemble a homogeneous population of cadaveric hips exhibiting acetabular dysplasia for in-vitro testing.
In contrast, subject-specific finite element (FE) modeling provides a viable approach for the study of chondrolabral mechanics in normal and pathomorphologic hips [8–10], thus offering the potential to obtain insight into the mechanics of dysplastic hips. Previous subject-specific evaluations have primarily focused on cartilage contact mechanics and have omitted the acetabular labrum [11, 12]. This is a reasonable approach for the study of activities of daily living in the normal hip, since labral load support under these conditions would be expected to be small [9, 13]. The dysplastic hip has been evaluated on a subject-specific basis, but without the acetabular labrum [11]. In this previous study, hips with symptomatic residual dysplasia had elevated contact stresses in comparison to non-symptomatic hips and in comparison to a single normal hip. Idealized FE models of the dysplastic hip have also suggested elevated cartilage stresses in this population [14]. However, idealized geometry provides inaccurate predictions of cartilage contact stress [15, 16], so these results may not accurately reflect the in-vivo contact patterns in the dysplastic hip.
In addition to evaluation of the mechanical environment in the dysplastic hip, morphological features may be important and useful predictors of the pathogenesis of OA and may help in the interpretation of population differences in joint contact mechanics [6]. The dysplastic hip has been described qualitatively as less congruent than the normal hip based on two-dimensional radiographs [17, 18]. This qualitative description has resulted in poor inter- and intra-observer reliability in reporting congruency in dysplastic hips [19, 20]. Further, congruency has previously been described globally and only on the bony surfaces [17, 18]. However, local features of subject-specific geometry drive cartilage contact mechanics in the normal hip [12, 15], suggesting that local congruency on the articular surfaces may have greater consequences than global congruency on the bony surfaces for chondrolabral mechanics.
While previous studies have begun to evaluate the mechanical and morphologic characteristics of the dysplastic hip that may lead to OA, analyses that include the labrum and quantitatively measure congruency may provide valuable insight into the chondrolabral mechanics that precede OA in this patient population. Therefore, the aims of this study were to evaluate chondolabral mechanics in dysplastic hips in comparison to normal hips using a validated approach to subject-specific FE modeling [8], and to quantitatively evaluate congruency in dysplastic hips in comparison to normal hips.
Methods
A total of twenty subject-specific FE models were generated. Subjects were recruited and imaged with IRB approval (University of Utah IRB #10983; the procedures followed were also in accordance with the Helsinki Declaration). Ten patients with lateral CEA less than 25 degrees, acetabular indices greater than 10 degrees and hip pain secondary to acetabular dysplasia were modeled (3 male and 7 female, BMI 23.4 ± 5.9 kg·m−2, age 26 ± 6 years, CEA 14.8 ± 4.6 degrees, acetabular index 18.1 ± 6.9 degrees). Ten healthy control subjects with CEA greater than 25 degrees, acetabular indices less than 10 degrees and without a history of hip pain were drawn from a previous study (5 male and 5 female, BMI 23.0 ± 3.9 kg·m−2, age 26 ± 4 years, CEA 33.5 ± 5.4 degrees, acetabular index 7.6 ± 1.7 degrees) [12]. Models were generated from volumetric CT arthrography data using previously described methods (Fig. 1) [9, 12]. The CT arthrography data for the 10 subjects with traditional acetabular dysplasia have been made available for download for use by other investigators (Supplementary Data). Cartilage and labrum mesh density and the position of the chondrolabral boundary were based on the results of previous validation and sensitivity studies [8, 9]. Models were analyzed for the activities of heel strike during walking (WH, 233% BW), mid-stance during walking (WM, 203% BW), heel strike during stair descent (DH, 261% BW) and heel strike during stair ascent (AH, 252% BW) to capture a range of anatomical orientations and loads [21]. The femur was translated along the loading axis until the desired load was achieved, but was free to translate in the plane normal to the loading axis in order to settle properly into the acetabulum [12]. While the labrum in the normal hip was not expected to support a significant amount of load during these routine activities, the role of the labrum in the dysplastic hip during routine activities is unknown. All FE models were analyzed with NIKE3D [22].
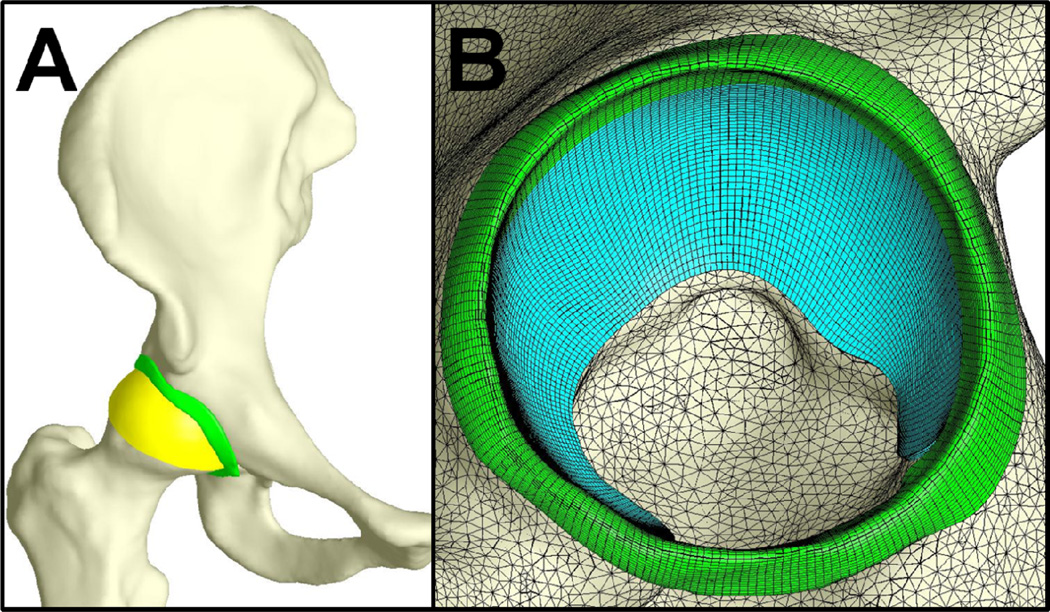
Figure 1.
Representative model showing the bones in white, the acetabular labrum in green, the acetabular cartilage in blue and the femoral cartilage in yellow. A – whole model. B – saggital view of the acetabulum, with lines indicating the discretization of the cartilage, labrum and bones.
Regional contact stress and contact area were evaluated on the labrum and acetabular cartilage [9]. Contact area, average contact stress and peak contact stress were evaluated in the anteromedial (AM), anterolateral (AL), superomedial (SM), superolateral (SL), posteromedial (PM) and posterolateral (PL) regions of the acetabular cartilage. For the labrum, contact area and peak contact stress were evaluated in the anterior, superior and posterior regions. Additionally, the percent of the total load supported by the labrum was evaluated. Model results were extracted using PostView [23].
Local and global congruencies of the articular surfaces were calculated. Local congruency was calculated at points of contact using established methods [24, 25], implemented in PostView [23]. Congruency between two curved surfaces at a point of contact can be defined as the curvature of a single curved surface contacting a plane, where the single surface represents the difference in profiles of the two original contacting surfaces [24]. To make this calculation, the magnitude and orientation of the principal curvatures of each surface were estimated at each node using quadric methods for triangulated meshes [25, 26]. The nodal magnitudes and orientations of principal curvature were used to calculate the curvature of the single surface contacting a plane [24]. To present one value for the congruency at each point, congruency was defined as the root-mean-square (RMS) curvature [24]. A larger value of RMS curvature indicates a less congruent surface, while a smaller value of RMS curvature indicates a more congruent surface. The position of the articular surfaces was determined from the final position of the FE models by aligning the undeformed and deformed bone meshes using the AlignSurfaces module in Amira® (Visualization Sciences Group, Burlington, MA), which uses an iterative closest point method [27]. The undeformed cartilage geometry was then transformed into the final position. Thus, the congruency that is reported is for the undeformed cartilage geometry, but in the final position where the femoral head has settled into the acetabulum. Evaluating congruency with the undeformed cartilage geometry prevented the deformation of the cartilage during loading from affecting the results. Congruency was calculated at all points that were in contact in the final position. Congruency was evaluated in the AM, AL, SM, SL, PM and PL regions, as well as the pooled anterior, superior, posterior, medial and lateral regions. The most congruent point, defined by the minimum RMS curvature, was evaluated in each anatomical region. Average local congruency was also evaluated in each region. Local congruency was calculated for WM and AH because these activities represent the positions with the least and most extreme kinematics angles, respectively, of the activities that were analyzed.
Global congruency was calculated by fitting the femoral and acetabular articular surfaces to spheres using the PreView software [28]. Each fit resulted in a radius and an objective value. The objective value was defined as the least squares sum of the distances from the fit sphere to the closest point on the native surface. Global congruency was evaluated as the RMS curvature of two spheres with the fit sphere radii, thus yielding a single value for each subject.
Differences between groups as a function of region and loading scenario were assessed using unpaired t tests. Differences between activities as a function of region and group were assessed using paired t tests. All statistical analysis was completed using SigmaPlot (Version 11.0, Systat Software, Inc., San Jose, CA). Significance was set at P ≤ 0.05.
Results
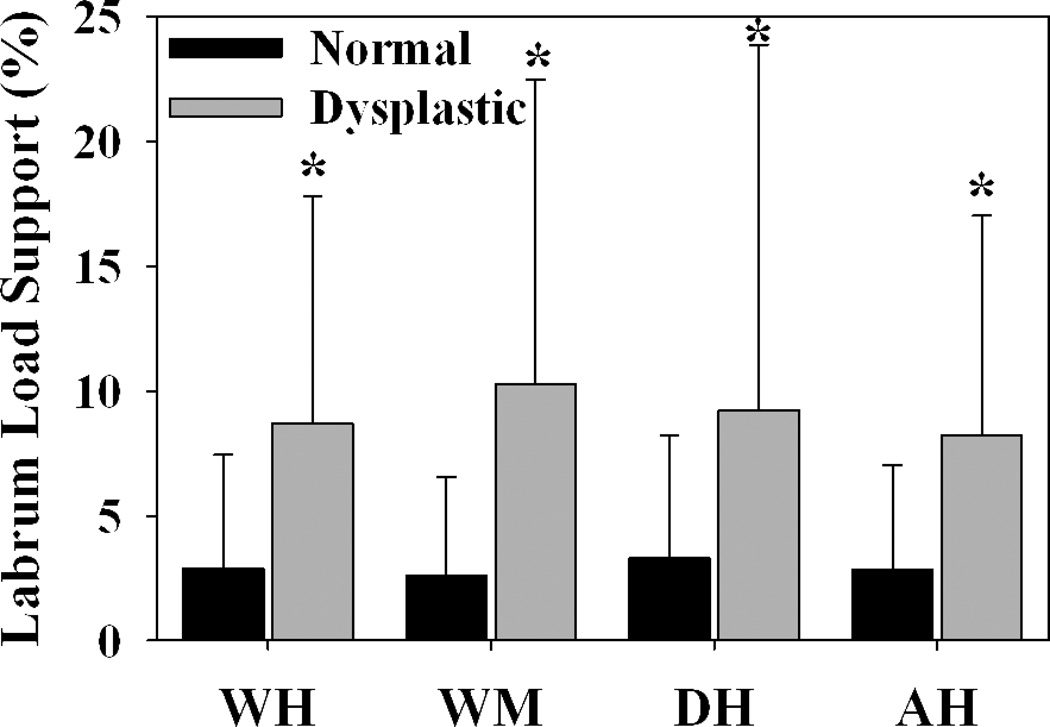
The labrum supported significantly more load in dysplastic hips than in normal hips during all loading scenarios (Fig. 2) (P = 0.002, 0.001, 0.026 and 0.003 for WH, WM, DH and AH, respectively). There was qualitatively more lateral loading in dysplastic hips in comparison to normal hips (Fig. 3).
Figure 2.
Load supported by the labrum was significantly larger for dysplastic hips than normal hips during all loading scenarios. Error bars indicate upper confidence bounds (at 95%). * indicates p ≤ 0.05 in comparison to normal hips during the same loading scenario (n = 10 in each group).
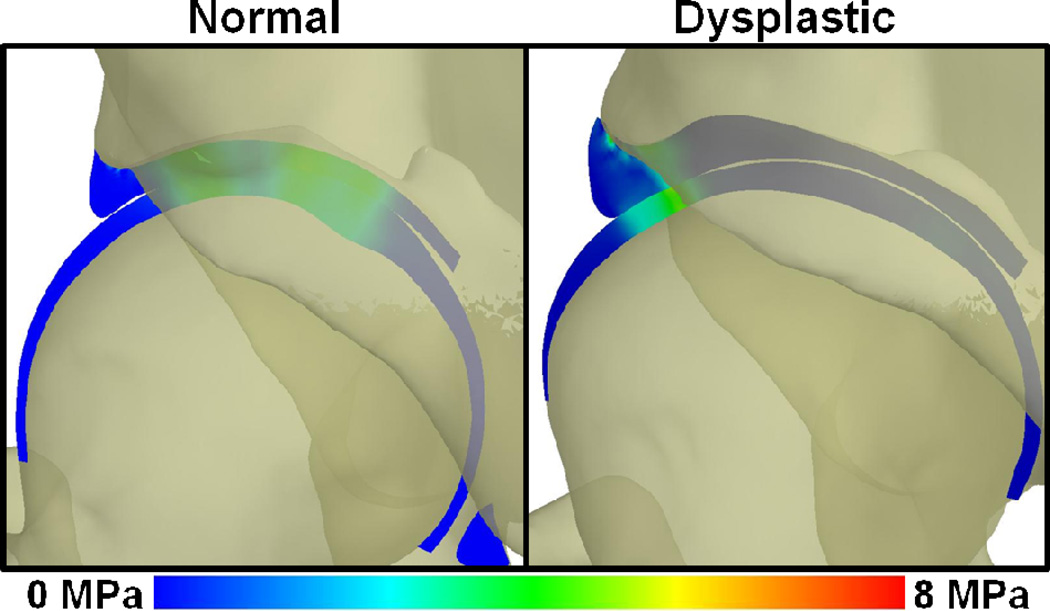
Figure 3.
Coronal cross-sectional images of contact stress in a representative normal and a representative dysplastic hip, with the three dimensional bones shown transparent. Lateral loading in the dysplastic hip results in higher contact stress in the acetabular labrum, and thus larger loads (Figure 2).
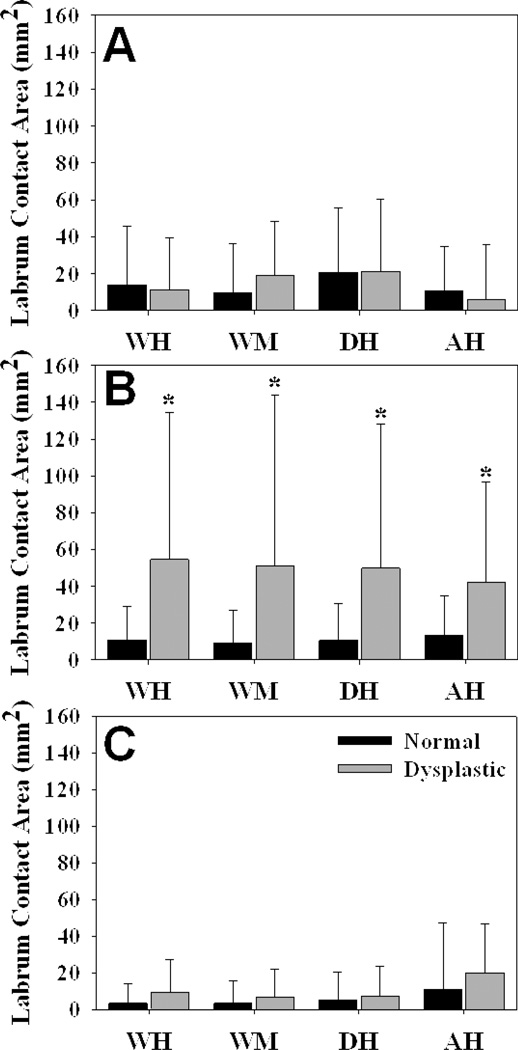
Contact area on the superior labrum was significantly larger in dysplastic hips than in normal hips for all loading scenarios (Fig. 4) (P = 0.003, 0.008, 0.004 and 0.003 for WH, WM, DH and AH, respectively). Peak contact stress on the superior labrum was significantly larger in the dysplastic hips than in the normal hips during WM only (P = 0.014 in WM; P = 0.100, 0.147 and 0.122 for WH, DH and AH, respectively). There were no significant differences in contact area on the anterior or posterior labrum (P = 0.671, 0.219, 0.983 and 0.325 for WH, WM, DH and AH, respectively, in the anterior region; P = 0.136, 0.320, 0.797 and 0.337 for WH, WM, DH and AH, respectively, in the posterior region). There were also no significant differences in peak contact stress on the anterior or posterior labrum (P = 0.356, 0.372, 0.904 and 0.252 for WH, WM, DH and AH, respectively, in the anterior region; P = 0.417, 0.938, 0.555 and 0.656 for WH, WM, DH and AH, respectively, in the posterior region).
Figure 4.
Contact area on the acetabular labrum. A – anterior region. B – superior region. C – posterior region. Contact area in the superior acetabular labrum was larger in dysplastic hips than in normal hips, but there were no differences in the anterior or posterior regions during the simulated activities. Error bars indicate upper confidence bounds (at 95%). * = p < 0.05 in comparison to normal hips during the same loading scenario (n = 10 in each group).
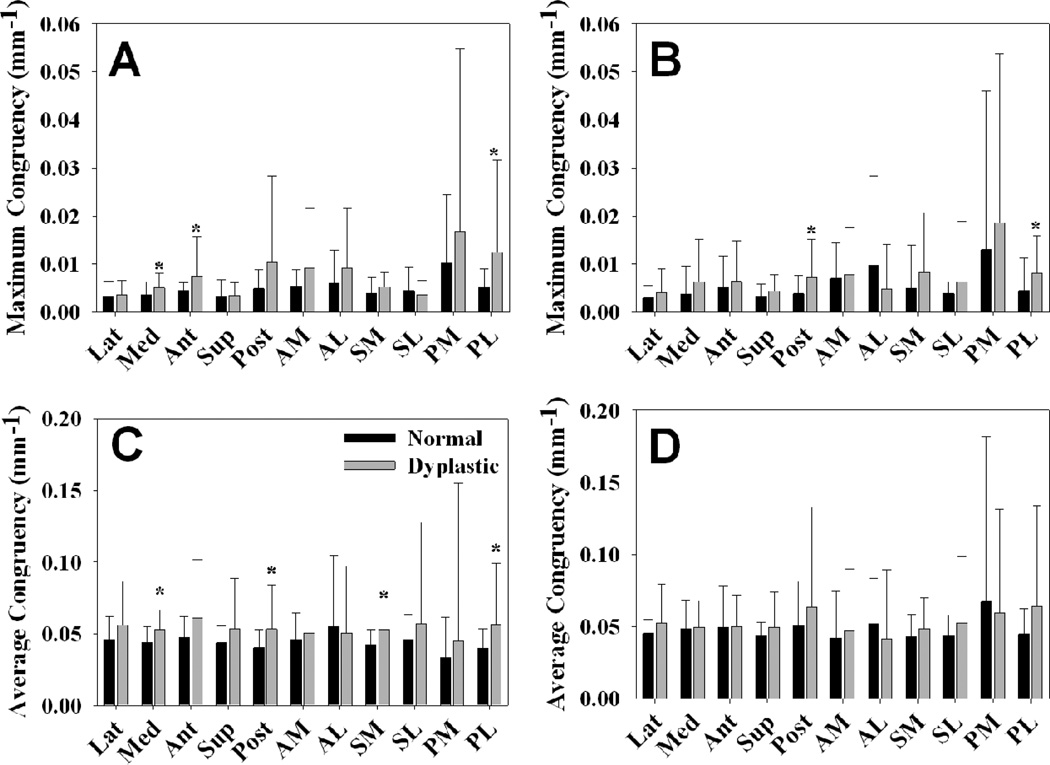
Dysplastic hips were significantly less congruent than normal hips in several non-weight bearing regions (Fig. 5). In particular, the most congruent point (as represented by the smallest RMS value) was less congruent in dysplastic hips than in normal hips in the medial, anterior and PL regions during WM, and in the posterior and PL regions during AH (P = 0.030, 0.036, 0.029, 0.025 and 0.044, respectively). Average congruency was less congruent in dysplastic hips than in normal hips during WM in the medial, posterior, SM and PL regions (P = 0.004, 0.025, P <0.001 and P = 0.028, respectively). Global congruency, as evaluated using best-fit spheres to the articular surfaces, was nearly identical between the two groups, at 0.08818 ± 0.00726 mm−1 in the dysplastic hips and 0.08817 ± 0.00734 mm−1 in the normal hips (P = 0.995). There were no significant differences in the radii of the femoral or acetabular cartilage between the two groups (P = 1.000 and 0.992, respectively). However, the objective value for both the femoral and acetabular cartilage best-fit spheres was significantly larger in the dysplastic hips than in the normal hips (P < 0.001 and P = 0.022, respectively), indicating that the dysplastic hips fit the idealized geometry more poorly than the normal hips fit the idealized geometry.
Figure 5.
Congruency during WM and AH in all anatomical regions. Smaller values indicate more congruent surfaces. A – most congruent point (minimum RMS value) during WM. B – most congruent point (minimum RMS value) during AH. C – average congruency (average RMS value) during WM. D – average congruency (average RMS value) during AH. Dysplastic hips were significantly less congruent in some unloaded regions. However, there were no significant differences in the primary load-bearing regions. Error bars indicate upper confidence bounds (at 95%). * = p < 0.05 in comparison to normal hips during the same loading scenario (n = 10 in each group).
There were few differences in cartilage contact stress or cartilage contact area between the two groups. When differences were significant for regional comparisons, the normal hips had larger contact stress or contact area than the dysplastic hips (Fig. 6 and Supplementary Figures S1, S2, S3 and S4). Specifically, peak contact stress was larger in the normal hips than in the dysplastic hips during WM in the AM region and during AH in the PM region (P = 0.049 and 0.019, respectively). Average contact stress was larger in the normal hips than in the dysplastic hips during WM in the PL and AM regions, during AH in the AL and PL regions and during DH in the SM region (P = 0.046, 0.013, 0.046, 0.044 and 0.042, respectively). Contact area as a percentage of the regional area was larger in normal hips than in dysplastic hips during WM in the PL and AM regions, during AH in the AL and PM regions and during DH in the SM region (P = 0.015, 0.003, 0.007, 0.004 and 0.028, respectively). There were fewer inter-activity differences in contact stress and contact area in the normal hips than in the dysplastic hips (Supplementary Figures S1, S2 and S3).
Figure 6.
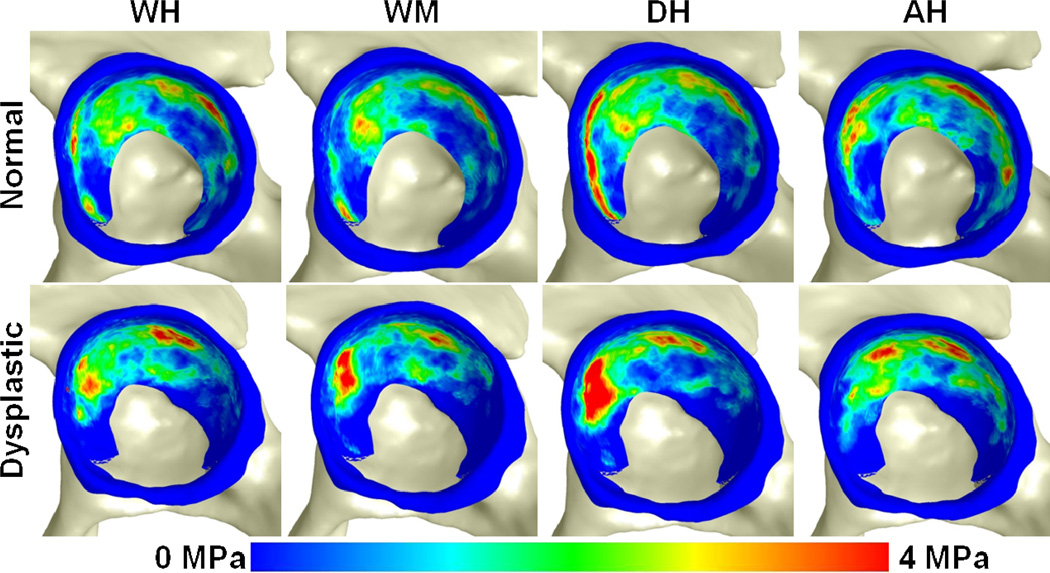
Average contact stress fringe plots during all simulated activities. Qualitative differences in the pattern of contact stress in the two groups can be seen, with more lateral contact and less medial contact in the dysplastic hips than in the normal hips (n =10 in each group).
Discussion
This study demonstrated the distinct role of the acetabular labrum as a load-bearing structure in patient-specific mechanics of dysplastic hips in comparison to normal hips during activities of daily living. While the labrum is known to provide additional contact area and volume to the morphologically normal human hip [29], contact pressure analysis of normal cadaveric hips and an animal model of labrum removal suggested that the labrum was relatively unimportant to the mechanics of the hip [13, 30]. The findings of the present study in the normal hips are consistent with previous studies. However, the labrum in the dysplastic hip provided significantly more mechanical support, experiencing loads 2.8 to 4.0 times larger than that of the normal hips. Thus, the present study adds new insight into the mechanical role of the labrum and how this role varies in the dysplastic hip versus the normal hip.
The location of differences in labral contact area between the two groups may have important implications for interpreting the location of initial chondrolabral damage in dysplastic hips. In particular, patient-specific FE predictions of contact area showed that only the superior region exhibited significantly different labral contact areas between normal and dysplastic subjects. This suggests that the superior labrum is loaded more than other portions of the acetabular labrum in the dysplastic hip during activities of daily living. This is consistent with clinical observations of labral hypertrophy, as well as the superior or anterosuperior location of labral tears in the dysplastic hip [7, 31, 32]. Clinical findings of anterosuperior labral tears represent a minor mismatch with results of the present study, since we did not find significant differences in anterior labrum contact stress or contact area. Activities other than those considered in this study may contribute to labral tears in the dysplastic hip, or additional variables may more accurately predict the onset of labral tears. For example, shear stress at the chondrolabral boundary or altered labrum material behavior in the dysplastic hip may be more relevant to predicting the location of labral tears.
The results of this study suggest that the pathogenesis of OA may occur in an “outside-in” manner in dysplastic hips, as observed clinically [33, 34]. Labral tears, labral hypertrophy and cartilage damage in the anterosuperior acetabulum often precede OA in the pathomorphologic hip [33, 34]. The lack of differences in cartilage mechanics between the two groups coupled with the drastic differences in labral mechanics suggest a pathway whereby the dysplastic hip is subjected to damage along the periphery of the acetabulum, initially on the acetabular labrum. This would account for the outside-in pathogenesis of disease in these hips, in contrast to the progressive joint space narrowing that characterizes OA as observed in the older patient without signs of hip pathomorphology [35].
The mechanical role of the acetabular labrum in dysplastic hips may have important clinical implications, as a strong indication against arthroscopic labral debridement in this patient population. Since arthroscopy is less invasive than open hip preservation surgeries that correct the underlying pathomorphology, it is an attractive option to the patient and surgeon [36]. However, labral debridement in the dysplastic hip can cause poor outcomes such as accelerated osteoarthritis, hip instability with lateral femoral head migration and increased need for re-operation [37, 38]. Nevertheless, there have also been reports of good outcomes [39, 40]. The present study suggests that clinical findings of poor outcomes following surgical debridement of the labrum in the dysplastic hip may be due to the loss of the load support by the intact labrum, but cannot account for the reported good outcomes.
Quantitative evaluation of local congruency provides unique insight into the morphology of dysplastic hips. In this study, there were no significant differences in local congruency between normal and dysplastic hips in the primary load-bearing areas. This contradicts previous reports that dysplastic hips are less congruent than normal hips [17, 18]. This study also found that a spherical fit of the articular surfaces provided a less accurate fit for dysplastic hips than for normal hips, which is consistent with previously reported elliptical femoral heads associated with dysplasia [41]. Combined, these findings indicate that the less spherical shape of the dysplastic hip does not result in reduced congruency in the primary load-bearing areas, because both the acetabulum and the femur are less spherical than in the normal hip.
The congruency findings in this study may have implications for the open hip preservation surgeries used to treat acetabular dysplasia. For example, during the periacetabular osteotomy procedure, the acetabulum is reoriented laterally to increase the lateral CEA. In this study, dysplastic hips were less congruent in the medial region during WM. Because this was not a primary load-bearing region in the native dysplastic hip, the relatively incongruent medial acetabulum did not affect cartilage contact stresses. However, surgery may place the medial region into additional loading in comparison to the pre-surgical hip, which may lead to iatrogenic outcomes.
Our results for cartilage contact stress are in contrast with the findings of previous mathematical and FE studies of the dysplastic hip. Previous mathematical and FE studies have assumed spherical, concentric geometry [14, 42, 43], and have mostly omitted the acetabular labrum [42, 43]. By simple description of stress as force divided by area, it would follow that any global reduction in coverage would cause an increase in cartilage contact stress for idealized hip geometry. Conversely, the subject-specific geometry used in the present study allowed the mechanical effects of local congruency to be included in the calculation of chondrolabral mechanics. Subject-specific geometry has previously been shown to be required for accurate FE predictions in the normal hip [15, 16]. In a previous FE study that omitted the labrum, but included subject-specific geometry, predictions of contact stress for dysplastic hips were larger than those for a single normal hip [11]. In the present study, the labrum provided additional contact surface through which load could be transferred, reducing the necessary support by the cartilage. Therefore, although these findings contrast previous reports, by including subject-specific geometry for bone, cartilage and labrum, we believe the results of the present study are the most physiologically representative of dysplastic patients presented to date.
There are several limitations in the present study that warrant discussion. Cartilage was represented as nearly-linear and nearly-incompressible hyperelastic, which is a simplification of the known material behavior of articular cartilage in the hip [10, 44]. However, nearly-incompressible hyperelasticity is an appropriate simplification when cartilage is deformed under fast loading rates, such as those applied in the present study [45, 46]. Additionally, our validation studies have demonstrated that our subject-specific modeling protocol can provide accurate predictions of experimentally measured contact stress and contact area when nearly-linear hyperelasticity and linear elasticity are used to represent articular cartilage [8, 10]. For the labrum, the constitutive representation was consistent with the known fiber structure [47], but material coefficients were derived from bovine labra [48]. In a previous study, we evaluated the effects of altering the labrum constitutive model and coefficients on FE predictions, demonstrating that the mechanical role of the labrum was affected more by the assumed constitutive model than the material coefficients [9]. When combined with the fact that there is insufficient data in the literature to curve fit a three dimensional transversely isotropic constitutive model using human data, drawing the material coefficients from bovine labra provided a reasonable and justified approach. In addition to the limitations regarding the selected constitutive models, normal hips and dysplastic hips were assumed to have identical cartilage and labrum material coefficients. Clinical evidence of labral hypertrophy suggests that the labrum in dysplastic hips may be stiffer than in normal hips. Also, cartilage in early OA is often softer than healthy cartilage, so the cartilage in dysplastic hips may be softer than in normal hips. Modeling the dysplastic subjects with softer cartilage and stiffer labra would increase the magnitudes of the differences found in the present study. However, we recruited patients without major chondrolabral changes that were candidates for hip preservation surgery. This reduced the likelihood of altered material behavior in the cartilage and labrum of dysplastic hips. Similarly, identical kinematics were used for both subject groups. In previous studies, dysplastic patients exhibited subtle alternations in kinematics during gait, including increased pelvic drop, increased pelvic rotation, reduced hip extension and a reduction in ground reaction force in comparison to normal hips [49, 50]. Increased pelvic drop may cause increased labral loading, while increased pelvic rotation may shift contact patterns posteriorly. Alterations in hip extension would occur at the end of the stance phase, a position not evaluated in the present study. A decrease in the total load transferred across the joint may slightly decrease contact stresses, but would not be expected to affect the percentage of load transferred across the labrum. In the present study, evaluation of all subjects with identical kinematics provided the ability to focus on changes in chondrolabral mechanics due to geometry of the hip, independent of potential differences in gait. Finally, the selection of homogeneous subject groups is challenging, and may present confounding factors for this study. The criteria for selecting patients in the dysplastic group were based on CEA and acetabular index in the anteroposterior plane. Femoral morphology is abnormal in hips with acetabular dysplasia [3, 41], but the extent of abnormal femoral morphology was not a part of the subject selection criteria in this study. Therefore, it is possible that differences in femoral geometry, or other geometric features that were not considered, could have affected the results.
In conclusion, the labrum in dysplastic hips has a far more significant role in hip mechanics than it does in normal hips. This study supports the concept of the outside-in pathogenesis of osteoarthritis in dysplastic hips and that the labrum in these patients should be preserved when possible. When performing rotational osteotomies to correct dysplasia, surgeons should be aware that originally non-loadbearing regions are less congruent than the originally loadbearing regions. Contrary to previous findings, this study shows that cartilage contact stresses in dysplastic hips are not increased compared to normal hips because the labrum supports a large percentage of the load transferred across the joint.
Supplementary Material
Acknowledgements
Financial support from NIH #R01AR053344 and #R01GM083925 is gratefully acknowledged.
Role of Funding Source
Funding was provided by the NIH. The funding agency had no role in study design; data collection, analysis or interpretation; manuscript writing; or the decision to submit the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
CRH: patient recruitment, image data collection, image data segmentation, finite element modeling, statistical analysis, manuscript preparation. CLA: patient recruitment, image data collection, congruency analysis, statistical analysis, manuscript editing. AEA: study design, patient recruitment, image data collection, manuscript editing. SAM: implementation of congruency algorithms into PostView. BJE: study design, manuscript preparation. CLP: study design, patient recruitment, manuscript editing. JAW: study design, manuscript preparation, manuscript editing. CRH and JAW take responsibility for the integrity of this work.
Conflict of Interest
The authors have no conflicts of interest.
Contributor Information
Corinne R Henak, Email: corinne.henak@gmail.com.
Christine L Abraham, Email: c.abraham@utah.edu.
Andrew E Anderson, Email: Andrew.Anderson@hsc.utah.edu.
Steve A Maas, Email: steve.maas@utah.edu.
Benjamin J Ellis, Email: ben@sci.utah.edu.
Christopher L Peters, Email: Chris.Peters@hsc.utah.edu.
References
- 1.Harris-Hayes M, Royer NK. Relationship of acetabular dysplasia and femoroacetabular impingement to hip osteoarthritis: a focused review. PM & R : the journal of injury, function, and rehabilitation. 2011;3:1055–1067. doi: 10.1016/j.pmrj.2011.08.533. e1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klaue K, Durnin CW, Ganz R. The acetabular rim syndrome. A clinical presentation of dysplasia of the hip. The Journal of bone and joint surgery. 1991;73:423–429. doi: 10.1302/0301-620X.73B3.1670443. British volume. [DOI] [PubMed] [Google Scholar]
- 3.Kosuge D, Yamada N, Azegami S, Achan P, Ramachandran M. Management of developmental dysplasia of the hip in young adults: current concepts. The bone & joint journal. 2013;95-B:732–737. doi: 10.1302/0301-620X.95B6.31286. [DOI] [PubMed] [Google Scholar]
- 4.Solomon L. Patterns of osteoarthritis of the hip. The Journal Of Bone And Joint Surgery. 1976;58:176–183. doi: 10.1302/0301-620X.58B2.932079. British Volume. [DOI] [PubMed] [Google Scholar]
- 5.Reijman M, Hazes JM, Pols HA, Koes BW, Bierma-Zeinstra SM. Acetabular dysplasia predicts incident osteoarthritis of the hip: the Rotterdam study. Arthritis and rheumatism. 2005;52:787–793. doi: 10.1002/art.20886. [DOI] [PubMed] [Google Scholar]
- 6.Cooperman D. What is the evidence to support acetabular dysplasia as a cause of osteoarthritis? Journal of pediatric orthopedics. 2013;33(Suppl 1):S2–S7. doi: 10.1097/BPO.0b013e3182770a8d. [DOI] [PubMed] [Google Scholar]
- 7.Peelle MW, Della Rocca GJ, Maloney WJ, Curry MC, Clohisy JC. Acetabular and femoral radiographic abnormalities associated with labral tears. Clinical orthopaedics and related research. 2005;441:327–333. doi: 10.1097/01.blo.0000181147.86058.74. [DOI] [PubMed] [Google Scholar]
- 8.Anderson AE, Ellis BJ, Maas SA, Peters CL, Weiss JA. Validation of finite element predictions of cartilage contact pressure in the human hip joint. Journal of biomechanical engineering. 2008;130:051008. doi: 10.1115/1.2953472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henak CR, Ellis BJ, Harris MD, Anderson AE, Peters CL, Weiss JA. Role of the acetabular labrum in load support across the hip joint. Journal of biomechanics. 2011;44:2201–2206. doi: 10.1016/j.jbiomech.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henak CR, Kapron AL, Anderson AE, Ellis BJ, Maas SA, Weiss JA. Specimen-specific predictions of contact stress under physiological loading in the human hip: validation and sensitivity studies. Biomechanics and modeling in mechanobiology. 2013 doi: 10.1007/s10237-013-0504-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell ME, Shivanna KH, Grosland NM, Pedersen DR. Cartilage contact pressure elevations in dysplastic hips: a chronic overload model. Journal Of Orthopaedic Surgery And Research. 2006;1:6–6. doi: 10.1186/1749-799X-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris MD, Anderson AE, Henak CR, Ellis BJ, Peters CL, Weiss JA. Finite element prediction of cartilage contact stresses in normal human hips. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2012;30:1133–1139. doi: 10.1002/jor.22040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konrath GA, Hamel AJ, Olson SA, Bay B, Sharkey NA. The role of the acetabular labrum and the transverse acetabular ligament in load transmission in the hip. The Journal of bone and joint surgery. 1998;80:1781–1788. doi: 10.2106/00004623-199812000-00008. American volume. [DOI] [PubMed] [Google Scholar]
- 14.Chegini S, Beck M, Ferguson SJ. The effects of impingement and dysplasia on stress distributions in the hip joint during sitting and walking: a finite element analysis. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2009;27:195–201. doi: 10.1002/jor.20747. [DOI] [PubMed] [Google Scholar]
- 15.Anderson AE, Ellis BJ, Maas SA, Weiss JA. Effects of idealized joint geometry on finite element predictions of cartilage contact stresses in the hip. Journal of biomechanics. 2010;43:1351–1357. doi: 10.1016/j.jbiomech.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu DY, Hu F, Wei JH, Dai KR, Chen YZ. Contributions of non-spherical hip joint cartilage surface to hip joint contact stress. Conference proceedings : … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference. 2011;2011:8166–8169. doi: 10.1109/IEMBS.2011.6092014. [DOI] [PubMed] [Google Scholar]
- 17.Okano K, Yamada K, Takahashi K, Enomoto H, Osaki M, Shindo H. Joint congruency in abduction before surgery as an indication for rotational acetabular osteotomy in early hip osteoarthritis. International orthopaedics. 2010;34:27–32. doi: 10.1007/s00264-009-0734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasunaga Y, Takahashi K, Ochi M, Ikuta Y, Hisatome T, Nakashiro J, et al. Rotational acetabular osteotomy in patients forty-six years of age or older: comparison with younger patients. The Journal of bone and joint surgery. 2003;85-A:266–272. doi: 10.2106/00004623-200302000-00013. American volume. [DOI] [PubMed] [Google Scholar]
- 19.Larson AN, Rabenhorst B, De La Rocha A, Sucato DJ. Limited intraobserver and interobserver reliability for the common measures of hip joint congruency used in dysplasia. Clinical orthopaedics and related research. 2012;470:1414–1420. doi: 10.1007/s11999-011-2136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clohisy JC, Carlisle JC, Trousdale R, Kim YJ, Beaule PE, Morgan P, et al. Radiographic evaluation of the hip has limited reliability. Clinical orthopaedics and related research. 2009;467:666–675. doi: 10.1007/s11999-008-0626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergmann G, Deuretzbacher G, Heller M, Graichen F, Rohlmann A, Strauss J, et al. Hip contact forces and gait patterns from routine activities. Journal of biomechanics. 2001;34:859–871. doi: 10.1016/s0021-9290(01)00040-9. [DOI] [PubMed] [Google Scholar]
- 22.Puso MA, Maker Bradley N, Ferencz Robert M, Hallquist John O. NIKE3D: A Nonlinear, Implicit, Three-Dimensional Finite Element Code For Solid and Structural Mechanics. User's Manual. 2007 [Google Scholar]
- 23.Maas SA. PostView Version 1.4: User's Manual. 2012 [Google Scholar]
- 24.Ateshian GA, Rosenwasser MP, Mow VC. Curvature characteristics and congruence of the thumb carpometacarpal joint: differences between female and male joints. Journal of biomechanics. 1992;25:591–607. doi: 10.1016/0021-9290(92)90102-7. [DOI] [PubMed] [Google Scholar]
- 25.Garimella RV, Swartz BK. Curvature estimation for unstructured triangulations of surfaces. Los Alamos National Laboratory. 2003 [Google Scholar]
- 26.Petitjean S. A survey of methods for recovering quadrics in triangle meshes. ACM Computing Surveys (CSUR) 2002;34:211–262. [Google Scholar]
- 27.Best PJ, McKay ND. A method for registration of 3-D shapes. IEEE Trans. on Pattern Analysis and Machine Intelligence. 1992;14:239–256. [Google Scholar]
- 28.Maas SA, Rawlins DS, Weiss JA. PreView Version 1.8: User's Manual. 2012 [Google Scholar]
- 29.Tan V, Seldes RM, Katz MA, Freedhand AM, Klimkiewicz JJ, Fitzgerald RH., Jr Contribution of acetabular labrum to articulating surface area and femoral head coverage in adult hip joints: an anatomic study in cadavera. American journal of orthopedics. 2001;30:809–812. [PubMed] [Google Scholar]
- 30.Miozzari HH, Clark JM, Jacob HA, von Rechenberg B, Notzli HP. Effects of removal of the acetabular labrum in a sheep hip model. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2004;12:419–430. doi: 10.1016/j.joca.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Fujii M, Nakashima Y, Jingushi S, Yamamoto T, Noguchi Y, Suenaga E, et al. Intraarticular findings in symptomatic developmental dysplasia of the hip. Journal of pediatric orthopedics. 2009;29:9–13. doi: 10.1097/BPO.0b013e318190a0be. [DOI] [PubMed] [Google Scholar]
- 32.Leunig M, Podeszwa D, Beck M, Werlen S, Ganz R. Magnetic resonance arthrography of labral disorders in hips with dysplasia and impingement. Clinical orthopaedics and related research. 2004:74–80. doi: 10.1097/00003086-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Jessel RH, Zurakowski D, Zilkens C, Burstein D, Gray ML, Kim YJ. Radiographic and patient factors associated with pre-radiographic osteoarthritis in hip dysplasia. The Journal of bone and joint surgery. 2009;91:1120–1129. doi: 10.2106/JBJS.G.00144. American volume. [DOI] [PubMed] [Google Scholar]
- 34.Noguchi Y, Miura H, Takasugi S, Iwamoto Y. Cartilage and labrum degeneration in the dysplastic hip generally originates in the anterosuperior weight-bearing area: an arthroscopic observation. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 1999;15:496–506. doi: 10.1053/ar.1999.v15.015049. [DOI] [PubMed] [Google Scholar]
- 35.Gupta KB, Duryea J, Weissman BN. Radiographic evaluation of osteoarthritis. Radiologic clinics of North America. 2004;42:11–41. doi: 10.1016/S0033-8389(03)00169-6. v. [DOI] [PubMed] [Google Scholar]
- 36.Matsuda DK, Khatod M. Rapidly progressive osteoarthritis after arthroscopic labral repair in patients with hip dysplasia. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2012;28:1738–1743. doi: 10.1016/j.arthro.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Kalore NV, Jiranek WA. Save the torn labrum in hips with borderline acetabular coverage. Clinical orthopaedics and related research. 2012;470:3406–3413. doi: 10.1007/s11999-012-2499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parvizi J, Bican O, Bender B, Mortazavi SM, Purtill JJ, Erickson J, et al. Arthroscopy for labral tears in patients with developmental dysplasia of the hip: a cautionary note. The Journal of arthroplasty. 2009;24:110–113. doi: 10.1016/j.arth.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 39.Byrd JW, Jones KS. Hip arthroscopy in the presence of dysplasia. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2003;19:1055–1060. doi: 10.1016/j.arthro.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto Y, Ide T, Nakamura M, Hamada Y, Usui I. Arthroscopic partial limbectomy in hip joints with acetabular hypoplasia. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2005;21:586–591. doi: 10.1016/j.arthro.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Steppacher SD, Tannast M, Werlen S, Siebenrock KA. Femoral morphology differs between deficient and excessive acetabular coverage. Clinical orthopaedics and related research. 2008;466:782–790. doi: 10.1007/s11999-008-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iglic A, Kralj-Iglic V, Daniel M, Macek-Lebar A. Computer determination of contact stress distribution and size of weight bearing area in the human hip joint. Computer methods in biomechanics and biomedical engineering. 2002;5:185–192. doi: 10.1080/10255840290010300. [DOI] [PubMed] [Google Scholar]
- 43.Mavcic B, Iglic A, Kralj-Iglic V, Brand RA, Vengust R. Cumulative hip contact stress predicts osteoarthritis in DDH. Clinical orthopaedics and related research. 2008;466:884–891. doi: 10.1007/s11999-008-0145-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mow V, Gu W, Chen F. Structure and Function of Articular Cartilage and Meniscus. In: Mow V, Huiskes R, editors. Basic Orthopaedic Biomechanics and Mechano-Biology. Philadephia: Lippincott; 2005. pp. 181–258. [Google Scholar]
- 45.Ateshian GA, Ellis BJ, Weiss JA. Equivalence between short-time biphasic and incompressible elastic material responses. Journal of biomechanical engineering. 2007;129:405–412. doi: 10.1115/1.2720918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong M, Ponticiello M, Kovanen V, Jurvelin JS. Volumetric changes of articular cartilage during stress relaxation in unconfined compression. Journal of biomechanics. 2000;33:1049–1054. doi: 10.1016/s0021-9290(00)00084-1. [DOI] [PubMed] [Google Scholar]
- 47.Petersen W, Petersen F, Tillmann B. Structure and vascularization of the acetabular labrum with regard to the pathogenesis and healing of labral lesions. Archives of orthopaedic and trauma surgery. 2003;123:283–288. doi: 10.1007/s00402-003-0527-7. [DOI] [PubMed] [Google Scholar]
- 48.Ferguson SJ, Bryant JT, Ito K. The material properties of the bovine acetabular labrum. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2001;19:887–896. doi: 10.1016/S0736-0266(01)00007-9. [DOI] [PubMed] [Google Scholar]
- 49.Romano CL, Frigo C, Randelli G, Pedotti A. Analysis of the gait of adults who had residua of congenital dysplasia of the hip. The Journal of bone and joint surgery. 1996;78:1468–1479. doi: 10.2106/00004623-199610000-00003. American volume. [DOI] [PubMed] [Google Scholar]
- 50.Jacobsen JS, Nielsen DB, Sorensen H, Soballe K, Mechlenburg I. Changes in walking and running in patients with hip dysplasia. Acta orthopaedica. 2013;84:265–270. doi: 10.3109/17453674.2013.792030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.