Abstract
A novel strategy to improve the therapeutic index of chemotherapy has been developed by the integration of nanotechnology with phage technique. The objective of this study was to combine phage display, identifying tumor-targeting ligands, with a liposomal nanocarrier for targeted delivery of doxorubicin. Following the proof of concept in cell-based experiments, this study focused on in vivo assessment of antitumor activity and potential side-effects of phage fusion protein-modified liposomal doxorubicin. MCF-7-targeted phage-Doxil treatments led to greater tumor remission and faster onset of antitumor activity than the treatments with non-targeted formulations. The enhanced anticancer effect induced by the targeted phage-Doxil correlated with an improved tumor accumulation of doxorubicin. Tumor sections consistently revealed enhanced apoptosis, reduced proliferation activity and extensive necrosis. Phage-Doxil-treated mice did not show any sign of hepatotoxicity and maintained overall health. Therefore, MCF-7-targeted phage-Doxil seems to be an active and tolerable chemotherapy for breast cancer treatment.
Keywords: Breast Cancer Targeting, Phage Display, Cancer Nanomedicines, Liposomes, Drug Delivery
1. Background
Breast cancer is a major public health problem worldwide. Among all cancer types, the incidence of breast cancer is the second most common after lung cancer and it is the most frequently occurring cancer among women (1). Breast cancer is the fifth most common cause of cancer death and the second leading cause of cancer deaths after lung cancer in women (1).
Among the therapeutical modalities currently available for breast cancer treatment, chemotherapy has been a primary option for managing advanced-stage breast cancer including metastatic (stage IV) or recurrent breast cancer. The major advantage of the clinical use of chemotherapy over surgery and radiation is its systemic action which kills cancer cells in both primary and metastatic tumors. Still, non-selective biodistribution and dose-limiting toxicity lead to suboptimal therapeutic outcomes for conventional chemotherapy. Doxorubicin, one of the most widely-used chemotherapeutics in the treatment of a wide range of cancers including breast cancer, has long been known to produce severe side-effects including cardiac toxicity and myelosuppression (2). Its dose-limiting toxicity has strongly influenced its clinical use. As a result, improvement of the therapeutic index of doxorubicin has been long sought.
One of the successful efforts has involved the encapsulation of doxorubicin within liposomes and has led to the clinical approvals of Myocet and Caelyx (Doxil in the United States) by regulatory authority (3). Caelyx is currently marketed for metastatic breast cancer, advanced ovarian cancer, multiple myeloma and Kaposi's sarcoma. Those liposomal doxorubicin formulations demonstrated their ability to improve the pharmacokinetic profile and to reduce drug-related toxicity including cardiomyopathy, bone marrow depression, alopecia and nausea. However, the clinical data did not suggest that liposomal doxorubicin enhances antitumor efficacy when compared with free doxorubicin (4-6).
Conceptually, the use of ligand-mediated targeted liposomes is a promising idea to make doxorubicin more effective. The site-specific delivery of doxorubicin to a tumor can enhance antitumor activity, while its toxicity can be decreased as a result of reduction of its delivery to non-target normal tissues. Still, the current ligand-mediated targeted strategy has produced mixed results (7, 8). In an attempt to improve ligand-targeted liposomal delivery systems, we have taken advantage of phage display techniques for the identification of new target ligands (9-12), and have developed a new approach for self-assembly of the targeted ligands with a liposomal drug carrier (13, 14). A 55-mer landscape phage fusion coat protein was identified from an 8-mer landscape library f8/8 using a biopanning protocol against MCF-7 cells (13, 15). A simple post-insertion protocol (13, 16, 17) without chemical conjugation has been developed for the modification of liposomes with a MCF-7 cancer cell-specific landscape phage protein (termed MCF-7-targeted phage-Doxil). Our recent in vitro results showed that MCF-7-targeted phage-Doxil enhanced the cytotoxicity and apoptotic activity against MCF-7 breast cancer cells (13, 18). The primary objective of this study was to evaluate the potential for its in vivo tumor delivery and antitumor activity. Both subcutaneous and orthotopic MCF-7 xenografted nude mouse models were developed to follow the effect of MCF-7-specific phage protein-modified Doxil on tumor size and growth. Control formulations included non-modified Doxil (or a generic Doxil - LipoDox) and Doxil (or LipoDox) modified with a non-targeted phage fusion protein (termed non-targeted phage-Doxil). The antitumor activity was further verified by the histological / immunohistochemical examination of changes associated with necrosis, apoptosis and proliferation in tumor sections after the treatments. Finally, we evaluated the potential side-effects of multiple doses of MCF-7-specific phage-Doxil by monitoring changes of mouse body weight, histological changes in tissue sections of vital organs and plasma liver enzyme levels.
2. Methods
2.1. Propagation of phage and isolation of phage fusion protein
Phages were propagated and phage fusion proteins were isolated using the protocol described by us (19) (Method S 1).
2. 2. Preparation of doxorubicin-loaded liposomes modified with phage-derived proteins (phage-Doxil)
Phage-Doxil was prepared by incubating Doxil (Ben Venue Laboratories Inc, Bedford, OH) and LipoDox (SUN Pharmaceutical ind. Itd. Gujaat, India) with the cholate-stabilized phage pVIII coat fusion protein at a lipid-to-protein weight ratio of 200: 1 at 37°C. After overnight incubation, the crude formulation was dialyzed overnight at 4°C against the cholate-free PBS buffer to remove sodium cholate.
Loading of doxorubicin into liposomes was examined using a size-exclusion HPLC system (Hitachi, Japan) equipped with a diode array and Shodex Protein KW-G column. The elution was performed at a rate of 1.0 ml/min with PBS (pH 7.4) as a mobile phase. Liposomes were detected at 254 nm and doxorubicin at 470 nm.
Entrapped doxorubicin was determined using a microplate reader (Bio-Tek, Winooski, VT) at 492 nm. Encapsulation efficiency is defined as the doxorubicin amount in the final formulation divided by the inputted doxorubicin.
2.3. Size distribution and stability of liposomal preparations
Liposomal size was detected using dynamic light scattering. Briefly, the preparations were diluted using PBS (pH 7.4) and a Beckman Coulter N4 Plus Particle Analyzer (Beckman Coulter, Fullerton, CA) was used to measure sizes with a scattering angle of 90° with a size range of 1-1000nm in triplicate.
For colloidal stability assessment, liposomes were stored at 4°C and their sizes were determined from aliquots at predetermined times. For serum stability assessment, liposomes were incubated in a final 50% fetal bovine serum (FBS) at 37°C followed by size measurement.
2.4. Zeta potential of liposomal preparations
Liposomal preparations were diluted with distilled water and zeta-potential was analyzed with a ZetaPLUS apparatus (Brookhaven, Holtsville, NY) in triplicate.
2.5. Transmission electron microscopy
MCF-7 targeted phage-Doxil were negatively stained using 0.5% uranyl acetate and images were taken using a JEOL JEM-1010 transmission electron microscope (JEOL USA, Inc., Peabody, MA) operating at an acceleration voltage of 80 kV.
2.6. Cell culture
MCF-7 breast adenocarcinoma (HTB 22™) cells (ATCC, Manassas, VA) were cultured in MEM supplemented with 10% FBS at 37 °C, 5% CO2. For inoculation into nude mice, cells were washed with PBS and detached with trypsin. After centrifugation, cells were re-suspended (1:1) with Matrigel HC (BD Biosciences, San Jose, CA) in MEM.
2.7. Development of the nude mouse MCF-7 tumor xenografted model
Female, 6-8week, nu/nu (athymic) mice (Charles River Laboratories, Wilmington, MA) were housed and kept on a 12:12 light: dark cycle in sterilized cages with ad libitum access to sterile food and water. All animal treatments were carried out in accordance with the guidelines of Northeastern University's IACUC.
To establish and maintain an estrogen-responsive MCF-7 tumor in vivo, the estradiol-containing silastic implants were prepared (Methods S2) and inserted subcutaneously over the dorsal thorax. 2 × 106 MCF-7 cells mixed with Matrigel HC were injected subcutaneously into the left flank of the lightly anesthetized mice (subcutaneous MCF-7 tumor xenografts), or into the mammary fat pad near the fourth nipple (orthotopic MCF-7 tumor xenografts).
2.8. Antitumor activity
For subcutaneous MCF-7 xenografts (n=3-4), mice were treated when tumors reached an estimated mean volume of 360 mm3. For orthotopic MCF-7 xenografts (n=5), mice were treated when tumors reached an estimated mean volume of 250 mm3. Mice were randomly assigned to each group. Tail vein injections with formulations at a total doxorubicin dosage of 15 mg/kg were divided into five doses given at day 0, +2, +4, +6 and +8. The tumor volume for each mouse was estimated with calipers and calculated using the formula: Tumor volume by caliper (mm3) = [length × (width) 2]/2.
Antitumor activity was assessed with parameters including the tumor volume from day 0 (%) and tumor growth inhibition (%) at the study endpoint. Definitions of these parameters are described below.
Tumor volume from day 0 (%) = (Tumor volume after treatment / Tumor volume at day 0) ×100.
Tumor growth inhibition (%) = [(Tumor volume in untreated control group - Tumor volume in treated group)at Endpoint / (Tumor volume in untreated control group) at Endpoint] ×100.
At the end of experiments, blood was collected by cardiac puncture of anesthetized mice into a heparinized (1 U) tube. The blood was centrifuged at 1000g for 10min at 4°C following by collection of the upper plasma layer and storage at −80°C until use. Liver, kidney, spleen, lung, heart and tumors were harvested for histological analysis.
2.9. Tumor volume estimation by MRI
At the end of experiments, mice bearing subcutaneous MCF-7 xenografts were anesthetized using 2% isoflurane. The animals were scanned on a 7 T preclinical MRI system (BioSpec 70/20 USR, Bruker BioSpin Corp, Billerica, MA). A multislice T1 RARE spin-echo sequence was used with repetition time (TR) of 3900 ms, echo time (TE) of 10ms. Image J software (National Institutes of Health) was used to estimate tumor volume.
2.10. H&E staining
Formalin-fixed paraffin embedded tissue- or tumor samples (5μm) were stained with Harris modified hematoxylin (Fisher Scientific, Fair Lawn, NJ) followed by rinsing in running tap water, and then re-stained with eosin Y (Sigma-Aldrich, St. Louis, MO), dehydrated, cleared and slide-mounted. The slides were visualized by light microscopy (Nikon Japan) at 20× magnification (tumor), 100× magnification (tumor, lung and spleen) or 200 × magnifications (liver, kidney and heart).
2.11. Tumor delivery of doxorubicin by Doxil and phage-Doxil
Doxorubicin-loaded liposomes were intravenously injected into MCF-7 tumor-bearing mice at single dose of 2mg/kg (imaging) or 5mg/kg (quantitation). Tumor localization of doxorubicin was visualized using a fluorescence microscopy (Zeiss Germany) with DAPI and Texas Red filters at 200× magnification. The doxorubicin biodistribution was estimated by measuring the fluorescence of doxorubicin after its extraction from tissue homogenates (Methods S3). The tumor accumulation of the drug was expressed as ng doxorubicin per gram of tumor tissue. Tumor-specific delivery was defined as tumor-to-muscle ratio of doxorubicin.
2.12. Apoptosis assay
Apoptosis was evaluated using terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling (TUNEL) staining of tumor sections according to the manufacturer's manual of FragELTM DNA Fragmentation Detection Kits (Calbiochem, Billerica, MA). The images were acquired by fluorescence microscopy (Zeiss Germany) using DAPI and FITC filters at 200× magnification.
2.13. Immunohistochemical staining for proliferation marker Ki-67 antigens
Frozen tumor sections were fixed with 10% phosphate buffered formalin for 10min at RT, followed by TBS washing, 15min treatment with 0.3% H2O2 and 1h incubation in a blocking buffer. Sections were then incubated overnight with rabbit anti-Ki-67 antibody (1:125) (Enzo Life Sciences, Inc, Farmingdale, NY) at 4 °C in a humidified chamber, and incubated with a HRP conjugated anti-rabbit IgG (1:1000) (Cell Signaling, Inc, MA, USA) at RT for 30 min. After TBS washings, they were stained with diaminobenzidine (DAB) for 15min followed by counterstaining with hematoxylin and dehydration with ethanol and xylene. The slides were visualized by microscopy (Nikon, Japan) at 200× magnification.
2.14. Quantitation of plasma alanine transaminase (ALT) and aspartate aminotransferase (AST) activity
Plasma ALT and AST activity were determined according to manufacturer's manual of Alanine Transaminase Activity Assay Kit and Aspartate Aminotransaminase Activity Assay Kit (the Biomedical Research Center, State of University of New York at Buffalo) (Method S4).
2.15. Statistical analysis
Each experimental group contained 3-5 mice. Results were expressed as mean ± SEM or mean ± SD. The statistical analysis was performed using one-way ANOVA followed by LSD post hoc test. The statistically significant was considered if the p < 0.05.
3. Results
3.1 Characterization of phage-derived proteins
A fusion phage with high selectivity and affinity for breast cancer MCF-7 cells was screened from an 8-mer landscape phage library with standard phage display protocols (19). The phage fusion proteins derived from the phages were composed of the MCF-7 cancer cell-specific peptide fused to the N-terminal of wild-type phage coat protein (13). After the isolation of phage-derived protein by chromatography, their physicochemical properties were determined using Deleage & Roux Modification of Nishikawa & Ooi 1987 (20). The physicochemical characteristics of phage fusion protein and wild-type phage coat protein are compared in (Table 1).
Table 1.
Characterization of phage-derived proteins
| Characteristic | Wild-type phage coat protein | MCF-7-specific phage fusion protein |
|---|---|---|
| Molecular Weight (Dalton) | 5254.02 | 5447.72 |
| Amino Acid Residue (Number) | 50 | 55 |
| Molar Extinction Coefficient (M−1cm−1) | 8250 ± 5 | 8250 ± 5 |
| 1 unit absorbance at 280nm (mg/ml) | 0.64 | 0.70 |
| Isoelectric point (PI) | 6.47 | 8.54 |
| Charge at pH 7 (mV) | −0.09 | +0.91 |
3.2. Characterization of phage-Doxil
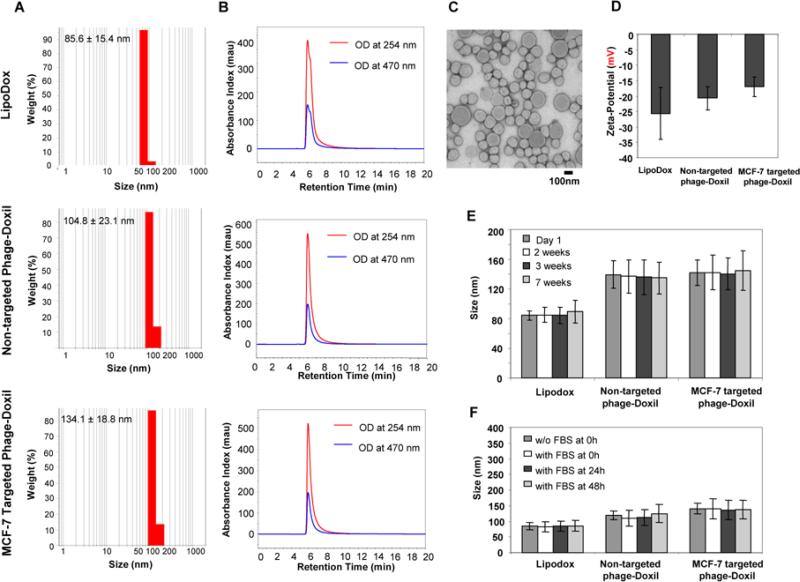
Phage-derived proteins were allowed to self-assemble with LipoDox using a post-insertion protocol we developed (13). In comparison with LipoDox, both non-targeted and targeted phage-Doxil formulations had a slight increase in particle size with narrow size distributions (Figure 1A). Both liposomes and doxorubicin co-eluted from the size-exclusion HPLC column at the same retention time (6 min), indicating the encapsulation of doxorubicin within liposomes (Figure 1B). Encapsulation efficiency of doxorubicin was 66.7±18.1% for non-targeted phage-Doxil and 71.8±15.4 % for targeted phage-Doxil (mean ± SD, n=3). The spherical morphology of LipoDox was retained after the incorporation of phage protein into liposomes (Figure 1C). Both LipoDox and phage-Doxil had negative charges (Figure 1 D). The phage-Doxil formulations also showed colloidal (Figure 1 E) and serum stability (Figure 1 F).
Figure 1. Characterization of LipoDox and phage-Doxil.
(A) Size and size distribution of LipoDox and Phage-Doxil measured by dynamic light scattering. (B) LipoDox and phage-Doxil elution profiles during SEC-HPLC. (C) Morphology of targeted phage-Doxil observed by transmission electron microscopy. (D) Zeta-potential of LipoDox and phage-Doxil. (E) Storage stability of LipoDox and phage-Doxil at 4°C. (F) Serum stability of LipoDox and phage-Doxil incubated in 50% FBS at 37°C.
3.3. Evaluation of antitumor efficacy of MCF-7-targeted phage-Doxil on subcutaneous xenografts by caliper-based estimation
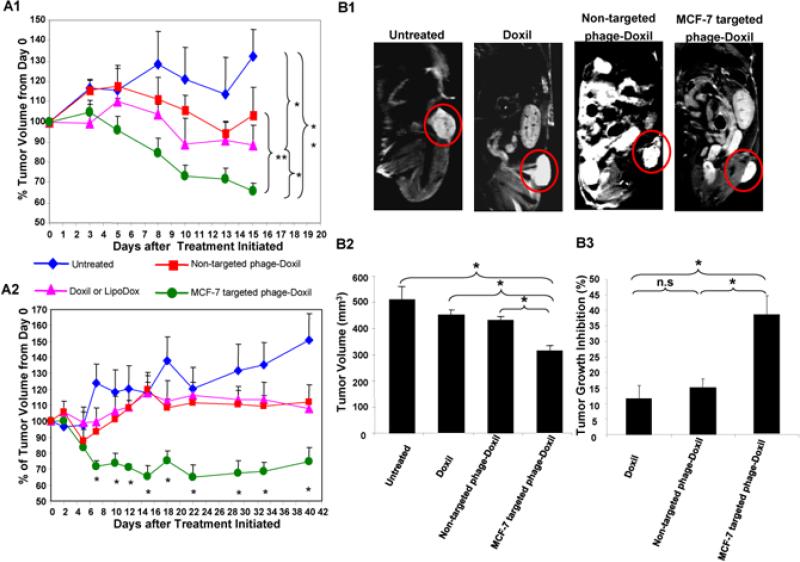
The tumor reduction study was initiated when the subcutaneous MCF-7 tumor xenografts (Figure S1A) reached a mean tumor volume of 362 ± 83 mm3. Mice showed the strongest tendency towards tumor reduction in response to the treatment with MCF-7-targeted phage-Doxil. Time-dependent tumor remission was observed as early as 5 to 8 days after the initiation of this targeted therapy. Tumor volume decreases continued from ~ 17% on day 8, to ~ 28% on day 10, and to ~34% on day 15 after treatments’ initiation (Figure 2A1). By contrast, mice in the untreated group showed continued tumor growth over time with a tumor volume increase of ~32% at 15 days after treatment was initiated. Two-weeks after initiation of treatment, average tumor volume in the MCF-7- targeted phage-Doxil group was 262 ± 83 mm3 versus 479 ± 127mm3 in the untreated group (p<0.005). In addition, the treatment with the non-modified Doxil produced a modest tumor growth inhibition with a slight 11% decrease in tumor volume two weeks after initiation of treatment (p<0.05, compared to untreated or targeted phage-Doxil). Doxil modified with a non-targeting phage fusion protein stabilized the tumor burden (p<0.005, compared to targeted phage-Doxil) (Figure 2A1).
Figure 2. Antitumor activity of Doxil and phage-Doxil.
(A) Tumor volume was estimated using a caliper. Tumor volume changes following the treatment in (A1) subcutaneous and (A2)orthotopic MCF-7 tumor-bearing nude mice. % Tumor volume from day 0 = (Tumor volumeafter treatment) / (Tumor volume at day 0) ×100; (B) Determination of tumor volume at the endpoint by MRI imaging with subcutaneous xenografts. (B1) The representative set of regular MRI images of tumors untreated and treated with Doxil, non-targeted phage-Doxil and MCF-7 targeted phage-Doxil. (B2) Tumor volume. (B3) % Tumor growth inhibition, defined as the difference between the tumor volume of the untreated group and the tumor volume of the treated group divided by the tumor volume in untreated group ×100. A one-way ANOVA was followed by LSD post hoc tests. Mean ± SEM, n=3-5. * p<0.05; ** p<0.005.
3.4 Evaluation of antitumor efficacy of MCF-7-targeted phage-Doxil on orthotopic xenografts using caliper-based estimation
The antitumor activity of phage-Doxil was also investigated using orthotopic MCF-7 tumor xenografts (Figure S1B). After treatment was initiated at a mean tumor size of 250 mm3, MCF-7-targeted phage-Doxil consistently induced a faster and stronger tumor reduction with a mean tumor volume decrease of ~ 16% at day 5; ~ 28% between day 7 and 12; and up to 35% between day 15 and 39, (p<0.005, between day 7 to day 39, compared to untreated mice). Even after therapy was discontinued, the tumor volume remained low for a month; Tumor volume reduction was between 25% and 35%. Mice in the untreated group had continued tumor growth from ~28% at day 7 to ~51% at day 40. In addition, the treatment with both non-modified LipoDox and LipoDox modified with a non-targeting phage fusion protein led to modest tumor growth inhibition with a slight tumor volume increase of ~10% within the 40-day observation period. Tumor remission in MCF-7-targeted phage Doxil treated mice was differed significantly from that of both LipoDox and non-targeted phage Doxil treated mice between days 10 and 33 (p<0.05) (Figure 2A2).
3.5. Evaluation of antitumor efficacy of MCF-7-targeted phage-Doxil on subcutaneous xenografts using MRI imaging
To independently evaluate tumor sizes, magnetic resonance imaging (MRI) was performed to acquire a series of multislice MRI images to determine tumor morphology as well as tumor volume. Figure 2B1 shows a representative set of regular MRI images. For calculation of the whole tumor volume, we summed the total tumor area from selected regions of interest (ROI) of each tumor slice for a consecutive set of tumor images. The results revealed markedly smaller tumors with treatment with MCF-7-targeted phage-Doxil compared with those from untreated mice and with non-targeted Doxil treatments. The mean tumor volumes were 510 ± 49 mm3 (untreated), 450 ± 21 mm3 (Doxil), 432 ± 77 mm3 (non-targeted phage-Doxil) and 314 ± 21mm3 (MCF-7-targeted phage-Doxil) (Figure 2B2), corresponding to tumor growth inhibitions of 11.6%, 15.2% and 38.4%, respectively (Figure 2B3). The targeted phage-Doxil treatment effect was clearly greater than with Doxil alone or non-targeted phage-Doxil (p<0.05).
3.6. Necrosis induced by MCF-7-targeted phage-Doxil
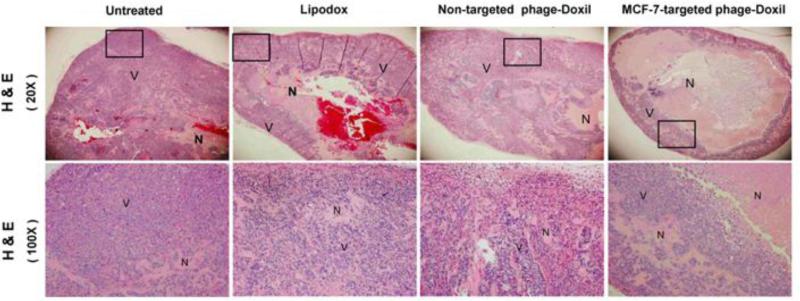
To confirm the antitumor efficacy induced by this targeted therapy, tumor sections were subjected to H&E staining, which has demonstrated that tumors from all drug-treated groups had increased areas with eosinophillic cytosol (pink) accompanied with the absence of hemotoxylin-stained nuclei (blue) when compared to the untreated group, indicating induction of necrosis by drug treatment. Notably, MCF-7-targeted phage-Doxil-treatment induced remarkable necrosis in the tumors, which contained the most extensive necrotic centers and the least viable tumor cells only existing in the tumor boundary. Conversely, much more viable cells heterogeneously appeared throughout the whole tumor sections in the groups treated with non-targeted formulations (Figure 3).
Figure 3. The confirmation of antitumor activity by H & E staining.
Representative images of tumor sections. Necrotic cells (N) showing eosinophillic cytosol (pink) accompanied by the absence of hemotoxylin-stained nuclei (blue); viable cells (V) showing eosinophillic cytosol (pink) accompanied by hemotoxylin-stained nuclei (blue).
3.7. Improved drug tumor delivery by MCF-7-targeted phage-Doxil
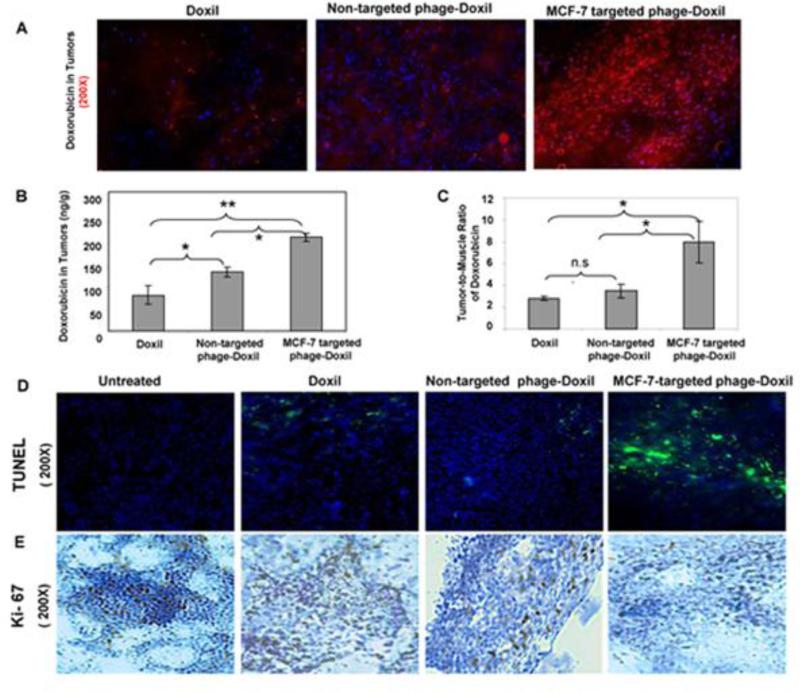
Doxorubicin biodistribution at 24h post-injection was analyzed to investigate whether or not the enhanced antitumor activity is attributable to preferential tumor delivery of doxorubicin. The fluorescence images clearly showed that the targeted group had the most intensive red fluorescence derived from doxorubicin, and noticeable co-localization of red doxorubicin fluorescence with DAPI-stained blue nuclei, suggesting effective nuclear targeting of the drug (Figure 4A). Doxorubicin quantitative analysis showed that the targeted phage-Doxil by 2-fold increased tumor accumulation of the drug compared to Doxil (p<0.005), and 1.4-fold increase compared to non-targeted phage-Doxil (p<0.05) (Figure 4B). Also, the tumor-selective delivery of doxorubicin was improved by the targeted therapy with tumor-to-muscle ratio of 8, compared to tumor-to-muscle ratio of 2.8 for Doxil and of 3.5 for non-targeted phage-Doxil (p<0.05) (Figure 4C).
Figure 4. Enhancement in tumor delivery of the drug, apoptosis, and anti-proliferative activity by MCF-7 targeted phage-Doxil.
(A) Tumor accumulation of doxorubicin observed by fluorescence microscopy, Red fluorescence: Doxorubicin; Blue fluorescence: DAPI-staining nuclei. (B) Quantification of doxorubicin tumor deposition expressed by ng doxorubicin per g of tumor tissue. (C) Tumor-to-muscle ratio of doxorubicin. (D) TUNEL staining of tumor sections. (E) Immunohistochemical staining of the Ki-67 proliferation marker on the tumor sections.
3.8. Enhanced apoptosis by MCF-7-targeted phage-Doxil
Improved tumor delivery of doxorubicin led to an enhanced apoptosis of cancer cells. While apoptosis was undetected in the untreated MCF-7-xenografted mice, apoptotic cells were observed in all drug-treated groups as indicated by the green fluorescence-staining nuclei (TUNEL-positive cells). Treatment with targeted phage-Doxil clearly produced more pronounced apoptotic cells than the treatment with the non-targeted formulations (Figure 4D).
3.9. Inhibition proliferation by MCF-7-targeted phage-Doxil
Improved tumor delivery of doxorubicin inhibited also the proliferation of cancer cells. Immunohistochemical examination of tumor sections associated with proliferation marker-Ki67 clearly indicated that a greater number of actively proliferating tumor cells existed in tumor sections from the untreated and non-targeted groups, but tumors treated with MCF-7 targeted phage-Doxil had the lowest expression of Ki67 antigen (Figure 4E).
3.10. Assessment of potential toxicity of MCF-7-targeted phage-Doxil
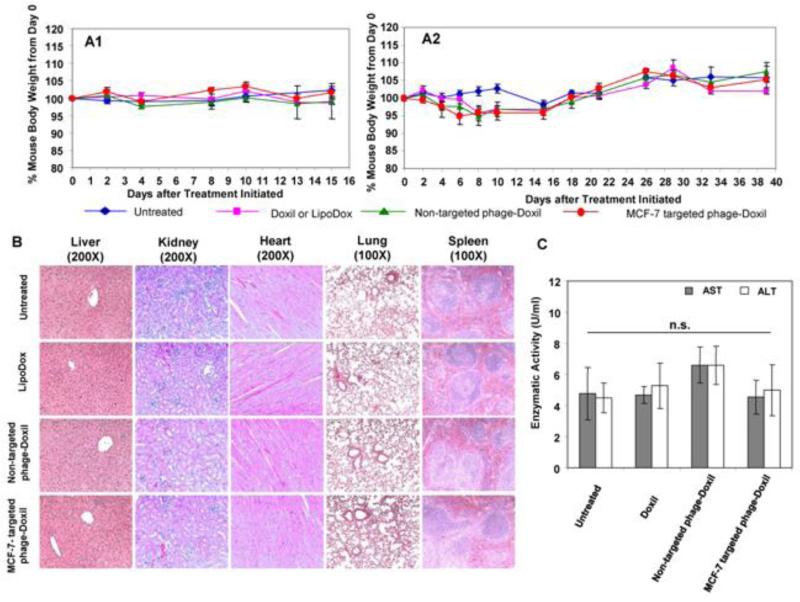
To assess the potential for adverse effects associated with treatments, mice were observed for changes in their body weight and appetite, for diarrhea and abnormal behavior over the course of treatments. Neither untreated nor drug-treated mice had significant changes in body weight (Figure 5A1, A2) and should no abnormalities in appetite and behavior.
Figure 5. Evaluation of potential side-effects of Doxil (or LipoDox) and Phage-Doxil.
(A) Body weight of mice following treatment in subcutaneous (A1), and orthotopic MCF-7 xenografts (A2). (B) Histology examination of tissue sections of vital organs following treatment. (C) The effect of treatment on liver enzyme activity of mice. AST: Aspartate Aminotransaminase Activity; ALT: Alanine Transaminase Activity. A one-way ANOVA was followed by LSD post hoc test to analyze the statistic. Mean ± SEM, n=3-5. * p >0.05.
Additionally, treatments with drug formulations produced no apparent histological changes based on the tissue sections of liver, kidney, heart, lung and spleen in comparison with those from untreated mice. H&E staining of the liver tissues revealed normal hepatocytes, central veins, portal triads and liver lobules. Striated cardiac muscles with the centrally placed nucleus were observed in heart sections. Kidney samples contained normal Bowman's capsule surrounding glomeruli as well as convoluted tubules. Normal alveoli without the sign of pulmonary fibrosis were seen in the lung sections. Red pulp and white pulp appeared in spleen samples (Figure 5B). Moreover, mice in all groups had normal ALT and AST levels (Figure 5C). Together, these results were consistent with the overall health of mice.
4. Discussion
Clinical application of targeting peptides has proven useful in the diagnosis and treatment of a variety of cancers (21). Phage display libraries can serve as powerful reservoirs for the screening and identification of tumor-targeted peptides (22, 23). Originally, we isolated a MCF-7 cancer cell-specific peptide fused to the N-terminal of a phage major coat protein, PVIII (13, 24). The membraneophilic property of the PVIII phage major coat protein was used to incorporate the phage-derived fusion protein into the liposomal bilayer without additional chemical conjugation.
In previous studies, wild-type phage major coat proteins were shown to readily translocate into liposomes and span the phospholipid bilayer (25-28). Its N-terminal amphipathic helix rests on the outer surface of the lipid bilayer membrane, while the 35-Å-long transmembrane helix spans the phospholipid bilayer (29). Since this “fusion phage” is produced as a result of an in-frame insertion of a foreign gene encoding for a targeting peptide into the gene of the phage major coat protein pVIII (30, 31), it was expected that even with the expression of the short MCF-7-targeted peptide fused to the N-terminal of the major coat protein, the resultant MCF-7 phage fusion protein would retain its membraneophilic property, translocate into the liposomal bilayer and make such liposomes targeted. Our results showed that the fusion of the MCF-7-specific targeting peptide to the phage coat protein changed its physicochemical properties only slightly. The MCF-7-specific phage fusion proteins self-assemble with doxorubicin-loaded liposomes and form spherical nanoparticles with a slight increase in size. Moreover, our early results showed that this targeted phage-Doxil improved MCF-7 cancer cell-binding and tumor cell killing (13, 18, 32).
To further evaluate the efficacy and potential toxicity of MCF-7-targeted phage-Doxil in vivo, both subcutaneous and orthotopic xenografts of human breast cancer were used in this study. The tumor response to therapy was evaluated by monitoring the dynamic change of tumor volume using both caliper measurement and MRI imaging (33). The results clearly indicated that treatment with MCF-7-targeted phage-Doxil produced a significant tumor remission compared to control non-targeted formulation in both subcutaneous and orthotopic tumor xenografts. The time to the onset of tumor reduction triggered by the targeted phage-Doxil was also shorter than that by non-targeted groups. Remarkably, we observed extensive necrotic cores with the lowest number of viable tumor cells remaining in the tumors treated with targeted therapy, further confirming the significantly enhanced antitumor activity induced by MCF-7 targeted phage-Doxil.
The enhanced antitumor activity correlates well with the improved tumor accumulation and nuclear delivery of the drug by the MCF-7-targeted phage-Doxil, suggesting that the integration of passive targeting of liposomal nanocarriers with tumor cell recognition and /or cell-internalizing systems represents a promising strategy for targeted tumor delivery of anti-cancer drugs in vivo (34). Liposomal carriers have proved to demonstrate beneficial pharmacokinetics/biodistribution of doxorubicin and, accordingly, reduced toxicity to normal organs (6, 35-37). Surface modification of liposomes with targeting ligands could further favor intratumoral distribution and intracellular delivery of drugs (7). The MCF-7 specific phage fusion proteins have been demonstrated not only to specifically recognize MCF-7 breast cancer cells, but also to facilitate the internalization into MCF-7 cells (13, 38). Their incorporation into liposomes promoted both tumor cell targeting and cytoplasmic delivery of liposome-carrying cargo in vitro (13, 17, 18). Consistently, the in vivo data further verified that MCF-7 targeted phage-Doxil improved both tumor localization and intracellular nuclear delivery of doxorubicin, and consequently demonstrated an enhanced antitumor activity (39).
Apoptosis and cell proliferation are commonly clinical indicators for the assessment of tumor prognosis and tumor response to therapy. A correlation between the proliferation index and malignancy has been recognized for many tumor types, including breast cancer (40). Our early in vitro results have showed that targeted phage-Doxil induced more pronounced apoptosis in MCF-7 cells (13). In the current animal study, the incidence of apoptosis and proliferation in MCF-7 breast cancer tumors suggests that the treatment by targeted phage-Doxil augmented the negative impact on the growth and survival of the breast tumors. Enhanced apoptosis combined with the reduced proliferation rate was correlated with the extensive tumor necrosis in the xenografted breast cancer tumors treated with MCF-7-targeted phage-Doxil and, accordingly, can be said to have contributed to the enhanced antitumor activity.
Plasma ALT and AST levels are common markers for liver disorders and for the assessment of liver injury. Animals treated with MCF-7 phage-Doxil had a normal serum ALT and AST activity when compared with the untreated control, suggesting that MCF-7 phage-Doxil produced no detectable hepatotoxicity (41). Consistently, histological examination of drug-treated vital organs showed no pathological change. The mice also showed no loss of body weight or behavior deficits during the study period, indicative of an absence of serious toxicity.
Overall, this study suggests that MCF-7-targeted phage-Doxil have the potential to serve as an active and tolerable chemotherapy for breast cancer treatment. MCF-7-targeted phage-Doxil reduced tumor volume and enhanced antitumor activity compared with non-targeted formulations without apparent toxicity.
Supplementary Material
Acknowledgments
Statements of funding: This work was supported by NIH grant # R01 CA125063-01 and the Animal Health and Disease Research grant 2006-9, College of Veterinary Medicine, Auburn University to Valery A. Petrenko and by NIH grant #1U54CA151881 to Vladimir P. Torchilin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Hutchinson L. Breast cancer: challenges, controversies, breakthroughs. Nat Rev Clin Oncol. 2010;7:669–670. doi: 10.1038/nrclinonc.2010.192. [DOI] [PubMed] [Google Scholar]
- 2.Singaland N PK. Iliskovic. Doxorubicin-induced cardiomyopathy. The New England journal of medicine. 1998;339:900–905. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 3.Lammers T, Hennink WE, Storm G. Tumour-targeted nanomedicines: principles and practice. Br J Cancer. 2008;99:392–397. doi: 10.1038/sj.bjc.6604483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drummond DC, Meyer O, Hong K, Kirpotin DB, Papahadjopoulos D. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol Rev. 1999;51:691–743. [PubMed] [Google Scholar]
- 5.Hofheinz RD, Gnad-Vogt SU, Beyer U, Hochhaus A. Liposomal encapsulated anti-cancer drugs. Anticancer Drugs. 2005;16:691–707. doi: 10.1097/01.cad.0000167902.53039.5a. [DOI] [PubMed] [Google Scholar]
- 6.Harris L, Batist G, Belt R, Rovira D, Navari R, Azarnia N, Welles L, Winer E. Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer. 2002;94:25–36. doi: 10.1002/cncr.10201. [DOI] [PubMed] [Google Scholar]
- 7.Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, Marks JD, Benz CC, Park JW. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66:6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 8.Gabizon A, Shmeeda H, Horowitz AT, Zalipsky S. Tumor cell targeting of liposome-entrapped drugs with phospholipid-anchored folic acid-PEG conjugates. Adv Drug Deliv Rev. 2004;56:1177–1192. doi: 10.1016/j.addr.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Petrenko V. Evolution of phage display: from bioactive peptides to bioselective nanomaterials. Expert Opin Drug Deliv. 2008;5:825–836. doi: 10.1517/17425247.5.8.825. [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Choi SP, La S, Seo JS, Kim KK, Nam SH, Kwon B. Constitutive expression of 4-1BB on T cells enhances CD4+ T cell responses. Exp Mol Med. 2003;35:509–517. doi: 10.1038/emm.2003.66. [DOI] [PubMed] [Google Scholar]
- 11.Cortese R, Monaci P, Luzzago A, Santini C, Bartoli F, Cortese I, Fortugno P, Galfre G, Nicosia A, Felici F. Selection of biologically active peptides by phage display of random peptide libraries. Curr Opin Biotechnol. 1996;7:616–621. doi: 10.1016/s0958-1669(96)80072-3. [DOI] [PubMed] [Google Scholar]
- 12.Krumpeand LR, Mori T. The Use of Phage-Displayed Peptide Libraries to Develop Tumor-Targeting Drugs. Int J Pept Res Ther. 2006;12:79–91. doi: 10.1007/s10989-005-9002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang T, D'Souza GG, Bedi D, Fagbohun OA, Potturi LP, Papahadjopoulos- Sternberg B, Petrenko VA, Torchilin VP. Enhanced binding and killing of target tumor cells by drug-loaded liposomes modified with tumor-specific phage fusion coat protein. Nanomedicine (Lond) 2010;5:563–574. doi: 10.2217/nnm.10.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayanna PK, Bedi D, Gillespie JW, DeInnocentes P, Wang T, Torchilin VP, Bird RC, Petrenko VA. Landscape phage fusion protein-mediated targeting of nanomedicines enhances their prostate tumor cell association and cytotoxic efficiency. Nanomedicine : nanotechnology, biology, and medicine. 2010;6:538–546. doi: 10.1016/j.nano.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang T, Petrenko VA, Torchilin VP. Paclitaxel-loaded polymeric micelles modified with MCF-7 cell-specific phage protein: enhanced binding to target cancer cells and increased cytotoxicity. Mol Pharm. 2010;7:1007–1014. doi: 10.1021/mp1001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang T, Kulkarni N, Bedi D, D'Souza GG, Papahadjopoulos-Sternberg B, Petrenko VA, Torchilin VP. In vitro optimization of liposomal nanocarriers prepared from breast tumor cell specific phage fusion protein. J Drug Target. 2011;19:597–605. doi: 10.3109/1061186X.2010.550920. [DOI] [PubMed] [Google Scholar]
- 17.Wang T, Kulkarni N, D'Souza GG, Petrenko VA, Torchilin VP. On the mechanism of targeting of phage fusion protein-modified nanocarriers: only the binding peptide sequence matters. Mol Pharm. 2011;8:1720–1728. doi: 10.1021/mp200080h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang T, Yang S, Petrenko VA, Torchilin VP. Cytoplasmic delivery of liposomes into MCF-7 breast cancer cells mediated by cell-specific phage fusion coat protein. Mol Pharm. 2010;7:1149–1158. doi: 10.1021/mp1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.S.T. Brigati JR, Jayanna PK, Petrenko VA, editors. Phage display for generating peptide reagents. John Wiley & Sons, Inc; New Jersey: 2008. [DOI] [PubMed] [Google Scholar]
- 20.Deleageand G, Roux B. An algorithm for protein secondary structure prediction based on class prediction. Protein Eng. 1987;1:289–294. doi: 10.1093/protein/1.4.289. [DOI] [PubMed] [Google Scholar]
- 21.Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev. 2003;24:389–427. doi: 10.1210/er.2002-0007. [DOI] [PubMed] [Google Scholar]
- 22.Aina OH, Liu R, Sutcliffe JL, Marik J, Pan CX, Lam KS. From combinatorial chemistry to cancer-targeting peptides. Mol Pharm. 2007;4:631–651. doi: 10.1021/mp700073y. [DOI] [PubMed] [Google Scholar]
- 23.Mori T. Cancer-specific ligands identified from screening of peptide-display libraries. Curr Pharm Des. 2004;10:2335–2343. doi: 10.2174/1381612043383944. [DOI] [PubMed] [Google Scholar]
- 24.Wang T, Petrenko VA, Torchilin VP. Optimization of Landscape Phage Fusion Protein-Modified Polymeric Peg-Pe Micelles for Improved Breast Cancer Cell Targeting. J Nanomedic Nanotechnol. 2012;S4:008. doi: 10.4172/2157-7439.S4-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soekarjo M, Eisenhawer M, Kuhn A, Vogel H. Thermodynamics of the membrane insertion process of the M13 procoat protein, a lipid bilayer traversing protein containing a leader sequence. Biochemistry. 1996;35:1232–1241. doi: 10.1021/bi951087h. [DOI] [PubMed] [Google Scholar]
- 26.Kuhn A. Major coat proteins of bacteriophage Pf3 and M13 as model systems for Sec-independent protein transport. FEMS Microbiol Rev. 1995;17:185–190. doi: 10.1111/j.1574-6976.1995.tb00201.x. [DOI] [PubMed] [Google Scholar]
- 27.Kieferand D, Kuhn A. Hydrophobic forces drive spontaneous membrane insertion of the bacteriophage Pf3 coat protein without topological control. Embo J. 1999;18:6299–6306. doi: 10.1093/emboj/18.22.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stopar D, Spruijt RB, Hemminga MA. Anchoring mechanisms of membrane-associated M13 major coat protein. Chem Phys Lipids. 2006;141:83–93. doi: 10.1016/j.chemphyslip.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 29.Spruijt RB, Wolfs CJ, Hemminga MA. Aggregation-related conformational change of the membrane-associated coat protein of bacteriophage M13. Biochemistry. 1989;28:9158–9165. doi: 10.1021/bi00449a030. [DOI] [PubMed] [Google Scholar]
- 30.Petrenko VA, Smith GP, Gong X, Quinn T. A library of organic landscapes on filamentous phage. Protein Eng. 1996;9:797–801. doi: 10.1093/protein/9.9.797. [DOI] [PubMed] [Google Scholar]
- 31.Kuzmicheva GA, Jayanna PK, Sorokulova IB, Petrenko VA. Diversity and censoring of landscape phage libraries. Protein Eng Des Sel. 2009;22:9–18. doi: 10.1093/protein/gzn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang T. Landscape phage fusion protein-modified pharmaceutical nanocarriers for targeted and cytoplasmic delivery of chemotherapeutics to breast cancer cells in vitro and in vivo. Pharmaceutical Science Dissertations. 2012 [Google Scholar]
- 33.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 34.Noble CO, Kirpotin DB, Hayes ME, Mamot C, Hong K, Park JW, Benz CC, Marks JD, Drummond DC. Development of ligand-targeted liposomes for cancer therapy. Expert Opin Ther Targets. 2004;8:335–353. doi: 10.1517/14728222.8.4.335. [DOI] [PubMed] [Google Scholar]
- 35.Huober J, Fett W, Nusch A, Neise M, Schmidt M, Wischnik A, Gerhardt S, Goehler T, Luck HJ, Rost A. A multicentric observational trial of pegylated liposomal doxorubicin for metastatic breast cancer. BMC Cancer. 2010;10:2. doi: 10.1186/1471-2407-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, Catane R, Kieback DG, Tomczak P, Ackland SP, Orlandi F, Mellars L, Alland L, Tendler C. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15:440–449. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 37.Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies. Clin Pharmacokinet. 2003;42:419–436. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 38.Fagbohun OA, Bedi D, Grabchenko NI, Deinnocentes PA, Bird RC, Petrenko VA. Landscape phages and their fusion proteins targeted to breast cancer cells. Protein Eng Des Sel. 2012;25:271–283. doi: 10.1093/protein/gzs013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park JW. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. 2002;4:95–99. doi: 10.1186/bcr432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colozza M, Azambuja E, Cardoso F, Sotiriou C, Larsimont D, Piccart MJ. Proliferative markers as prognostic and predictive tools in early breast cancer: where are we now? Ann Oncol. 2005;16:1723–1739. doi: 10.1093/annonc/mdi352. [DOI] [PubMed] [Google Scholar]
- 41.Lynch ED, Gu R, Pierce C, Kil J. Combined oral delivery of ebselen and allopurinol reduces multiple cisplatin toxicities in rat breast and ovarian cancer models while enhancing anti-tumor activity. Anticancer Drugs. 2005;16:569–579. doi: 10.1097/00001813-200506000-00013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.