Abstract
Background
B-cell activating factor of the TNF family (BAFF) promotes the maturation and survival of B cells. Because BAFF levels are elevated in systemic lupus erythematosus (SLE) patients, BAFF has been the target of emerging therapies for SLE, such as belimumab. Levels of BAFF and its receptors in discoid lupus erythematosus (DLE) patients are unknown.
Objective
To compare skin and blood mRNA and protein levels of BAFF and its receptors BAFF-R, TACI, and BCMA in DLE subjects with (DLE+/SLE+ (N=28)) and without SLE (DLE+/SLE− (N=35)), psoriasis subjects (N=11), and normal subjects (N=42).
Methods
We used quantitative real-time PCR to measure blood and skin BAFF, BAFF-R, TACI, and BCMA mRNA, sandwich ELISAs to measure sera BAFF, and immunohistochemistry to evaluate BAFF and BAFF-R skin protein expression.
Results
BAFF mRNA and protein levels were highest in DLE+/SLE+ blood, followed by DLE+/SLE−, psoriasis, and normal blood. BAFF protein also correlated with anti-nuclear antibodies, and autoantibodies against double-stranded DNA, single-stranded DNA, and ribonucleoprotein, and Systemic Lupus Erythematosus Disease Activity Index scores in DLE patients. While showing no difference between DLE+/SLE+ and DLE+/SLE− skin, BAFF and its receptors mRNA were up-regulated in DLE skin versus normal and psoriasis skin. DLE skin had higher percentages of BAFF-R+ inflammatory cells, likely T cells and macrophages, than psoriasis and normal skin.
Conclusions
BAFF may be a serologic marker of systemic disease in DLE patients. BAFF and its receptors are elevated in DLE skin, suggesting that targeted therapies against these proteins could treat refractory DLE patients.
Keywords: discoid lupus erythematosus, systemic lupus erythematosus, BAFF, BAFF-R, T cell, macrophage
1. Introduction
B-cell activating factor (BAFF) aids in B cell survival and homeostasis by interacting with three receptors, BAFF-receptor (BAFF-R), transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI), and B cell maturation antigen (BCMA) [1]. Binding of BAFF and BAFF-R, the predominant BAFF receptor in human B cells [2], facilitates the maturation of immature B cells to naïve B cells [3]. BAFF-TACI binding can enhance survival of activated B cells and IgM production [4]. BCMA can enhance B cell antigen presentation [5].
Systemic lupus erythematosus (SLE) patients have elevated BAFF levels [6–8], which drive abnormal B cell activation [9]. These studies prompted development of belimumab, a monoclonal antibody against BAFF, to treat SLE. Clinical improvement in SLE patients with mucocutaneous manifestations taking belimumab [10] prompted investigation of their lesional skin, which displayed up-regulation of BAFF mRNA [11].
Thus, we compared BAFF protein and mRNA levels in the blood and skin of discoid lupus erythematosus (DLE) patients, who were subdivided into those with (DLE+/SLE+) and without SLE (DLE+/SLE−), versus those in psoriasis and normal patients. We examined mRNA levels of BAFF-R, TACI, and BCMA in DLE, psoriasis, and normal skin and blood, and skin protein levels of BAFF-R. We hypothesized that mRNA and protein levels of BAFF and its receptors would be increased in DLE patients.
2. Materials and Methods
2.1. Patient population
63 DLE patients, 11 psoriasis patients, and 42 gender- and age-matched normal controls gave informed consent in outpatient dermatology and rheumatology clinics at University of Texas Southwestern (UTSW) Medical Center and Parkland Health and Hospital System in Dallas, TX. The study was approved by the UTSW Institutional Board and complied with Declaration of Helsinki Principles. Patients with DLE were divided into two groups, depending on presence or absence of systemic lupus erythematosus (SLE). SLE was defined by meeting at least four American College of Rheumatology SLE diagnostic criteria [12, 13]. Healthy controls were excluded if they had a history of autoimmune diseases. Clinical information was collected from each patient, who additionally underwent blood draws, skin biopsies, and/or cutaneous surgeries. Blood was collected in serum separator tubes (DLE (N=63), psoriasis (N=11), normal (N=32)) and/or PAXgene tubes (DLE (N=55), psoriasis (N=11), normal (N=17)) (PreAnalytiX, Hombrechtikon, Switzerland). 6 mm skin biopsies taken from lesional skin (DLE (N=20), psoriasis (N=5)) or normal skin (N=10) were stored in RNALater Solution (Ambion, Austin, TX). A subset was bisected and transferred to 10 % formalin (DLE (N=14), psoriasis (N=4), normal (N=6)). Sun-exposed sites (e.g. head, neck, upper body) were favored for DLE, psoriasis, and normal skin.
2.2. Quantitative real-time PCR (qRT-PCR)
Skin and blood RNA were isolated using RNeasy Lipid Tissue Mini kit (Qiagen, Hilden, Germany) and PAXgene blood RNA system kit (PreAnalytiX), respectively. RNA was reverse transcribed into cDNA using the iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA). We amplified cDNA of GAPDH, BAFF, BAFF-R, BCMA, and TACI using forward and reverse primers (Supplemental Table 1) and SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA), per the manufacturers’ instructions. Multiple qRT-PCR cycles were performed in a CFX96 qRT-PCR machine (Bio-Rad) with the following cycling variables: 3 mins at 95°C, then 40 cycles of 20 secs at 95°C, 1 min at 55°C, and 30 secs at 72°C. Cycle threshold (CT) values were standardized to the housekeeping gene GAPDH, and converted to fold change using the 2−ΔΔCT formula [14].
2.3. Immunoassays
We measured sera BAFF protein levels using commercially available sandwich enzyme-linked immunosorbant assay (ELISA) kits (R&D Systems, Minneapolis, MN). ELISAs were also performed to assess IgG anti-nuclear antibodies (ANAs), anti-double-stranded DNA (dsDNA) antibodies, anti-ribonucleoprotein (RNP) antibodies (INOVA Diagnostics, Inc., San Diego, CA), anti-single-stranded DNA (ssDNA) antibodies (ORGENTEC Diagnostika, Mainz-Germany)), total IgG, and total IgM (eBiosciences, San Diego, CA), according to manufacturers’ instructions. Concentrations were extrapolated from standard curves. Fluorescent immunoassays using QUANTA Plex™ (Luminex®) kits (INOVA Diagnostics, Inc.) were executed to measure anti-SS-A (52 kDa), -SS-A (60 kDa), -SS-B, -Smith (Sm), and -Scl-70 IgG autoantibodies.
Immunohistochemistry
Formalin-fixed paraffin-embedded tissues [15] were sectioned at four microns and mounted on adhesive slides. After drying, the slides were deparaffinized in xylene and rehydrated in graded alcohols to distilled water. Endogenous peroxidase activity was quenched for 10 minutes at room temperature, using 0.3 % H2O2 and 0.1 % sodium azide.
For BAFF, BAFF-R, CD3, CD20, and CD163 immunohistochemistry, for epitope retrieval, slides were placed in 0.25 M Tris base buffer, pH 9.0, in a pressure cooker (BAFF) [16] or 1 mM EDTA, pH 8.5, for 30 minutes in a steamer, followed by a 10 minute cool-down time (BAFF-R, CD3, CD20, CD163). After PBS rinse, incubation with primary antibody (rat monoclonal anti-BAFF IgM antibody (GenWay Biologics, San Diego, CA); mouse monoclonal anti-BAFF-R antibody (Abcam, Cambridge, MA); rabbit monoclonal anti-CD3 antibody (Neomarkers/Thermo Fisher Scientific, Fremont, CA); mouse monoclonal anti-CD20 antibody (Leica Novocastra, Buffalo Grove, IL); and mouse monoclonal anti-CD163 antibody (Neomarkers/Thermo Fisher Scientific) or isotype control was performed for 50 minutes at 25°C [17]. Following PBS rinse, slides were incubated with horseradish peroxidase-conjugated goat anti-rat IgM antibody (Southern Biotech, Birmingham, AL), for 60 minutes at 37°C, or anti-mouse or anti-rabbit horseradish peroxidase-conjugated IgG antibody (Leica Novocastra) [18] for 45 minutes at 25°C [17]. Finally, the slides were immersed for 8 minutes in 25°C diaminobenzidine (Invitrogen, Carlsbad, CA), enhanced with 0.5 % copper sulfate in PBS for 1–3 minutes at 25°C, counterstained in hematoxylin, dehydrated in graded alcohols, cleared in xylene, and coverslipped.
Two independent evaluators (BFC, GAH) assessed immunoreactivity based on a three-point scale (1 (mild), 2 (moderate), 3 (marked)) of staining intensity in inflammatory cells, epidermis, follicles, sebaceous glands, and eccrine glands. Percentages of inflammatory cells with positive staining were estimated by a three-point scale (1: <10 %, 2: 10–50 %, 3: >50 %). Gradings from both investigators were averaged and compared.
2.4. Statistical analysis
Since this was a pilot study, sample size was not calculated. Skin and blood mRNA and protein expressions were compared using Kruskal-Wallis tests with Dunn’s multiple comparison post hoc tests. Correlations were carried out by calculating Spearman’s correlation coefficients.
3. Results
3.1. Patients
63 DLE (28 DLE+/SLE+, 35 DLE+/SLE−) patients, 11 psoriasis patients, and 42 normal controls were enrolled. Tables 1 (blood) and 2 (skin) summarize their demographics and disease information.
Table 1.
Subject demographic data (blood)
| DLE+/SLE+ | DLE+/SLE− | Psoriasis | Normal | |
|---|---|---|---|---|
| N | 28 | 35 | 11 | 32 |
| Age at visit, yr (SD) | 45 (13) | 45 (11) | 53 (10) | 43 (11) |
| Gender (M/F) | 5/23 | 7/28 | 2/9 | 7/35 |
| Ethnicity, No. (%) | ||||
| Caucasian | 6 (21%) | 8 (23%) | 8 (73%) | 16 (38%) |
| African American | 15 (54%) | 23 (66%) | 0 (0%) | 22 (52%) |
| Hispanic | 4 (14%) | 3 (9%) | 2 (18%) | 4 (10%) |
| Asian | 3 (11%) | 0 (0%) | 1 (9%) | 0 (0%) |
| Other | 0 (0%) | 1 (3%) | 0 (0%) | 0 (0%) |
| CLASI activity score, mean (SD) | 9 (9) | 6 (5) | N/A | N/A |
| CLASI damage score, mean (SD) | 12 (12) | 8 (5) | N/A | N/A |
| SLEDAI score, mean (SD) | 3 (3) | 1 (2) | N/A | N/A |
| PASI score, mean (SD) | N/A | N/A | 5 (4) | N/A |
| Disease Duration, yr (SD) | 9 (12) | 8 (10) | 10 (11) | N/A |
| Lupus medications at study visit, No. (%) | ||||
| Topical/Intralesional Corticosteroids | 12 (43%) | 18 (51%) | 3 (27%) | N/A |
| Topical Immunomodulators | 2 (7%) | 2 (6%) | 0 (0%) | N/A |
| Hydroxychloroquine | 16 (57%) | 18 (51%) | 0 (0%) | N/A |
| Chloroquine | 3 (11%) | 3 (9%) | 0 (0%) | N/A |
| Quinacrine | 2 (7%) | 4 (11%) | 0 (0%) | N/A |
| Methotrexate | 0 (0%) | 2 (6%) | 0 (0%) | N/A |
| Prednisone | 9 (32%) | 1 (3%) | 0 (0%) | N/A |
| Mycophenolate mofetil | 7 (25%) | 1 (3%) | 0 (0%) | N/A |
| Acitretin | 0 (0%) | 0 (0%) | 1 (9%) | N/A |
| Ustekinumab | 0 (0%) | 0 (0%) | 1 (9%) | N/A |
| None | 0 (0%) | 8 (24%) | 8 (73%) | N/A |
| SLE criteria, No. (%)a | ||||
| Malar rash | 12 (43%) | 1 (3%) | N/A | N/A |
| Discoid rash | 28 (100%) | 35 (100%) | N/A | N/A |
| Photosensitivity | 25 (89%) | 24 (69%) | N/A | N/A |
| Oral ulcers | 12 (43%) | 5 (14%) | N/A | N/A |
| Arthritis | 16 (57%) | 4 (11%) | N/A | N/A |
| Serositis | 3 (11%) | 0 (0%) | N/A | N/A |
| Renal disorder | 6 (21%) | 0 (0%) | N/A | N/A |
| Hematological disorder | 19 (68%) | 4 (11%) | N/A | N/A |
| Anti-nuclear antibody | 27 (96%) | 8 (23%) | N/A | N/A |
| Immunological disorder | 21 (75%) | 0 (0%) | N/A | N/A |
| Mean # of SLE criteria (SD) | 6 (1) | 2 (1) | N/A | N/A |
None of the DLE patients had neurological disorder.
Abbreviations: CLASI = Cutaneous Lupus Disease Activity and Severity Index; DLE = discoid lupus erythematosus; PASI = Psoriasis Activity and Severity Index; SLE = Systemic Lupus Erythematosus; SLEDAI = Systemic Lupus Erythematosus Disease and Activity Index
Table 2.
Subject demographic data (skin)
| DLE+/SLE+ | DLE+/SLE− | Psoriasis | Normal | |
|---|---|---|---|---|
| N | 10 | 10 | 5 | 10 |
| Age at visit, yr (SD) | 46 (13) | 41 (11) | 58 (6) | 44 (8) |
| Gender (M/F) | 2/8 | 2/8 | 1/4 | 1/9 |
| Ethnicity, No. (%) | ||||
| Caucasian | 2 (20%) | 0 (0%) | 5 (100%) | 8 (80%) |
| African American | 6 (60%) | 8 (80%) | 0 (0%) | 2 (20%) |
| Hispanic | 2 (20%) | 2 (20%) | 0 (0%) | 0 (0%) |
| Asian | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Other | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| CLASI activity score, mean (SD) | 12 (12) | 8 (6) | N/A | N/A |
| CLASI damage score, mean (SD) | 14 (14) | 12 (5) | N/A | N/A |
| SLEDAI score, mean (SD) | 4 (3) | 0 (0) | N/A | N/A |
| PASI score, mean (SD) | N/A | N/A | 7 (5) | N/A |
| Disease Duration, yr (SD) | 10 (9) | 7 (8) | 5 (7) | N/A |
| Lupus medications at study visit, No. (%) | ||||
| Topical/Intralesional Corticosteroids | 5 (50%) | 7 (70%) | 2 (40%) | N/A |
| Topical Immunomodulators | 1 (10%) | 0 (0%) | 0 (0%) | N/A |
| Hydroxychloroquine | 4 (40%) | 5 (50%) | 0 (0%) | N/A |
| Chloroquine | 1 (10%) | 0 (0%) | 0 (0%) | N/A |
| Prednisone | 3 (30%) | 0 (0%) | 0 (0%) | N/A |
| Mycophenolate mofetil | 1 (10%) | 1 (10%) | 0 (0%) | N/A |
| Acitretin | 0 (0%) | 0 (0%) | 1 (20%) | N/A |
| None | 0 (0%) | 0 (0%) | 3 (60%) | N/A |
| SLE criteria, No. (%)a | ||||
| Malar rash | 4 (40%) | 0 (0%) | N/A | N/A |
| Discoid rash | 10 (100%) | 10 (100%) | N/A | N/A |
| Photosensitivity | 9 (90%) | 7 (70%) | N/A | N/A |
| Oral ulcers | 5 (50%) | 3 (30%) | N/A | N/A |
| Arthritis | 6 (60%) | 0 (0%) | N/A | N/A |
| Serositis | 2 (20%) | 0 (0%) | N/A | N/A |
| Renal disorder | 3 (30%) | 0 (0%) | N/A | N/A |
| Hematological disorder | 7 (70%) | 2 (20%) | N/A | N/A |
| Anti-nuclear antibody | 10 (100%) | 3 (30%) | N/A | N/A |
| Immunological disorder | 7 (70%) | 0 (0%) | N/A | N/A |
| Mean # of SLE criteria (SD) | 6 (1) | 3 (1) | N/A | N/A |
None of the DLE patients had neurological disorder.
Abbreviations: CLASI = Cutaneous Lupus Disease Activity and Severity Index; DLE = discoid lupus erythematosus; PASI = Psoriasis Activity and Severity Index; SLE = Systemic Lupus Erythematosus; SLEDAI = Systemic Lupus Erythematosus Disease and Activity Index
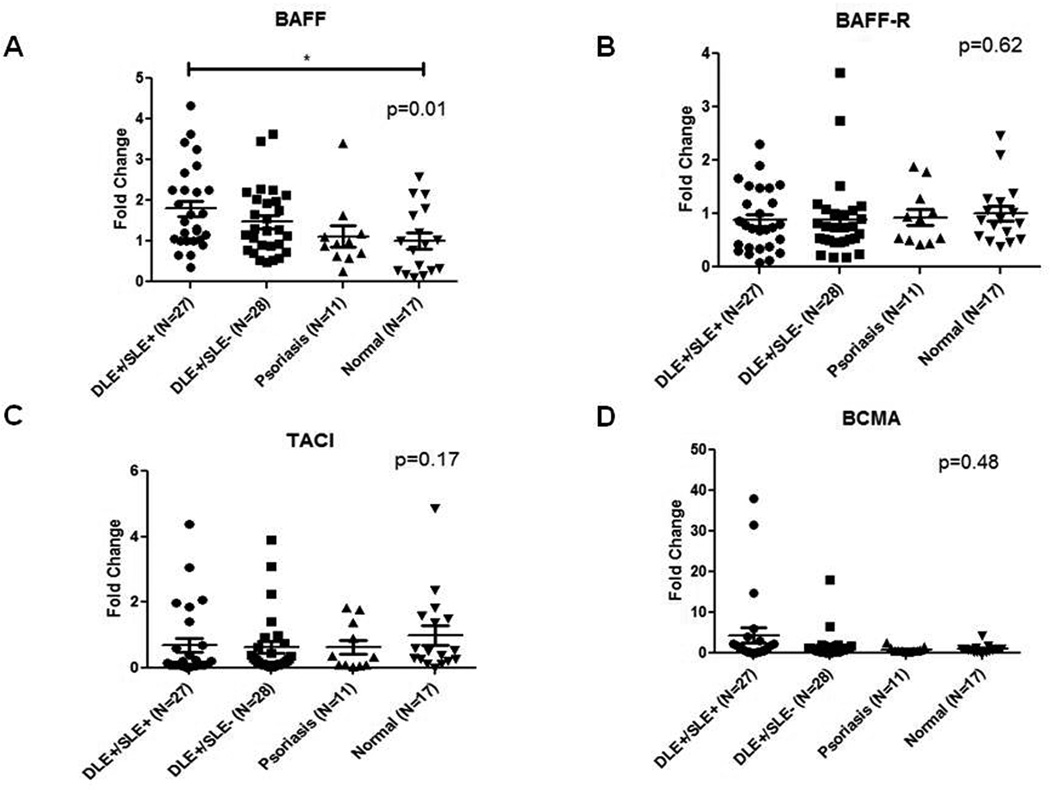
3.2. BAFF mRNA levels were highest in DLE+/SLE+ blood
Blood mRNA levels of BAFF, BAFF-R, TACI, and BCMA in 27 DLE+/SLE+, 28 DLE+/SLE−, 11 psoriasis, and 17 normal patients were evaluated by qRT-PCR. There were fewer mRNA than sera samples because of poor mRNA quality or sample unavailability. DLE+/SLE+ blood showed higher expression of BAFF mRNA than DLE+/SLE− blood (1.2-fold higher), psoriasis blood (1.6-fold higher) and normal blood (1.8-fold higher) (p=0.01) (Fig. 1A). The four patient groups did not differentially express BAFF-R, TACI, and BCMA (Fig. 1B–D).
Fig. 1.
mRNA levels of BAFF, but not its receptors, are elevated in DLE+/SLE+ blood versus DLE+/SLE−, psoriasis, and normal blood. Quantitative real-time PCR was performed to evaluate mRNA levels of BAFF (A), and its receptors, BAFF-R (B), TACI (C), and BCMA (D) in the whole blood of DLE+/SLE+ (N=27), DLE+/SLE− (N=28), psoriasis (N=11), and normal patients (N=17). We performed secondary analyses using Kruskal Wallis test. Capped bars between groups with astericks represent significant differences between groups based on Dunn’s test for multiple comparisons. *: p<0.05.
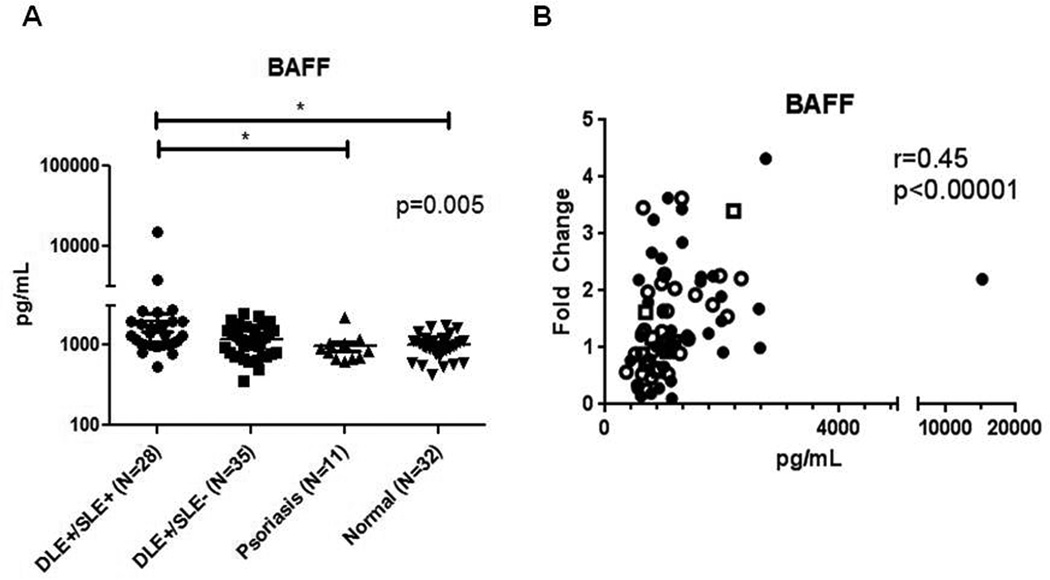
3.3. BAFF protein levels were highest in DLE+/SLE+ sera
We then investigated BAFF sera protein levels in 28 DLE+/SLE+, 35 DLE+/SLE−, 11 psoriasis, and 32 normal patients using sandwich ELISAs. DLE+/SLE+ sera (2008.1±2685.6 pg/mL) contained higher BAFF than DLE+/SLE− (1183.0±538.8), normal (1019.7±336.5 pg/mL), and psoriasis sera (975.5±441.8 pg/mL) (p=0.005) (Fig. 2A). There was direct correlation of mRNA and protein BAFF blood levels in 27 DLE+/SLE+, 28 DLE+/SLE−, 11 psoriasis, and 17 normal patients (r=0.45, p<0.0001) (Fig. 2B).
Fig. 2.
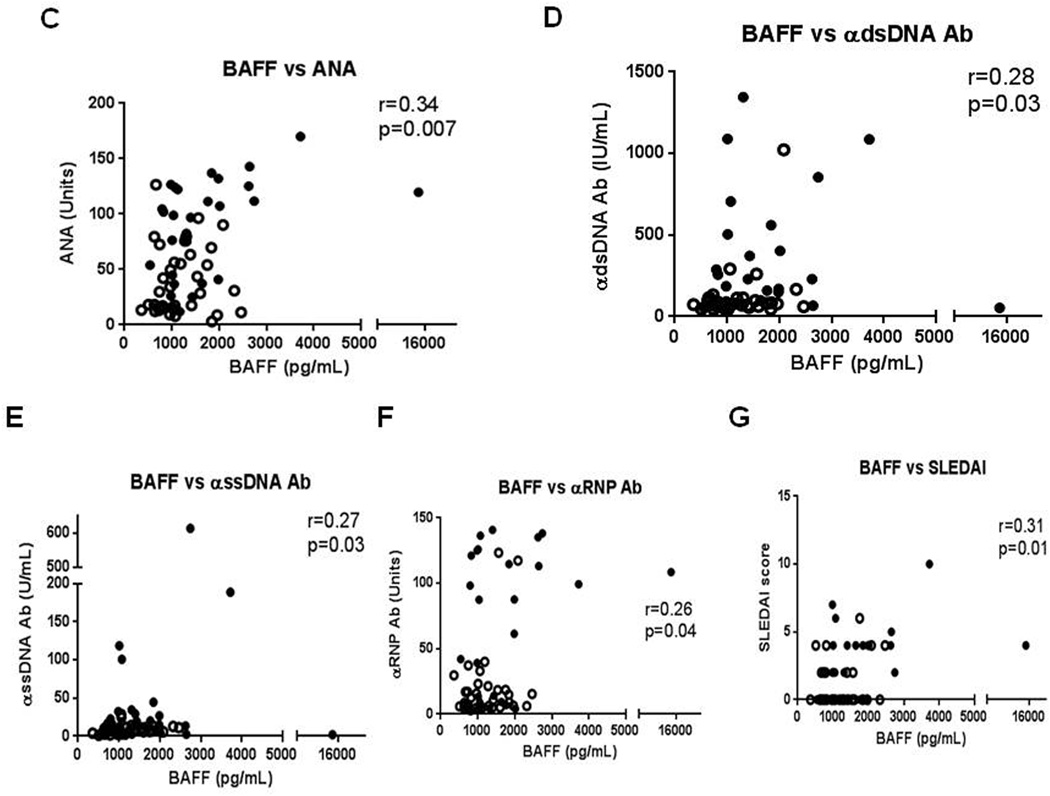
BAFF protein levels are elevated in DLE+/SLE+ sera versus DLE+/SLE−, normal and blood sera and correlate with levels of BAFF mRNA, various autoantibodies, and SLEDAI scores. Sandwich ELISAs were performed to evaluate sera levels of BAFF in DLE+/SLE+ (N=28), DLE+/SLE− (N=35), psoriasis (N=11), and normal (N=32) patients (A). We performed secondary analyses using Kruskal Wallis test. Capped bars between groups with astericks represent significant differences between groups based on Dunn’s test for multiple comparisons. Protein (pg/mL) and mRNA levels (fold change) of BAFF in the sera of DLE+/SLE+ (N=27) (dark circles), DLE+/SLE− (N=28) (white circles), psoriasis (dark squares) (N=11), and normal (white squares) (N=17) patients were correlated (B). BAFF protein and levels of ANA (C), anti-dsDNA (D), anti-ssDNA (E), and anti-RNP (F) antibodies and SLEDAI scores (G) in the sera of 28 DLE+/SLE+ (dark circles) and 35 DLE+/SLE− (white circles) patients were correlated. Spearman’s correlation coefficients and corresponding p-values were calculated. *: p<0.05.
Autoantibodies against various nuclear antigens and disease activity markers were analyzed against BAFF levels in DLE patients (N=63). BAFF levels directly correlated with ANA (r=0.34, p=0.007), anti-dsDNA (r=0.28, p=0.03), anti-ssDNA (r=0.27, p=0.03), and anti-RNP antibodies (r=0.26, p=0.04), and Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) scores (r=0.31, p=0.01) (Fig. 2C–G). There were no significant correlations with BAFF and total IgG and IgM, IgG autoantibodies against Scl-70, SS-A (52 kDa), SS-A (60 kDa), SS-B, and Sm, and Cutaneous Lupus Activity and Severity Index scores (data not shown).
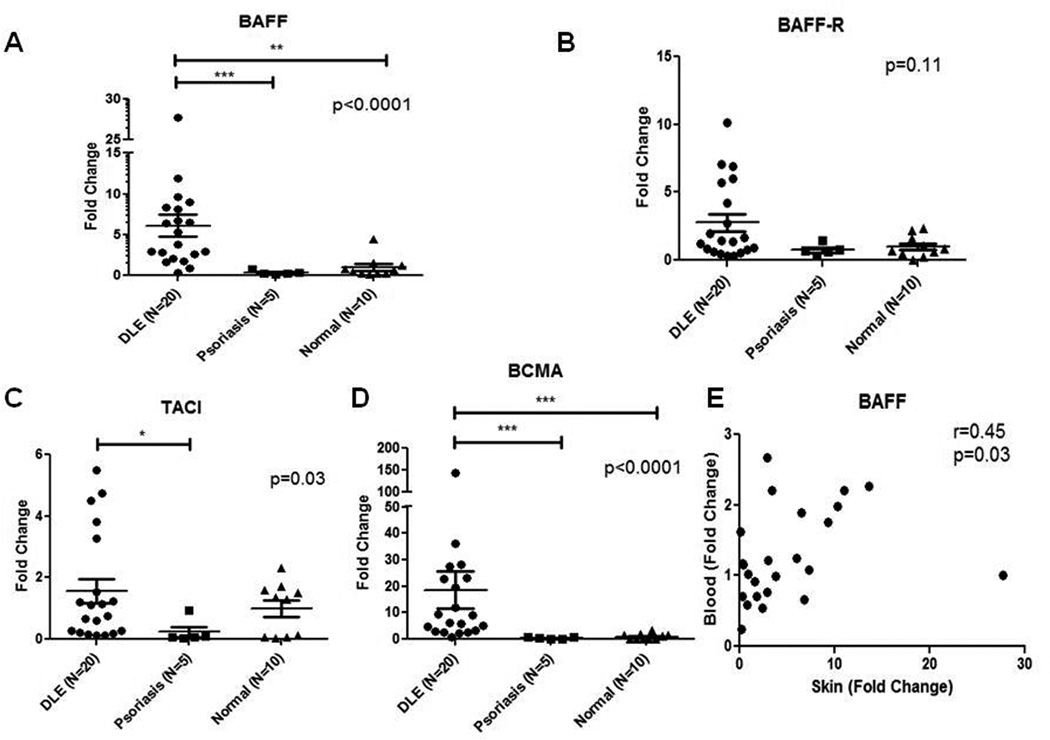
3.3. mRNA levels of BAFF and its receptors are elevated in DLE skin
We then investigated mRNA levels of BAFF, BCMA, TACI, and BAFF-R in DLE (N=20, 10 DLE+/SLE+, 10 DLE+/SLE−), psoriasis (N=5), and normal skin (N=10). DLE skin contained significantly higher BAFF mRNA levels than psoriasis (18.0-fold higher) and normal skin (6.1-fold higher) (p<0.0001) (Fig. 3A). BAFF-R mRNA levels were higher in DLE skin versus psoriasis (3.7-fold higher) and normal skin (2.8-fold higher), but did not reach statistical significance (p=0.11) (Fig. 3B). Compared with psoriasis (6.8-fold higher) and normal (1.6-fold higher) skin, DLE skin expressed higher TACI mRNA levels (p=0.03) (Fig. 3C). BCMA mRNA levels were greatly augmented in DLE skin versus psoriasis (41.1-fold higher) and normal skin (18.5-fold higher) (p<0.0001) (Fig. 3D). DLE+/SLE+ and DLE+/SLE− skin had no significant differences in BAFF, BAFF-R, TACI, and BCMA mRNA. There was correlation between BAFF mRNA blood and skin levels in 19 DLE and 5 psoriasis patients (r=0.45, p=0.03), but not BCMA, BAFF-R, and TACI (p>0.05) (Fig. 3E).
Fig. 3.
mRNA levels of BAFF and its receptors are up-regulated in DLE skin versus psoriasis and normal skin. Quantitative real-time PCR was performed to evaluate mRNA levels of BAFF (A), and its receptors, BAFF-R (B), TACI (C), and BCMA (D) in the skin of DLE (N=20), psoriasis (N=5), and normal (N=10) patients. We performed secondary analyses using Kruskal Wallis test. Capped bars between groups with astericks represent significant differences between groups based on Dunn’s test for multiple comparisons. mRNA levels of BAFF in the skin and blood of DLE (N=19) and psoriasis (N=5) patients were correlated (E). Spearman’s correlation coefficient and corresponding p-value were calculated. *: p<0.05, **: p<0.005, ***: p<0.0005.
3.4. BAFF-R protein levels are pronounced in the inflammatory cell infiltrate of DLE skin
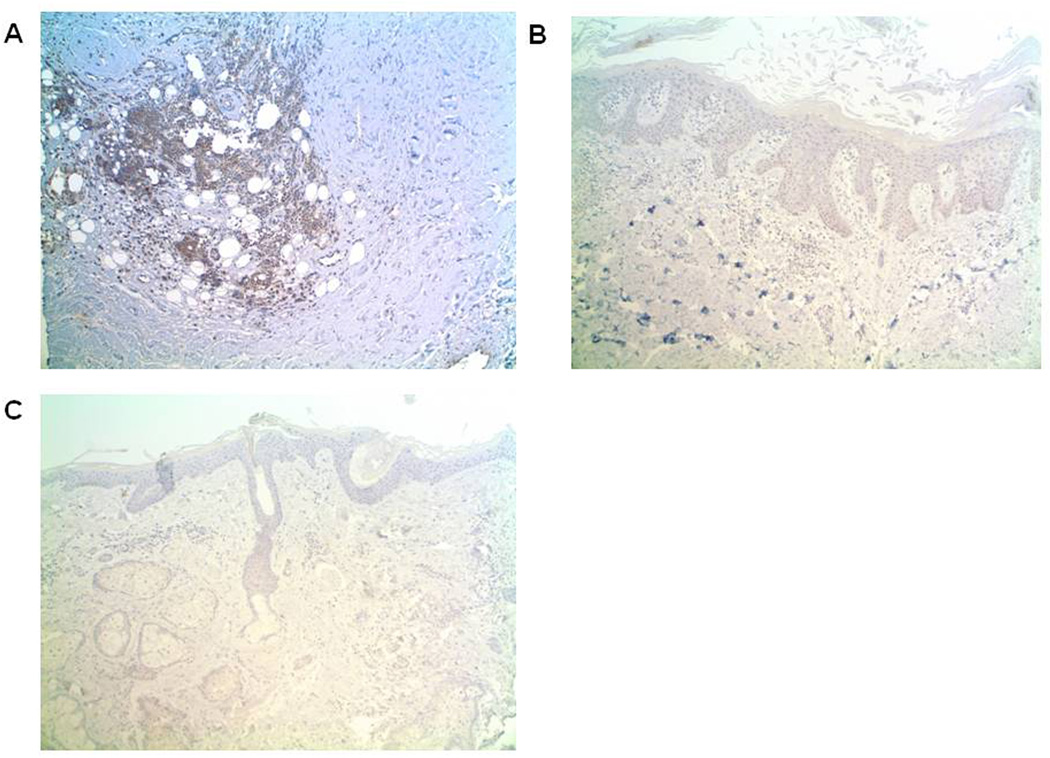
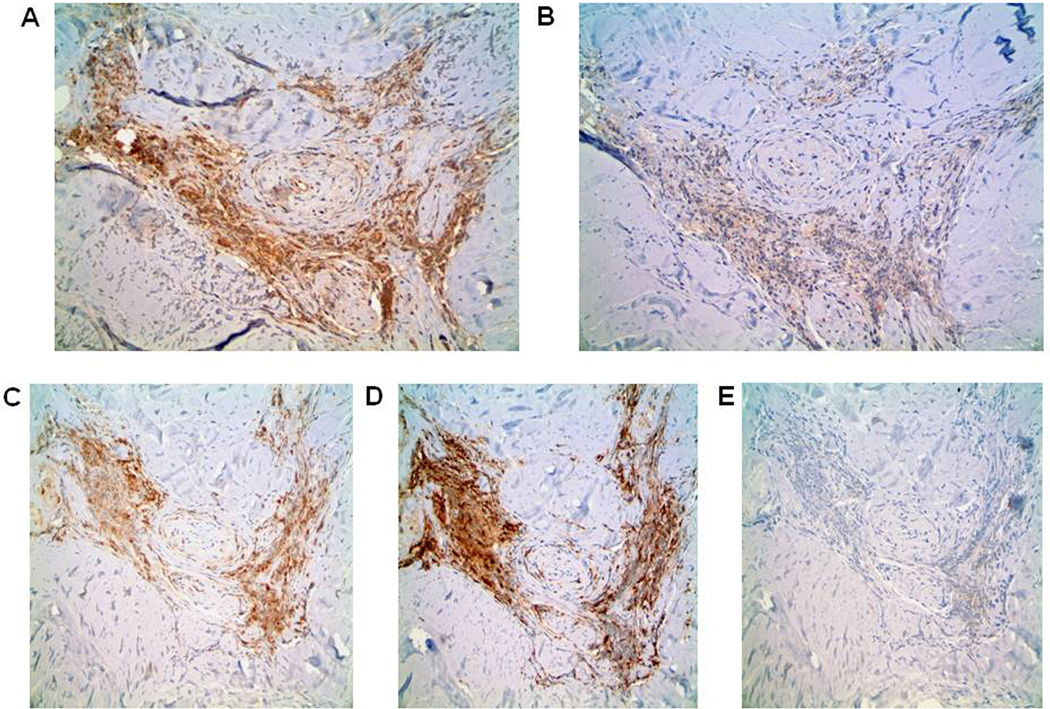
To confirm these mRNA findings, we utilized immunohistochemistry to examine BAFF and BAFF-R protein levels in DLE (N=14, 8 DLE+/SLE+, 6 DLE+/SLE−), psoriasis (N=4), and normal (N=6) skin samples. Based on average staining intensities and percentages of positively-staining inflammatory cells, BAFF levels trended higher for DLE skin than psoriasis and normal skin, although this did not reach statistical significance (p=0.08 (intensity), p=0.10 (percentage)) (Table 3). The epidermis, hair follicles, sebaceous glands, and eccrine glands of all skin groups showed similar BAFF patterns (data not shown). Of the BAFF receptors, we focused on BAFF-R, given its high affinity for and specificity with BAFF [19]. While absent in epidermis, hair follicles, sebaceous glands, and eccrine glands, BAFF-R was expressed in significantly more inflammatory cells in DLE skin versus psoriasis and normal skin (p=0.0004) (Table 3, Fig. 4A–C). Serial sections of DLE skin staining demonstrated that BAFF and BAFF-R staining patterns closely follow those of CD3+ T cells and/or CD163+ macrophages in DLE skin, but not CD20+ B cells (Fig. 5A–E). No differences were found in BAFF and BAFF-R expression between DLE+/SLE+ and DLE+/SLE− lesional skin.
Table 3.
BAFF and BAFF-R inflammatory cell expression in DLE, psoriasis, and normal skin.
| DLE (N=14) |
Psoriasis (N=4) |
Normal (N=6) |
p-valuea | |
|---|---|---|---|---|
| BAFF (inflammation %)b | 2.3+ | 1.6+ | 2.0+ | 0.10 |
| BAFF (inflammation)c | 2.1+ | 1.5+ | 1.7+ | 0.08 |
| BAFF-R (inflammation %)b | 2.1+ | 1.1+ | 1.2+ | 0.0004 |
| BAFF-R (inflammation)c | 1.6+ | 1.4+ | 1.3+ | 0.08 |
p-values were calculated using Kruskal-Wallis test (all groups) or t-test with Welch’s correction (two groups).
1 = <10% positive cells, 2 = 10–50% positive cells, 3 = >50% positive cells. Grading was performed by two independent investigators (BFC, GAH) and averaged.
1 = weak, 2 = moderate, 3 = strong. Grading was performed by two independent investigators (BFC, GAH) and averaged.
Fig. 4.
BAFF-R is expressed by a greater percentage of inflammatory cells in DLE skin (N=14) versus psoriasis (N=4) and normal (N=6) skin. Immunohistochemical analysis of BAFF-R (brown) were performed on representative samples of DLE (A), psoriasis (B), and normal (C) skin. Magnification: 100×.
Fig. 5.
BAFF and BAFF-R expression correspond to cells expressing CD3 and CD163 in DLE skin. Immunohistochemical stainings of BAFF (A), BAFF-R (B), T-cell marker CD3 (C), macrophage marker CD163 (D), and B-cell marker CD20 (E) were performed on another representative sample of DLE skin. Magnification: 100×.
4. Discussion
We demonstrated up-regulated mRNA and protein levels of BAFF in DLE+/SLE+ blood. We found higher mRNA levels of BAFF and its receptors in DLE skin. Furthermore, BAFF and BAFF-R protein levels were expressed by higher percentages of inflammatory cells in DLE skin, with BAFF almost approaching statistical significance. Serial immunohistochemical analysis on DLE skin showed BAFF and BAFF-R expression mirroring those of CD3+ T cells and CD163+ macrophages.
The increase in BAFF in DLE+/SLE+ blood is consistent with previous literature showing higher BAFF levels in SLE sera versus controls [7, 8, 20]. We also found that BAFF blood levels trended higher in DLE+/SLE+ than DLE+/SLE− patients. While this did not reach statistical significance, there were significant correlations of BAFF with SLEDAI scores and with ANAs, anti-dsDNA, anti-ss-DNA, and anti-RNP antibodies. These findings suggest BAFF as a potential marker of systemic disease in DLE patients. As BAFF levels increase in DLE patients, this results in enhanced B cell survival, and increased autoantigen exposure and autoantibody production [21]. SLE patients have previously demonstrated direct correlations of BAFF and dsDNA antibodies [8, 21, 22]. Consequently, this rise in autoantibodies can exacerbate systemic disease activity, as evidenced by BAFF’s correlation with SLEDAI scores in SLE patients [23]. To verify BAFF’s potential as a biomarker that traces progression to systemic disease, prospective studies following DLE patients who progress to SLE need to be performed.
The prominent elevations in mRNA levels of BAFF and its receptors in DLE skin may be linked to exogenous sources, such as viruses, to which the skin is exposed. Viruses may provide the impetus of increased BAFF production. The viral dsRNA complex polyinosinic:polycytidylic acid (poly I:C) can induce dendritic cells and salivary gland epithelial cells to enhance BAFF production [24, 25]. Since poly I:C is a TLR3 ligand, it has been postulated that abnormal TLR3 signaling results in increased BAFF levels [26]. While virus-like structures have been previously described in DLE skin by electron microscopy [27, 28], a virus has yet to be identified.
Despite showing increased BAFF mRNA levels in DLE skin, we found modest elevations in BAFF protein in DLE skin versus normal and psoriasis skin, particularly in the inflammatory cell infiltrate. This discrepancy may be related to the nature of the techniques used. Immunohistochemical analyses draw data from selected skin sections, while qRT-PCR encompasses data from whole skin biopsies. Additionally, BAFF can be converted from a membrane-bound to soluble form by protease cleavage [29], which is triggered by FcγRI cross-linkage with IgG or C-reactive protein [30], and may impact immunohistochemical interpretation.
This study also confirmed elevated mRNA expression of BAFF-R in DLE skin where greater numbers of inflammatory cells expressed BAFF-R protein. Based on serial sections, T cells appear to display BAFF-R on their surface. Although present in B cells [2], BAFF-R on T cells may bear greater relevance to the pathogenesis of DLE because T cells are the predominant cell type in the inflammatory infiltrate [31, 32]. BAFF can co-stimulate T cells expressing BAFF-R [5, 8], resulting in T cell proliferation and enhanced IFN-γ production [33]. Moreover, more inflammatory cells in DLE skin expressed BAFF protein, but this did not reach statistical significance. BAFF and CD3 had similar expression patterns by immunohistochemistry in DLE skin. As T cells can also produce BAFF [34], this finding may be due to either BAFF-expressing T cells or co-localization of BAFF and BAFF-R-expressing T cells.
BAFF and BAFF-R expression by immunohistochemistry appeared to mirror CD163 expression in DLE skin. While CD163 has not previously been studied in DLE skin, other macrophage markers such as CD68 [35], 27E10 [36], and CD14 [37], were enhanced in DLE skin lesions. Macrophages produce BAFF to stimulate B cell proliferation [38]. Peripheral blood monocytes in SLE patients have generated higher levels of BAFF under the influence of IFN-γ [39]. Moreover, activated monocytic cell lines can up-regulate BAFF-R [40], and their stimulation resulted in amplification of inflammatory mediators such as MMP-9 and IL-8 [40, 41].
This study did have limitations. As patients were recruited from one medical center, several psoriasis patients had mild disease. However, patients with the highest PASI scores had amongst the lowest BAFF mRNA levels. Medications may alter BAFF levels, as patients taking antimalarials [42] or immunosuppressants (e.g. prednisone, methotrexate) had decreased BAFF levels in prior studies [22, 43]. Because this was a cross-sectional study, we did not assess BAFF mRNA or protein levels before and after DLE patients initiated their oral medications. Additionally, our immunohistochemical analysis was not completely blinded. Although evaluators were instructed to focus on staining patterns only, features of the underlying condition could be ascertained in the background. Furthermore, the study does not address whether or not abnormal expressions of BAFF and its receptors are critical to the pathogenesis of DLE. Future studies involving murine cutaneous lupus models with limited BAFF or BAFF-R expression will help evaluate their roles.
In conclusion, we demonstrated that DLE+/SLE+ blood had the highest BAFF mRNA and protein levels, implying that elevated BAFF levels could indicate systemic spread in DLE patients. mRNA levels of BAFF and its receptors and BAFF-R protein levels were elevated in DLE skin. T cells and macrophages were more likely involved in amplifying BAFF-R expression in DLE. Further projects involving dual chromagen immunohistochemistry, immunofluorescence, and/or flow cytometry to assess co-localization of BAFF and BAFF-R to inflammatory cells may confirm these findings. As clinical implications are unclear, studies involving topical therapies against BAFF and its receptors could be pursued in DLE patients.
Supplementary Material
Acknowledgements
We would like to thank Jack Cohen, Melissa Costner, Rebecca Vasquez, Julie Song, Sandra Victor, Azza Mutwally, and Valerie Branch for aiding in the recruitment of patients.
Funding Sources: The research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number K23AR061441 (BFC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- ANA
anti-nuclear antibody
- BAFF
B-cell activating factor of the tumor necrosis family (BAFF)
- BAFF-R
BAFF-receptor
- BCMA
B cell maturation antigen
- DLE
discoid lupus erythematosus
- dsDNA
double-stranded DNA
- ELISAs
enzyme-linked immunosorbant assays
- poly I:C
polyinosinic:polycytidylic acid
- qRT-PCR
quantitative real-time PCR
- RNP
ribonucleoprotein
- SLE
systemic lupus erythematosus
- Sm
Smith
- ssDNA
single-stranded DNA
- TACI
transmembrane activator and calcium modulator and cyclophilin ligand interactor
- UTSW
University of Texas Southwestern
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Dr. Chong is an investigator for Celgene Corporation, Amgen Incorporated, and Daavlin Corporation. Dr. Yancey has served in the advisory boards for Stiefel/Glaxo-Smith Kline, and Mary Kay Inc.
References
- 1.Davidson A. Targeting BAFF in autoimmunity. Curr Opin Immunol. 2010;22:732–739. doi: 10.1016/j.coi.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng LG, Sutherland AP, Newton R, Qian F, Cachero TG, Scott ML, et al. B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. J Immunol. 2004;173:807–817. doi: 10.4049/jimmunol.173.2.807. [DOI] [PubMed] [Google Scholar]
- 3.Mackay F, Browning JL. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol. 2002;2:465–475. doi: 10.1038/nri844. [DOI] [PubMed] [Google Scholar]
- 4.Bossen C, Cachero TG, Tardivel A, Ingold K, Willen L, Dobles M, et al. TACI, unlike BAFF-R, is solely activated by oligomeric BAFF and APRIL to support survival of activated B cells and plasmablasts. Blood. 2008;111:1004–1012. doi: 10.1182/blood-2007-09-110874. [DOI] [PubMed] [Google Scholar]
- 5.Yang M, Hase H, Legarda-Addison D, Varughese L, Seed B, Ting AT. B cell maturation antigen, the receptor for a proliferation-inducing ligand and B cell-activating factor of the TNF family, induces antigen presentation in B cells. J Immunol. 2005;175:2814–2824. doi: 10.4049/jimmunol.175.5.2814. [DOI] [PubMed] [Google Scholar]
- 6.Pers JO, Daridon C, Devauchelle V, Jousse S, Saraux A, Jamin C, et al. BAFF overexpression is associated with autoantibody production in autoimmune diseases. Ann N Y Acad Sci. 2005;1050:34–39. doi: 10.1196/annals.1313.004. [DOI] [PubMed] [Google Scholar]
- 7.Stohl W, Metyas S, Tan SM, Cheema GS, Oamar B, Xu D, et al. B lymphocyte stimulator overexpression in patients with systemic lupus erythematosus: longitudinal observations. Arthritis Rheum. 2003;48:3475–3486. doi: 10.1002/art.11354. [DOI] [PubMed] [Google Scholar]
- 8.Cheema GS, Roschke V, Hilbert DM, Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 2001;44:1313–1319. doi: 10.1002/1529-0131(200106)44:6<1313::AID-ART223>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 9.Chu VT, Enghard P, Schurer S, Steinhauser G, Rudolph B, Riemekasten G, et al. Systemic activation of the immune system induces aberrant BAFF and APRIL expression in B cells in patients with systemic lupus erythematosus. Arthritis Rheum. 2009;60:2083–2093. doi: 10.1002/art.24628. [DOI] [PubMed] [Google Scholar]
- 10.Manzi S, Sanchez-Guerrero J, Merrill JT, Furie R, Gladman D, Navarra SV, et al. Effects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: combined results from two phase III trials. Ann Rheum Dis. 2012;71:1833–1838. doi: 10.1136/annrheumdis-2011-200831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao Y, Richman L, Higgs BW, Morehouse CA, de los Reyes M, Brohawn P, et al. Neutralization of interferon-alpha/beta-inducible genes and downstream effect in a phase I trial of an anti-interferon-alpha monoclonal antibody in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1785–1796. doi: 10.1002/art.24557. [DOI] [PubMed] [Google Scholar]
- 12.Tan EM, Cohen AS, Fries JF, Masi AT, Mcshane DJ, Rothfield NF, et al. Special Article - the 1982 Revised Criteria for the Classification of Systemic Lupus-Erythematosus. Arthritis and Rheumatism. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 13.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Miller RT. Multitumor "Sandwich" Blocks in Immunohistochemistry. Simplified Method of Preparation and Practical Uses. Appl Immunohistochem. 1993;1:156–159. [Google Scholar]
- 16.Miller RT, Estran C. Heat-Induced Epitope Retrieval with a Pressure Cooker - Suggestions for Optimal Use. Appl Immunohistochem. 1995;3:190–193. [Google Scholar]
- 17.Butz WR, Clark KC, Miller RT. Improved Manual Immunohistochemistry Employing Orbital Mixing of Reagents during Incubations. Appl Immunohistochem. 1994;2:65–67. [Google Scholar]
- 18.Shi SR, Guo J, Cote RJ, Young LL, Hawes D, Shi Y, et al. Sensitivity and detection efficiency of a novel two-step detection system (PowerVision) for immunohistochemistry. Appl Immunohisto M M. 1999;7:201–208. [Google Scholar]
- 19.Day ES, Cachero TG, Qian F, Sun Y, Wen D, Pelletier M, et al. Selectivity of BAFF/BLyS and APRIL for binding to the TNF family receptors BAFFR/BR3 and BCMA. Biochemistry. 2005;44:1919–1931. doi: 10.1021/bi048227k. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Roschke V, Baker KP, Wang Z, Alarcon GS, Fessler BJ, et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol. 2001;166:6–10. doi: 10.4049/jimmunol.166.1.6. [DOI] [PubMed] [Google Scholar]
- 21.Petri M, Stohl W, Chatham W, McCune WJ, Chevrier M, Ryel J, et al. Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis Rheum. 2008;58:2453–2459. doi: 10.1002/art.23678. [DOI] [PubMed] [Google Scholar]
- 22.Zen M, Canova M, Campana C, Bettio S, Nalotto L, Rampudda M, et al. The kaleidoscope of glucorticoid effects on immune system. Autoimmun Rev. 2011;10:305–310. doi: 10.1016/j.autrev.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Becker-Merok A, Nikolaisen C, Nossent HC. B-lymphocyte activating factor in systemic lupus erythematosus and rheumatoid arthritis in relation to autoantibody levels, disease measures and time. Lupus. 2006;15:570–576. doi: 10.1177/0961203306071871. [DOI] [PubMed] [Google Scholar]
- 24.Douagi I, Gujer C, Sundling C, Adams WC, Smed-Sorensen A, Seder RA, et al. Human B cell responses to TLR ligands are differentially modulated by myeloid and plasmacytoid dendritic cells. J Immunol. 2009;182:1991–2001. doi: 10.4049/jimmunol.0802257. [DOI] [PubMed] [Google Scholar]
- 25.Ittah M, Miceli-Richard C, Gottenberg JE, Sellam J, Eid P, Lebon P, et al. Viruses induce high expression of BAFF by salivary gland epithelial cells through TLR- and type-I IFN-dependent and -independent pathways. Eur J Immunol. 2008;38:1058–1064. doi: 10.1002/eji.200738013. [DOI] [PubMed] [Google Scholar]
- 26.Xu W, Santini PA, Matthews AJ, Chiu A, Plebani A, He B, et al. Viral double-stranded RNA triggers Ig class switching by activating upper respiratory mucosa B cells through an innate TLR3 pathway involving BAFF. J Immunol. 2008;181:276–287. doi: 10.4049/jimmunol.181.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashimoto K, Thompson DF. Discoid lupus erythematosus. Electron microscopic studies of paramyxovirus-like structures. Arch Dermatol. 1970;101:565–567. doi: 10.1001/archderm.101.5.565. [DOI] [PubMed] [Google Scholar]
- 28.Nagy E, Nagy IZ, Nagy-Vezekenyi C. Virus-like structures in lupus erythematosus discoides. Acta Derm Venereol. 1977;57:211–215. [PubMed] [Google Scholar]
- 29.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Su K, Ji C, Szalai AJ, Wu J, Zhang Y, et al. Immune opsonins modulate BLyS/BAFF release in a receptor-specific fashion. J Immunol. 2008;181:1012–1018. doi: 10.4049/jimmunol.181.2.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grassi M, Capello F, Bertolino L, Seia Z, Pippione M. Identification of granzyme B-expressing CD-8-positive T cells in lymphocytic inflammatory infiltrate in cutaneous lupus erythematosus and in dermatomyositis. Clin Exp Dermatol. 2009;34:910–914. doi: 10.1111/j.1365-2230.2009.03297.x. [DOI] [PubMed] [Google Scholar]
- 32.Magro CM, Segal JP, Crowson AN, Chadwick P. The phenotypic profile of dermatomyositis and lupus erythematosus: a comparative analysis. J Cutan Pathol. 2010;37:659–671. doi: 10.1111/j.1600-0560.2009.01443.x. [DOI] [PubMed] [Google Scholar]
- 33.Ye Q, Wang L, Wells AD, Tao R, Han R, Davidson A, et al. BAFF binding to T cell-expressed BAFF-R costimulates T cell proliferation and alloresponses. Eur J Immunol. 2004;34:2750–2759. doi: 10.1002/eji.200425198. [DOI] [PubMed] [Google Scholar]
- 34.Mackay F, Leung H. The role of the BAFF/APRIL system on T cell function. Semin Immunol. 2006;18:284–289. doi: 10.1016/j.smim.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Flier J, Boorsma DM, van Beek PJ, Nieboer C, Stoof TJ, Willemze R, et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398–405. doi: 10.1002/1096-9896(200108)194:4<397::aid-path899>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 36.Kunz M, Henseleit-Walter U, Sorg C, Kolde G. Macrophage marker 27E10 on human keratinocytes helps to differentiate discoid lupus erythematosus and Jessner's lymphocytic infiltration of the skin. Eur J Dermatol. 1999;9:107–110. [PubMed] [Google Scholar]
- 37.de Jong EM, van Erp PE, Ruiter DJ, van de Kerkhof PC. Immunohistochemical detection of proliferation and differentiation in discoid lupus erythematosus. J Am Acad Dermatol. 1991;25:1032–1038. doi: 10.1016/0190-9622(91)70303-j. [DOI] [PubMed] [Google Scholar]
- 38.Craxton A, Magaletti D, Ryan EJ, Clark EA. Macrophage- and dendritic cell-- dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood. 2003;101:4464–4471. doi: 10.1182/blood-2002-10-3123. [DOI] [PubMed] [Google Scholar]
- 39.Harigai M, Kawamoto M, Hara M, Kubota T, Kamatani N, Miyasaka N. Excessive production of IFN-gamma in patients with systemic lupus erythematosus and its contribution to induction of B lymphocyte stimulator/B cell-activating factor/TNF ligand superfamily-13B. J Immunol. 2008;181:2211–2219. doi: 10.4049/jimmunol.181.3.2211. [DOI] [PubMed] [Google Scholar]
- 40.Jeon ST, Kim WJ, Lee SM, Lee MY, Park SB, Lee SH, et al. Reverse signaling through BAFF differentially regulates the expression of inflammatory mediators and cytoskeletal movements in THP-1 cells. Immunol Cell Biol. 2010;88:148–156. doi: 10.1038/icb.2009.75. [DOI] [PubMed] [Google Scholar]
- 41.Lee SM, Kim EJ, Suk K, Lee WH. BAFF and APRIL induce inflammatory activation of THP-1 cells through interaction with their conventional receptors and activation of MAPK and NF-kappaB. Inflamm Res. 2011;60:807–815. doi: 10.1007/s00011-011-0336-3. [DOI] [PubMed] [Google Scholar]
- 42.Toubi E, Kessel A, Rosner I, Rozenbaum M, Paran D, Shoenfeld Y. The reduction of serum B-lymphocyte activating factor levels following quinacrine add-on therapy in systemic lupus erythematosus. Scand J Immunol. 2006;63:299–303. doi: 10.1111/j.1365-3083.2006.01737.x. [DOI] [PubMed] [Google Scholar]
- 43.Scuderi F, Alboini PE, Bartoccioni E, Evoli A. BAFF serum levels in myasthenia gravis: effects of therapy. J Neurol. 2011;258:2284–2285. doi: 10.1007/s00415-011-6092-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.