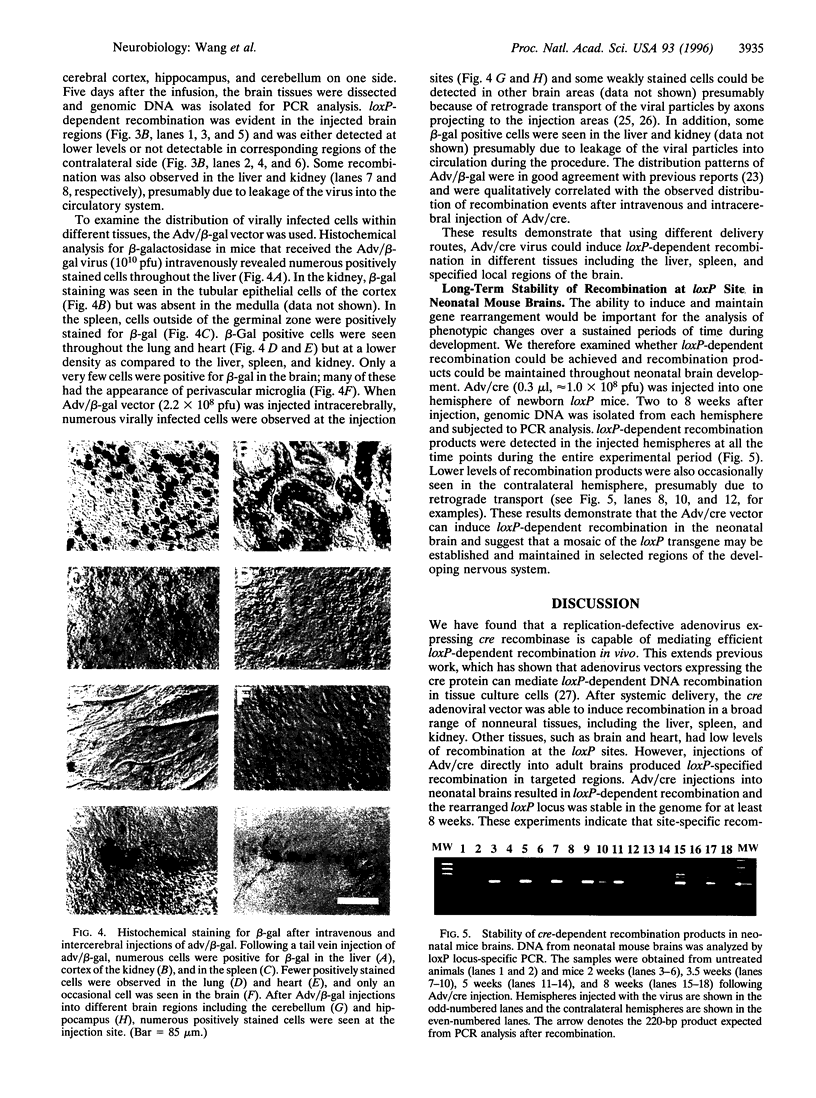
Abstract
Conditional gene expression and gene deletion are important experimental approaches for examining the functions of particular gene products in development and disease. The cre-loxP system from bacteriophage P1 has been used in transgenic animals to induce site-specific DNA recombination leading to gene activation or deletion. To regulate the recombination in a spatiotemporally controlled manner, we constructed a recombinant adenoviral vector, Adv/cre, that contained the cre recombinase gene under regulation of the herpes simplex virus thymidine kinase promoter. The efficacy and target specificity of this vector in mediating loxP-dependent recombination were analyzed in mice that had been genetically engineered to contain loxP sites in their genome. After intravenous injection of the Adv/cre vector into adult animals, the liver and spleen showed the highest infectivity of the adenovirus as well as the highest levels of recombination, whereas other tissues such as kidney, lung, and heart had lower levels of infection and recombination. Only trace levels of recombination were detected in the brain. However, when the Adv/cre vector was injected directly into specific regions of the adult brain, including the cerebral cortex, hippocampus, and cerebellum, recombination was detectable at the injection site. Furthermore, when the Adv/cre vector was injected into the forebrains of neonatal mice, the rearranged toxP locus from recombination could be detected in the injected regions for at least 8 weeks. Taken together, these results demonstrate that the Adv/cre vector expressing a functional cre protein is capable of mediating loxP-dependent recombination in various tissues and the recombined gene locus may in some cases be maintained for an extended period. The use of the adenovirus vector expressing cre combined with localized delivery to specific tissues may provide an efficient means to achieve conditional gene expression or knockout with precise spatiotemporal control.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anton M., Graham F. L. Site-specific recombination mediated by an adenovirus vector expressing the Cre recombinase protein: a molecular switch for control of gene expression. J Virol. 1995 Aug;69(8):4600–4606. doi: 10.1128/jvi.69.8.4600-4606.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki K., Araki M., Miyazaki J., Vassalli P. Site-specific recombination of a transgene in fertilized eggs by transient expression of Cre recombinase. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):160–164. doi: 10.1073/pnas.92.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody S. L., Crystal R. G. Adenovirus-mediated in vivo gene transfer. Ann N Y Acad Sci. 1994 May 31;716:90–103. doi: 10.1111/j.1749-6632.1994.tb21705.x. [DOI] [PubMed] [Google Scholar]
- Byrnes A. P., Rusby J. E., Wood M. J., Charlton H. M. Adenovirus gene transfer causes inflammation in the brain. Neuroscience. 1995 Jun;66(4):1015–1024. doi: 10.1016/0306-4522(95)00068-t. [DOI] [PubMed] [Google Scholar]
- Chen S. H., Chen X. H., Wang Y., Kosai K., Finegold M. J., Rich S. S., Woo S. L. Combination gene therapy for liver metastasis of colon carcinoma in vivo. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2577–2581. doi: 10.1073/pnas.92.7.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisaka O., Capecchi M. R. Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene hox-1.5. Nature. 1991 Apr 11;350(6318):473–479. doi: 10.1038/350473a0. [DOI] [PubMed] [Google Scholar]
- Davidson B. L., Allen E. D., Kozarsky K. F., Wilson J. M., Roessler B. J. A model system for in vivo gene transfer into the central nervous system using an adenoviral vector. Nat Genet. 1993 Mar;3(3):219–223. doi: 10.1038/ng0393-219. [DOI] [PubMed] [Google Scholar]
- Doran S. E., Ren X. D., Betz A. L., Pagel M. A., Neuwelt E. A., Roessler B. J., Davidson B. L. Gene expression from recombinant viral vectors in the central nervous system after blood-brain barrier disruption. Neurosurgery. 1995 May;36(5):965–970. doi: 10.1227/00006123-199505000-00012. [DOI] [PubMed] [Google Scholar]
- Engelhardt J. F., Ye X., Doranz B., Wilson J. M. Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6196–6200. doi: 10.1073/pnas.91.13.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushige S., Sauer B. Genomic targeting with a positive-selection lox integration vector allows highly reproducible gene expression in mammalian cells. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7905–7909. doi: 10.1073/pnas.89.17.7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Marth J. D., Orban P. C., Mossmann H., Rajewsky K. Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science. 1994 Jul 1;265(5168):103–106. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- Huard J., Lochmüller H., Acsadi G., Jani A., Massie B., Karpati G. The route of administration is a major determinant of the transduction efficiency of rat tissues by adenoviral recombinants. Gene Ther. 1995 Mar;2(2):107–115. [PubMed] [Google Scholar]
- Kass-Eisler A., Falck-Pedersen E., Elfenbein D. H., Alvira M., Buttrick P. M., Leinwand L. A. The impact of developmental stage, route of administration and the immune system on adenovirus-mediated gene transfer. Gene Ther. 1994 Nov;1(6):395–402. [PubMed] [Google Scholar]
- Kühn R., Schwenk F., Aguet M., Rajewsky K. Inducible gene targeting in mice. Science. 1995 Sep 8;269(5229):1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- Lakso M., Sauer B., Mosinger B., Jr, Lee E. J., Manning R. W., Yu S. H., Mulder K. L., Westphal H. Targeted oncogene activation by site-specific recombination in transgenic mice. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6232–6236. doi: 10.1073/pnas.89.14.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gal La Salle G., Robert J. J., Berrard S., Ridoux V., Stratford-Perricaudet L. D., Perricaudet M., Mallet J. An adenovirus vector for gene transfer into neurons and glia in the brain. Science. 1993 Feb 12;259(5097):988–990. doi: 10.1126/science.8382374. [DOI] [PubMed] [Google Scholar]
- McGrory W. J., Bautista D. S., Graham F. L. A simple technique for the rescue of early region I mutations into infectious human adenovirus type 5. Virology. 1988 Apr;163(2):614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- Meyer D., Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995 Nov 23;378(6555):386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- Orban P. C., Chui D., Marth J. D. Tissue- and site-specific DNA recombination in transgenic mice. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6861–6865. doi: 10.1073/pnas.89.15.6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Norstedt G., Gelinas R. E., Hammer R. E., Brinster R. L. Metallothionein-human GH fusion genes stimulate growth of mice. Science. 1983 Nov 18;222(4625):809–814. doi: 10.1126/science.6356363. [DOI] [PubMed] [Google Scholar]
- Ridoux V., Robert J. J., Zhang X., Perricaudet M., Mallet J., Le Gal La Salle G. Adenoviral vectors as functional retrograde neuronal tracers. Brain Res. 1994 Jun 13;648(1):171–175. doi: 10.1016/0006-8993(94)91919-4. [DOI] [PubMed] [Google Scholar]
- Sauer B., Henderson N. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5166–5170. doi: 10.1073/pnas.85.14.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B. Manipulation of transgenes by site-specific recombination: use of Cre recombinase. Methods Enzymol. 1993;225:890–900. doi: 10.1016/0076-6879(93)25056-8. [DOI] [PubMed] [Google Scholar]
- Smith A. J., De Sousa M. A., Kwabi-Addo B., Heppell-Parton A., Impey H., Rabbitts P. A site-directed chromosomal translocation induced in embryonic stem cells by Cre-loxP recombination. Nat Genet. 1995 Apr;9(4):376–385. doi: 10.1038/ng0495-376. [DOI] [PubMed] [Google Scholar]
- Smith L. C., Eisensmith R. C., Woo S. L. Gene therapy in heart disease. Adv Exp Med Biol. 1995;369:79–88. doi: 10.1007/978-1-4615-1957-7_8. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Hamilton D. Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. J Mol Biol. 1981 Aug 25;150(4):467–486. doi: 10.1016/0022-2836(81)90375-2. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Hamilton D., Hoess R. Bacteriophage P1 site-specific recombination. II. Recombination between loxP and the bacterial chromosome. J Mol Biol. 1981 Aug 25;150(4):487–507. doi: 10.1016/0022-2836(81)90376-4. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Van Deursen J., Fornerod M., Van Rees B., Grosveld G. Cre-mediated site-specific translocation between nonhomologous mouse chromosomes. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7376–7380. doi: 10.1073/pnas.92.16.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Jones F. S., Krushel L. A., Edelman G. M. Embryonic expression patterns of the neural cell adhesion molecule gene are regulated by homeodomain binding sites. Proc Natl Acad Sci U S A. 1996 Mar 5;93(5):1892–1896. doi: 10.1073/pnas.93.5.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham T. J., Filardo E. J., Cheresh D. A., Nemerow G. R. Integrin alpha v beta 5 selectively promotes adenovirus mediated cell membrane permeabilization. J Cell Biol. 1994 Oct;127(1):257–264. doi: 10.1083/jcb.127.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Nunes F. A., Berencsi K., Furth E. E., Gönczöl E., Wilson J. M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]