Abstract
Using several tumor models we demonstrate that mice deficient in Bcl11b in T cells, though having reduced numbers of T cells in the peripheral lymphoid organs, developed significantly less tumors compared to wild type mice. Bcl11b−/− CD4+ T cells, with elevated TNFα levels, but not the Bcl11b−/− CD8+ T cells, were required for the reduced tumor burden, as were NK1.1+ cells, found in increased numbers in Bcl11bF/F/CD4-Cre mice. Among NK1.1+ cells, the NK cell population was predominant in number and was the only population displaying elevated Granzyme B levels and increased degranulation, however not increased proliferation. Though the number of myeloid derived suppressor cells (MDSCs) was increased in the lungs with metastatic tumors of Bcl11bF/F/CD4-Cre mice, their Arginase 1 levels were severely reduced. The increase in NK cell and MDSC numbers was associated with increased bone marrow and splenic hematopoiesis. Finally, the reduced tumor burden, increased numbers of NK cells in the lung and increased hematopoiesis in Bcl11bF/F/CD4-Cre mice, were all dependent on TNFα. Moreover, TNFα treatment of wild type mice also reduced the tumor burden, increased hematopoiesis and the numbers and activity of NK cells in the lung. In vitro treatment with TNFα of lineage negative hematopoietic progenitors increased NK and myeloid differentiation, further supporting a role of TNFα in promoting hematopoiesis. These studies reveal a novel role for TNFα in the anti-tumor immune response, specifically in stimulating hematopoiesis and increasing the numbers and activity of NK cells.
Keywords: Melanoma, Bcl11b, TNFα, NK cells, hematopoiesis
Introduction
Several immune populations are known to play key roles in anti-tumor immune responses, including CD8+ T or cytotoxic T cells (CTLs) and NK cells. CTLs act through recognition of tumor-associated antigens, presented in the context of MHC class I, based on which vaccines have been developed ((1–4) and reviewed in (5)). However, melanoma cells and other tumor cells downregulate MHC class I, making the CTL-mediated immune response inefficient (6). NK cells with enhanced effector activity were shown to prevent metastasis (7). Following activation through engagement of activating NK receptors, NK cells lyse targeted tumor cells predominantly using perforin and granzymes (8, 9). Several activating NK receptors critical in the anti-tumor immune response have been described, including NKG2D belonging to natural killer group 2, important in tumor surveillance (11, 12), and NKp46, part of the natural cytotoxicity receptors (NCRs)(10), critical in preventing tumor progression (13). Conversely, other tumor infiltrating populations, such as tumor-associated macrophages (TAMs) and myeloid derived suppressor cells (MDSCs) restrain the anti-tumor immune response (14–17).
Tumor necrosis factor α (TNFα) is currently used for the treatment of advanced soft tissue sarcomas and metastatic melanomas (reviewed in (18, 19)). TNFα is a potent inhibitor of tumor-associated vasculature (20, 21) and (reviewed in (18, 19)), however its impact on the anti-tumor immune response is less characterized.
Bcl11b is a C2H2 zinc finger transcriptional regulator (22, 23) important for positive selection and survival of DN3 and double-positive thymocytes (24, 25). It has been demonstrated that deletion of Bcl11b at the DN3 stage of thymic development or from DP thymocytes with the use of an inducible ERCre system resulted in generation of induced T-to–natural killer (ITNK) cells that possess anti-tumor activity (26), which opens an exciting avenue to develop potent anti-tumor immune cells. Following these findings, in this study we directly tested the anti-tumor immune response of in vivo generated Bcl11b−/− T cells using Bcl11bF/F/CD4-Cre mice, in which the gene is removed at the DP stage of T cell development (25). We demonstrate that Bcl11bF/F/CD4-Cre mice, despite the reduced numbers of T cells in the periphery (25), developed significantly fewer metastatic lung nodules compared to wild type mice and showed lower tumor burdens in flank melanoma and flank Tramp tumor models. The reduction in the tumor burden was dependent on NK1.1+ cells and CD4+ T cells, but not on CD8+ T cells. The NK cells predominated and were the only NK1.1+ population upregulating Granzyme B and exhibiting elevated degranulation. The increase in the NK population was dependent on TNFα produced by Bcl11b−/− CD4+ T cells. Bcl11bF/F/CD4-Cre mice showed increased bone marrow and splenic hematopoiesis which was also dependent on TNFα. TNFα treatment of wild type mice with metastatic tumors reduced the tumor burden and caused increased NK cell numbers and increased splenic hematopoiesis, supporting a novel role for TNFα in anti-tumor immune response.
MATERIALS AND METHODS
Mice
Bcl11bF/F/CD4-Cre mice have been previously described (25, 27). Mice were housed under specific pathogen-free conditions. All the experiments were performed in accordance with animal protocols approved by the Institutional Animal Care and Use Committee of Albany Medical Center.
Metastatic melanoma and other tumors
0.5 ×106 B16–F10 (B16) melanoma cells were transferred intravenously (i.v.) into 8–10-weeks old Bcl11bF/F/CD4-Cre and wild type mice. On Day 10 post tumor post transfer, mice were sacrificed. The lungs were flushed with PBS and collected into Fekete’s solution for counting melanoma nodules. Flank melanoma and flank Tramp tumors were induced by injecting of 5 ×106 B16 melanoma cells or Tramp C-2 tumor cells, subcutaneously. The tumor size was measured from day 9 to day 25 for B16 melanoma and for 10 weeks for Tramp C-2 tumors.
In vivo cell depletion, cytokine neutralization and TNF treatment
Mice were intraperitoneally (i.p.) injected with 200µg anti-CD8a (53-6.72, BioXcell), anti-CD4 (GK1.5, BioXcell), anti-NK1.1 (PK-136, BioXcell), anti-TNFα (XT3.11, BioXcell), anti-IFN (XMG1.2, BioXcell), anti-IL-17a (17F3, BioXcell) antibodies or IgG one day before tumor cell injection, and further the treatment was continued on days 2, 5, and 8 with 150µg antibodies. 1µg recombinant murine TNF (Peprotech) or vehicle were i.p. injected as above.
In vitro NK and myeloid cell differentiation
Lineage negative (lin−) bone marrow (BM) cells were enriched twice with the mouse lineage cell depletion kit (Miltenyi Biotec). Cells were cultured first in complete RPMI medium with 50 ng/ml SCF, 5 ng/ml Flt3-L, 20 ng/ml IL-6, 0.5 ng/ml IL-7, +/− 50 ng/ml TNF for 6 days, following which cells were transferred in media with 20 ng/ml IL-15 (28) (29), +/− TNF. For myeloid cell differentiation, lin− BM cells were cultured on OP9 cells in -MEM medium with 10 ng/ml IL-3, 10 ng/ml IL-7, 100 ng/ml SCF, 100 ng/ml M-CSF and 5 ng/ml Flt3L +/− 20 ng/ml TNF for 10 days.
Flow cytometry
Cellular suspensions were stained as previously described (30), using the following fluorophore-conjugated antibodies: CD3ε (145-2c11), CD4 (GK1.5), CD8a (53-6.7), CD27 (LG.7F9), CD107a (ebio1D4B), CD127 (A7R34), NK1.1 (PK-136), NKp46 (29A1.1), c-Kit (2B8), Sca-1 (D7), Flt3 (A2F10), IFNγ (XMG1.2), IL-17A (17B7), and TNFα (MP6-XT22) from eBiosciene. Anti–granzyme B (GB11) was from Biolegend. Intracellular cytokine staining was conducted as previously described (31). FACS was performed on an upgraded (Cytek) eight-color FACSCalibur using FlowJo acquisition software and the data were analyzed by FlowJo.
Progenitor cells in spleen and bone marrow
Lineage positive populations were stained with the following mix of biotinylated anti-mouse antibodies for: CD3, CD4, CD8, CD11b/Mac1 (M1/70), CD11c (N418), CD19 (eBio1D3), B220 (RA3-6B2), GR-1 (RB6-8C5), NK1.1, Ter119 (TER-119), TCRβ (H57–597), γTCR (eBioGL3) and FcR1 (MAR-1), followed by streptavidin-conjugated APC-eFluor® 780. Cells were further stained with fluorophore specific antibodies and the following populations were defined as: Lin−/c-kit+/Sca-1− (LK), Lin−/c-kit+Sca-1+ (LSK), granulocyte macrophage progenitors (GMP), based on CD16 and CD34 within the LK population, multipotent progenitors (MPP) and hematopoietic stem/progenitor cells (HSPC) based on Flt3 in the LSK population, PrePro NKa and PreProNKb cells within the c-kitlowSca-1+ and c-kit− Sca-1+ cells, respectively, based on Flt3 and CD127 (32–34).
Statistical analysis
The statistical significance between experimental groups was determined by unpaired two-tailed Student’s t test. p values = or< 0.05 were considered statistically significant. Results are presented as Mean ± SD.
Results
Bcl11bF/F/CD4-Cre mice exhibit increased control of tumors, independent of Bcl11b−/− CD8+ T cells
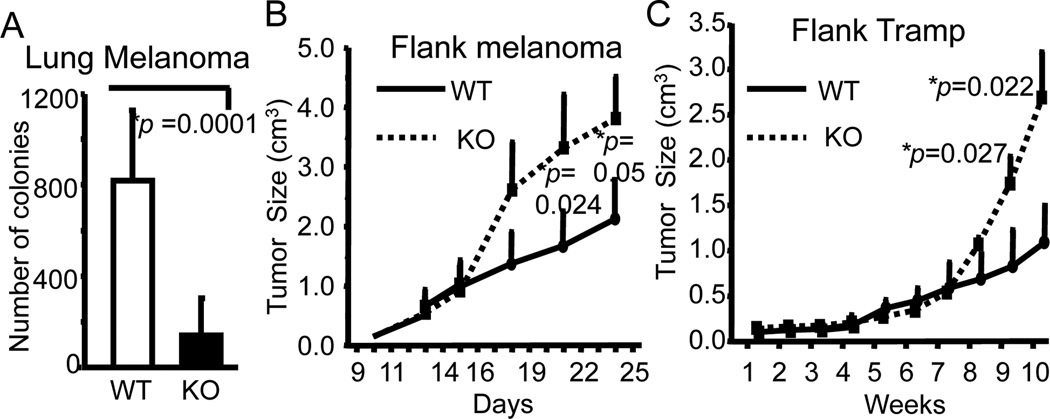
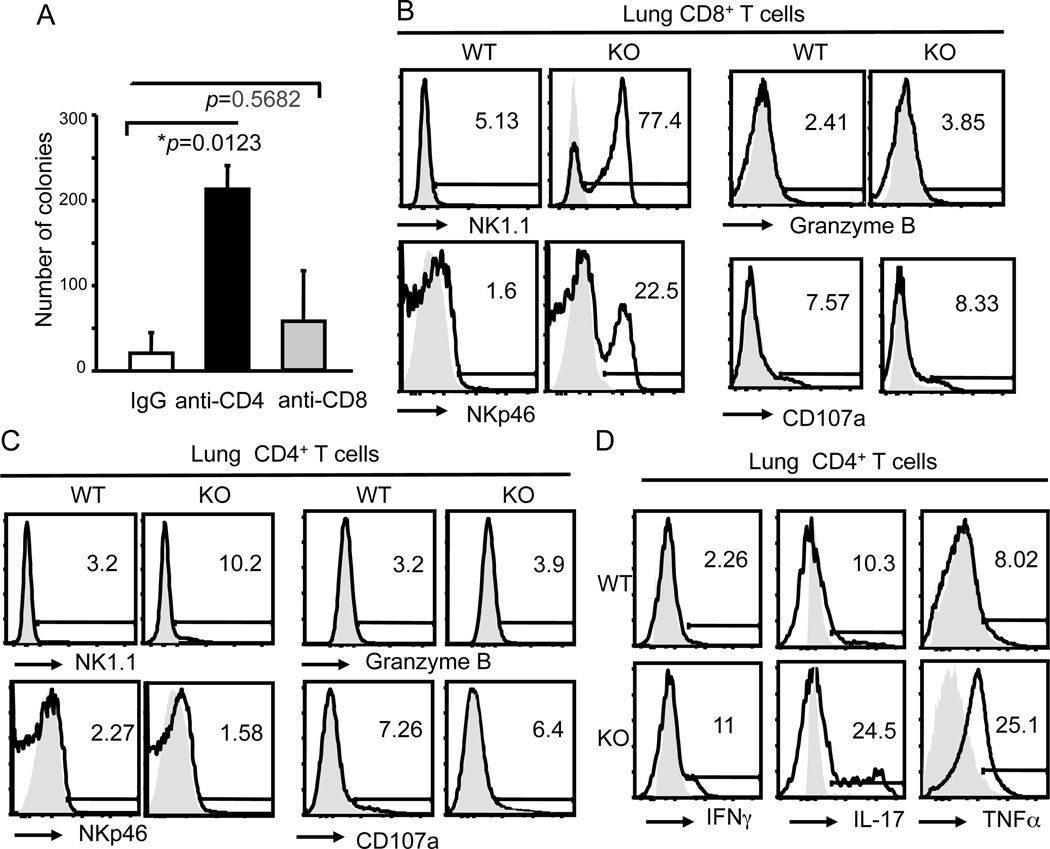
We employed several tumor models, specifically, metastatic B16-F10 (B16) melanoma, and flank grafts of B16 melanoma and Tramp prostate cancer cells, to test in vivo the contribution of Bcl11b−/− T cell populations in anti-tumor immune response, using Bcl11bF/F/CD4-Cre mice (25). Bcl11bF/F/CD4-Cre mice were previously shown to have reduced numbers of T cells in the periphery (25), yet the number of the tumor nodules in the lung was approximately eight times reduced in Bcl11bF/F/CD4-Cre mice i.v. transferred with B16 melanoma cells, compared to wild type mice (Fig. 1A and Supplementary Fig. 1). Similarly, the tumor volumes were significantly reduced in the grafts initiated with melanoma or Tramp prostate cancer cells (Fig. 1B and C). Ex vivo removal of Bcl11b in DP thymocytes has been shown previously to generate CD8+ T cells reprogrammed to ITNK cells highly efficient in anti-tumor immune response when transferred into Rag2−/−gc−/− mice (26). To test the contribution of Bcl11b−/− CD8+ T cells to the anti-tumor immune response we antibody-depleted CD8+ T cells (Supplementary Fig. 1B), which did not lead to a statistically distinguishable increase in lung metastases (Fig. 2A). Despite the fact that a large percentage of Bcl11b−/− CD8+ T cells in the lungs with metastatic tumors of Bcl11bF/F/CD4-Cre mice upregulated NK1.1 and NKp46 (Fig. 2B), these cells expressed only modest Granzyme B levels and exhibited reduced degranulation based on surface expression of CD107a (Fig. 2B). For comparison, see levels of Granzyme B and CD107a in NK cells (Fig. 3B). The reduced levels of Granzyme B and surface CD107a could explain the modest contribution of Bcl11b−/− CD8+ T cells in the anti-tumor immune response, compared to the previously described reprogrammed Bcl11b−/− ITNK CD8+ T cells which were shown to express elevated levels of effector molecules (26). Thus, in the Bcll1bF/F/CD4-Cre system we observed enhanced anti-tumor control that did not depend on CD8+ T cells.
Figure 1. Bcl11bF/F/CD4-Cre mice have significantly reduced tumors in metastatic melanoma, and flank melanoma and Tramp syngeneic graft models.
(A) Numbers of tumor nodules in lungs of Bcl11bF/F/CD4-Cre (KO) and wild type (WT) mice on Day 10 post tumor injection. N=9. (B) Size of the flank melanomas in Bcl11bF/F/CD4-Cre (KO) and wild type (WT) mice measured on the indicated days. Five pairs of mice were evaluated. (C) Size of the flank Tramp tumors in Bcl11bF/F/CD4-Cre (KO) and wild type (WT) mice measured for 10 weeks. N=8. (A–C) p values are indicated. Mean ± SD.
Figure 2. Bcl11b−/− CD4+ T cells, but not CD8+ T cells, have critical role in reduced metastatic lung melanoma tumor burden.
(A) Numbers of tumor colonies in lungs of Bcl11bF/F/CD4-Cre mice treated with anti-CD4, anti-CD8 antibodies or IgG. p values are indicated. Mean ± SD. (B and C) Frequencies of NK1.1, NKp46, Granzyme B and surface CD107a expressing CD8+ T (B) and CD4+ T (C) cells from lungs of Bcl11bF/F/CD4-Cre (KO) and wild type (WT) mice on Day 10 post tumor injection, evaluated by FACS. Data are representative of six pairs of mice. (D) Frequencies of IFNγ-, IL17- and TNF-producing CD4+ T cells in the lungs of Bcl11bF/F/CD4-Cre (KO) and wild type (WT) mice, evaluated by FACS. Data are representative of six pairs of mice.
Figure 3. NK cells express elevated levels of Granzyme B and degranulation and are critical for reduced tumor burden in Bcl11bF/F/CD4-Cre mice.
(A) Representative FACS data of NK1.1+CD3i−CD4−CD8− NK cells and NK1.1+CD3i+CD4−CD8− iTNK cells; (B) absolute numbers of NK1.1+CD3i−CD4−CD8− NK cells and NK1.1+CD3i+CD4−CD8− iTNK cells in lungs with metastatic melanoma from Bcl11bF/F/CD4-Cre (KO) and wild type (WT) mice. Data is representative of five independent experiments. (C) BrdU incorporation in lung NK1.1+CD3i−CD4−CD8− NK cells and NK1.1+CD3i+CD4−CD8− iTNK cells of mice with metastatic lung tumors. Mice were i.p. injected with 1mg BrdU 6 hrs before being sacrificed. Data are representative of three independent experiments. (D and E) Levels of Granzyme B and surface CD107a in NK1.1+CD3i−CD4−CD8− NK cells (D) and NK1.1+CD3i+CD4−CD8− iTNK cells (E) from lungs with metastatic melanoma of Bcl11bF/F/CD4-Cre (KO) and wild type (WT) mice. (F) Numbers of tumor colonies in the lungs of Bcl11bF/F/CD4-Cre mice treated with anti-NK1.1 antibodies or IgG. p values are indicated. Mean ± SD. CD3i+ and CD3i− cells are positive and negative, respectively, for intracellular CD3.
Bcl11b−/− CD4+ T cells play a critical role in protection against metastatic lung melanoma
It has been reported that tumor specific CD4+ T cells are able to eradicate tumors by developing cytotoxic activity (35). To test the contribution of CD4+ T cells in control of tumor growth, we antibody-depleted CD4+ T cells (Supplementary Fig. 1B) which caused a significant increase in the numbers of tumor nodules compared to IgG-treated Bcl11bF/F/CD4-Cre mice (Fig. 2A). Bcl11b−/− CD4+ T cells only minimally upregulated NK1.1, but not NKp46, and they neither expressed Granzyme B nor evidenced degranulation (Fig. 2C). We previously demonstrated that CD4+ T cells of Bcl11bF/F/CD4-Cre mice had an activated phenotype (25, 31) and produced elevated levels of proinflammatory cytokines, specifically TNFα, IFNγ and IL-17 (31). Elevated levels of TNFα, IFNγ and IL-17 were also produced by Bcl11b−/− CD4+ T cells in the lungs of mice with metastatic lung tumors (Fig. 2D). These results taken together demonstrate that Bcl11b−/− CD4+ T cells play an important role in antitumor immune response, however without upregulation of cytotoxic markers.
NK cells are increased in Bcl11bF/F/CD4-Cre mice and essential for reduced tumor burden
NK cells are known to play a critical role in the anti-tumor immune response to B16 melanoma (36). This is because B16 melanoma cells express reduced levels of MHC class I (37), however they express ligands for NKp46 and NKG2D receptors, which are recognized by, and engage NK cells (reviewed in (10)). Our previous results showed that Bcl11bF/F/CD4-Cre mice have a 3-fold increase in splenic NK cells (25). To identify the NK population, we used a gating strategy that eliminates not only CD8+ T cells, but also cells that still have intracellular CD3. In this way we defined two populations: (1) NK1.1+CD4−CD8− CD3i− cells, considered real NK cells, and (2) NK1.1+CD4−CD8− CD3i+, which are CD8+ T cells that downregulated the co-receptor CD8, express CD3 only intracellularly, and are likely a subpopulation of ITNKs. Both populations were increased in lungs with metastatic tumors of Bcl11bF/F/CD4-Cre mice, compared to control wild type mice (Fig. 3A and B), however the NK1.1+CD4−CD8−CD3i+ cells only represented approximately 25% of the NK1.1+CD4−CD8−CD3i− NK population (Fig. 3A and B). Both populations had increased levels of the activating receptors NKp46 and NKG2D and reduced levels of inhibitory receptor Ly49C (Supplementary Fig. 2A and B). In addition, both populations showed modest incorporation of BrdU, suggesting limited proliferation (Fig. 3C). However only the NK1.1+CD4−CD8−CD3i− NK population expressed elevated levels of Granzyme B and showed high degranulation (Fig. 3D and E). To further demonstrate the role of NK1.1+ cells in the antitumor immune response in Bcl11bF/F/CD4-Cre mice, we antibody-depleted NK1.1+ cells (Supplementary Fig. 1B) which caused a significant increase in tumor burden (Fig. 3F). Collectively, these results demonstrate that NK1.1+ cells play a critical role in the anti-tumor immune response in Bcl11bF/F/CD4-Cre mice, within which the NK1.1+CD4−CD8−CD3i− NK population is predominant in number and is the only population that exhibited elevated Granzyme B levels and degranulation.
TAMs and MDSCs of Bcl11bF/F/CD4-Cre mice have reduced Arginase 1 levels
TAMs and MDSCs are known to suppress the antitumor immune response and one mechanism responsible for this effect is production of elevated levels of Arginase-1 (17, 38). We therefore evaluated the CD11b+F4/80+CD11c− cells in the metastatic lungs of Bcl11bF/F/CD4-Cre mice and found that these cells were similar numerically between Bcl11bF/F/CD4-Cre and wild type mice, however the levels of Arginase-1 were reduced in Bcl11bF/F/CD4-Cre mice compared to wild mice (Supplementary Fig. 3A). MDSCs were elevated in numbers in the lung with metastatic melanoma, however their Arginase-1 levels were severely reduced (Supplementary Fig. 3B). These results collectively demonstrate that though MDSCs are increased in the metastatic lungs of Bcl11bF/F/CD4-Cre mice, their Arginase 1 levels, as well as that of TAMs, was substantially reduced, suggesting an altered suppressive function.
Hematopoietic stem and progenitor cells (HSPCs), as well as other progenitor populations are increased in the spleen and BM of Bcl11bF/F/CD4-Cre mice
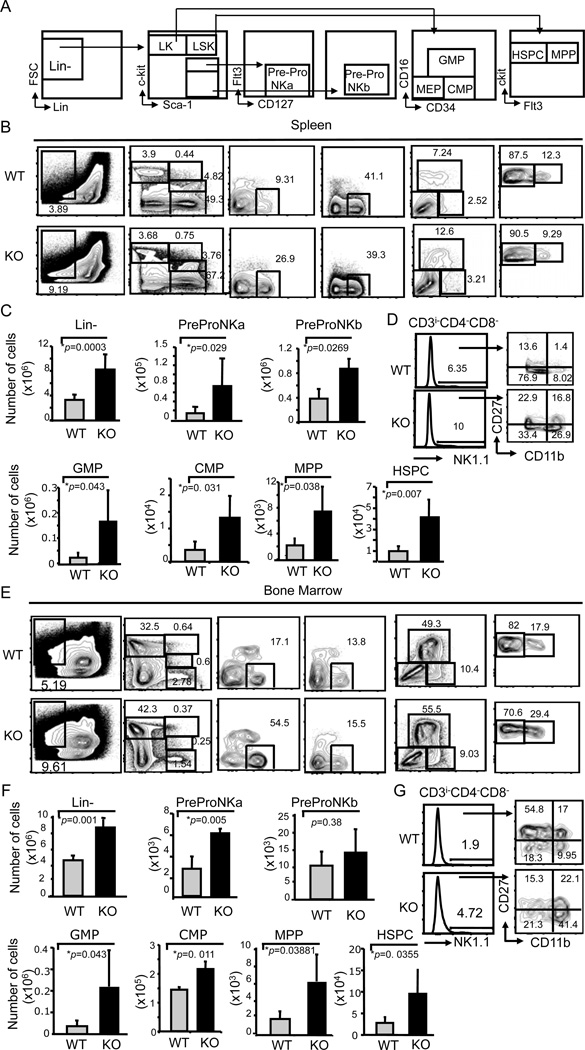
Bcl11b is not expressed in myeloid cells, however it is expressed in NK cells (26). Importantly, the CD4-Cre system does not remove floxed alleles in NK cells, thus excluding a direct effect of Bcl11b deficiency on increased NK cell numbers. Considering the increased numbers of NK1.1+CD4−CD8−CD3i− NK cells, however in the absence of proliferation, and of MDSCs in Bcl11bF/F/CD4-Cre mice and the fact that previously we observed that these mice had splenomegaly (25) and exhibited extramedullary hematopoiesis (Albu and Avram, 2007, unpublished observation), we investigated hematopoiesis both in BM and spleen in the mice with lung metastatic tumors and found an increase in the lin− population both in the spleen and BM (Fig. 4A–C and E–F). The lin− population was definied as CD3−CD4−CD8−CD11b/Mac1−CD11c−CD19−B220−GR-1−NK1.1−Ter119−TCRβ−TCRγ−FcR1−. Specifically, HSPCs, multipotent progenitors (MPPs), common myeloid progenitors (CMPs) and granulocyte macrophage progenitors (GMPs) were all increased in numbers (Fig. 4A–C, and E–F), as well as the NK cell progenitor populations, predominantly the PrePro NKa (Fig. 4A–C and E–F). PrePro NKa and PreProNKb cells were defined within the c-kitlowSca-1+ and c-kit− Sca-1+ cells, respectively, as Flt3−CD127hi (32–34). Mature NK cells, comprising NK1.1+CD27+/−CD11b+ cells (39, 40), as well as total NK cells, had higher frequencies as well, both in BM and spleen of Bcl11bF/F/CD4-Cre mice compared to control wild type mice (Fig. 4D and G). Thus, these results suggest that the increased numbers of NK1.1+CD4−CD8−CD3i− NK cells and MDSCs in the lung of Bcl11bF/F/CD4-Cre mice with metastatic tumors, is likely a consequence of increased hematopoiesis in the BM and extramedullary in the spleen.
Figure 4. Bcl11bF/F/CD4-Cre mice have increased bone marrow and splenic hematopoiesis.
(A) Diagram illustrating the gating strategy. Progenitor populations were evaluated in the lineage negative (lin−) CD3−CD4−CD8−CD11b/Mac1−CD11c−CD19−B220− GR-1− NK1.1−Ter119− TCRβ−, γTCR−FcR1− cells. LK are Lin−/c-kit+/Sca-1−, LSK are Lin−/c-kit+Sca-1+. NK precursors, Pre-Pro-NKa and Pre-Pro-NKb, are CD127+Flt3− within the gated c-kitlosca-1+ and c-kit−sca-1+, respectively, populations. Multipotent progenitors (MPP) and hematopoietic stem and progenitor cells (HSPC) are Flt3+ and Flt3−, respectively, within the LSK population. Granulocyte macrophage progenitors (GMP) and common myeloid progenitors (CMP) were defined based on CD16 and CD34 in the LK population. Frequencies (B and E) and absolute numbers (C and F) of lin−, Pre-Pro-NKa, Pre-Pro-NKb, GMP, CMP, MPP and HSPC populations in spleen (B and C) and bone marrow (E and F) of Bcl11bF/F/CD4-Cre (KO) and wild type (WT) mice with lug metastatic melanoma. (D and G) Frequencies of NK1.1+CD3i−CD4−CD8− cells and mature NK cells (CD11b+ CD27+/−) in the spleen (D) and bone marrow (G). Data are representative of five independent experiments. p values are indicated. Mean ± SD.
TNFα plays a key role in reduced tumor burden in Bcl11bF/F/CD4-Cre mice
Given the elevated levels of proinflammatory cytokines in Bcl11bF/F/CD4-Cre mice, we investigated their roles in the protection against tumors by treatment of Bcl11bF/F/CD4-Cre mice with neutralizing antibodies against TNFα, IFNγ and IL-17. While the treatment with TNFα-neutralizing antibodies resulted in a major increase in the tumor burden, treatment with anti-IFNγ or anti-IL17 antibodies had only a minimal, and statistically nonsignificant effect (Fig. 5A), demonstrating that TNFα has a critical role in reduced tumor burden in Bcl11bF/F/CD4-Cre mice.
Figure 5. TNFα is critical for reduced tumor burden, increased numbers of NK cells and increased hematopoiesis in Bcl11bF/F/CD4-Cre mice.
(A) Fold increase of tumor colonies in lungs of Bcl11bF/F/CD4-Cre mice treated with anti-TNF, anti-IL-17a, anti-IFN- antibodies or IgG. (B) Absolute numbers of NK1.1+CD3i−CD4−CD8− in the lung of Bcl11bF/F/CD4-Cre mice treated with anti-TNFα antibodies or IgG. (C) Absolute numbers of lin−, Pre-Pro-NKa, Pre-Pro-NKb, GMP, CMP, MPP and HSPC populations in the spleen of Bcl11bF/F/CD4-Cre mice with metastatic lung tumors treated with anti-TNFα antibodies or IgG. The gating strategies for progenitor cells are as described in Fig. 4. Data are representative of five independent experiments. p values are indicated. Mean ± SD.
TNFα is critical for the increase in the NK cell numbers and elevated splenic hematopoiesis in Bcl11bF/F/CD4-Cre mice with lung metastatic tumors
Both the increased numbers of NK cells and TNFα were required for reduced tumor burden in Bcl11bF/F/CD4-Cre mice. Considering that increased NK cells is likely to occur through a bystander effect, we next investigated whether TNFα plays a role in the increase in NK cell numbers and elevated hematopoiesis. Treatment with TNFα-neutralizing antibodies caused a marked decrease in the NK1.1+CD4−CD8−CD3i− NK cells in the lung of Bcl11bF/F/CD4-Cre mice with metastatic tumors (Fig. 5B), suggesting that TNFα is essential for the elevated numbers of NK cells. Further evaluation of the effect of anti-TNFα treatment on hematopoiesis revealed a reduction in the lin− population, including HSPCs, MMPs, CMPs and GMPs, as well as of the PrePro NKa/b populations in the spleen (Fig. 5C). However the treatment caused a less pronounced effect in the BM (data not shown). The more pronounced effect of TNFα on extramedullary hematopoieisis may be due to the known role of TNFα in mobilization (41). These results taken together demonstrate that TNFα is critical for reduced tumor burden, elevated numbers of NK cells in the lung and increased splenic hematopoiesis in Bcl11bF/F/CD4-Cre mice.
TNFα treatment of wild type mice with lung metastatic melanoma ameliorates tumor burden, increases NK cell numbers and elevates splenic hematopoiesis
To further demonstrate the implication of TNFα in the anti-tumor immune response, we treated wild type mice with lung metastatic melanoma with recombinant TNFα. TNFα treatment caused a significant reduction in the tumor burden, increased the numbers of NK cells (Fig. 6A and B) and MDSCs in the lung, however the percentages of Arginase 1+ TAMs and MDSCs were reduced (Supplementary Fig. 3C and D). Importantly, lung NK cells of TNFα-treated mice had elevated Granzyme B and degranulation (Fig. 6C). Additionally, lin− cells, HSPCs, MPPs, CMPs, GMPs, as well as NK progenitors were increased in the spleen, however not significantly in the BM (Fig. 6D and data not shown). We further investigated whether TNFα treatment caused increased proliferation of HSPCs and progenitors, and our results demonstrated that HSPCs, MPPs, GMPs, as well as PrePro NKa/b populations of TNFα treated wild mice with lung metastatic melanoma incorporated more BrdU than untreated controls (Fig. 6E). Similarly, HSPCs and progenitors of Bcl11bF/F/CD4-Cre mice had also increased BrdU incorporation (Fig. 6E). Collectively, these results suggest that TNFα promotes increased hematopoiesis through increased formation of progenitors cells, as well as increased proliferation of HSPCs and progenitor populations.
Figure 6. TNFα treatment of wild type mice with lung metastatic melanoma inhibits tumor development, increases numbers and activity of NK cells in the lung and splenic hematopoiesis.
(A) Numbers of tumor colonies in lungs of wild type mice treated with 1µg of TNF± or vehicle. (B) Absolute numbers of NK1.1+CD3i−CD4−CD8− in the lung of wild type mice treated with TNFα or vehicle. (C) Levels of Granzyme B and surface CD107a in NK1.1+CD3i−CD4−CD8− NK cells in TNFα-treated mice with metastatic tumors versus controls. (D) Absolute numbers of lin−, Pre-Pro-NKa, Pre-Pro-NKb, GMP, CMP, MPP and HSPC populations in the spleen of wild type mice with lung metastatic melanoma treated with TNFα or vehicle. Data are representative of five independent experiments. p values are indicated. Mean ± SD. (E) Frequencies of cells which incorporated BrdU within HSPC, Pre-Pro-NKa and Pre-Pro-NKb, MPP, GMP and CMP populations in the spleen of wild type mice treated with TNFα or vehicle and in Bcl11bF/F/CD4-Cre mice. Mice were i.p. injected with 1mg BrdU 6 hrs before being sacrificed. The gating strategy for progenitor cells is described in Fig. 4. Data are representative of three independent experiments.
In vitro TNFα treatment of HSPCs and progenitors accelerates formation of NK and myeloid cells
We further investigated the impact of TNF treatment on differentiation of lin− HSPCs and progenitors, and their potential to form NK and myeloid cells in vitro. In vitro TNF- treatment of the lin− cells derived from wild type and Bcl11bF/F/CD4-Cre mice with lung metastatic tumors enhanced the NK cell and myeloid cell formation, under the NK-promoting or myeloid-promoting conditions, respectively, compared to no treatment (Supplementary Fig. 4), further demonstrating the direct role of TNF in promoting the differentiation of HSPCs and progenitors.
Discussion
Here we demonstrate that Bcl11bF/F/CD4-Cre mice have reduced tumor burden in three tumor models. We further demonstrate that the reduced tumor burden was dependent on the NK1.1+ cells and CD4+ T cells, but not on CD8+ T cells. This was surprising, as it was previously demonstrated that ex vivo removal of Bcl11b in DP thymocytes resulted in generation of CD8+ T cells reprogrammed to ITNK cells efficient in anti-tumor immune response when transferred into Rag2−/−gc−/− mice (26). In our study, Bcl11b−/− CD8+ T cells neither expressed elevated Granzyme B levels nor exhibited enhanced degranulation. There are several possibilities which can explain why Bcl11b−/− CD8+ T cells of Bcl11bF/F/CD4-Cre mice failed in anti-tumor immune response compared to Bcl11b−/− CD8+ T cells generated ex vivo from DP thymocytes of Bcl11bF/F/ER-Cre mice followed by transfer in Rag2−/−gc−/− mice. Perhaps the ex vivo generation of ITNK cells is more efficient, as it does not have to pass the rigorous control of thymic selection, which is likely to cause deletion of numerous reactive cells. In addition, the transfer in Rag2−/−gc−/− mice offers the opportunity of expansion related to lymphopenia in these mice, which is more strictly controlled in Bcl11bF/F/CD4-Cre mice. In addition, in Bcl11bF/F/CD4-Cre mice the CD8+ T cells are also exposed to other T cell populations and a different environment, which further can shape them differently than in Rag2−/−gc−/− mice. Interestingly, when we removed Bcl11b from mature CD8+ T cells using the dLck-iCre system, Bcl11b−/− CD8+ T cells not only had reduced expansion in response to Listeria monocytogenes and influenza challenge, but they also exhibited reduced Granzyme B levels and reduced cytotoxicity (42). However, when effector Bcl11b−/− CD8+ T cells were generated in vitro in the presence of IL-2, starting with naïve CD8+ T cells of Bcl11bF/F/ER-Cre mice, in conditions in which the activation preceded the tamoxifen treatment, Bcl11b−/− CD8+ T cells did not downregulate perforin and Granzyme B mRNA levels (42). Taken together, these data suggest that a specific in vivo environment, versus the more controlled in vitro environment, is likely to change the phenotype of the cells.
Bcl11bF/F/CD4-Cre mice had two populations of NK1.1+CD4−CD8− cells: the real NK cells, negative for intracellular CD3 (NK1.1+CD3i−), and a population which expressed CD3 intracellularly (NK1.1+CD3i+). Importantly, of these two NK1.1+ populations, only the NK1.1+CD3i− exhibited increased Granzyme B and elevated degranulation, and also represented the numerically predominant population. Both populations expressed NKG2D and NKp46 activating receptors with roles in tumor surveillance (11, 12) and tumor progression, respectively (13). These findings suggest that NK1.1+CD3i− NK cells constitute the predominant NK1.1+ population responsible for the reduced tumor burden in Bcl11bF/F/CD4-Cre mice. Interestingly, NK cell numbers were found increased in the spleen (25) and in the lungs with metastatic tumor of Bcl11bF/F/CD4-Cre mice, as demonstrated here. Bcl11b is expressed in NK cells, however the CD4Cre system does not remove the gene in NK cells, suggesting that the increase in the NK population was caused by a bystander effect. The fact that Bcl11b−/− CD4+ T cells were shown to produce elevated levels of proinflammatory cytokines (31) prompted our investigation of their contribution to anti-tumor immune response. Whereas IFNγ and IL-17 were dispensable, TNFα was found critical for the reduced tumor burden in Bcl11bF/F/CD4-Cre mice and for the increased numbers of NK cells. Our results further demonstrated that TNFα was responsible for the elevated hematopoiesis, including extramedullary hematopoiesis in the spleen, which explains the increased numbers of NK and MDSCs cells in the lungs with metastatic tumors of Bcl11bF/F/CD4-Cre mice. In support of this notion TNFα treatment of wild type mice with lung metastatic tumors, not only reduced their tumor burden, but increased the NK cells in the lung and caused elevated splenic hematopoiesis. Thus TNFα contributes to generation of immune populations, by increasing hematopoiesis, particularly extramedullary, increasing therefore generation of NK cells. Though the numbers of MDSCs were increased too, their Arginase 1 levels were reduced, suggesting decreased suppressive activity. Additionally, TNFα increased formation of NK and myeloid cells in vitro starting from lin− HSPCs and progenitors. Whether TNFα exerts this effect by simply promoting increased proliferation of HSPCs and progenitors or accelerates formation of progenitors from HSPCs requires further investigation. It will be also important to determine whether the reduced Arginase 1 levels in MDSCs in Bcl11bF/F/CD4-Cre mice and in TNFα-treated wild type mice with tumors is a consequence of the tumor environment or altered generation of MDSCs.
We reason that a direct effect of TNFa on killing tumors is less likely to occur, as it has been previously demonstrated that B16 cells are resistant to direct TNFa-mediated killing (43). Moreover, knockdown of TNFaR1 in B16 cells had no impact on tumor burden in Bcl11bF/F/CD4-Cre mice or wild type mice treated with TNFa (data not shown). Thus, the major role of TNFa in this system, in preventing tumor growth, appears to be mediated through enhanced hematopoiesis.
We previously demonstrated that the Treg cells of Bcl11bF/F/CD4-Cre mice are less suppressive (31). Though we cannot exclude that their phenotype participated in reduced tumor burden in Bcl11bF/F/CD4-Cre mice, it was ultimately TNFa which was responsible for the reduced tumor burden, increased NK cell numbers, and their generation through increased splenic hematopoiesis. If Treg cells rather than TNFa were responsible for the reduced tumor burden in Bcl11bF/F/CD4-Cre mice, TNFa neutralization alone would not have caused such a dramatic change in tumor burden, as in these conditions the Treg cells were still present in Bcl11bF/F/CD4-Cre mice. Additionally we would not have expected to observe an impact of TNFa treatment on wild type mice, in which Treg cells were unaltered. Based on all these findings we propose that TNFa is effective in protection against tumors not only in blocking angiogenesis (20, 21) (and reviewed in (18, 19)), but also in shaping the anti-tumor immune response, by increasing splenic hematopoiesis and generation of NK cells. Previously TNFa was shown to increase mobilization of progenitor populations from BM, particularly of B cell progenitors, likely through suppression of CXCL12 (41, 44), and to enhance generation of dendritic cells in vitro (45). Reports in the literature suggest a complex role of TNFα on HSCs. While in vitro studies mostly demonstrated a suppressive effect on HSC renewal and maintenance (46, 47), in vivo studies suggested a stimulatory role of TNFa on HSC maintenance (48), but a suppressive impact on actively cycling HSCs (49). Other studies further showed a beneficial effect on hematopoietic progenitors (44) and a favorable effect on engraftment (50). Our findings support the idea that in mice with lung metastatic tumors TNFa production or its administration increases HSPC and progenitor cell numbers, as well as their proliferation, resulting in increased NK numbers and efficient anti-tumor immune response. Importantly, the dose of TNFa used in our treatments was much less than the doses observed to have toxicity (51, 52) and none of the treated mice died (data not shown). Our observation that TNFa increases formation of NK cells from Lin− HSPC and progenitor cells under NK differentiation conditions, opens an avenue for therapeutic generation of NK cells for transplantation to promote anti-tumor immune responses.
Supplementary Material
Acknowledgments
We acknowledge Drs. Hung Le and Chandra S. Bapanpally for the flank melanoma and the flank TRAMP prostate grafts experiments. We acknowledge Dr. Diana Albu for the preliminary observations of splenic extramedullary hematopoiesis. We greatly acknowledge Drs. Neal G. Copeland and Nancy A. Jenkins (MHRI) for mice. We acknowledge Mr. Adrian Avram for graphical presentation, and Dr. Douglas Cohn, Ms. Victoria Boppert and Ms. Hattie Wang for care of the mice.
Abbreviations
- NK cell
natural killer cell
- TNFα
tumor necrosis factor alpha
- IL-17
interleukin-17
- IFNγ
interferon gamma
- CTLs
cytotoxic T cells
- TAMs
tumor-associated macrophages
- MDSCs
myeloid derived suppressor cells
- ITNK
induced T-to–natural killer
- GMP
granulocyte macrophage progenitors
- CMP
common myeloid progenitors
- MPP
multipotent progenitors
- HSPC
hematopoietic stem and progenitor cells
Footnotes
The authors disclose no potential conflicts of interest.
Grant support: AI067846 and AI078273 to Dorina Avram
Contributor Information
Mohammad N. Uddin, Email: UddinM@mail.amc.edu.
Yubin Zhang, Email: zhangy3@mail.amc.edu.
Jonathan A. Harton, Email: HartonJ@mail.amc.edu.
Katherine C. MacNamara, Email: MacnamK@mail.amc.edu.
Dorina Avram, Email: avramd@mail.amc.edu.
References
- 1.Perez-Diez A, Joncker NT, Choi K, Chan WF, Anderson CC, Lantz O, Matzinger P. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood. 2007;109:5346–5354. doi: 10.1182/blood-2006-10-051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, Muranski P, Restifo NP, Antony PA. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J Exp Med. 2010;207:651–667. doi: 10.1084/jem.20091921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schietinger A, Philip M, Liu RB, Schreiber K, Schreiber H. Bystander killing of cancer requires the cooperation of CD4(+) and CD8(+) T cells during the effector phase. J Exp Med. 2010;207:2469–2477. doi: 10.1084/jem.20092450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fransen MF, Sluijter M, Morreau H, Arens R, Melief CJ. Local activation of CD8 T cells and systemic tumor eradication without toxicity via slow release and local delivery of agonistic CD40 antibody. Clin Cancer Res. 2011;17:2270–2280. doi: 10.1158/1078-0432.CCR-10-2888. [DOI] [PubMed] [Google Scholar]
- 5.Vujanovic L, Butterfield LH. Melanoma cancer vaccines and anti-tumor T cell responses. J Cell Biochem. 2007;102:301–310. doi: 10.1002/jcb.21473. [DOI] [PubMed] [Google Scholar]
- 6.Baitsch L, Baumgaertner P, Devevre E, Raghav SK, Legat A, Barba L, Wieckowski S, Bouzourene H, Deplancke B, Romero P, Rufer N, Speiser DE. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest. 2011;121:2350–2360. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuda T, Maekawa K, Asano K, Hisamitsu T. Suppressive effect of juzen-taiho-to on lung metastasis of b16 melanoma cells in vivo. Evid Based Complement Alternat Med. 2011;2011:743153. doi: 10.1093/ecam/nen081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voskoboinik I, Smyth MJ, Trapani JA. Perforin-mediated target-cell death and immune homeostasis. Nat Rev Immunol. 2006;6:940–952. doi: 10.1038/nri1983. [DOI] [PubMed] [Google Scholar]
- 9.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 10.Burke S, Lakshmikanth T, Colucci F, Carbone E. New views on natural killer cell-based immunotherapy for melanoma treatment. Trends Immunol. 2010;31:339–345. doi: 10.1016/j.it.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Smyth MJ, Swann J, Cretney E, Zerafa N, Yokoyama WM, Hayakawa Y. NKG2D function protects the host from tumor initiation. J Exp Med. 2005;202:583–588. doi: 10.1084/jem.20050994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NM, Raulet DH. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–580. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lakshmikanth T, Burke S, Ali TH, Kimpfler S, Ursini F, Ruggeri L, Capanni M, Umansky V, Paschen A, Sucker A, Pende D, Groh V, Biassoni R, Hoglund P, Kato M, Shibuya K, Schadendorf D, Anichini A, Ferrone S, Velardi A, Karre K, Shibuya A, Carbone E, Colucci F. NCRs and DNAM-1 mediate NK cell recognition and lysis of human and mouse melanoma cell lines in vitro and in vivo. J Clin Invest. 2009;119:1251–1263. doi: 10.1172/JCI36022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 2011;7:651–658. doi: 10.7150/ijbs.7.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32:19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Horssen R, Ten Hagen TL, Eggermont AM. TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist. 2006;11:397–408. doi: 10.1634/theoncologist.11-4-397. [DOI] [PubMed] [Google Scholar]
- 19.ten Hagen TL, Seynhaeve AL, Eggermont AM. Tumor necrosis factor-mediated interactions between inflammatory response and tumor vascular bed. Immunol Rev. 2008;222:299–315. doi: 10.1111/j.1600-065X.2008.00619.x. [DOI] [PubMed] [Google Scholar]
- 20.Baxevanis CN, Voutsas IF, Tsitsilonis OE, Tsiatas ML, Gritzapis AD, Papamichail M. Compromised anti-tumor responses in tumor necrosis factor-alpha knockout mice. Eur J Immunol. 2000;30:1957–1966. doi: 10.1002/1521-4141(200007)30:7<1957::AID-IMMU1957>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 21.Ostensen ME, Thiele DL, Lipsky PE. Tumor necrosis factor-alpha enhances cytolytic activity of human natural killer cells. J Immunol. 1987;138:4185–4191. [PubMed] [Google Scholar]
- 22.Avram D, Fields A, Senawong T, Topark-Ngarm A, Leid M. COUP-TF (chicken ovalbumin upstream promoter transcription factor)-interacting protein 1 (CTIP1) is a sequence-specific DNA binding protein. Biochem J. 2002;368:555–563. doi: 10.1042/BJ20020496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avram D, Fields A, Pretty On Top K, Nevrivy DJ, Ishmael JE, Leid M. Isolation of a novel family of C(2)H(2) zinc finger proteins implicated in transcriptional repression mediated by chicken ovalbumin upstream promoter transcription factor (COUP-TF) orphan nuclear receptors. J Biol Chem. 2000;275:10315–10322. doi: 10.1074/jbc.275.14.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wakabayashi Y, Watanabe H, Inoue J, Takeda N, Sakata J, Mishima Y, Hitomi J, Yamamoto T, Utsuyama M, Niwa O, Aizawa S, Kominami R. Bcl11b is required for differentiation and survival of alphabeta T lymphocytes. Nat Immunol. 2003;4:533–539. doi: 10.1038/ni927. [DOI] [PubMed] [Google Scholar]
- 25.Albu DI, Feng D, Bhattacharya D, Jenkins NA, Copeland NG, Liu P, Avram D. BCL11B is required for positive selection and survival of double-positive thymocytes. J Exp Med. 2007;204:3003–3015. doi: 10.1084/jem.20070863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li P, Burke S, Wang J, Chen X, Ortiz M, Lee SC, Lu D, Campos L, Goulding D, Ng BL, Dougan G, Huntly B, Gottgens B, Jenkins NA, Copeland NG, Colucci F, Liu P. Reprogramming of T cells to natural killer-like cells upon Bcl11b deletion. Science. 2010;329:85–89. doi: 10.1126/science.1188063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albu DI, VanValkenburgh J, Morin N, Califano D, Jenkins NA, Copeland NG, Liu P, Avram D. Transcription factor Bcl11b controls selection of invariant natural killer T-cells by regulating glycolipid presentation in double-positive thymocytes. Proc Natl Acad Sci U S A. 2011;108:6211–6216. doi: 10.1073/pnas.1014304108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams NS, Moore TA, Schatzle JD, Puzanov IJ, Sivakumar PV, Zlotnik A, Bennett M, Kumar V. Generation of lytic natural killer 1.1+, Ly-49-cells from multipotential murine bone marrow progenitors in a stroma-free culture: definition of cytokine requirements and developmental intermediates. J Exp Med. 1997;186:1609–1614. doi: 10.1084/jem.186.9.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aliahmad P, de la Torre B, Kaye J. Shared dependence on the DNA-binding factor TOX for the development of lymphoid tissue-inducer cell and NK cell lineages. Nat Immunol. 2010;11:945–952. doi: 10.1038/ni.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albu DI, Califano D, Avram D. Flow cytometry analysis of transcription factors in T lymphocytes. Methods Mol Biol. 2010;647:377–390. doi: 10.1007/978-1-60761-738-9_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vanvalkenburgh J, Albu DI, Bapanpally C, Casanova S, Califano D, Jones DM, Ignatowicz L, Kawamoto S, Fagarasan S, Jenkins NA, Copeland NG, Liu P, Avram D. Critical role of Bcl11b in suppressor function of T regulatory cells and prevention of inflammatory bowel disease. J Exp Med. 2011;208:2069–2081. doi: 10.1084/jem.20102683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynaud D, Pietras E, Barry-Holson K, Mir A, Binnewies M, Jeanne M, Sala-Torra O, Radich JP, Passegue E. IL-6 controls leukemic multipotent progenitor cell fate and contributes to chronic myelogenous leukemia development. Cancer Cell. 2011;20:661–673. doi: 10.1016/j.ccr.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carotta S, Pang SH, Nutt SL, Belz GT. Identification of the earliest NK-cell precursor in the mouse BM. Blood. 2011;117:5449–5452. doi: 10.1182/blood-2010-11-318956. [DOI] [PubMed] [Google Scholar]
- 34.Ramirez K, Chandler KJ, Spaulding C, Zandi S, Sigvardsson M, Graves BJ, Kee BL. Gene deregulation and chronic activation in natural killer cells deficient in the transcription factor ETS1. Immunity. 2012;36:921–932. doi: 10.1016/j.immuni.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, Restifo NP, Allison JP. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207:637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27:5932–5943. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- 37.Kawano Y, Taniguchi K, Toshitani A, Nomoto K. Synergistic defense system by cooperative natural effectors against metastasis of B16 melanoma cells in H-2-associated control: different behavior of H-2+ and H2− cells in metastatic processes. J Immunol. 1986;136:4729–4734. [PubMed] [Google Scholar]
- 38.Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, Antonia S, Ochoa JB, Ochoa AC. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 39.Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113:5488–5496. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- 40.Kim S, Iizuka K, Kang HS, Dokun A, French AR, Greco S, Yokoyama WM. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol. 2002;3:523–528. doi: 10.1038/ni796. [DOI] [PubMed] [Google Scholar]
- 41.Ueda Y, Yang K, Foster SJ, Kondo M, Kelsoe G. Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression. J Exp Med. 2004;199:47–58. doi: 10.1084/jem.20031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang S, Rozell M, Verma RK, Albu DI, Califano D, VanValkenburgh J, Merchant A, Rangel-Moreno J, Randall TD, Jenkins NA, Copeland NG, Liu P, Avram D. Antigen-specific clonal expansion and cytolytic effector function of CD8+ T lymphocytes depend on the transcription factor Bcl11b. J Exp Med. 2010;207:1687–1699. doi: 10.1084/jem.20092136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeda K, Nakayama M, Sakaki M, Hayakawa Y, Imawari M, Ogasawara K, Okumura K, Smyth MJ. IFN-gamma production by lung NK cells is critical for the natural resistance to pulmonary metastasis of B16 melanoma in mice. J Leukoc Biol. 2011;90:777–785. doi: 10.1189/jlb.0411208. [DOI] [PubMed] [Google Scholar]
- 44.Mizrahi K, Stein J, Yaniv I, Kaplan O, Askenasy N. TNF-alpha has tropic rather than apoptotic activity in human hematopoietic progenitors: involvement of TNF receptor-1 and caspase-8. Stem Cells. 2013;31:156–166. doi: 10.1002/stem.1259. [DOI] [PubMed] [Google Scholar]
- 45.Santiago-Schwarz F, McCarthy M, Tucci J, Carsons SE. Neutralization of tumor necrosis factor activity shortly after the onset of dendritic cell hematopoiesis reveals a novel mechanism for the selective expansion of the CD14-dependent dendritic cell pathway. Blood. 1998;92:745–755. [PubMed] [Google Scholar]
- 46.Bryder D, Ramsfjell V, Dybedal I, Theilgaard-Monch K, Hogerkorp CM, Adolfsson J, Borge OJ, Jacobsen SE. Self-renewal of multipotent long-term repopulating hematopoietic stem cells is negatively regulated by Fas and tumor necrosis factor receptor activation. J Exp Med. 2001;194:941–952. doi: 10.1084/jem.194.7.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dybedal I, Bryder D, Fossum A, Rusten LS, Jacobsen SE. Tumor necrosis factor (TNF)-mediated activation of the p55 TNF receptor negatively regulates maintenance of cycling reconstituting human hematopoietic stem cells. Blood. 2001;98:1782–1791. doi: 10.1182/blood.v98.6.1782. [DOI] [PubMed] [Google Scholar]
- 48.Rebel VI, Hartnett S, Hill GR, Lazo-Kallanian SB, Ferrara JL, Sieff CA. Essential role for the p55 tumor necrosis factor receptor in regulating hematopoiesis at a stem cell level. J Exp Med. 1999;190:1493–1504. doi: 10.1084/jem.190.10.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pronk CJ, Veiby OP, Bryder D, Jacobsen SE. Tumor necrosis factor restricts hematopoietic stem cell activity in mice: involvement of two distinct receptors. J Exp Med. 2011;208:1563–1570. doi: 10.1084/jem.20110752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pearl-Yafe M, Mizrahi K, Stein J, Yolcu ES, Kaplan O, Shirwan H, Yaniv I, Askenasy N. Tumor necrosis factor receptors support murine hematopoietic progenitor function in the early stages of engraftment. Stem Cells. 2010;28:1270–1280. doi: 10.1002/stem.448. [DOI] [PubMed] [Google Scholar]
- 51.Duprez L, Takahashi N, Van Hauwermeiren F, Vandendriessche B, Goossens V, Vanden Berghe T, Declercq W, Libert C, Cauwels A, Vandenabeele P. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity. 2011;35:908–918. doi: 10.1016/j.immuni.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 52.Huys L, Van Hauwermeiren F, Dejager L, Dejonckheere E, Lienenklaus S, Weiss S, Leclercq G, Libert C. Type I interferon drives tumor necrosis factor-induced lethal shock. J Exp Med. 2009;206:1873–1882. doi: 10.1084/jem.20090213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.