Abstract
Up-regulated expression of telomerase reverse transcriptase (TERT) and subsequent maintenance of telomere length are essential in tumor development. Recent studies have implicated somatic gain-of-function mutations at the TERT promoter as one of the mechanisms that promote transcriptional activation of TERT; however, it remains unclear whether this genetic abnormality is prevalent in gynecologic neoplasms. We performed mutational analysis in a total of 525 gynecological cancers, and correlated TERT promoter mutations with clinicopathological features. With the exception of ovarian clear cell carcinomas, in which mutations were found in 37 (15.9%) of 233 cases, the majority of gynecologic malignancies were wild-type. TERT promoter mutation does not appear to be an early event during oncogenesis, as it was not detected in the contiguous endometriosis associated with ovarian clear cell carcinoma. Ovarian clear cell carcinoma cell lines with TERT promoter mutations exhibited higher TERT mRNA expression than those with wild-type sequences (p = 0.0238). TERT promoter mutation tended to be mutually exclusive with loss of ARID1A protein expression (p= 4.4×10−9) and PIK3CA mutation (p= 0.0019) in ovarian clear cell carcinomas. No associations with disease-specific survival were observed for ovarian clear cell carcinoma. The above results, in conjunction with our previous report showing longer telomeres in ovarian clear cell carcinomas relative to other types of ovarian cancer, suggests that aberrations in telomere biology may play an important role in the pathogenesis of ovarian clear cell carcinoma.
Keywords: clear cell carcinoma, telomerase, TERT promoter, telomere, ARID1A, PIK3CA
Introduction
Telomeres are DNA-protein complexes that protect the ends of eukaryotic chromosomes by preventing them from being recognized as DNA double-strand breaks, which inappropriately trigger DNA damage response pathways and may lead to cellular senescence or illegitimate fusion of chromosomal ends [1]. The termini of a linear DNA template cannot be completely replicated by conventional DNA-dependent DNA polymerases, and thus, in the absence of mechanisms to counter this effect, telomeres of eukaryotic cells shorten every round of DNA replication. Telomere shortening in somatic cells limits the life span of somatic cells and acts as a natural barrier to oncogenic transformation [2]. To maintain unlimited replication potential, cancer cells evolve mechanisms to maintain telomere length [3]. Most cancers, especially carcinomas, maintain their telomere length by increasing the activity of telomerase reverse transcriptase (TERT), the catalytic unit of the telomerase that adds the six-nucleotide telomere sequences to the end of chromosomes [4]. This is made possible through a variety of mechanisms, including amplification of the TERT gene [5], expression of transcriptional activators of TERT [6], and CpG methylation at the TERT promoter [7]. Some cancers maintain telomere length through a telomerase-independent mechanism called alternative lengthening of telomeres [8], which is thought to be dependent on homologous recombination [9].
Recently, somatic mutations at the TERT promoter in human cancer have been reported in two independent studies using whole genome sequencing on sporadic melanomas and multipoint linkage analysis in melanoma-prone families [10, 11]. Both studies demonstrated an unusually high frequency of TERT promoter mutations in sporadic melanomas; more than 70% of cases studied harbored such mutations [10, 11]. Subsequent studies reported TERT promoter mutations in other malignancies including glioma, urinary bladder carcinoma, tongue squamous cell carcinoma, and hepatocellular carcinoma [12–14]. The majority of reported mutations are located at two hot-spots, both of which create an 11-bp sequence, resembling the binding motif for ETS-domain transcription factors [10, 11]. Mutations in these hot-spots were shown to enhance transcriptional activity of the TERT promoter in vitro, and were thought to increase the expression of TERT in cancer cells [11].
Cancer genomes have been recently sequenced in a number of gynecologic neoplasms including high-grade ovarian serous carcinoma, low-grade ovarian serous carcinoma, ovarian clear cell carcinoma, uterine endometrioid carcinoma, and uterine serous carcinoma [15–19]. However, the prevalence and clinical significance of TERT promoter mutations in gynecologic malignancies remains largely unclear because non-coding regions, including promoter sequences, were not included in the previous analyses. In this study, we analyzed TERT promoter mutations in a total of 525 gynecological malignancies, and evaluated the clinical significance of TERT promoter mutations in those tumors.
Materials and Methods
Screening TERT Promoter Mutations in Gynecological Cancers
A total of 250 anonymous fresh frozen tissues were obtained from the Johns Hopkins Hospital (Baltimore, USA), and 275 anonymous formalin-fixed paraffin-embedded (FFPE) tissues were obtained from Asan Medical Center (Seoul, Korea), National Taiwan University Hospital (Taipei, Taiwan), Seirei Mikatahara General Hospital (Hamamatsu, Japan), Toronto General Hospital (Toronto, Canada), and University of Tokyo Hospital (Tokyo, Japan). All samples were procured under appropriate approval of Institutional Review Board. Hematoxylin and eosin stained sections were re-reviewed by pathologists (RC, AA, IS) to confirm the diagnosis before experiments were performed. Genomic DNA from frozen tissue was extracted by the DNeasy blood and tissue kit (Qiagen, Valencia, CA). For FFPE tissues, tumor components were manually dissected from 10 µm sections to reduce normal tissue contamination. Genomic DNA of dissected tumor tissue was then extracted with the QIAmp DNA FFPE tissue kit (Qiagen, Valencia, CA). We obtained genomic DNA from a total of 525 gynecological malignancies, including 389 ovarian carcinomas, 58 uterine corpus malignancies and 78 uterine cervical carcinomas. More specifically, the ovarian carcinomas included 233 clear cell carcinomas (36 fresh frozen and 197 FFPE), 43 endometrioid carcinomas (fresh frozen), 80 high-grade serous carcinomas (fresh frozen), and 33 low-grade serous carcinomas (fresh frozen). The uterine corpus malignancies included 24 uterine endometrioid carcinomas (fresh frozen), 12 uterine serous carcinomas (fresh frozen), and 22 leiomyosarcomas (fresh frozen). The uterine cervical carcinomas included 53 squamous carcinomas (FFPE) and 25 endocervical adenocarcinomas (FFPE). The source and type of each tissue specimen are specified in the supplemental dataset S1.
The TERT promoter region containing the two mutation hot spots (chr5: 1,295,228 and 1,295,250; hg19) were amplified by polymerase chain reaction (PCR) using the following primers: 5’-M13-CAGCGCTGCCTGAAACTC-3’ and 5’-GTCCTGCCCCTTCACCTT-3’, where M13 is a universal sequencing primer with sequence 5’-GTAAAACGACGGCCAGT-3’. PCR was performed using the following conditions: 94°C for 2 minutes; 3 cycles at 94°C for 15 seconds, 64°C for 30 seconds, and 70°C for 30 seconds; 3 cycles at 94°C for 15 seconds, 61°C for 30 seconds, and 70°C for 30 seconds; 3 cycles at 94°C for 15 seconds, 58°C for 30 seconds, and 70°C for 30 seconds; and 30 cycles at 94°C for 15 seconds, 57°C for 30 seconds, and 70°C for 30 seconds, followed by 70°C for 5 minutes. Sanger DNA sequencing was performed by either Macrogen (Rockville, MD) or Beckman Coulter Genomics (Danvers, MA). Mutational analysis was performed using a software package (Mutation Surveyor 4.0; SoftGenetics LLC, PA). All detected TERT promoter mutations were confirmed by re-sequencing.
TERT Promoter Mutation in Precursor Lesions of Ovarian Clear Cell Carcinoma
Nine ovarian clear cell carcinomas with TERT promoter mutations contained normal-appearing or atypical endometriotic cyst epithelium adjacent to the carcinoma. The cyst epithelium was carefully micro-dissected with 27-gauge needles and the genomic DNA of dissected tissue was then extracted with the QIAmp DNA FFPE tissue kit (Qiagen, Valencia, CA). TERT promoter sequences were analyzed separately for matched samples of clear cell carcinoma and adjacent normal/atypical cystic epithelium.
TERT Promoter Mutation and mRNA Expression in Ovarian Clear Cell Carcinoma Cell Lines
Cell lines derived from ovarian clear cell carcinomas were cultured in RPMI 1640 medium (Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (Atlanta Biological, Lawrenceville, GA) (JHOC5, KK, KOC-7C, OVCA429, OVISE, and OVTOKO) or DMEM medium (Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (OV207, ES-2, and TOV21G). Genomic DNA was isolated from cell lines using the DNeasy blood and tissue kit (Qiagen, Valencia, CA), and RNA was extracted with the RNeasy blood and tissue kit (Qiagen, Valencia, CA). The TERT promoter region was amplified and sequenced and quantitative reverse-transcription PCR was performed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA), Maxima SYBR green qPCR master mix (Thermo Fisher Scientific, Waltham, MA), and 500 nM primers. TERT primers used were: 5’-CAGGATCTCCTCACGCAGAC-3’ and 5’-GAGCTGACGTGGAAGATGAG-3’. APP was used as an internal expression control because of its stable expression among ovarian cancers. APP primers used were: 5’-CTGAAGATGGATGCAGAATTCC-3’ and 5’-AAAGAACTTGTAGGTTGGATTT-3’.
Telomere Length Evaluation in Ovarian Clear Cell Carcinoma
Telomere length in ovarian clear cell carcinoma was assessed in our previous study [20] by telomere fluorescence in situ hybridization. 32 ovarian clear cell carcinomas from this previous cohort had quality DNA available for sequencing analysis and were included in the current study. The mean relative telomere length of carcinoma cells normalized to telomere length of stromal cells was calculated as the telomere index [20]. Telomere indices were compared between ovarian clear cell carcinomas with TERT promoter mutation and those without.
Immunohistochemical Staining for ARID1A in Ovarian Clear Cell Carcinoma
Immunohistochemistry for ARID1A was performed on a total of 191 ovarian clear cell carcinoma samples. Among them, 99 cases had been previously stained and reported, including 84 from Tokyo [21] and 15 from Hamamatsu, Japan [22]; 92 additional cases were analyzed in this report. Immunohistochemical staining for ARID1A was performed with the following protocol. Antigen retrieval was performed on rehydrated tissue sections in a pH 6.0 citrate buffer (Dako, Carpinteria, CA) at 92 °C for 30 minutes. The sections were incubated overnight at 4°C with a rabbit polyclonal anti-ARID1A antibody (Sigma-Aldrich HPA005456) at a dilution of 1:1000, and visualized by the EnVision+ System (Dako, Carpinteria, CA). For each section, only nuclear staining was scored and positive nuclear staining in stromal cells was used as positive control. The tissues were examined in a blinded fashion without knowledge of clinical information or TERT mutation status.
Sequencing of PIK3CA in Ovarian Clear Cell Carcinoma
45 of the ovarian clear cell carcinoma samples in this study have been previously sequenced for PIK3CA [23]. An additional 35 ovarian clear cell carcinomas were sequenced for PIK3CA (coding exon 1, 9, and 20) following the aforementioned protocol. The amplification primers used for PIK3CA sequencing were as follows. Proximal coding exon 1: 5’-TGCTTTGGGACAACCATACATC-3’ and 5’-CTTGCTTCTTTAAATAGTTCATGCTTT-3’; distal coding exon 1: 5’-CCCCTCCATCAACTTCTTCAA-3’ and 5’-ATTGTATCATACCAATTTCTCGATTG-3’; coding exon 9: 5’-TTTTCTGTAAATCATCTGTGAATCC-3’ and 5’-TCTCCATTTTAGCACTTACCTGTGA-3’; proximal coding exon 20: 5’-M13-TTTGCTCCAAACTGACCAAAC-3’ and 5’-ACTCCAAAGCCTCTTGCTCA-3’; distal coding exon 20: 5’-ACATTCGAAAGACCCTAGCC-3’ and 5’-M13-GGTCTTTGCCTGCTGAGAGT-3’. The sequencing primers for PIK3CA were as follows. Proximal coding exon 1: 5’-ACCATCATCAGGTGAACTGTGG-3’; distal coding exon 1: 5’-CTCAAGAAGCAGAAAGGGAAG-3’; coding exon 9: 5’-AAAATATGACAAAGAAAGCTATAT-3’; proximal and distal coding exon 20: 5’-GTAAAACGACGGCCAGT-3’ (M13).
Statistical Analysis
Two-tailed Fisher’s exact test and unpaired t test were performed to determine the association between mutation status at the TERT promoter and clinico-pathological features in ovarian clear cell carcinoma. Confidence intervals of mutation frequencies were determined using the Agresti-Coull method, and mutation frequencies of different cancer types were compared using two-tailed Fisher’s exact test. Disease-specific overall survival of ovarian clear cell carcinoma with and without TERT promoter mutations was compared using the Kaplan-Meier method followed by the log-rank test to determine significance. Comparison of TERT mRNA expression levels between mutant and wild-type ovarian clear cell carcinoma cell lines was performed using one-tailed Mann-Whitney U test to determine whether TERT promoter mutation was associated with increased TERT mRNA expression. For comparison of telomere length between mutant and wild-type ovarian clear cell carcinomas, one-tailed Mann-Whitney U test was used when the telomere index was treated as a continuous variable and Fisher’s exact test used when the telomere index was treated as a binary variable. Two-tailed Fisher’s exact test was used to determine the association between TERT promoter mutation and loss of ARID1A immunoreactivity, as well as the association between TERT promoter mutation and PIK3CA mutation in ovarian clear cell carcinoma. P < 0.05 was considered significant for all tests. All statistical analyses were performed with R version 2.14.
Results
TERT Promoter Mutations in Gynecologic Malignancies
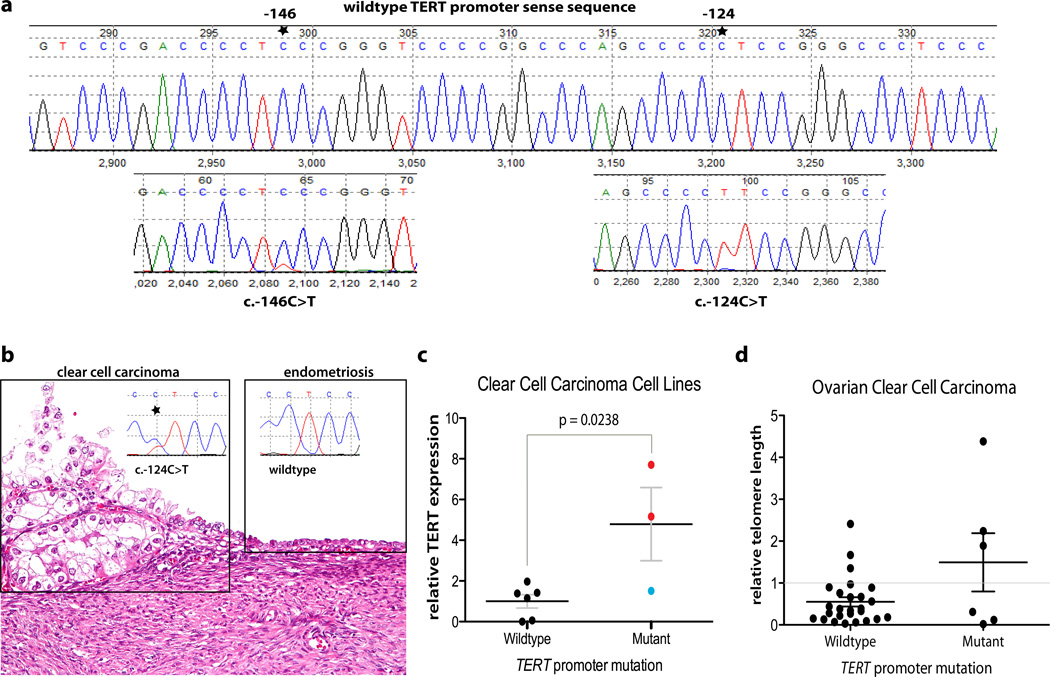
Sequencing analysis was performed in the TERT promoter region containing the two previously reported mutation hot spots. The frequencies of TERT promoter mutations in various gynecologic cancers are summarized in Table 1. TERT promoter mutations were detected in 40 of 525 tumor samples (7.6%; 95%CI: 5.6 – 10.2%), the majority occurring in ovarian clear cell carcinomas. While present in only 3 of 292 other tumors (1.0%; 95%CI: 0.2 – 3.1%), 37 of 233 (15.9%; 95%CI: 11.7 – 21.2%) ovarian clear cell carcinoma specimens harbored TERT promoter mutations. The difference in mutation frequency was significant between clear cell carcinoma and other gynecologic neoplasms (p< 0.0001, two-tailed Fisher’s exact test). Among the 40 mutations, 37 were located at −124 bp from the TERT translation start site while only 3 were at −146 bp (Fig. 1a). Since ovarian clear cell carcinoma and endometrioid carcinoma are thought to arise from endometriotic cysts (endometriomas) [24], we compared the TERT promoter mutation frequency between these two cancer types, and found a significantly higher frequency of TERT promoter mutation in ovarian clear cell carcinoma than in ovarian endometrioid carcinoma (p = 0.0024, two-tailed Fisher’s exact test). There was no significant difference in TERT promoter mutation frequency among the ovarian clear cell carcinoma samples collected from different institutions (p = 0.3137, Fisher’s exact test) or from different countries (p = 0.9278, Fisher’s exact test).
Table 1.
Prevalence of TERT promoter mutation in gynecologic malignancie
| mutated/total | percentage | Specific mutations * | |
|---|---|---|---|
| Ovarian Cancers | |||
| clear cell carcinoma | 40/243 | 16.5% | |
| clinical sample | 37/233 | 15.9% | c.−124C>T (n=34); c.−146C>T (n=3) |
| cell line | 3/10 | 30.0% | c.−124C>T(n=2); c.[−138C>T;−139C>T]**(n=1) |
| endometrioid carcinoma | 0/43 | 0.0% | |
| high-grade serous carcinoma | 0/80 | 0.0% | |
| low-grade serous carcinoma | 1/33 | 3.3% | c.−124C>T (n=1) |
| Uterine Corpus Cancers | |||
| endometrioid carcinoma | 0/24 | 0.0% | |
| serous carcinoma | 0/12 | 0.0% | |
| leiomyosarcoma | 0/22 | 0.0% | |
| Uterine Cervix Cancer | |||
| squamous cell carcinoma | 2/53 | 3.7% | c.−124C>T (n=2) |
| endocervical adenocarcinoma | 0/25 | 0.0% | |
TERT reference sequence: NM_001193376;
c.−139C>T is a rare reported polymorphism (rs35550267).
Fig. 1.
(a) TERT promoter mutations in ovarian clear cell carcinoma. Shown are representative chromatograms of the wild-type TERT promoter and two mutated TERT promoters at two different hot spots. Both mutations create an identical 11bp sequence: CCCCTTCCGGG. (b) An example of ovarian clear cell carcinoma arising from an endometriotic cyst. TERT promoter mutation occurred in clear cell carcinoma but not in the adjacent endometriotic cyst epithelium. (c) Ovarian clear cell carcinoma cell lines with mutated TERT promoter have increased expression of TERT mRNA. Red circles: OV207 and JHOC5 cells with c.−124C>T mutation; blue circle: ES-2 cell with c.[−139C>T;−138C>T] tandem mutation; black horizontal bar: mean; error bar: SEM. (d) Comparison of telomere length between ovarian clear cell carcinomas with and without TERT promoter mutation. Black horizontal bar: mean; error bar: SEM; gray horizontal bar: cutoff for binary analysis.
TERT Promoter Mutations Are Not Present in Endometriotic Cyst Epithelium Adjacent to Ovarian Clear Cell Carcinoma
To determine if TERT promoter mutation occurs early in the development of clear cell carcinoma, we analyzed 9 ovarian clear cell carcinomas with TERT promoter mutations in which there was sufficient endometriotic cyst epithelium for mutational analysis. TERT promoter mutations were not identified in any of the 9 specimens (Fig. 1b). This finding suggests that TERT promoter mutations do not occur early in the tumor progression from endometriotic cyst to clear cell carcinoma.
TERT Promoter Mutation Is Associated with Elevated mRNA Expression in Ovarian Clear Cell Carcinoma Cell Lines
In a screen of 9 ovarian clear cell carcinoma cell lines, we found that three (33.3%; 95% CI: 11.7 – 64.9%) contained mutations at the TERT promoter. Cell lines JHOC5 and OV207 carried the hot spot mutation c.−124C>T, whereas the ES-2 cell line harbored the less common tandem mutation c.[−139C>T; −138C>T], which has been reported in a few melanomas [10]. Cell lines harboring the c.−124C>T mutation showed a 5 to 8 fold increase of TERT mRNA expression as compared to the cell lines without TERT promoter mutation (Fig. 1c). Overall, clear cell carcinoma cell lines with TERT promoter mutations expressed higher levels of TERT mRNA than those with wild-type sequence(p = 0.0238, one-tailed Mann-Whitney U test, Fig. 1c).
Ovarian Clear Cell Carcinomas with TERT Promoter Mutation Tend to Have Longer Telomere Length
In a previous study [20], we assessed the telomere length in different types of ovarian carcinomas and found that clear cell carcinoma had the longest average telomere length compared to other histologic types of ovarian carcinomas. To examine the relationship between TERT promoter mutation and telomere length, we compared TERT promoter mutation status and telomere length data in 32 ovarian clear cell carcinomas, including 6 with TERT promoter mutation and 26 without. There was a trend towards longer telomere lengths in ovarian clear cell carcinomas with TERT promoter mutation relative to those without, though statistical significance was not reached (median, 1.1 vs. 0.36; p = 0.2278, one-tailed Mann-Whitney U test, Fig. 1d). Similarly, when telomere length was coded as a binary variable (telomere index > 1 vs. ≤ 1), clear cell carcinomas with TERT promoter mutation showed higher, though not statistically significant, frequency of possessing long telomere length (index > 1) than those without TERT promoter mutation (3/6 vs. 3/26; p = 0.0629, Fisher’s exact test, Fig. 1d).
TERT Promoter Mutation Is Inversely Associated with Loss of ARID1A Protein Expression in Ovarian Clear Cell Carcinomas
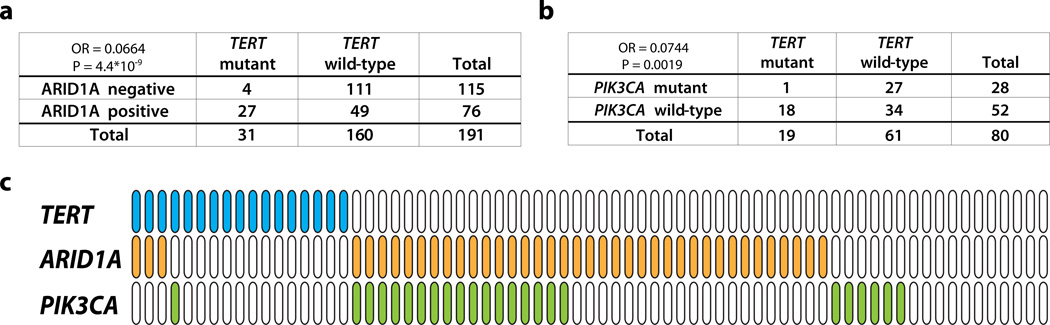
ARID1A, a tumor suppressor gene, is the most frequently mutated gene in ovarian clear cell carcinoma [17, 25]. It has been shown that loss of ARID1A expression is a surrogate marker for its mutation [21]. To examine the correlation between TERT promoter mutation and ARID1A aberration, we compared the TERT promoter mutation status and ARID1A protein expression data in 191 of the analyzed ovarian clear cell carcinomas. Loss of ARID1A protein expression, by immunohistochemistry, was detected in 115/191 (60.4%) ovarian clear cell carcinoma samples: in 4 of 31 (12.9%) cases harboring the TERT promoter mutation compared to 111 of 160 (69.4%) cases without (Fig. 2a). This result indicates a strong tendency towards mutual exclusivity between TERT promoter mutation and lack of ARID1A expression (odds ratio = 0.066, p = 4.4×10−9, two-tailed Fisher’s exact test).
Fig. 2.
(a) Correlation between TERT promoter mutation and ARID1A immunoreactivity in ovarian clear cell carcinomas. (b) Correlation between TERT promoter mutation and PIK3CA mutation in ovarian clear cell carcinomas. (c) Mutation status of TERT promoter and PIK3CA, and immunostaining result of ARID1A in 71 clear cell carcinoma tissues. Blue: TERT promoter mutation; orange: loss of ARID1A expression; green: PIK3CA mutation.
TERT Promoter Mutation and PIK3CA Mutation Tend to Be Mutually Exclusive in Ovarian Clear Cell Carcinoma
PIK3CA is the second most commonly mutated gene in ovarian clear cell carcinoma [23]. To determine the correlation between TERT promoter mutation and PIK3CA mutation, we sequenced PIK3CA hotspot coding exon 1, 9, and 20 for 80 ovarian clear cell carcinomas, 45 of which, including 9 ovarian clear cell carcinoma cell lines, had been reported previously [23]. Interestingly, we observed a strong tendency towards mutual exclusivity between TERT promoter and PIK3CA mutations (odds ratio = 0.074, p = 0.0019, two-tailed Fisher’s exact test), with only one of the 80 samples harboring both TERT promoter and PIK3CA mutations (Fig. 2b). All clinical samples that had PIK3CA mutation, TERT promoter mutation, and ARID1A immunostaining data (n = 71) were summarized in Fig. 2c.
TERT Promoter Mutation Has No Impact on Prognosis in Ovarian Clear Cell Carcinoma
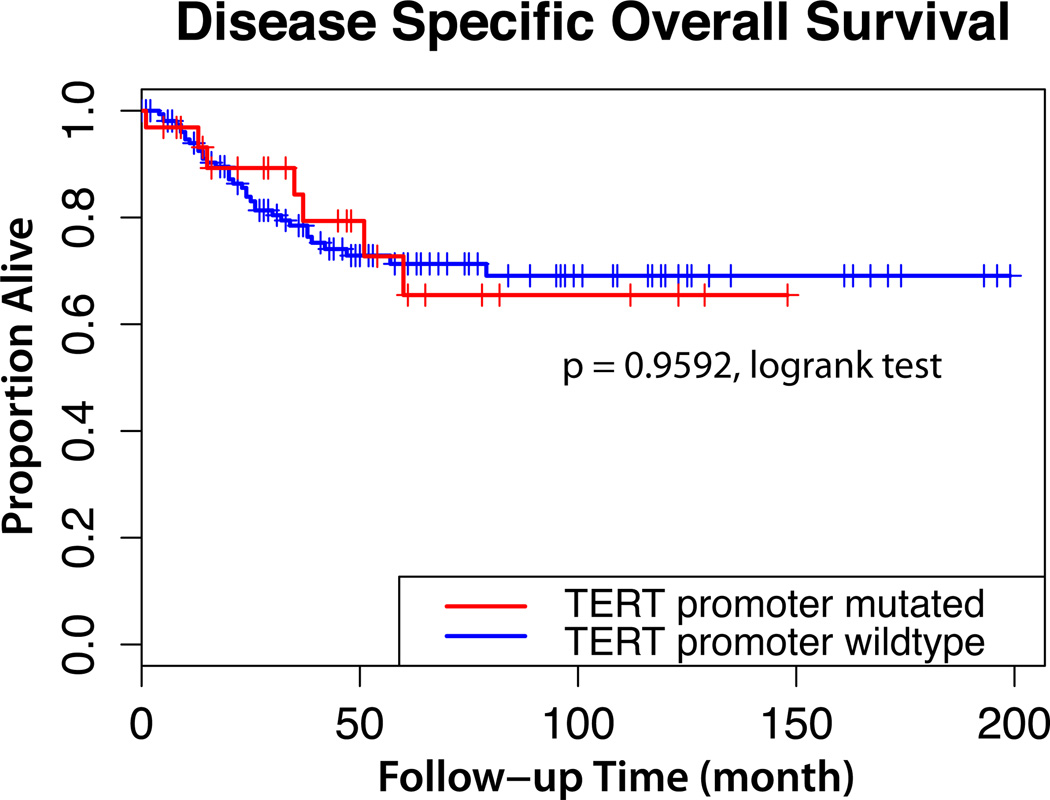
Next, we determined correlations between TERT promoter mutation status and clinico-pathological features of ovarian clear cell carcinoma (Table 2). We found no association between TERT promoter mutation and patients’ age, FIGO stage, lymph node metastasis, nuclear atypia, and histological features. In 196 ovarian clear cell carcinomas with available survival data, there was no significant difference in disease-specific overall survival between patients with TERT promoter mutations and those without (hazard ratio, 0.9790; 95% CI: 0.4347 to 2.205; p = 0.9592, log-rank test; Fig.3).
Table 2.
Clinicopathological features of ovarian clear cell carcinoma
| Mutant | Wild-type | P value | |
|---|---|---|---|
| Age (n = 196) | |||
| mean ± SD | 51.6 ± 10.4 | 51.8 ± 9.3 | 0.9106 |
| median | 52 | 51 | |
| range | 33–73 | 25–76 | |
| FIGO stage (n = 175) | |||
| I / II | 22 | 112 | 1.0000 |
| III / IV | 7 | 34 | |
| Lymph node metastasis (n = 88) | |||
| positive | 0 | 15 | 0.1992 |
| negative | 11 | 62 | |
| Nuclear atypia (n = 83) | |||
| mild | 3 | 25 | 0.8999 |
| moderate | 5 | 33 | |
| severe | 1 | 16 | |
| Predominant histological pattern (n = 83) | |||
| papillary | 3 | 36 | 0.6623 |
| tubulocystic | 3 | 21 | |
| solid | 3 | 17 | |
Fig. 3.
Kaplan-Meier survival curves of ovarian clear cell carcinoma patients. Overall survival time of patients with TERT promoter mutation is similar to patients with wild-type sequence.
Discussion
In this study, we found that among all types of gynecologic cancers examined, ovarian clear cell carcinomas had the highest frequency of TERT mutations. Ovarian clear cell carcinoma is a unique type of ovarian cancer. Advanced stage clear cell carcinoma is associated with a poorer prognosis because the tumor is highly resistant to platinum-based chemotherapy [26]. Ovarian clear cell carcinoma has been thought to develop from endometriotic cyst (endometrioma) of the ovary, which has also been considered a precursor lesion of ovarian endometrioid carcinoma [24]. Ovarian clear cell carcinoma and endometrioid carcinoma share several molecular genetic alterations including activating mutations in PIK3CA, and inactivating mutations and loss of expression in ARID1A and PTEN [23, 25]. Despite these similarities, ovarian clear cell carcinoma, as compared to ovarian endometrioid carcinoma, is characterized by distinct molecular features including up-regulation of hepatocyte nuclear factor-1β and less frequent mutation in CTNNB1 [18, 23, 27]. The higher mutation frequency in TERT promoters as reported here underscores the difference in the pathogenesis of ovarian clear cell carcinoma and ovarian endometrioid carcinoma.
Analysis of the mutation types reveals the likely mechanism by which TERT promoter mutations contribute to tumor development. Both hot spot mutations (c.−124C>T and c.−146C>T) at the TERT promoter create an 11-bp sequence that contains the binding motif (CCGGAA) of ETS-domain transcription factor family members, and both mutations have been shown to increase transcriptional activity of the TERT promoter [11]. Likewise, in this study, we observed that TERT promoter mutation was associated with higher TERT mRNA expression in the ovarian clear cell carcinoma cell lines examined. The less common tandem mutation c.[−139C>T; −138C>T] also generates an ETS binding motif. ETS-domain transcription factors act as activators or repressors of a compendium of genes that are involved in diverse biological processes, many of which are cancer-related, including cellular proliferation, inhibition of apoptosis, regulation of cellular senescence, and angiogenesis [28]. Abnormalities of ETS-domain family members have been implicated in a variety of human cancers through different mechanisms, including amplification, mutation, and translocation that generates fusion proteins [29]. In addition, several ETS-domain transcription factors are activated by mitogen-activated protein kinase (MAPK), which is commonly up-regulated in cancer cells [28]. Since all hotspot mutations result in creation of a new ETS binding motif that is accessible to ETS factors, it is likely that these mutations turn the TERT promoter into a target of ETS-domain transcription factors.
Several molecular genetic abnormalities including ARID1A mutation, PIK3CA mutation, and PTEN deletion (loss of heterozygosity) have been thought to be early events in the development of ovarian clear cell carcinoma, as these alterations are frequently detected in both carcinoma and adjacent benign epithelium within the endometriotic cyst, the precursor lesion of ovarian clear cell carcinoma [22, 30–32]. In this study, we did not detect TERT promoter mutations in the contiguous endometriotic cyst epithelium associated with ovarian clear cell carcinomas that harbored TERT promoter mutations. This result indicates that TERT promoter mutation is not an early event in tumorigenesis. This finding can be explained by the telomere crisis theory during tumor evolution [33, 34], which proposes that incipient tumor clones slowly evolve while gradually acquiring somatic mutations until reaching a sufficient number of cell divisions when accumulated telomere erosion triggers senescence and/or deleterious genomic instability. Therefore, in order to survive, pre-cancer cells need to develop a strategy to escape from telomere crisis. TERT promoter mutation is dispensable during the very early stage of tumor initiation because it does not provide a survival advantage until telomere attrition becomes evident. During the telomere crisis stage, only the cells that acquire the ability to maintain telomere length through ways such as TERT promoter mutation will progress through this selection pressure.
Recent genome-wide analysis has revealed that ARID1A, a tumor suppressor gene, is the most frequently mutated gene in ovarian clear cell carcinoma; somatic mutation of ARID1A was found in approximately half of ovarian clear cell carcinomas [17, 25]. Because ARID1A mutations correlate with loss of expression, immunohistochemistry has been used to assess ARID1A mutation status on tissue sections [21]. In this study, we found that TERT promoter mutation was inversely associated with loss of ARID1A protein expression (presumably through mutations) in ovarian clear cell carcinoma. This implies that intact ARID1A-containing BAF chromatin remodeling complexes may be required for TERT re-expression mediated by a mutated TERT promoter. Alternatively, loss of ARID1A may facilitate incipient tumor cells to achieve replicative immortality. Thus, it is not essential for those tumor cells to acquire TERT promoter mutation.
PIK3CA, an oncogene, was found to be mutated in about one third of ovarian clear cell carcinoma [23]. Interestingly, in this study, we demonstrated that TERT promoter mutation and PIK3CA mutation showed strong tendency towards mutual exclusivity in 80 ovarian clear cell carcinoma samples examined. PI3K/AKT signaling pathway plays a role in maintaining self-renewal in embryonic stem cells [35] and cancer stem cells [36–39]. Moreover, PI3K/AKT pathway is involved in transcriptional up-regulation of TERT and activation of telomerase through phosphorylation [40–43]. Therefore, like TERT promoter mutation, activating PIK3CA mutation confers cancer cells a growth advantage by overcoming the hurdle of replicative senescence. Consequently, TERT promoter mutation is not selected in ovarian clear cell carcinomas with PIK3CA mutations.
Ovarian clear cell carcinoma patients with TERT promoter mutation have no difference in disease-specific overall survival as compared to those without TERT promoter mutation. This finding contrasts with a previous study, in which primary gliobastoma with TERT promoter mutations had a worse prognosis than patients without TERT mutations [12]. We note that most glioblastomas that do not have TERT promoter mutations exhibit the alternative lengthening of telomere (ALT) phenotype, which has been reported as an independent indicator of better prognosis [44]; whereas, only a small proportion of ovarian clear cell carcinomas exhibit the ATL phenotype [8]. Therefore, the discrepancy is not surprising. To learn more about the effect of TERT promoter mutation on patients’ outcome, it would be helpful to compare the prognosis between TERT promoter mutants and wildtypes in other cancers in which ALT is uncommon, such as hepatocellular carcinomas, urothelial carcinomas, or squamous cell carcinomas of the tongue [8, 12, 13].
In summary, we found that TERT promoter mutations were most common in ovarian clear cell carcinoma among gynecological malignancies. TERT promoter mutation was associated with higher TERT mRNA levels in ovarian clear cell carcinoma cell lines. TERT promoter mutation showed strong tendency towards mutual exclusivity with loss of ARID1A expression and PIK3CA mutation in ovarian clear cell carcinoma. These results, in conjunction with our previous report showing longer telomeres in ovarian clear cell carcinomas compared to other types of ovarian cancer, suggest an important role of telomere biology in the development of ovarian clear cell carcinoma.
Supplementary Material
A list of all samples with results of TERT promoter and PIK3CA sequencing and ARID1A immunohistochemistry.
Acknowledgements
This work was supported by NIH grants (R21CA165807, RO1CA129080) and a CDMRP grant (W81XWH-11-2-0230) from US Department of Defense.
Footnotes
All authors declare no conflicts of interest.
Statement of author contributions
RW and AA conceived and carried out experiments and analyzed data. AA, DM, KK, BC, PS, MHC, and BR collected specimens and analyzed data. IS and TW designed studies and experiments. All authors were involved in writing the paper and had final approval of the submitted and published versions.
References
- 1.de Lange T. How telomeres solve the end-protection problem. Science. 2009;326:948–952. doi: 10.1126/science.1170633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prieur A, Peeper DS. Cellular senescence in vivo: a barrier to tumorigenesis. Curr Opin Cell Biol. 2008;20:150–155. doi: 10.1016/j.ceb.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Artandi SE, DePinho RA. Telomeres and telomerase in cancer. Carcinogenesis. 2010;31:9–18. doi: 10.1093/carcin/bgp268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harley CB. Telomerase and cancer therapeutics. Nat Rev Cancer. 2008;8:167–179. doi: 10.1038/nrc2275. [DOI] [PubMed] [Google Scholar]
- 5.Pirker C, Holzmann K, Spiegl-Kreinecker S, et al. Chromosomal imbalances in primary and metastatic melanomas: over-representation of essential telomerase genes. Melanoma Res. 2003;13:483–492. doi: 10.1097/00008390-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Dalla-Favera R, Wu K-J, Grandori C, et al. Direct activation of TERT transcription by c-MYC. Nat Genet. 1999;21:220–224. doi: 10.1038/6010. [DOI] [PubMed] [Google Scholar]
- 7.Castelo-Branco P, Choufani S, Mack S, et al. Methylation of the TERT promoter and risk stratification of childhood brain tumours: an integrative genomic and molecular study. Lancet Oncol. 2013;14:534–542. doi: 10.1016/S1470-2045(13)70110-4. [DOI] [PubMed] [Google Scholar]
- 8.Heaphy CM, Subhawong AP, Hong S-M, et al. Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am. J. Pathol. 2011;179:1608–1615. doi: 10.1016/j.ajpath.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henson J, Neumann A, Yeager T. Alternative lengthening of telomeres in mammalian cells. Oncogene. 2002;21:598–610. doi: 10.1038/sj.onc.1205058. [DOI] [PubMed] [Google Scholar]
- 10.Horn S, Figl A, Rachakonda PS, et al. TERT Promoter Mutations in Familial and Sporadic Melanoma. Science. 2013;339:959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 11.Huang FW, Hodis E, Xu MJ, et al. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957–959. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110:6021–6026. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Wu G, Shan Y, et al. Highly prevalent TERT promoter mutations in bladder cancer and glioblastoma. Cell Cycle. 2013;12:1637–1638. doi: 10.4161/cc.24662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vinagre J, Almeida A, Pópulo H, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun. 2013;4:2185. doi: 10.1038/ncomms3185. [DOI] [PubMed] [Google Scholar]
- 15.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones S, Wang T-L, Kurman RJ, et al. Low-grade serous carcinomas of the ovary contain very few point mutations. J Pathol. 2012;226:413–420. doi: 10.1002/path.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones S, Wang T-L, Shih I-M, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330:228–231. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhn E, Wu R-C, Guan B, et al. Identification of molecular pathway aberrations in uterine serous carcinoma by genome-wide analyses. J Natl Cancer Inst. 2012;104:1503–1513. doi: 10.1093/jnci/djs345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhn E, Meeker AK, Visvanathan K, et al. Telomere length in different histologic types of ovarian carcinoma with emphasis on clear cell carcinoma. Mod Pathol. 2011;24:1139–1145. doi: 10.1038/modpathol.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maeda D, Mao T-L, Fukayama M, et al. Clinicopathological significance of loss of ARID1A immunoreactivity in ovarian clear cell carcinoma. Int J Mol Sci. 2010;11:5120–5128. doi: 10.3390/ijms11125120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ayhan A, Mao T-L, Seckin T, et al. Loss of ARID1A expression is an early molecular event in tumor progression from ovarian endometriotic cyst to clear cell and endometrioid carcinoma. Int J Gynecol Cancer. 2012;22:1310–1315. doi: 10.1097/IGC.0b013e31826b5dcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuo K-T, Mao T-L, Jones S, et al. Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. Am J Pathol. 2009;174:1597–1601. doi: 10.2353/ajpath.2009.081000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viganó P, Somigliana E, Chiodo I, et al. Molecular mechanisms and biological plausibility underlying the malignant transformation of endometriosis: a critical analysis. Hum Reprod Update. 2006;12:77–89. doi: 10.1093/humupd/dmi037. [DOI] [PubMed] [Google Scholar]
- 25.Wiegand KC, Shah SP, Al-Agha OM, et al. ARID1A Mutations in Endometriosis-Associated Ovarian Carcinomas. N Engl J Med. 2010;363:1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan DSP, Kaye S. Ovarian clear cell adenocarcinoma: a continuing enigma. J Clin Pathol. 2007;60:355–360. doi: 10.1136/jcp.2006.040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuchiya A, Sakamoto M, Yasuda J, et al. Expression profiling in ovarian clear cell carcinoma: identification of hepatocyte nuclear factor-1 beta as a molecular marker and a possible molecular target for therapy of ovarian clear cell carcinoma. Am J Pathol. 2003;163:2503–2512. doi: 10.1016/s0002-9440(10)63605-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharrocks AD. The ETS-domain transcription factor family. Nat Rev Mol Cell Biol. 2001;2:827–837. doi: 10.1038/35099076. [DOI] [PubMed] [Google Scholar]
- 29.Seth A, Watson DK. ETS transcription factors and their emerging roles in human cancer. Eur J Cancer. 2005;41:2462–2478. doi: 10.1016/j.ejca.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Sato N, Tsunoda H, Nishida M, et al. Loss of heterozygosity on 10q23.3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res. 2000;60:7052–7056. [PubMed] [Google Scholar]
- 31.Yamamoto S, Tsuda H, Takano M, et al. Loss of ARID1A protein expression occurs as an early event in ovarian clear-cell carcinoma development and frequently coexists with PIK3CA mutations. Mod Pathol. 2011;25:615–624. doi: 10.1038/modpathol.2011.189. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto S, Tsuda H, Takano M, et al. PIK3CA mutation is an early event in the development of endometriosis-associated ovarian clear cell adenocarcinoma. J Pathol. 2011;225:189–194. doi: 10.1002/path.2940. [DOI] [PubMed] [Google Scholar]
- 33.Ishikawa F. Telomere Crisis, the Driving Force in Cancer Cell Evolution. Biochem Biophys Res Commun. 1997;230:1–6. doi: 10.1006/bbrc.1996.5928. [DOI] [PubMed] [Google Scholar]
- 34.Campbell PJ. Telomeres and cancer: from crisis to stability to crisis to stability. Cell. 2012;148:633–635. doi: 10.1016/j.cell.2012.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh AM, Reynolds D, Cliff T, et al. Signaling network crosstalk in human pluripotent cells: a Smad2/3-regulated switch that controls the balance between self-renewal and differentiation. Cell Stem Cell. 2012;10:312–326. doi: 10.1016/j.stem.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dubrovska A, Kim S, Salamone RJ, et al. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci U S A. 2009;106:268–273. doi: 10.1073/pnas.0810956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H, Gao Q, Guo L, et al. The PTEN/PI3K/Akt pathway regulates stem-like cells in primary esophageal carcinoma cells. Cancer Biol Ther. 2011;11:950–958. doi: 10.4161/cbt.11.11.15531. [DOI] [PubMed] [Google Scholar]
- 38.Bleau A-M, Hambardzumyan D, Ozawa T, et al. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4:226–235. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin Y, Yang Y, Li W, et al. Reciprocal regulation of Akt and Oct4 promotes the self-renewal and survival of embryonal carcinoma cells. Mol Cell. 2012;48:627–640. doi: 10.1016/j.molcel.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang SS, Kwon T, Kwon DY, et al. Akt protein kinase enhances human telomerase activity through phosphorylation of telomerase reverse transcriptase subunit. J Biol Chem. 1999;274:13085–13090. doi: 10.1074/jbc.274.19.13085. [DOI] [PubMed] [Google Scholar]
- 41.Bellon M, Nicot C. Central role of PI3K in transcriptional activation of hTERT in HTLV-I-infected cells. Blood. 2008;112:2946–2955. doi: 10.1182/blood-2008-01-134692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heeg S, Hirt N, Queisser A, et al. EGFR overexpression induces activation of telomerase via PI3K/AKT-mediated phosphorylation and transcriptional regulation through Hif1-alpha in a cellular model of oral-esophageal carcinogenesis. Cancer Sci. 2011;102:351–360. doi: 10.1111/j.1349-7006.2010.01796.x. [DOI] [PubMed] [Google Scholar]
- 43.Yang K, Zheng D, Deng X, et al. Lysophosphatidic acid activates telomerase in ovarian cancer cells through hypoxia-inducible factor-1α and the PI3K pathway. J Cell Biochem. 2008;105:1194–1201. doi: 10.1002/jcb.21919. [DOI] [PubMed] [Google Scholar]
- 44.Hakin-Smith V, Jellinek DA, Levy D, et al. Alternative lengthening of telomeres and survival in patients with glioblastoma multiforme. Lancet. 2003;361:836–838. doi: 10.1016/s0140-6736(03)12681-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A list of all samples with results of TERT promoter and PIK3CA sequencing and ARID1A immunohistochemistry.