Abstract
Our objective was to develop a non-invasive magnetic resonance (MR) method to predict the structural properties of a healing anterior cruciate ligament (ACL) using volume and T2* relaxation time. We also compared our T2*-based structural property prediction model to a previous model utilizing signal intensity, an acquisition-dependent variable. Surgical ACL transection followed by no treatment (i.e., natural healing) or bio-enhanced ACL repair was performed in a porcine model. After 52 weeks of healing, high-resolution MR images of the ACL tissue were collected. From these images, ligament volumes and T2* maps were established. The structural properties of the ligaments were determined via tensile testing. Using the T2* histogram profile, each ligament voxel was binned based on its T2* value into four discrete tissue sub-volumes defined by specific T2* intervals. The linear combination of the ligament sub-volumes binned by T2* value significantly predicted maximum load, yield load, and linear stiffness (R2 = 0.92, 0.82, 0.88; p<0.001) and were similar to the previous signal intensity based method. In conclusion, the T2* technique offers a highly predictive methodology that is a first step towards the development of a method that can be used to assess ligament healing across scanners, studies, and institutions.
Keywords: MRI, ligament healing, ACL, structural properties, biomechanics
INTRODUCTION
Biomechanical measurements of the structural properties of the anterior cruciate ligament (ACL) are frequently used to document functional healing after surgical ACL repair and reconstruction in pre-clinical animal models.1–5 Despite being a useful quantitative measure of graft healing,1–3 the current techniques to quantify the structural properties require harvesting the joint and testing the ligament to failure. Therefore, these current methods are inherently unsuitable for in vivo longitudinal assessment in both animal studies and human clinical trials.
Alternatively, magnetic resonance (MR) imaging is a widely available, non-invasive tool that has the potential to predict the biomechanical properties of ACL treatments.6 MR graft signal intensity has been found to correlate to the structural properties measured via ex vivo mechanical testing.3 Building on these initial studies, we found that the combination of MR ligament volume (a measure of tissue quantity) and the median ligament signal intensity (a surrogate measure of tissue quality) within that volume can be incorporated into a first order multiple regression model to improve the accuracy of the prediction of the structural properties.7 This new technique offered a more complete evaluation of graft integrity than either volume or signal intensity alone.7
However, the use of signal intensity as an outcome measure is limited by its dependence on image acquisition parameters and scanner manufacturer, rendering the predictions to be protocol, magnet, and hence, institution specific. One way to standardize MR results between scanners is to use relaxation time variables, such as T2 and T2*. These variables are inherent tissue properties that reflect specific tissue characteristics, and are much less sensitive to image acquisition parameters than conventional signal intensity data.8 T2* relaxation time is a MR parameter that has been shown to correlate with the level of tissue organization, and is thus well suited for imaging highly organized collagenous structures,9–12 such as ligaments and tendons. Thus, T2* relaxation time could provide a more universal prediction model of the structural properties of a healing ligament that would be applicable across scanners of the same strength and between institutions.
The purpose of this study was to establish the relationship between ligament volume, T2* relaxation time, and the structural properties of a healing ligament in a porcine model of ACL repair. We hypothesized that a multiple regression model based on ligament volume and its corresponding T2* values would provide a noninvasive predictor of the ligament's structural properties after 52 weeks of healing. As a secondary aim, we compared the proposed T2*-based structural properties prediction model to our previous model that incorporates signal intensity instead of T2*.7 We hypothesized that the coefficients of determination would be greater and that the standard errors would be less when using the T2* prediction method compared to the signal intensity method.
MATERIALS AND METHODS
Animal Model
Approval was obtained from the Institutional Animal Care and Use Committee prior to performing these studies. Fifteen adolescent Yucatan minipigs (approximately 15 weeks of age) underwent unilateral ACL transection surgery as previously described.13,14 Immediately following transection, eight of the animals received bio-enhanced ACL repair with an extracellular matrix-blood composite (Bio-Enhanced Repair, or BE-ACL group) and seven were left untreated to heal naturally without repair (ACL transection, or ACLT group).13 All animals made it to 52 weeks with no complications, at which point all fifteen operative knees were harvested and immediately imaged. Following imaging, the specimens were frozen and stored at −20 degrees Celsius until mechanical testing.
MR Imaging
A surface knee coil on a 3T MR scanner (TIM Trio; Siemens, Erlangen, Germany) was used to image the joints. Two separate imaging protocols were performed on each knee: 1) a dual echo protocol to determine T2* relaxation time, and 2) a manufacturer provided protocol to determine signal intensity.7 The images used for the signal intensity determination and analysis were a subset of those used in another study investigating the relationship between volume, signal intensity, and ligament structural properties over the course of healing.7 There was no intraarticular artifact found in any of the specimens. The BE-ACL group had a titanium button for suture fixation on the anterolateral cortical bone of the femur but was sufficiently far from the intra-articular space to avoid issues with artifact with the ACL.
MR Imaging: T2* determination
To determine T2*, a high-resolution T1-weighted gradient echo 3-D FLASH dataset (note: T1 weighted images are used to derive T2* relaxation time) utilizing two echo times (TR/TE/FA, 25/7.36 & 15.24/ 12°; FOV, 140 mm; matrix 512×512; slice length/gap, 0.85mm/0; avg, 3; bandwidth, 130; scan time 19 minutes) was acquired of the injured knee immediately after harvest. High-resolution 3-D image acquisition was required to optimally capture the relatively small structure of the healing ACL. The healing ligaments and associated peri-ligamentous scar tissue were then manually segmented from these T1-weighted MR images using commercially available software (Mimics 14.1; Materialize, Ann Arbor, MI). 3-D models of the healing ligaments were created using previously described methods.7 Summing the total number of ACL voxels provides an estimate of the whole ligament volume (16.1 voxels equaled one mm3). Using custom Matlab (R2012b; MathWorks, Natick, MA) code, T2* maps were calculated using the signal intensity (SI) relationship: ,15 where SI1 and SI2 are the signal intensities corresponding to the echo times TE1 and TE2 where TE2 > TE1 for each voxel (note: TR was the same for both echo times allowing for the determination of T2* using a two echo fit). To ensure the relaxation time maps used in this study were T2* weighted, the images used to create the maps were gradient echo acquisitions, and the echo times were significant compared to the T2* distribution of the tissue under examination.9
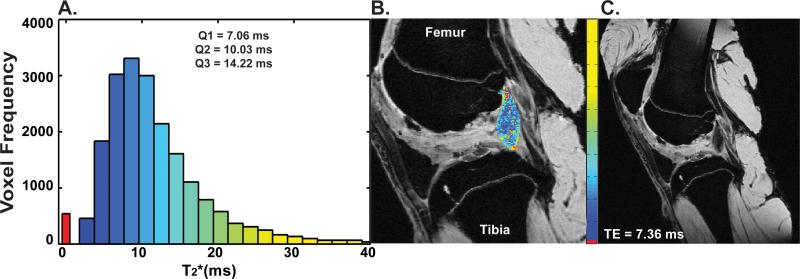
To produce ligament specific maps, the voxels corresponding to the ligament were extracted from the T2* maps using the 3-D models created from the segmented T1 weighted images (Fig. 1). Histograms of the voxel-wise T2* values were plotted using these ligament specific maps. Two distinct peaks of relaxation times were apparent with no overlap within each healing ligament (Fig. 1). Further, the voxels making up these peaks were spatially organized such that the first voxel peak represented those with T2*=0 ms and was generally located within the central portion of the ligament. Presumably, the voxels with T2*=0 ms have a range of short T2* values below our MR protocol's measurable limit (4.8 ms, the theoretical limit based on voxel signal to noise ratio)16, and would therefore fall between 0 and 4.8 ms. The second peak formed a lognormal distribution of relaxation times, where voxels with lower T2* values were primarily found in the central portion of the ligament while higher T2* values were identified towards the periphery (Fig. 1). The whole ligament volume was then binned into four separate tissue sub-volumes (Vol1, Vol2, Vol3, Vol4) with equal T2* intervals up to 50 ms (0-12.5; 12.6-25; 25.1-37.5; 37.6-50 ms, respectively) (Fig. 2). Tissue volume in terms of mm3 (note: for this MR protocol 16.1 voxels equaled one mm3) was calculated for each sub-volume.
Figure 1.
Example ligament histogram showing (A) the bimodal distribution for T2* with associated T2* first quartile (Q1), median (Q2) and third quartile (Q3) summary statistics, (B) the T2* ligament map, and (C) the original DICOM image. Note the ligament voxels illustrated in a red (B) represent voxels with a T2* value of 0 ms. The MR images are a sagittal view of the femoral notch with the femur at the top of the image and the tibia at the bottom. For the MR images shown TE = 7.36 ms.
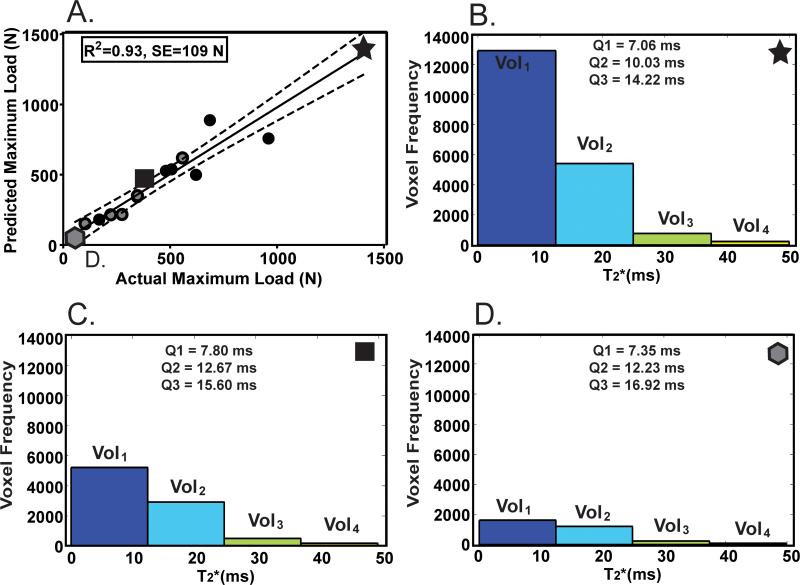
Figure 2.
T2* model: (A) Actual versus predicted maximum load calculated using the linear combination of Vol1, Vol2, Vol3 and Vol4. The dotted lines represent the 95% confidence intervals. Gray shapes represent transected ligaments while black shapes represent repaired ligaments. The highest (star, B), median (square, C) and lowest (hexagon, D) maximum load ligaments and their corresponding histogram profile are also represented with associated T2* first quartile (Q1), median (Q2) and third quartile (Q3) summary statistics.
MR Imaging: Signal intensity determination
During the same imaging session an additional single set of MR images was acquired to determine signal intensity. To accomplish this a T2* weighted 3-D-CISS sequence (note: signal intensity was derived from a single set of T2* weighted images) (TR/TE/FA, 12.9/6.5/ 35°; FOV, 140 mm; matrix 512×512, slice length/gap, 0.8mm/0; avg 1) was performed to establish the median signal intensity and volume of the whole ligament using our previously established multiple regression model.7 The healing ligaments were segmented from these MR images and 3-D models of the healing ligaments were created. From the models, the whole ligament volume (VWSI) in terms of mm3 (note: for this MR protocol 17.1 voxels equaled one mm3) was determined. Histograms of signal intensity in terms of grayscale values normalized to the signal of posterior femoral cortical bone (normalization standard) were plotted.9,17 Histograms of signal intensity were found to have a single uniform distribution for each ligament (Fig. 3). Signal intensity in terms of median gray scale value (MGVSI) was calculated from each distribution for the whole ligament.
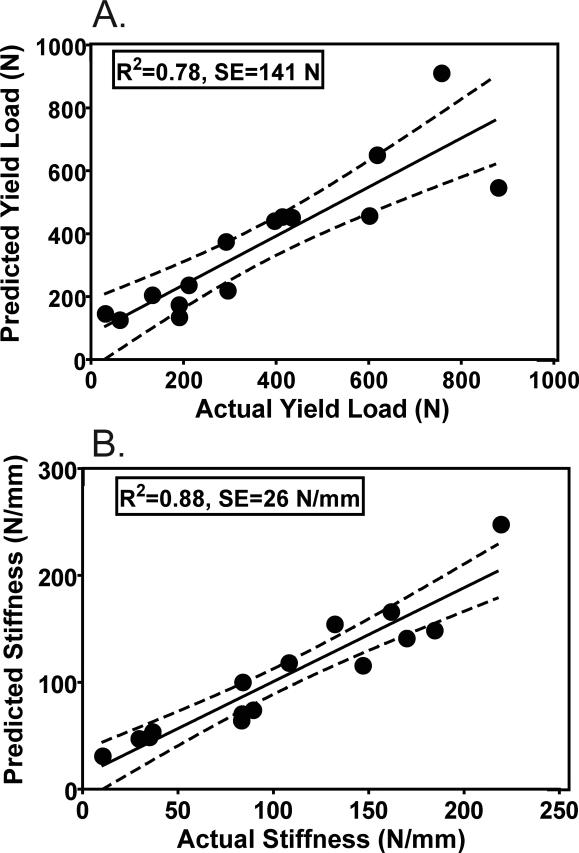
Figure 3.
T2* model: (A) Actual versus predicted yield load (B) and actual versus predicted linear stiffness plots calculated using the linear combination of Vol1, Vol2, Vol3 and Vol4. The dotted lines represent the 95% confidence intervals.
Structural Properties of the healing ACL
An established tensile testing protocol was used to determine the structural properties of the repaired and untreated transected ACLs after 52 weeks of healing.18,19 The specimens were thawed to room temperature. The proximal end of the femur and the distal end of the tibia were potted in PVC pipe using a urethane resin. The joint was carefully dissected, leaving only the femur-ligament-tibia complex intact and all associated peri-ligamentous scar tissue. Using a servohydraulic material testing system (MTS 810; Prairie Eden, MN), the tensile loads were applied at 20 mm/min to failure as previously reported.18 Initially, the joint was placed so that the mechanical axis of the ligament was collinear with the direction of pull of the actuator. Starting with a tibiofemoral compressive pre-load of 5 N, the entire tensile load-displacement curve was recorded until a precipitous drop in load occurred. The maximum load, yield load, and linear stiffness values of the ligaments were calculated from the load-displacement data.18
Data Analysis
First order multiple linear regression analyses (SigmaPlot 12.0; Systat Software Inc., San Jose, CA) were used to find the best-fit parameters and test the relationship between each ligament's sub-volume and the respective structural properties in the T2* model. The resulting model included a volume term (Vol1, Vol2, Vol3, Vol4) representing each of the four bins. Each bin was defined by its associated interval of T2* (0-12.5; 12.6-25; 25.1-37.5; 37.6-50 ms, respectively). Note that the voxels with a T2* of 0 ms were included in Vol1 because their relaxation times fall into the 0-12.5 ms bin range. The R-square values were reported as indicators of the relationship strength and goodness of fit. The p-values of the covariates (Vol1, Vol2, Vol3, Vol4) in the regression tested the contribution of the T2* defined sub-volumes to the model. The predicted structural properties (maximum load, yield load, and linear stiffness) across specimens were plotted against the actual experimental structural properties to visualize the standard error of the regressions as an additional check of the T2* model fit.
Additionally the volume of the whole ligament (VWSI) and signal intensity (MGVSI) values from each ligament were used in a first order multiple linear regression analysis to predict the structural properties as previously described.7 The predictions of the T2* model and the signal intensity model were compared using the mean R-square values and their respective 95% confidence limits (CL).20 Percent overlap of the confidence limits was tested using a z test statistic (alpha=0.05) to evaluate the differences between models.21,22
RESULTS
T2* Model Prediction
Of the T2* derived parameters evaluated, the best prediction of mechanical properties was a linear combination of the ligament sub-volumes (Vol1, Vol2, Vol3, Vol4) defined by their respective intervals of T2* values (Table 1). The R-squared values of the T2* prediction equation for maximum load, yield load and linear stiffness were 0.93, 0.78, and 0.88, respectively (p<0.001 for all). The 95% confidence limits for these R-squared values of maximum load, yield load and linear stiffness were [0.92, 0.94]; [0.75, 0.81]; [0.86, 0.90], respectively. Standard errors for the prediction of maximum load (Fig. 2), yield load (Fig. 3A), and linear stiffness (Fig. 3B) were 109 N, 141 N and 26 N/mm respectively (Table 1).
Table 1.
T2* model: Summary of ligament structural property prediction equations as a function of ligament sub-volumes (Vol1, Vol2, Vol3, Vol4) defined by range of T2* values.
| Dependent Variable (Y) | Predictor Term | T2* Range (ms) | P | R2 | R2 95% CL | Standard Error | Predictor Term Contribution per unit volume (mm3) | Equati0n |
|---|---|---|---|---|---|---|---|---|
| Max Load (N) | Vol1 | 0 -12.5 | <0.001 | 0.93 | [0.92, 0.94] | 109 N | 1.55 N | Max Load = −161.5 + (1.55 × Vol1) + (1.20 × Vol2) - (1.62 × V0l3) - (5.48 × Vol4) |
| Vol2 | 12.6 - 25 | 0.396 | 1.20 N | |||||
| Vol3 | 25.1 - 37.5 | 0.891 | −1.62 N | |||||
| Vol4 | 37.6 - 50 | 0.746 | −5.48 N | |||||
| Yield Load (N) | Vol1 | 0 -12.5 | 0.005 | 0.78 | [0.75, 0.81] | 141 N | 1.13 N | Yield Load = 55.3 + (1.13 × Vol1) + (0.04 × Vol2) - (1.24 × Vol3) - (1.64 x Vol4) |
| Vol2 | 12.6 - 25 | 0.983 | 0.04 N | |||||
| Vol3 | 25.1 - 37.5 | 0.936 | −1.24 N | |||||
| Vol4 | 37.6 - 50 | 0.940 | −1.64 N | |||||
| Stiffness (N/mm) | Vol1 | 0 -12.5 | <0.001 | 0.88 | [0.86, 0.90] | 26 N/mm | 0.28 N/mm | Stiffness = −1.8 + (0.28 × Vol1) + (0.02 × Vol2) + (0.55 × Vol3) - (0.70 × Vol4) |
| Vol2 | 12.6 - 25 | 0.961 | 0.02 N/mm | |||||
| Vol3 | 25.1 - 37.5 | 0.845 | 0.55 N/mm | |||||
| Vol4 | 37.6 - 50 | 0.860 | −0.70 N/mm |
Signal Intensity Model Prediction
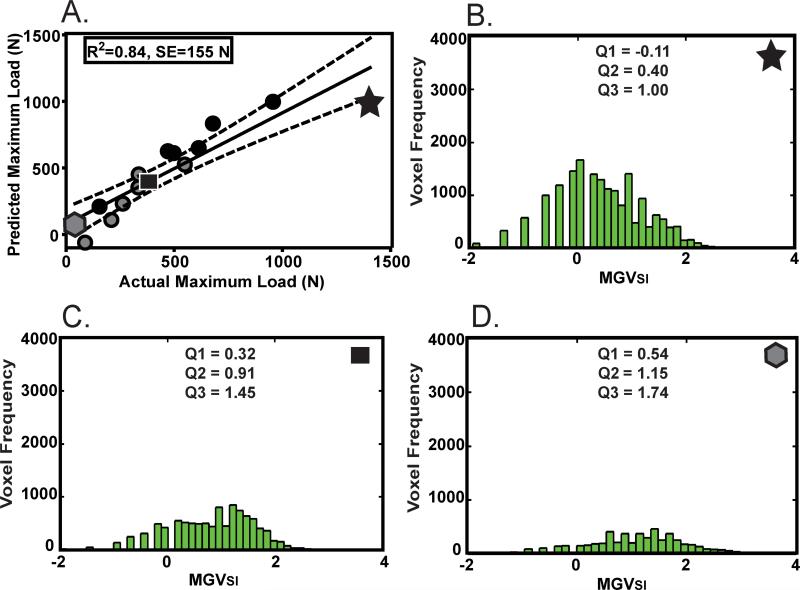
Of the signal intensity derived parameters studied, the best predictors of mechanical properties were a linear combination of the whole ligament volume (VWSI) and the signal intensity (MGVSI) (Table 2). The R-squared values of the signal intensity prediction equations for maximum load, yield load, and linear stiffness were 0.84, 0.92, and 0.88 respectively (p<0.001 for all) (Fig. 4). The 95% confidence limits for these R-squared values of maximum load, yield load and linear stiffness were [0.80, 0.88]; [0.90, 0.94]; [0.85, 0.91], respectively. Standard errors for the prediction of maximum load, yield load, and linear stiffness were 155 N, 75 N and 24 N/mm respectively (Table 2).
Table 2.
Signal Intensity model: Summary of ligament structural property prediction equations as a function of ligament whole volume (VWSI) and signal intensity (MGVsi).
| Dependent Variable (Y) | Predictor Term | p | R2 | R2 95% CL | Standard Error | Equation |
|---|---|---|---|---|---|---|
| Max Load (N) | VWsI | <0.001 | 0.84 | [0.80, 0.88] | 155 N | Max Load = 299.8 + (0.8 × VWsI) - (424.7 × MGVsI) |
| MGVsI | 0.006 | |||||
| Yield Load (N) | VWsI | <0.001 | 0.92 | [0.90, 0.94] | 75 N | Yield Load = 340.2 + (0.5 × VWsI) - (372.4 × MGVsI) |
| MGVsI | <0.001 | |||||
| Stiffness (N/mm) | VWsI | <0.001 | 0.88 | [0.85, 0.91] | 24 N/mm | Stiffness = 73.6 + (0.1 × VWsI) - (75.9 × MGVsI) |
| MGVsI | 0.002 |
Figure 4.
Signal intensity model: (A) Actual versus predicted maximum load calculated using linear combination of VWSI and MGVSI. The dotted lines represent the 95% confidence intervals. Gray shapes represent transected ligaments while black shapes represent repaired ligaments. The highest (star, B), median (square, C) and lowest (hexagon, D) maximum load ligaments and their corresponding histogram profile are also represented with associated SI first quartile (Q1), median (MGVSI, Q2) and third quartile (Q3) summary statistics.
There was no overlap of the R-squared confidence limits between the T2* and signal intensity models for the maximum load prediction. For maximum load the T2* model displayed significantly higher R-squared confidence limits than the signal intensity model. There was also no overlap of R-squared confidence limits between the T2* and signal intensity models for the yield load prediction. In this case the signal intensity model displayed significantly higher R-squared confidence limits than the T2* model. There was a 100% overlap of R-squared confidence limits for linear stiffness with the T2* prediction interval nested within the signal intensity model interval making them statistically equivalent (Tables 1 & 2).
DISCUSSION
A non-invasive tool that can predict the biomechanical properties of a healing ligament would be highly valuable in a research and clinical setting for evaluating outcomes of different ACL treatments. Our objective was to develop a magnetic resonance (MR) method to predict structural properties of a healing anterior cruciate ligament (ACL) using volume and T2* relaxation time. We found the linear combination of the ligament sub-volumes defined by increasing T2* intervals (Vol1, Vol2, Vol3, Vol4) significantly predicted structural properties of a healing porcine ACL at 52 weeks post-operatively.
There are two parameters that contribute to the structural properties of a ligament; 1) the amount of tissue (as represented by the volume) and 2) the quality of the tissue (as represented by T2*). Using a T2* histogram profile, each ligament was partitioned into four tissue sub-volumes with equal intervals of increasing T2*. In our regression model, we determined the relative proportions of the total ligament volume made up of high quality tissue (Vol1) down to the lowest quality tissue (Vol4) to determine how these proportions contribute to the overall ligament structural properties. The amount of tissue with a T2* value between 0-12.5 ms (Vol1) was found to be the most significant in predicting structural properties (p<0.001; Table 1). This would be expected as highly organized tissue has been associated with short T2* values10. Contribution of this sub-volume to structural properties can be observed with its associated slope in the prediction equations (Table 1). Per unit volume of Vol1 (T2* values 0-12.5 ms), 1.55 N, 1.13 N, and 0.28 N/mm are contributed to maximum load, yield load and linear stiffness, respectively. Generally less organized tissue in the T2* range of 12.6-25 ms (Vol2) contributed less to structural properties per unit volume with 1.20 N, 0.04 N and 0.02 N/mm for maximum load, yield load and linear stiffness respectively. While Vol2, Vol3 and Vol4 were not found to be significant predictors in this model (p>0.3) they were kept as terms in the model to accommodate a broader range of T2* values that would be expected with phases of healing earlier (<52 weeks) and to include the whole ligament volume in the analysis. The composition of the voxels in Vol3 and Vol4 with higher ranges of T2* values is likely a combination of less densely packed collagen fibrils and periligamentous tissue of scar formation.
Additional analyses were performed to determine the effect of partitioning the ligaments into more sub-volumes (bins = 8) with smaller ranges of T2* intervals defining each sub-volume (see Supplement 1). The four-bin model was the simplest linear regression model that yielded relatively high R-squared and low standard errors for determining structural properties when compared to other bin numbers.
The distribution of T2* values for each ligament was found to be bimodal and exhibited two discrete peaks. The voxels in this first peak displayed a single T2* value of 0 ms. Presumably there is variability in this tissue type, however the relaxation times approaching zero were too short to be acquired using our current MR protocol. The impact of this first peak is relatively small, however, as the median percentage of ACL voxels with T2*=0 to the total number of voxels for the whole ACL was only 3.8% (1st Quartile = 2.5%; 3rd Quartile =8.3%). Nonetheless, we know that the zero T2* values fall within the range of 0 and 4.8 ms. Despite not being able to capture the exact T2* value of the voxels under the theoretical 4.8 ms limit16, the current approach of partitioning or binning each ligament into four sub-volumes based on the T2* ranges helps to reduce the effects of minor observation error and allows these voxels to be accounted for in the prediction model. Unlike previous studies using a single average value for volume, signal intensity, or T2;7,23 quantifying sub-volumes (Vol1, Vol2, Vol3, Vol4) of tissue (with different levels of organization) based on their associated T2* values offers a more detailed evaluation of tissue composition and its relationship to structural properties. Models that neglect the heterogenous composition of the healing ACL tissues do not account for the fact that these tissues likely have different material properties. Additionally, the histograms of individual ligaments (Fig. 2) can be observed for the contribution of the sub-volumes (Vol1, Vol2, Vol3, Vol4) to the structural properties prediction. The ligaments with the highest structural properties displayed larger sub-volumes of organized tissue represented by low T2* times (Vol1), while ligaments with the lowest structural properties displayed smaller sub-volumes of organized tissue (Fig. 2).
The T2* model was found to be comparable to the original signal intensity prediction model. While the T2* model did not display higher R-squared values for all structural properties as hypothesized, the 100% overlap seen in linear stiffness confidence limits between the T2* and signal intensity methods, along with the marginal differences in maximum load and yield load confidence limits, suggest that these two prediction models offer similar certainty when determining structural properties.
However, the T2* prediction model offers the benefit of using the T2* variable, which is an inherent tissue property and much less sensitive to image acquisition parameters than conventional signal intensity data. T2* is influenced by susceptibility gradients at a microscopic level (reflecting the quantity and distribution of free water) where signal intensity is influenced by sequence parameters and hardware effects that can vary between scanners and even between scan sessions.8 Furthermore, the SI values must be normalized to a standard in the field of view, in this case posterior femoral cortical bone. With the SI method, finding an easy to identify and repeatable structure in the field of view, which is not potentially influenced by treatment effects, is difficult. Conversely, T2* is an inherent tissue property so a normalization standard is unnecessary, eliminating any associated confounding effects. Thus, T2*, with additional validation, may provide a more consistent assessment of the healing ligament across scanners of the same magnet strength than examination of signal intensity and could make the T2* predictions for scans performed across scanning intervals, studies, and institutions readily comparable.
Our findings are supported by previous research showing that volume normalized to a different relaxation time parameter (T2) correlates to ACL reconstruction graft structural properties.23 However, this previous study was limited by a non-significant correlation between structural properties and T2 values as an independent quantifiable variable. The lack of correlation found between T2 and structural properties was likely the result of relatively long echo times (>10ms) used to collect T2 and may have been too long for gathering the short relaxation times of graft tissue.24 Building on these earlier findings, the current study found that by utilizing T2*, we were able to better capture the shorter relaxation times that are inherent to organized structures such as ligaments and tendons.10 Furthermore, we were able to identify a significant individual contribution of T2* in terms of ligament sub-volume defined by ranges of T2* values (see Supplement 2). Additionally, a study using Ultra-short echo time (UTE) imaging found a correlation between level of collagen organization in the meniscus and T2*. Short T2* was correlated with more densely packed collagen fibers and longer T2* values were associated with less densely packed collagen and meniscal damage.10 While our study did not utilize UTE imaging, tissues with shorter T2* values were linked to higher ligament structural properties. This suggests that T2* can serve as a proxy for tissue organization and remodeling can be identified with the MR methodology presented herein.
This study was limited by the use of only two echo times which adds uncertainty to the determination of relaxation time.8 A two-point determination can overestimate relaxation time and is more sensitive to SI variability.8,25 However, the correlation analysis between the median T2* and structural properties incorporates the inter-specimen variability in T2*. Additionally, this approach served to limit scan time (19 min), an important factor when developing a technique for clinical use. The standard errors for the structural properties predictions are comparable to the previous methods using signal intensity,7 indicating an effective determination of T2* (Fig. 2). A two point determination of T2 has also been used in cartilage imaging15,26 to limit scan time and maximize resolution when considering geometry was paramount, similar to the goal of our study. Further refinement of our imaging protocol could be achieved by collecting additional shorter echo times, which would allow for improved certainty in the T2* estimation using a nonlinear least-squares fit of voxel intensity versus echo time. Including additional shorter echo times could minimize the population of tissue with a zero ms relaxation time and make the T2* estimation less sensitive to signal noise. Also, as with any MR study, the tradeoffs between resolution, scan time and image acquisition parameters, such as echo time, must be considered.26 Due to the relatively small size of the healing ACL, the imaging protocol was optimized to reconstruct the whole volume of the ligament, opposed to a single mid-substance slice,3,23 and to minimize the influence of partial volume effects. To minimize these concerns we employed high resolution 3-D MR images with minimal voxel size (0.85×0.27×0.27 mm) and no slice gap (0 mm). Also, this study did not include histological confirmation of tissue organization level within the healing ligament. However, a previous meniscal study reported a link between T2* and collagen organization.10 Lastly, the MR images collected in this study were collected postmortem eliminating problems with motion artifact. Future work will incorporate histological confirmation of tissue organization and evaluate the efficacy of using a T2* imaging method to predict structural properties in vivo for longitudinal studies.
The results reported here provide a critical step toward the improvement of a noninvasive method for predicting the structural properties of a healing ligament for in vivo use. Furthermore, the use of relaxation time instead of signal intensity specifically makes this approach independent of image acquisition parameters and is the first step to making this approach comparable across institutions. This will be advantageous for reducing the number of animals used in pre-clinical studies, and provide a noninvasive outcome measure of ligament healing in multicenter clinical trials.
Supplementary Material
ACKNOWLEDGEMENTS
This study made possible by Grants from the National Institutes of Health (RO1-AR056834; RO1-AR054099; P20-GM104937), National Football League Charities and the Lucy Lippitt Endowed Professorship. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIAMS, NIH or NFL Charities. Two of the authors (MMM, BCF) have patents related to the bio-enhanced ACL repair procedure.
REFERENCES
- 1.Murray MM, Magarian EM, Harrison SL, et al. The effect of skeletal maturity on functional healing of the anterior cruciate ligament. J Bone Joint Surg Am. 2010;92(11):2039–2049. doi: 10.2106/JBJS.I.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hashemi J, Mansouri H, Chandrashekar N, et al. Age, sex, body anthropometry, and ACL size predict the structural properties of the human anterior cruciate ligament. J. Orthop. Res. 2011;29(7):993–1001. doi: 10.1002/jor.21245. [DOI] [PubMed] [Google Scholar]
- 3.Weiler A, Peters G, Mäurer J, et al. Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast-enhanced magnetic resonance imaging. A two-year study in sheep. Am J Sports Med. 2001;29(6):751–761. doi: 10.1177/03635465010290061401. [DOI] [PubMed] [Google Scholar]
- 4.Chandrashekar N, Slauterbeck J, Hashemi J. Re: Sex-based differences in the anthropometric characteristics of the anterior cruciate ligament and its relation to intercondylar notch geometry: a cadaveric study. Am J Sports Med. 2009;37(2):423. doi: 10.1177/0363546508330152. [DOI] [PubMed] [Google Scholar]
- 5.Noyes FR, Grood ES. The strength of the anterior cruciate ligament in humans and rhesus monkeys. J Bone Joint Surg Am. 1976;58(8):1074–1081. [PubMed] [Google Scholar]
- 6.Gold GE, Pauly JM, Macovski A, et al. MR spectroscopic imaging of collagen: tendons and knee menisci. Magn Reson Med. 1995;34(5):647–654. doi: 10.1002/mrm.1910340502. [DOI] [PubMed] [Google Scholar]
- 7.Biercevicz AM, Miranda DL, Machan JT, et al. In situ, noninvasive, T2*-weighted MRI-derived parameters predict ex vivo structural properties of an anterior cruciate ligament reconstruction or bioenhanced primary repair in a porcine model. Am J Sports Med. 2013;41(3):560–566. doi: 10.1177/0363546512472978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deoni SCL, Williams SCR, Jezzard P, et al. Standardized structural magnetic resonance imaging in multicentre studies using quantitative T1 and T2 imaging at 1.5 T. Neuroimage. 2008;40(2):662–671. doi: 10.1016/j.neuroimage.2007.11.052. [DOI] [PubMed] [Google Scholar]
- 9.Chavhan GB, Babyn PS, Thomas B, et al. Principles, Techniques, and Applications of T2*-Based MR Imaging and Its Special Applications. Radiographics. 2009;29(5):1433–1449. doi: 10.1148/rg.295095034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams A, Qian Y, Golla S, et al. UTE-T2 mapping detects sub-clinical meniscus injury after anterior cruciate ligament tear. Osteoarthrit is Cartilage. 2012;20(6):486–494. doi: 10.1016/j.joca.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krasnosselskaia LV, Fullerton GD, Dodd SJ, et al. Water in tendon: orientational analysis of the free induction decay. Magn Reson Med. 2005;54(2):280–288. doi: 10.1002/mrm.20540. [DOI] [PubMed] [Google Scholar]
- 12.Koff MF, Shah P, Pownder S, et al. Correlation of meniscal T2* with multiphoton microscopy, and change of articular cartilage T2 in an ovine model of meniscal repair. Osteoarthritis Cartilage. 2013;21(8):1083–1091. doi: 10.1016/j.joca.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vavken P, Fleming BC, Mastrangelo AN, et al. Biomechanical outcomes after bioenhanced anterior cruciate ligament repair and anterior cruciate ligament reconstruction are equal in a porcine model. Arthroscopy. 2012;28(5):672–680. doi: 10.1016/j.arthro.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray MM, Fleming BC. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med. 2013;41(8):1762–1770. doi: 10.1177/0363546513483446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haacke EM, Brown RW, Thompson MR, et al. Magnetic resonance imaging: Physical principles and sequence design. A John Wiley and Sons; New York, NY: 1999. [Google Scholar]
- 16.Gudbjartsson H, Patz S. The Rician distribution of noisy MRI data. Magn Reson Med. 1995;34(6):910–914. doi: 10.1002/mrm.1910340618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sansome M, Aprile F, Fusco R, et al. A study on reference based time intensity curves quantification in DCE-MRI monitoring of Rectal Cancer. IFMBE Proceedings World Congress on Medical Physics and Biomedical Engineering. 2009;25(2):38–41. [Google Scholar]
- 18.Murray MM, Magarian E, Zurakowski D, et al. Bone-to-bone fixation enhances functional healing of the porcine anterior cruciate ligament using a collagen-platelet composite. Arthroscopy. 2010;26(9, Suppl):S49–S57. doi: 10.1016/j.arthro.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleming BC, Spindler KP, Palmer MP, et al. Collagen-platelet composites improve the biomechanical properties of healing anterior cruciate ligament grafts in a porcine model. Am J Sports Med. 2009;37(8):1554–1563. doi: 10.1177/0363546509332257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kromrey J, Melinda H. Interval Estimates of R2: An Empirical Investigation of the Influence of Fallible Regressors. Multiple Linear Regression Viewpoints. 2005;31(1):22–41. [Google Scholar]
- 21.Austin PC, Hux JE. A brief note on overlapping confidence intervals. J Vascular Surg. 2002;36(1):194–195. doi: 10.1067/mva.2002.125015. [DOI] [PubMed] [Google Scholar]
- 22.Cumming G, Fidler F. Interval estimates for statistical communication: problems and possible solutions. Refereed Proceedings of Statistics Education and the Communication of Statistics. International Association for Statistics Education; Sydney, Australia: 2005. [Google Scholar]
- 23.Fleming BC, Vajapeyam S, Connolly SA, et al. The use of magnetic resonance imaging to predict ACL graft structural properties. J Biomech. 2011;44(16):2843–2846. doi: 10.1016/j.jbiomech.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gatehouse PD, Bydder GM. Magnetic Resonance Imaging of Short T2 Components in Tissue. Clin Radiol. 2003;58(1):1–19. doi: 10.1053/crad.2003.1157. [DOI] [PubMed] [Google Scholar]
- 25.Kingsley PB, Ogg RJ, Reddick WE, et al. Correction of errors caused by imperfect inversion pulses in MR imaging measurement of T1 relaxation times. Magn Reson Imaging. 1998;16(9):1049–1055. doi: 10.1016/s0730-725x(98)00112-x. [DOI] [PubMed] [Google Scholar]
- 26.Dunn TC, Lu Y, Jin H, et al. T2 Relaxation Time of Cartilage at MR Imaging: Comparison with Severity of Knee Osteoarthritis1. Radiol. 2004;232(2):592–598. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.