Summary
We and others have previously shown that IL-12 is indispensable for immunity and is required for optimal antiparasitic activity of antimonials in experimental visceral leishmaniasis caused by Leishmania donovani. In this study we investigated the role of STAT4 in immunity against L. donovani using STAT4 knockout mice and also determined the effect of STAT4 deficiency in response to antimonial therapy. Upon infection with L. donovani, stat4−/− BALB/c and C57BL/6 mice showed enhanced susceptibility to Leishmania during late time points of infection which was associated with a marked reduction in Th1 responses and hepatic immunopathology. Interestingly, these defects in Th1 responses in stat4−/− did not impair the antimonial chemotherapy as both stat4−/− and WT mice showed comparable levels of parasite clearance from the liver and spleen. These findings highlight the role of STAT4 in immunity to L. donovani infection and also provide evidence that STAT4 is dispensable for antimonial based chemotherapy.
Keywords: Leishmania donovani, IL-12, IFN-γ, STAT4 chemotherapy and sodium stibogluconate
Introduction
Visceral leishmaniasis (VL) affects millions of people worldwide and is a major health concern. This fatal disease is caused by the intracellular protozoan parasite, Leishmania donovani, which infects host macrophages. In experimental VL, L. donovani infection targets the hosts’ spleen and liver resulting in the development of organ specific immunity [1]. Strain dependent resistance profiles of mice to L. donovani have been linked to the ability of the host to mount a strong Th1 immune response with high amounts of IFN-γ early in infection. As such, while C57BL/6 mice clear L. donovani rapidly within the first 30 days of infection, BALB/c mice have a delayed recovery and develop chronic infection [2].
IL-12 is a critical Th1 cytokine associated with the host protection in VL [3] which stimulates the production of IFN-γ from NK cells [4, 5], CD4+ T cells [6, 7] and CD8+ T cells [8]. IL-12 deficient C57BL6 [9] and BALB/c [10] mice are highly susceptible to L. donovani infection exhibiting high hepatic parasite burdens. IL-12 exerts its biological activity through STAT4 dependent and STAT4 independent pathways [11]. However, it is not known whether STAT4 pathway is required for resistance to VL. Furthermore, we and others have previously shown that IFN-γ, which signals via STAT1, promotes Th1 responses and mediates immunity against L. donovani, but stat1−/− mice are highly resistant to L. donovani and develop less liver immunopathology despite impaired Th1 immunity [12]. These findings indicate that a cytokine and its signaling mediators could play distinct roles in determining the outcome of L. donovani infection and that STAT4 may or may not be required for resistance against VL.
Host immune responses are also crucial for determining the outcome of antimonial therapy during VL as it is less effective in immunocompromised individuals and SCID mice [13]. Studies have shown that sodium stibogluconate (SSG) based therapy are also less effective in mice deficient in IL-10 [14], IL-4 [15], IFN-γ [16] and IL-12 [17]. STAT4 is involved in IL-12 signaling, which could subsequently regulate the production of other cytokines, but the role of STAT4 in antimonial therapy during experimental VL has not been investigated.
In this study, we investigate the role of STAT4 in immunity against L. donovani using stat4 deficient C57BL/6 and BALB/c mice. We also examine the effect of stat4 deficiency during antimonial based chemotherapy of VL.
Results
STAT4 is indispensable for protection against VL caused by Leishmania donovani
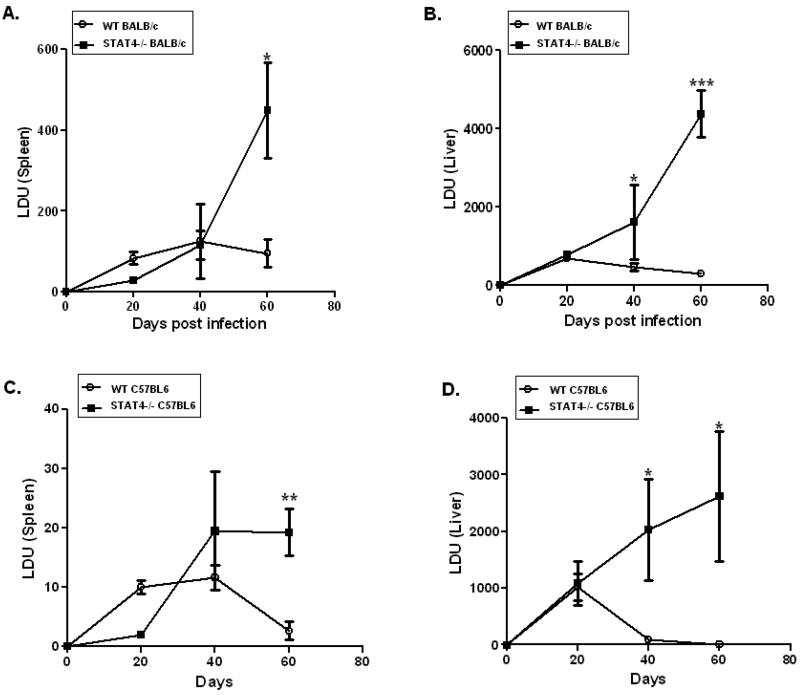
To determine the role of STAT4 in immunity against VL, we examined the course of L. donovani infection in stat4−/− mice of BALB/c and C57BL/6 backgrounds and compared these to their WT counterparts following intravenous infection with 2 × 107 L. donovani amastigotes. At day 20 post-infection, WT and stat4−/− mice (BALB/c or C57BL/6 background) showed similar levels of parasite loads in their spleens and livers (Figures 1A-D). At day 40 post-infection, both stat4−/− BALB/c and C57BL/6 mice had significantly higher parasite loads in their livers compared to WT BALB/c and C57BL/6 mice respectively, but not in spleens (Figures 1A-1D). At day 60 post infection, both stat4−/− BALB/c and C57BL/6 mice had more elevated parasite loads in their livers and spleens compared to WT (Figures 1A-D). These data demonstrate that the absence of STAT4 results in the failure of both C57BL/6 and BALB/c mice to control L. donovani infection in spleens and livers.
Figure 1. stat4−/− mice are highly susceptible to L. donovani infection.
Spleen and liver parasite loads in WT and stat4−/− BALB/c (A-B) or C57BL/6 (C-D) mice were determined 20, 40 and 60 days following intravenous inoculation of 107 Leishmania donovani amastigotes. Parasite burdens in spleen of and liver were expressed as mean LDU ±SE. The data are the mean values from four or five individual mice per group at each time point in three independent experiments pooled with similar results. *, P < 0.05, **, P < 0.01 and ***, P < 0.001 using unpaired t test.
STAT4 is critical for mounting an efficient Th1 response during Leishmania donovani infection
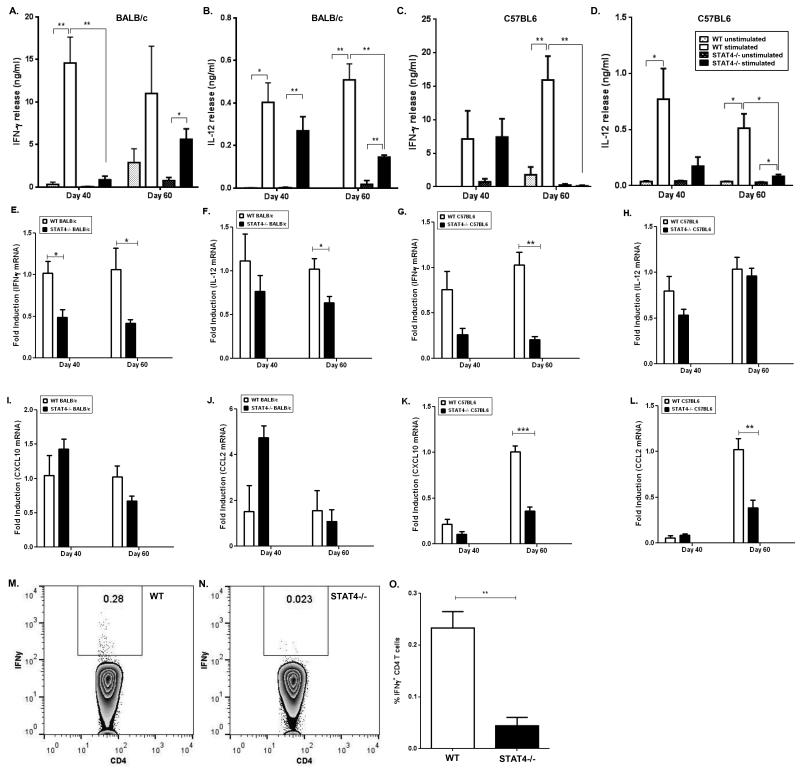
IL-12, a critical Th1 cytokine which induces IFN-γ, can function via a STAT4 dependent or independent pathway [18]. To determine whether stat4−/− mice infected with L. donovani were able to mount a Th1 response, we measured IL-12 and IFN-γ production from splenocytes of WT and stat4−/− mice following in vitro stimulation with L. donovani antigen (LdAg). At day 40, IFN-γ levels were impaired in stat4−/− BALB/c mice and at day 60 the production of IFN-γ was impaired in both stat4−/− BALB/c and C57BL/6 mice compared to WT counterparts (Figures 2A and 2C). IFN-γ producing CD4+ T cell populations were also significantly reduced in spleens of L. donovani infected stat4−/− mice compared to WT mice (Figures 2M-O). Although IL-12 production from both stat4−/− and WT BABL/c or C57BL/6 mice were comparable at day 40 (Figures 2B and 2D), it was impaired in stat4−/− BALB/c and C57BL/6 mice at day 60 compared to their respective WT counterparts (Figures 2B and 2D). Real time PCR analysis of splenic RNA from WT and stat4−/− BALB/c and C57BL/6 mice showed similar Th1 cytokine profiles (Figures 2E-H). These findings show that STAT4 is required for mounting efficient Th1 immune responses in L. donovani infected BALB/c and C57BL/6 mice.
Figure 2. stat4−/− mice are defective in generating a Th1 response during L. donovani infection.
Th1 cytokine production by splenocytes from L. donovani infected WT and stat4−/− BALB/c mice or WT and stat4−/− C57BL/6 mice stimulated with 20μg/ml LdAg. IFN-γ (A and C) and IL-12p70 (B and D) were measured by ELISA. Data shown were the mean ± SE of triplicates from four or five individual mice per group and are representative of three individual experiments. *, P < 0.05 and **, P < 0.01 using unpaired t test.
Th1 cytokine and chemokine mRNA expression by spleen tissue from L. donovani infected WT and stat4−/− BALB/c mice or WT and stat4−/− C57BL/6 mice. IFN-γ mRNA (E and G), IL-12p35 mRNA (F and H), CXCL10 mRNA (I and K) and CCL2 mRNA (J and L) were measured by Real Time PCR. Intracellular IFN-γ production by CD4+ T cells isolated from spleens of L. donovani infected WT (M) and stat4−/− (N) mice. Average percentage of IFN-γ producing CD4+ T cells of L. donovani infected WT and stat4−/− (O) mice. (n=5 mice per group). *, P < 0.05, **, P < 0.01 and ***, P < 0.001 using unpaired t test.
We also determined the role of STAT4 in the induction of the inflammatory chemokines CCL2 and CXCL10. Both CCL2 and CXCL10 are required for the recruitment of Th1 cells to sites of inflammation [19-21] and activation of host immune response pathways against Leishmania [22-24]. Although there were no significant differences in CXCL10 and CCL2 mRNA expression in WT and stat4−/− BALB/c mice, there was a significant decrease in both CXCL10 and CCL2 mRNA expression in stat4−/− C57BL/6 mice compared to WT at day 60 post-infection (Figures 2I-L).
Stat4 deficiency does not increase the production of IL-4 and IL-10 during L. donovani infection
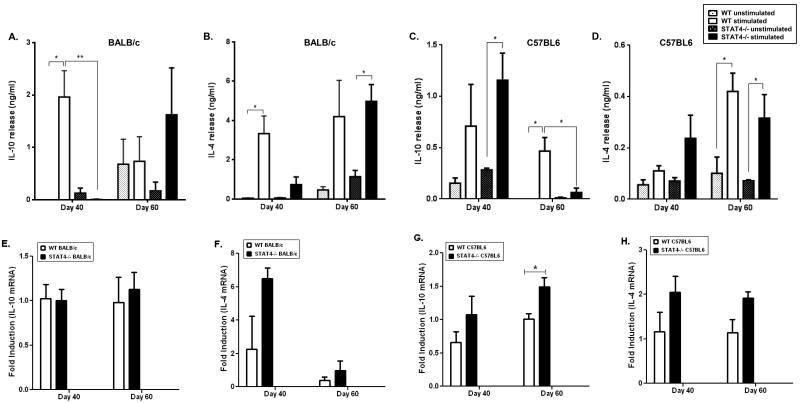
Previous studies using stat4−/− mice have shown that the absence of STAT4 enhances Th2 development [25]. We therefore analyzed the production of the Th2 cytokine IL-4, as well as the anti-inflammatory cytokine IL-10 in L. donovani infected stat4−/− BALB/c and C57BL/6 mice. At day 40 post infection, stat4−/− BALB/c mice showed significantly low levels of IL-10 compared to WT BALB/c mice (Figure 3A), while stat4−/− C57BL/6 mice showed slightly increased IL-10 levels compared to WT C57BL/6 mice although not statistically significant (Figure 3C). Furthermore, at day 60 post-infection there was no significant difference in the levels of IL-10 and IL-4 in stat4−/− and WT BALB/c mice while stat4−/− C57BL/6 mice showed a reduction in the production of IL-10 and IL-4 at day 60 post-infection compared to its WT counterpart (Figures 3A-D). We obtained similar results using quantitative PCR of splenic mRNA (Figures 3E-H). Taken together our results show that STAT4 is not involved in suppressing Th2 cytokine production during VL.
Figure 3. Th2 responses in stat4−/− mice during L. donovani infection.
Th2 cytokine production by splenocytes from L. donovani infected WT and stat4−/− BALB/c mice or WT and stat4−/− C57BL/6 mice stimulated with 20μg/ml LdAg. IL-10 (A and C) and IL-4 (B and D) were measured by ELISA. Data shown are the mean ± SE of triplicates from four or five individual mice per group and are representative of three individual experiments. ** P < 0.01, * P < 0.05 using unpaired t test.
Th2 cytokine mRNA expression by spleen tissue from L. donovani infected WT and stat4−/− BALB/c mice or WT and stat4−/− C57BL6 mice. IL-10 mRNA (E and G) and IL-4 mRNA (F and H) were measured by Real Time PCR. *, P < 0.05 and **, P < 0.01 using unpaired t test.
Stat4−/− mice have delayed formation of granulomas in the liver
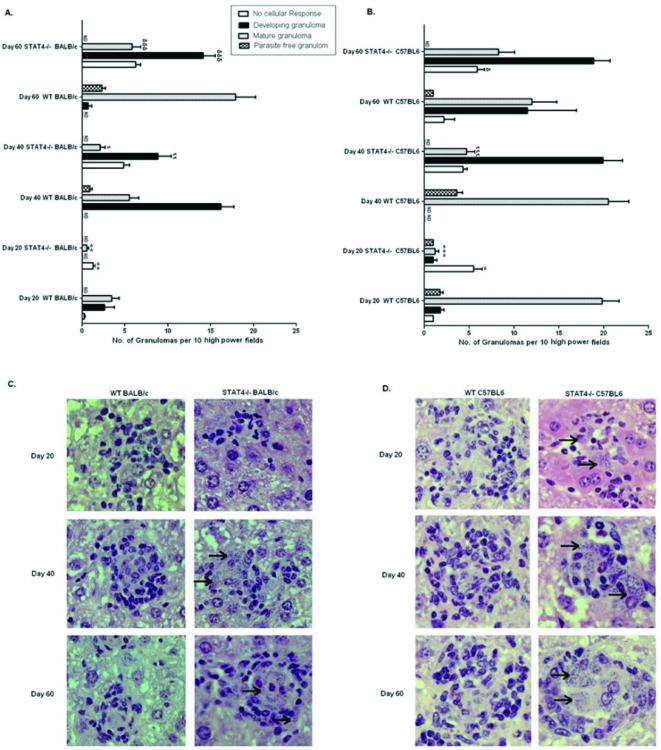
The hallmark of L. donovani infection is the formation of granulomas, comprised of macrophages and T cells, aimed at controlling the spread of the parasite [26]. We therefore analyzed granuloma formation in stat4−/− mice and determined its correlation with hepatic parasite burdens during experimental VL. We observed an inefficient and delayed granulomatous response in stat4−/− BALB/c and C57BL/6 mice compared to WT counterparts. At day 40, while WT BALB/c and C57BL/6 mice exhibited well-formed granulomas, stat4−/− mice did not show foci of inflammation (Figures 4C and D). Further, at day 60 hepatic granulomas in stat4−/− mice were still ill-formed (Figures 4A-D). This inability to mount an efficient granulomatous response correlated with increased hepatic parasite burdens in stat4−/− mice infected with L. donovani (Figures 1B and D).
Figure 4. Immunopathology of stat4−/− mice during L. donovani infection.
Liver sections from L. donovani infected WT and stat4−/− BALB/c mice (A) or WT and stat4−/− C57BL/6 mice (B) at days 20, 40 and 60 post infection were scored for the extent of granuloma formation. Results were from one representative experiment of three with similar findings. Data were expressed as mean granuloma number per 10 high-power fields (magnification 200×) ±SE. ND, not determined.*, P < 0.05, **, P < 0.01 and ***, P < 0.001 compared to their respective infected Day 20 WT control; ι, P < 0.05, ιι, P < 0.01 and ιιι, P < 0.001 compared to their respective infected Day 40 WT control; φ, P < 0.05, φφ, P < 0.01 and φφφ, P < 0.001 compared to their respective infected Day 60 WT control.
Histopathology of infected livers from WT and stat4−/− BALB/c mice (C), or WT and stat4−/− C57BL/6 mice (D). WT BALB/c and C57BL/6 mice showed developing granuloma at day 20 post infection and contained matured and parasite free granulomas at days 40 and 60 post infection. In contrast, stat4−/− BALB/c and C57BL/6 mice showed marked delay in granuloma formation as there was lack of cellular reaction at day 20 post infection and even at days 40 and 60 post infection, the granulomas were poorly developed and were highly parasitized (arrow). Magnifications 400× for panels C and D.
STAT4 is not required for sodium stibogluconate based chemotherapy of Leishmania donovani infected mice
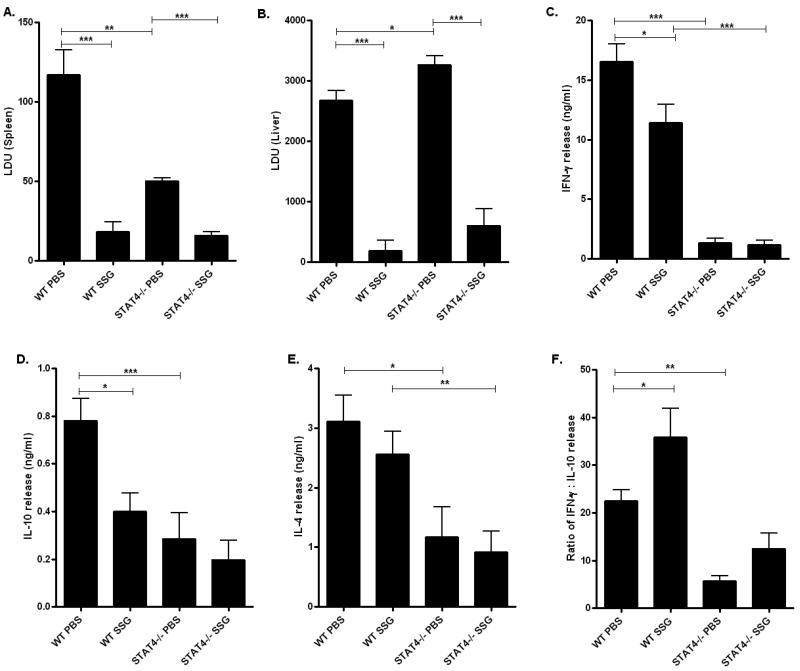
Previous studies have shown that IL-12 is important for mediating antimonial therapy of L. donovani infected mice [27]. To determine whether STAT4 is required for the anti-parasitic activity of SSG based chemotherapy, we evaluated the parasite loads in the spleen and liver of L. donovani infected WT and stat4−/− BALB/c mice treated with SSG. BALB/c mice were chosen for this treatment study because they present a more susceptible phenotype to L. donovani infection. L. donovani infected WT mice treated with 500mg per kg body weight of SSG showed about 85% and 94% clearance of parasites from the spleen and the liver respectively in comparison to PBS treated WT infected controls (Figures 5A, B and 6A). Interestingly, stat4−/− infected mice showed similar rates of parasite clearance (about 69% and 82% clearance in the spleen and the liver respectively) compared to PBS treated stat4−/− infected mice (Figures 5A, B and 6A).
Figure 5. Activity of SSG based chemotherapy in stat4−/− mice.
Spleen (A) and liver (B) parasite loads in SSG or PBS treated L. donovani infected WT and stat4−/− BALB/c mice. Parasite burdens in spleen of and liver were expressed as mean LDU ±SE. The data were the mean values from four or five individual mice per group at each time point in three independent experiments pooled with similar results. *, P < 0.05, **, P < 0.01 and ***, P < 0.001 using unpaired t test.
Th1 and Th2 cytokine production by splenocytes from SSG or PBS treated L. donovani infected WT and stat4−/− BALB/c mice stimulated with 20μg/ml LdAg. IFN-γ (C), IL-10 (D) and IL-4 (E) were measured by ELISA. Ratios of IFN-γ to IL-10 production (F) are also shown. Data shown were the mean ± SE of triplicates from four or five individual mice per group and are representative of three individual experiments. *, P < 0.05 and **, P < 0.01 using unpaired t test.
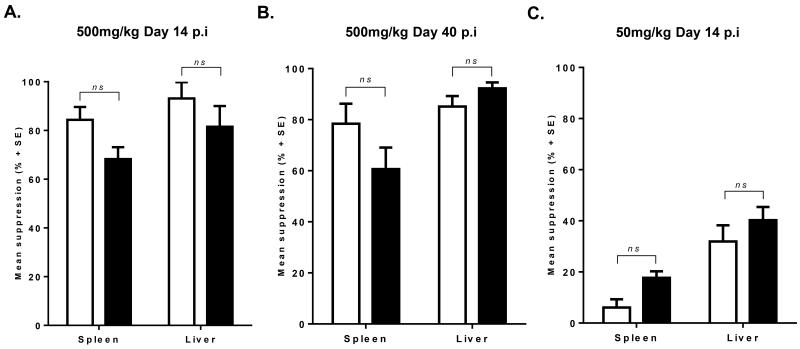
Figure 6. Mean suppression of parasite burdens in SSG treated WT and stat4−/− mice.
The mean suppression (± SE) of parasite burdens in spleen and liver of L. donovani infected WT and stat4−/− BALB/c mice treated with different doses of SSG or at different times post infection. (A) Mice were treated with 500mg/kg at day 14 post infection and analyzed 14 days later. (B) Mice were treated with 500mg/kg at day 40 post infection and analyzed at day 14 days later. (C) Mice were treated with 50mg/kg at day 14 post infection and analyzed 14 days later. Parasite suppression was measured by comparing each experimental value with the mean control value. Data shown are the mean values from four to six individual mice per group in two independent experiments with similar results. (n.s: p < 0.1, p.i: post infection).
To expand our understanding of the role STAT4 plays in SSG based chemotherapy of L. donovani infected mice, we treated WT and stat4−/− BALB/c mice at either a later time point when STAT4 deficiency significantly affected parasite infection levels, or at suboptimal doses which would require a greater immune response involvement. L. donovani infected mice treated with 500mg per kg body weight of SSG at day 40 post infection resulted in a comparable reduction in parasite burdens between stat4−/− and WT infected mice (Figure 6B). Further, suppression of parasite burdens was similar to stat4−/− infected mice treated with SSG at day 14 (Figure 6A and B). Treatment of L. donovani infected BALB/c mice with the suboptimal dose of 50mg per kg SSG [28, 29] at day 14 post infection resulted in about 35% mean suppression of parasite burdens in the liver, while minimal effects were observed in splenic parasite burdens of SSG treated WT mice (Figure 6C). As in previous SSG treatment experiments, suppression of parasite burdens in stat4−/− mice was similar to that observed in WT mice (Figure (6C). These results suggest that STAT4 plays a negligible role in SSG based chemotherapy of L. donovani infected mice.
We also analyzed host cytokine responses of splenocytes restimulated with LdAg in vitro following SSG treatment of L. donovani infected WT and stat4−/− mice. In WT mice, although IFN-γ levels were lower in SSG treated mice compared to PBS treated controls (Figure 5C), IL-10 and IL-4 levels were also significantly lower in the SSG treated group compared to PBS treated controls (Figures 5D and E). More significantly, the ratio of IFN-γ to IL-10 was higher in SSG treated WT mice compared to PBS treated WT controls (Figure 5F), suggesting the existence of a protective balance between cytokines necessary for the eradication of the parasite. Similar to WT mice, stat4−/− mice displayed slightly lower levels of IL-10 and IL-4 (Figures 5D and E) and an increased ratio of IFN-γ to IL-10 in SSG treated stat4−/− mice infected with L. donovani compared to the PBS treated control group (Figure 5F). Taken together, these results suggest that STAT4 is dispensable for SSG based chemotherapy of L. donovani infected mice.
Discussion
This study clearly establishes a role for STAT4 in mediating protection against experimental VL caused by L. donovani. We and others have previously shown that IL-12 is essential for protective immunity during L. donovani infection [3, 9]. Although IL-12 can function independently of STAT4 [18], it is evident from this study that STAT4 independent pathways do not result in resistance to L. donovani infection in mice. This is true for both BALB/c and C57BL/6 mouse backgrounds which are genetically and immunologically distinct and display dissimilar phenotypes in response to VL. While C57BL/6 WT mice have elevated levels of IFN-γ during the initial acute phase of L. donovani infection leading to early recovery, BALB/c mice have a delayed IFN-γ response resulting in chronic infection [2]. It is noteworthy that during early time points of infection (day 20), STAT4 does not appear to contribute to immunity in both BALB/c and C57BL/6 mice. However, at later time points (day 40 and day 60), STAT4 deficiency led to a progressive increase in parasite loads in the spleens and livers of both BALB/c and C57BL6 mice. A similar trend was observed in previous studies using il-12−/− mice infected with L. donovani, which showed increased susceptibility during late time points of infection in both the spleen and liver [9].
IFN-γ produced mostly by Th1 cells during the adaptive phase of the immune response is critical for host antiparasitic activity and subsequent control of L. donovani infection in resistant mice [30]. Previous studies have shown that IL-12 signaling via STAT4 is essential for IFN-γ production and Th1 cell development [31], and IL-12 stimulated T cells from stat4−/− mice produce low levels of IFN-γ compared to WT T cells [25]. Our studies with L. donovani infected stat4−/− BALB/c and C57BL6 mice at days 40 and 60 post-infection also showed low levels of IFN-γ compared to WT counterparts. Similar defects in the production of IFN-γ were also observed in previous studies with L. donovani infected il-12−/− mice [9], L. major infected stat4−/− mice [32] and L. mexicana infected stat4/stat6−/− mice [33]. Furthermore, in this study, we observed low levels of IL-12 induction at days 40 and 60 post-infection in stat4−/− BALB/c and C57BL6 mice compared to WT counterparts. In the absence of STAT4, compromised IL-12 mediated IFN-γ production by Th1 cells could lead to a subsequent attenuation of IL-12 production by macrophages and B cells [34].
Stat4−/− mice also failed to develop mature hepatic granulomas which are critical to the containment of the Leishmania parasite. This is not surprising since the formation of granulomas during L. donovani infection requires the action of endogenous IFN-γ [30] and IL-12 [3, 9], which activate macrophages and promote the development of Th1 immune responses important in resistance against VL.
Although IFN-γ signaling is largely mediated by STAT1 [35], and stat1 −/− mice are highly susceptible to L. major infection [36], we previously showed that stat1−/− mice are surprisingly highly resistant to L donovani through mechanisms which are still not fully understood [12]. Interestingly, others have shown that both il-12−/− and stat4−/− mice are susceptible to L. major infection [32], and il12−/− mice are susceptible to L. donovani [10]. Our current study demonstrates that stat4−/− mice are also susceptible to L. donovani infection. It is therefore evident that cytokines and their signaling mediators may not always play similar roles in determining the outcome of experimental VL in mice, which highlights the importance of a thorough evaluation of the role of each signaling molecule in immunity to infection.
Previous studies by Kaplan et al showed that STAT4 deficiency favors the development of Th2 cells [25]. However, in L. donovani infection, we do not observe significant differences in IL-10 or IL-4 production by WT and stat4 −/− splenocytes from BALB/c mice restimulated with LdAg at day 60 post-infection. Further, in C57BL/6 mice, IL-4 and IL-10 production by restimulated splenocytes was higher in WT than in stat4−/− mice at the same late stage of infection. It appears from this study that STAT4 deficiency does not enhance the production of Th2 cytokines during L. donovani infection. Multiple factors, including the dynamics of host-pathogen interactions govern the development of Th2 cells. In WT C57BL/6 mice, a recovery from the acute stage of infection followed by the abrogation of L. donovani induced immune responses might lead to higher Th2 cytokine production than in stat4−/− C57BL/6 mice which still contain chronic infection [2]. However, it is the deficiency in the induction and maintenance of Th1 responses, and not the production of Th2 cytokines, that account for the development of progressive and non-healing disease in L. donovani infected stat4−/− mice.
Our evaluation of the role of STAT4 in SSG chemotherapy showed that STAT4 deficiency did not affect the outcome of SSG treatment in L. donovani infected BALB/c mice. Both SSG treated WT and stat4−/− mice showed similar levels of parasite clearance from the spleen and liver. The ability to respond to SSG chemotherapy has been shown to require a fully competent T cell response. In T cell deficient mice antimonial treatment is completely inactive [37]. Previous studies with il-12−/− and ifn-γ−/− mice have also shown that endogenous IL-12 and IFN-γ are essential for optimal activity of SSG based chemotherapy during L. donovani infection [27]. In contrast with IL-12, our results demonstrate that STAT4 is not required for optimal activity of SSG based chemotherapy in the management of VL. One possible explanation for this contrasting effect of SSG in stat4−/− and il12−/− mice could be the influence of the immunosuppressive cytokine IL-35 which has recently been shown to signal through STAT1 and STAT4 [38]. In the absence of IL-12, the immunosuppressive effect of IL-35 could abrogate the antileishmanial activity of SSG, whereas in stat4−/− mice, the effect of IL-35 is lost, rendering them more responsive to SSG chemotherapy. Not surprisingly, treatment with SSG lowered IL-4 and IL-10 production in stat4−/− mice at the same rate as in WT mice. Further, similar rates of increase in the ratio of IFN-γ to IL-10 were observed in SSG treated WT and stat4−/− mice compared to PBS treated controls. These factors could contribute to the optimal activity of SSG in L. donovani infected WT mice and such effects do not seem to be compromised in stat4−/− mice.
In conclusion, we demonstrate for the first time the role of STAT4 in disease progression during experimental VL. Our findings reveal that STAT4 is essential for immunity against L. donovani infection. During the late phases of infection, the absence of STAT4 results in progressive and non-curing infection in the spleen and liver of both BALB/c and C57BL6 mice due to defects in the induction of Th1 cytokines. On the other hand, unlike IL-12, STAT4 is dispensable for antimonial based chemotherapy in L. donovani infected mice.
Materials and Methods
Animals
Wild type BALB/c and C57BL/6 mice were purchased from Harlan Laboratories. Stat4−/− BALB/c and C57BL/6 mice were gift from Dr. Mark Kaplan (Indiana University). Mice were maintained and bred in hepa filter cages in a facility at the Ohio State University. The experiments were performed using 8-10 weeks old sex matched mice according to the institutional guidelines for animal research.
Parasites and Infection Protocol
Leishmania donovani (LV82 strain) was maintained by serial passage of amastigotes in Golden Syrian hamsters. Amastigotes were isolated from the spleen of sick hamsters and experimental mice were injected with 107 L. donovani amastigotes in 100μl by intravenous injection into the tail vein. Groups of three or five mice were sacrificed at various time points post infection for further analysis.
Treatment of mice
L. donovani infected wild type (WT) or stat4−/− mice on BALB/c background were treated (i.p) with SSG (Albert David, India) at a dose of 500mg/kg body weight of the animal or phosphate buffered saline (PBS) at 2 weeks post infection. Following two weeks of treatment, the animals were sacrificed for further analysis.
Parasite Burden Calculation
Livers and spleens were harvested, weighed and sectioned to prepare impression smears that were stained with Giemsa to enumerate the number of amastigotes per thousand nucleated cells. The parasite loads were expressed as Leishman-Donovan Units (LDU) = Number of amastigotes per 1000 nucleated cells × organ weight (grams). Effect of SSG therapy was expressed as mean parasite suppression which was determined by comparing percentage parasite reduction in SSG treated mice with the corresponding mean control value [15, 28].
Cytokine ELISA
Splenocytes isolated from WT and stat4−/− mice were plated at a concentration of 0.5 × 106 cells per well in quadruplicates in a sterile 96-well tissue culture plates. Cells were then stimulated with freeze thawed L. donovani antigen (20μg/ml). Supernatants were collected after 72h of incubation at 37°C and analyzed for the production of IFN-γ, IL-12p70, IL-4, and IL-10 by ELISA (BD Pharmingen).
Quantification of transcript levels by RT-PCR
Total RNA was extracted from 50mg of spleen tissue using TRIZOL Reagent. mRNA was reverse transcribed and cDNA was amplified by real-time PCR as described previously [39]. Primers and reaction conditions were found using the PRIMER BANK website (Massachusetts General Hospital. Primer Bank. http://pga.mgh.harvard.edu/primerbank). Data were normalized to the housekeeping gene β-actin and presented as fold induction over infected WT mice using the delta-delta CT method.
Histopathology
Tissue sections from the liver of L. donovani-infected mice were stained using H&E and subjected to histopathology. Liver granulomas were enumerated and scored as follows: 1) no reaction; 2) developing; 3) mature; and 4) empty [26]. At each time point, livers from at least 4–5 individual mice were analyzed in each group.
Intracellular cytokine staining
Spleens of infected mice were recovered, homogenized using 70-μM strainers and re-stimulated in the presence of brefeldin A for 5 hours. Cells were then stained with fluorescently labeled anti-CD4 antibody (Biolegend San Diego, CA), permeabilized and intracellular IFN-γ production was measured by flow cytometry using PE conjugated anti-IFN-γ antibody (BioLegend, San Diego, CA) on cells gated on CD4+ populations.
Statistical analysis
Student’s unpaired t test was used to determine statistical significance of differences in the values. A value of p < 0.05 was considered significant. The statistical significance of antibody titers and IFN-γ: IL-10 ratios were determined by non parametric tests using Mann-Whitney U-test.
Acknowledgements
This work was supported by National Institutes of Health grants R03AI090231, RC4AI092624, R34AI100789, R21AT004160 and R03CA164399 awarded to A.R.S and National Institute of Dental and Craniofacial Research Training Grant T32DE014320 awarded to S.O.
Abbreviations
- VL
visceral leishmaniasis
- WT
wild type
- SSG
sodium stibogluconate
Footnotes
Conflict of Interest: The authors do not have a commercial or other association that might pose a conflict of interest
References
- 1.Stanley AC, Engwerda CR. Balancing immunity and pathology in visceral leishmaniasis. Immunol Cell Biol. 2007;85:138–147. doi: 10.1038/sj.icb7100011. [DOI] [PubMed] [Google Scholar]
- 2.Lehmann J, Enssle KH, Lehmann I, Emmendörfer A, Lohmann-Matthes ML. The capacity to produce IFN-gamma rather than the presence of interleukin-4 determines the resistance and the degree of susceptibility to Leishmania donovani infection in mice. J Interferon Cytokine Res. 2000;20:63–77. doi: 10.1089/107999000312748. [DOI] [PubMed] [Google Scholar]
- 3.Murray HW. Endogenous interleukin-12 regulates acquired resistance in experimental visceral leishmaniasis. J Infect Dis. 1997;175:1477–1479. doi: 10.1086/516482. [DOI] [PubMed] [Google Scholar]
- 4.Gazzinelli RT, Wysocka M, Hayashi S, Denkers EY, Hieny S, Caspar P, Trinchieri G, Sher A. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- 5.Chan SH, Perussia B, Gupta JW, Kobayashi M, Pospísil M, Young HA, Wolf SF, Young D, Clark SC, Trinchieri G. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med. 1991;173:869–879. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manetti R, Parronchi P, Giudizi MG, Piccinni MP, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 8.Ahn HJ, Maruo S, Tomura M, Mu J, Hamaoka T, Nakanishi K, Clark S, Kurimoto M, Okamura H, Fujiwara H. A mechanism underlying synergy between IL-12 and IFN-gamma-inducing factor in enhanced production of IFN-gamma. J Immunol. 1997;159:2125–2131. [PubMed] [Google Scholar]
- 9.Satoskar AR, Rodig S, Telford SR, Satoskar AA, Ghosh SK, von Lichtenberg F, David JR. IL-12 gene-deficient C57BL/6 mice are susceptible to Leishmania donovani but have diminished hepatic immunopathology. Eur J Immunol. 2000;30:834–839. doi: 10.1002/1521-4141(200003)30:3<834::AID-IMMU834>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Murray HW, Tsai CW, Liu J, Ma X. Responses to Leishmania donovani in mice deficient in interleukin-12 (IL-12), IL-12/IL-23, or IL-18. Infect Immun. 2006;74:4370–4374. doi: 10.1128/IAI.00422-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy KM, Ouyang W, Szabo SJ, Jacobson NG, Guler ML, Gorham JD, Gubler U, Murphy TL. T helper differentiation proceeds through Stat1-dependent, Stat4-dependent and Stat4-independent phases. Curr Top Microbiol Immunol. 1999;238:13–26. doi: 10.1007/978-3-662-09709-0_2. [DOI] [PubMed] [Google Scholar]
- 12.Rosas LE, Snider HM, Barbi J, Satoskar AA, Lugo-Villarino G, Keiser T, Papenfuss T, Durbin JE, Radzioch D, Glimcher LH, Satoskar AR. Cutting edge: STAT1 and T-bet play distinct roles in determining outcome of visceral leishmaniasis caused by Leishmania donovani. J Immunol. 2006;177:22–25. doi: 10.4049/jimmunol.177.1.22. [DOI] [PubMed] [Google Scholar]
- 13.Murray HW, Oca MJ, Granger AM, Schreiber RD. Requirement for T cells and effect of lymphokines in successful chemotherapy for an intracellular infection. Experimental visceral leishmaniasis. J Clin Invest. 1989;83:1253–1257. doi: 10.1172/JCI114009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray HW, Moreira AL, Lu CM, DeVecchio JL, Matsuhashi M, Ma X, Heinzel FP. Determinants of response to interleukin-10 receptor blockade immunotherapy in experimental visceral leishmaniasis. J Infect Dis. 2003;188:458–464. doi: 10.1086/376510. [DOI] [PubMed] [Google Scholar]
- 15.Alexander J, Carter KC, Al-Fasi N, Satoskar A, Brombacher F. Endogenous IL-4 is necessary for effective drug therapy against visceral leishmaniasis. Eur J Immunol. 2000;30:2935–2943. doi: 10.1002/1521-4141(200010)30:10<2935::AID-IMMU2935>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 16.Murray HW, Delph-Etienne S. Roles of endogenous gamma interferon and macrophage microbicidal mechanisms in host response to chemotherapy in experimental visceral leishmaniasis. Infect Immun. 2000;68:288–293. doi: 10.1128/iai.68.1.288-293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray HW, Montelibano C, Peterson R, Sypek JP. Interleukin-12 regulates the response to chemotherapy in experimental visceral Leishmaniasis. J Infect Dis. 2000;182:1497–1502. doi: 10.1086/315890. [DOI] [PubMed] [Google Scholar]
- 18.Lund RJ, Chen Z, Scheinin J, Lahesmaa R. Early target genes of IL-12 and STAT4 signaling in th cells. J Immunol. 2004;172:6775–6782. doi: 10.4049/jimmunol.172.11.6775. [DOI] [PubMed] [Google Scholar]
- 19.Siveke JT, Hamann A. T helper 1 and T helper 2 cells respond differentially to chemokines. J Immunol. 1998;160:550–554. [PubMed] [Google Scholar]
- 20.Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, North R, Gerard C, Rollins BJ. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998;187:601–608. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonecchi R, Bianchi G, Bordignon PP, D’Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A, Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mannheimer SB, Hariprashad J, Stoeckle MY, Murray HW. Induction of macrophage antiprotozoal activity by monocyte chemotactic and activating factor. FEMS Immunol Med Microbiol. 1996;14:59–61. doi: 10.1111/j.1574-695X.1996.tb00268.x. [DOI] [PubMed] [Google Scholar]
- 23.Ritter U, Moll H. Monocyte chemotactic protein-1 stimulates the killing of leishmania major by human monocytes, acts synergistically with IFN-gamma and is antagonized by IL-4. Eur J Immunol. 2000;30:3111–3120. doi: 10.1002/1521-4141(200011)30:11<3111::AID-IMMU3111>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 24.Gupta G, Bhattacharjee S, Bhattacharyya S, Bhattacharya P, Adhikari A, Mukherjee A, Bhattacharyya Majumdar S, Majumdar S. CXC chemokine-mediated protection against visceral leishmaniasis: involvement of the proinflammatory response. J Infect Dis. 2009;200:1300–1310. doi: 10.1086/605895. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 26.Murray HW. Mononuclear cell recruitment, granuloma assembly, and response to treatment in experimental visceral leishmaniasis: intracellular adhesion molecule 1-dependent and -independent regulation. Infect Immun. 2000;68:6294–6299. doi: 10.1128/iai.68.11.6294-6299.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor AP, Murray HW. Intracellular antimicrobial activity in the absence of interferon-gamma: effect of interleukin-12 in experimental visceral leishmaniasis in interferon-gamma gene-disrupted mice. J Exp Med. 1997;185:1231–1239. doi: 10.1084/jem.185.7.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carter KC, Hutchison S, Boitelle A, Murray HW, Sundar S, Mullen AB. Sodium stibogluconate resistance in Leishmania donovani correlates with greater tolerance to macrophage antileishmanial responses and trivalent antimony therapy. Parasitology. 2005;131:747–757. doi: 10.1017/S0031182005008486. [DOI] [PubMed] [Google Scholar]
- 29.Murray HW, Berman JD, Wright SD. Immunochemotherapy for intracellular Leishmania donovani infection: gamma interferon plus pentavalent antimony. Journal of Infectious Diseases. 1988;157:973–978. doi: 10.1093/infdis/157.5.973. [DOI] [PubMed] [Google Scholar]
- 30.Squires KE, Schreiber RD, McElrath MJ, Rubin BY, Anderson SL, Murray HW. Experimental visceral leishmaniasis: role of endogenous IFN-gamma in host defense and tissue granulomatous response. J Immunol. 1989;143:4244–4249. [PubMed] [Google Scholar]
- 31.Thierfelder WE, van Deursen JM, Yamamoto K, Tripp RA, Sarawar SR, Carson RT, Sangster MY, Vignali DA, Doherty PC, Grosveld GC, Ihle JN. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- 32.Stamm LM, Satoskar AA, Ghosh SK, David JR, Satoskar AR. STAT-4 mediated IL-12 signaling pathway is critical for the development of protective immunity in cutaneous leishmaniasis. Eur J Immunol. 1999;29:2524–2529. doi: 10.1002/(SICI)1521-4141(199908)29:08<2524::AID-IMMU2524>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Sosa M, Monteforte GM, Satoskar AR. Susceptibility to Leishmania mexicana infection is due to the inability to produce IL-12 rather than lack of IL-12 responsiveness. Immunol Cell Biol. 2001;79:320–322. doi: 10.1046/j.1440-1711.2001.01014.x. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida A, Koide Y, Uchijima M, Yoshida TO. IFN-gamma induces IL-12 mRNA expression by a murine macrophage cell line, J774. Biochem Biophys Res Commun. 1994;198:857–861. doi: 10.1006/bbrc.1994.1122. [DOI] [PubMed] [Google Scholar]
- 35.Ramana CV, Gil MP, Schreiber RD, Stark GR. Stat1-dependent and - independent pathways in IFN-gamma-dependent signaling. Trends Immunol. 2002;23:96–101. doi: 10.1016/s1471-4906(01)02118-4. [DOI] [PubMed] [Google Scholar]
- 36.Späth GF, Schlesinger P, Schreiber R, Beverley SM. A novel role for Stat1 in phagosome acidification and natural host resistance to intracellular infection by Leishmania major. PLoS Pathog. 2009;5:e1000381. doi: 10.1371/journal.ppat.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray HW, Hariprashad J, Fichtl RE. Treatment of experimental visceral leishmaniasis in a T-cell-deficient host: response to amphotericin B and pentamidine. Antimicrob Agents Chemother. 1993;37:1504–1505. doi: 10.1128/aac.37.7.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collison LW, Delgoffe GM, Guy CS, Vignali KM, Chaturvedi V, Fairweather D, Satoskar AR, Garcia KC, Hunter CA, Drake CG, Murray PJ, Vignali DA. The composition and signaling of the IL-35 receptor are unconventional. Nat Immunol. 2012;13:290–299. doi: 10.1038/ni.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barbi J, Oghumu S, Lezama-Davila CM, Satoskar AR. IFN-gamma and STAT1 are required for efficient induction of CXC chemokine receptor 3 (CXCR3) on CD4+ but not CD8+ T cells. Blood. 2007;110:2215–2216. doi: 10.1182/blood-2007-03-081307. [DOI] [PMC free article] [PubMed] [Google Scholar]