Abstract
Estrogen receptors (ERs) including ERα and ERβ are known to regulate multiple biological responses in various cell-types. The expression of ERβ is lost in various cancers. ERβ-agonists were shown to modulate inflammation, cancer cell proliferation and differentiation. Here, we investigated the cancer chemopreventive properties of Erb-041, an ERβ agonist employing a model of UVB-induced photocarcinogenesis in SKH-1 mice. Erb-041 significantly reduced UVB-induced carcinogenesis. Tumor numbers and volume were reduced by 60% and 84%, respectively in Erb-041-treated group as compared to UVB (alone) control. This inhibition in tumorigenesis was accompanied by the decrease in PCNA, cyclin D1, VEGF and CD31; and increase in apoptosis. The lost ERβ expression in SCCs was significantly recovered by Erb-041 treatment. Additionally, the UVB-induced inflammatory responses were remarkably reduced. Myeloperoxidase activity; levels of cytokines IL1β, IL6 and IL10; and expression of p-ERK1/2, p-p38, p-IκB, iNOS, COX-2 and nuclear NFκBp65 were diminished. The number of tumor-associated inflammatory cells (GR-1+/CD11b+ and F4/80+) was also decreased. Tumors excised from Erb-041-treated animal were less invasive and showed reduced epithelial-mesenchymal transition (EMT). The enhanced expression of E-cadherin with the concomitantly reduced expression of N-Cadherin, Snail, Slug and Twist characterized these lesions. WNT/β-catenin signaling pathway, which underlies pathogenesis of skin cancer was found to be down-regulated by Erb-041 treatment. Similar but not identical changes in proliferation and EMT regulatory proteins were noticed following treatment of tumor cells with a WNT-signaling inhibitor XAV939. Our results show that Erb-041 is a potent skin cancer chemopreventive agent which acts by dampening WNT/β-catenin signaling pathway.
Keywords: Estrogen receptor, Erb-041, photocarcinogenesis, skin, UVB, inflammation, Wnt signaling
Introduction
Non-melanoma skin cancers (NMSCs), which include basal cell carcinoma (BCCs) and squamous cell carcinoma (SCCs) are the most commonly diagnosed cancers in the United States. Their incidence exceeds the combined incidence of cancers of the breast, prostate, lung and colon (1). Ultraviolet (UV) B radiation (280–320 nm) from the sun and tanning beds are the main etiologic cause of skin cancer (2). UVB induces DNA damage, inflammatory response, and alters multiple cell signaling events, which altogether lead to initiation, promotion and progression of epidermal neoplasm (3). During the past decade, a number of attempts have been made to understand the pathogenesis of these cancers and to identify novel molecular targets to intervene the disease progression. In this regard, we and others have demonstrated the involvement of p53, ornithine decarboxylase, cyclooxygenases, retinoid receptor signaling, oxidative stress etc, besides many others in the molecular pathogenesis of these cancers (3–8). Strategies have also been developed to modify these targets to prevent NMSCs both in humans and in experimental animals (5, 9, 10). However, these approaches have been only partially successful.
The modulation of estrogen receptors (ERs) activity has proved therapeutically valuable for the treatment of various epithelial cancers in experimental models (11, 12). The ERs exist in two distinct forms ERα and ERβ. Their splice variants, which are also biologically active, have been identified (13). Estrogens exert their tissue-specific responses via ERα or ERβ or their splice variants by activating diverse signaling pathways that mediate both genomic and non-genomic events (11). It is interesting that despite remarkable similarities in the two receptors, ERα and ERβ are often antagonistic in nature. Altered ratio of ERα/ERβ in a cell is the major determinant of responses of the cell to estrogen. ERα/ERβ-mediated activation or deactivation is dependent on the effects of co-activator and co-repressor proteins on estrogen responsive element (ERE) (14, 15).
ERβ is a member of the nuclear receptor superfamily (13) and is produced from eight exons. Upon ligand activation, it regulates gene expression by modulating transcription factors, such as nuclear factor kappa B (NFκB), activating protein-1 (AP-1) and stimulating protein-1 (SP-1) through transcription factor crosstalk (16, 17). The non-genomic effects of ERβ are regulated by the activation of PKA, PKC and MAPK signaling pathways (18). The expression of ERβ is considered an important determinant of tumor phenotype and has also been suggested as a useful biomarker in the rheumatoid disease progression (19). ERβ-selective agonists have been shown to possess anti-carcinogenetic and anti-inflammatory properties in experimental model systems (20, 21). Loss of ERβ expression has been reported in various cancers, such as prostate, colorectal, thyroid carcinoma etc. (22–24). Methylation of CpG islands in the promoter of ERβ is considered as one of the putative mechanisms involved in the loss of its expression (25). Erb-041, a selective ERβ-agonist has been reported to possess strong anti-inflammatory activity and is under clinical trial for its potential use in rheumatoid arthritis (20, 26, 27).
In this study, we investigated the cancer chemopreventive effects of Erb-041 on the UVB-induced skin photocarcinogenesis employing SKH-1 hairless mice. We observed a potent cancer chemopreventive activity of Erb-041 in this experimental animal model. Erb-041 affects the growth of UVB-induced murine SCCs. We show that the mechanism by which this ERβ-agonist manifests cancer chemopreventive effects, involves inhibition of WNT/β-catenin-dependent signaling pathway.
Materials and Methods
Reagents and Antibodies
Erb-041 (C15H10FNO3) was procured from IRIX Pharmaceuticals Inc. (Florence, SC). Details of antibodies used in this study are provided as supplemental table 1.
Human tissue
Fresh skin tumor samples were collected according to our approved IRB protocol (N081204004) for undesignated samples. Human samples were carefully handled according to IRB guidelines.
Animals
Six- to eight-weeks-old SKH-1 hairless female mice were used for this study. Animals were housed in groups of five in each cage under conditions of constant temperature of 24±2°C and relative humidity of 50±10%, and were maintained on a 12 h light/12 h dark cycle with food and drinking water ad libitum. The animal studies described here were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Alabama at Birmingham.
Cell culture and treatment
Human immortalized keratinocyte (HaCaT) and human epidermoid carcinoma (A431) cells were purchased from the American Type Culture Corporation (Manassas, VA, USA) and SCC13 cells were gifted by Dr. S. K. Katiyar (UAB). These cells were routinely cultured in the recommended growth medium containing 10% FBS, 100U/ml of penicillin, and 100µg/ml of streptomycin in humidified incubators at 37°C under 5% CO2. Cells (60–70% confluent) were treated with Erb-041 or WNT signaling inhibitor or vehicle (DMSO) in complete culture medium. After 24 h of treatment, medium was removed and the cells were washed and harvested to prepare cell lysates.
UV light source
The UVB light source was a UVA/UVB Research Irradiation Unit (Daavlin Co., Bryan, OH) which is fitted with an electronic controller to regulate dose of irradiated UVB. The UVB irradiation procedure was identical to that described earlier (7).
Experimental Protocol
Animals were randomly divided into three groups of 20 mice each. Group-I animals received topical treatment with ethanol and served as age-matched vehicle control (negative control). Group-II and -III animals were irradiated with UVB (180mJ/cm2; twice/week) for 30 weeks. In addition, while group-II received vehicle and group-III animals received topical treatments with Erb-041 (2mg/mouse in 200µl ethanol), 30 min prior to UVB irradiation. The tumor number and size were recorded weekly using electronic Vernier Caliper as described earlier (7). Data were presented as mean ±SE and plotted as a function of weeks on test. After 30 weeks, the experiment was terminated and all mice were euthanized as per IACUC recommendations. Skin and tumor tissues were harvested and processed for histological and biochemical analysis as described in the following sections.
Histology, Immunohistochemistry, Immunofluorescence staining and Terminal deoxynucleotidyl transferase–mediated nick end labeling (TUNEL) assay
10% neutral-buffered formalin fixed tissues were embedded, and cut in the serial sections of 5µm. For histological evaluation, tissues were stained with H&E. Immunohistochemical and immunofluorescence staining were performed as described earlier (7). Vector Red Alkaline Phosphatase Substrate Kit (Cat no. SK5100) was also used according to manufacturer’s guidelines for immunohistochemistry. TUNEL assay was done using an in situ cell death detection, fluorescein kit from Roche Applied Science (Cat. no.1684795) following manufacturer’s guidelines.
Myeloperoxidase (MPO) activity
MPO activity in the skin samples was determined as described earlier (28). The change in absorbance was recorded at 460 nm using a Perkin Elmer 1420 Multilabel Counter Victor 3. The data are expressed as mean MPO units/mg protein/min.
Western blot analysis
Tissues were lysed in ice-cold lysis buffer containing 50mM Tris pH, 1% Triton X 100, 0.25% NaF, 10mM β-glycerophosphate, 1mM EDTA, 5mM sodium pyrophosphate, 0.5mM Na3VO4, 10mM DTT, 1% PMSF and protease inhibitors cocktail. For western blot analysis, proteins (60–80µg) were resolved on 10–15% SDS-PAGE and transferred onto a nitrocellulose membrane (BioRad, CA, USA) as described previously (7). Membrane was stripped and re-probed with anti-β-actin antibody to confirm equal protein loading. In instances where a blot was stripped multiple times and probed with different antibodies but the data are presented as a part of more than one figure, the same β-actin image was placed to represent loading controls in the figures.
Qualitative and quantitative RT-PCR
Extraction of total RNA, cDNA preparation and RT-PCR were performed as described previously (29). Relative quantification of the steady state target mRNA levels was calculated after normalization of total amount of cDNA to GAPDH endogenous reference. List of primers used in this study are described in supplementary table 2.
Flow cytometry
A431 and SCC13 cells were treated with and without Erb-041 for 0, 24, 36 and 48 h. The cells were trypsinized, washed and fixed with ice-cold 70% ethanol at −20°C overnight. Thereafter, the cells were washed and incubated with 20 mg/ml RNase A and 200 mg/ml propidium iodide in PBS at room temperature for 30 min, and subjected to flow cytometry using the BD Accuri C6 or FACSCalibur flow cytometer (San Jose, California). Cell cycle distribution was analyzed and provided as percentage of G1, S, and G2/M phase of cells.
Colony forming assay
A431 and SCC13 cells (500 cells/well) were seeded into 6-well plates and were allowed to grow overnight. Cells were treated with and without Erb-041 for 24 h and incubated in humidified chamber at 37°C for additional 10 days. Cell colonies were fixed with 4% paraformaldehyde for 5 min and stained with 0.5% crystal violet for 30s, and cell colonies were counted (30).
Wound healing assay
Briefly, A431 and SCC13 cells were allowed to grow to 90–100% confluence, and a fine scratch was made using a sterile pipette tip. Then, these cells were treated with and without Erb-041 and incubated at 37°C for 24 h. The cell motility was observed at 12 h and 24 h using an Olympus CK2 microscope with Olympus DP20 digital camera (Tokyo, Japan).
Immunocytostaining
HaCaT, A431 and SCC13 cells were grown in 24-well plate on round glass cover slips with or without Erb-041 slides. The cells were fixed with 4% paraformaldehyde for 15 min at RT. Cells were permeabilized and blocked with 1% BSA, 10% goat serum, 0.3M glycine and 0.1% Tween X for 1 h at RT. Then, cells were incubated with primary antibodies for 2 h at RT. After washing, the cells were incubated with appropriate Dylight 488 or Alexa Fluor 594 secondary antibodies for 1 h at RT in humidified chamber, washed and mounted with DAPI, and observed using Olympus BX51TRF microscope with an Olympus DP71 digital camera (Tokyo, Japan).
Densitometry and statistical analysis
Relative density of western blot bands was analyzed by using IMAGE J software downloaded from http://rsbweb.nih.gov/ij/. All values are expressed as mean±SE. Statistical analysis was performed using Microsoft Excel software 2007. The significance between two test groups was determined using Student’s t-test. ‘p’ value <0.05 was considered to be significant.
Results
Erb-041 treatment reduces UVB-induced skin photocarcinogenesis
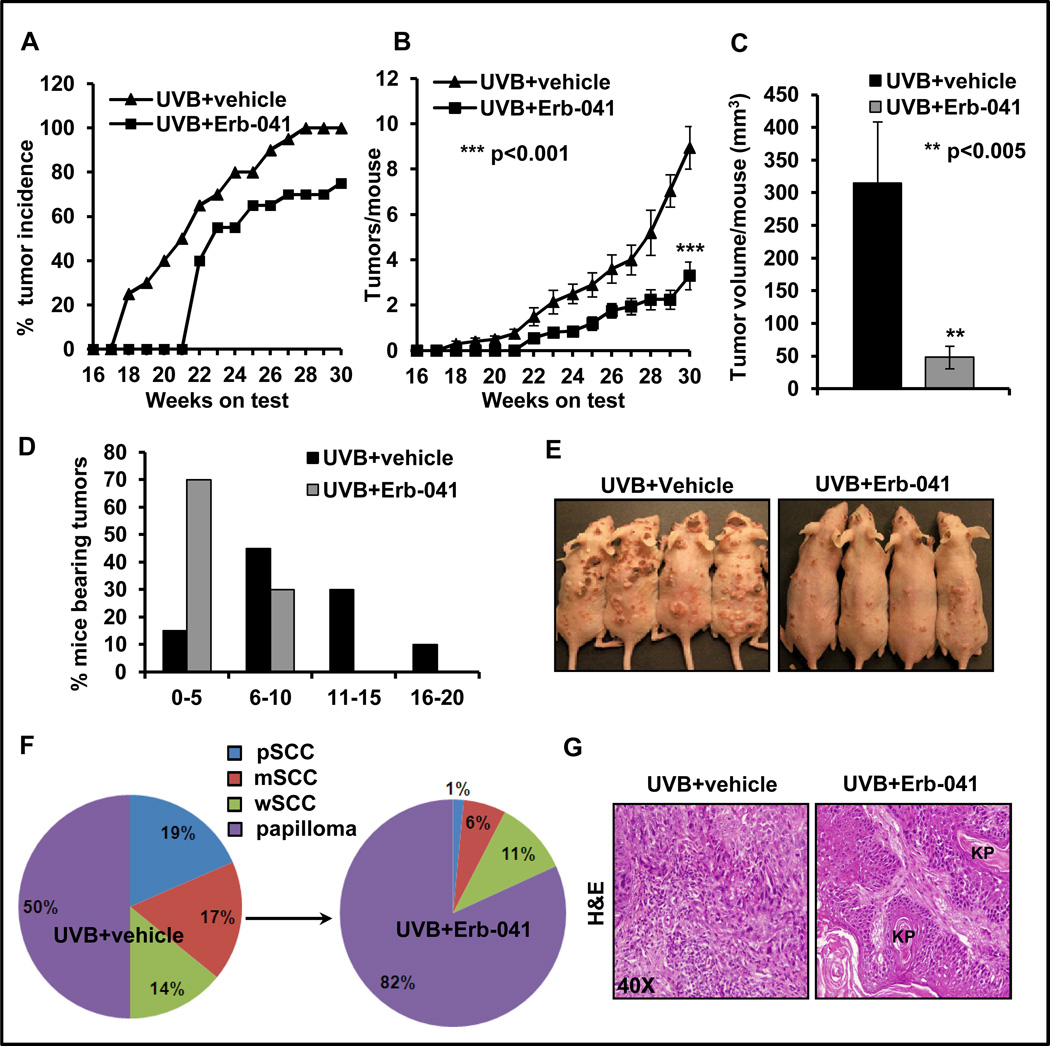
Topical treatment with Erb-041 substantially diminished UVB-induced skin tumor development in SKH-1 hairless mice as compared to vehicle-treated and UVB (alone)-irradiated mice. At the time of termination of experiment at week 30, the percentage of mice bearing tumors, tumors/mouse and tumor volume/mouse were significantly reduced in Erb-041-treated mice. The tumor incidence was 75% in Erb-041+UVB group whereas it was 100% in UVB-irradiated (alone) mice (Fig. 1A). The number of tumors/mouse was reduced to 3.3±0.62/mouse from 8.95±0.94/mouse in the UVB (alone) group, which represents >60% inhibition (Fig. 1B). Similarly, a 50% reduction (p<0.001) in the number of tumors/tumor-bearing mouse was observed (Fig. S1A). About 84% reduction in tumor volume (p<0.05) was noted in Erb-041-treated group (Fig. 1C). Erb-041 treatment increased latency period of tumor induction from 17 to 21 weeks. Overall, the number of SCCs/mouse was also reduced by 86% (p<0.001) (Fig. S1B). To analyze tumor burden in these animals, we divided each group with respect to the number of animals bearing 0–5, 6–10, 11–15 or 16–20 tumors/mouse. 15% of UVB-irradiated mice were bearing 0–5 tumors/mouse, 45% 6–10 tumors/mouse, 30% 10–15 tumors/mouse and 10% 16–20 tumors/mouse. However in Erb-041 treatment group, 70% of mice were bearing 0–5 tumors/mouse whereas 30% had 6–10 tumors/mouse (Fig. 1D and E). Histologically, SCCs at week 30 were characterized as a mix of poorly-differentiated SCCs (pSCC), moderately-differentiated SCCs (mSCC) and well-differentiated SCCs (wSCC). We also observed a few invasive keratoacanthomas. In UVB (alone)-group, SCC spectrum comprised of mice with 19% pSCC, 17% mSCC and 14% (wSCC) of the total tumors, whereas in Erb-041 treatment group, only 1% pSCC, 6% mSCC and 11% wSCC were observed (Fig. 1F). UVB-irradiated poorly differentiated SCCs were distinguished by the absence of keratin pearls, aggressive spindle cells with hyperchromatic pleomorphic nuclei and invasion of dermis. However, well-differentiated SCCs were characterized by the frequent presence of well-defined keratin pearls (Fig. 1G).
Figure 1. Estrogen receptor β agonist, Erb-041 suppresses development of squamous cell carcinoma in SKH-1 hairless mice.
SKH-1 hairless mice were topically treated with Erb-041 (2 mg/mouse in ethanol), 30 min prior to UVB (180mJ/cm2) irradiation for 30 weeks. Tumor number and size were recorded weekly. (A) percentage incidence of mice bearing tumor; (B) tumor/mouse; (C) tumor volume/mouse (mm3); (D) tumor spectrum of mice; (E) representative photograph of UVB-treated and Erb-041-treated SKH-1 hairless mice; (F) Pie chart showing % distribution of poorly differentiated SCCs (pSCC), moderately differentiated SCCs (mSCC) and well-differentiated SCCs (wSCC) and papilloma; (G) histology of tumor tissues showing poorly and well-differentiated SCCs. KP=keratin pearls.
Erb-041 reduces proliferation and angiogenesis and induces apoptosis in UVB-induced skin tumors
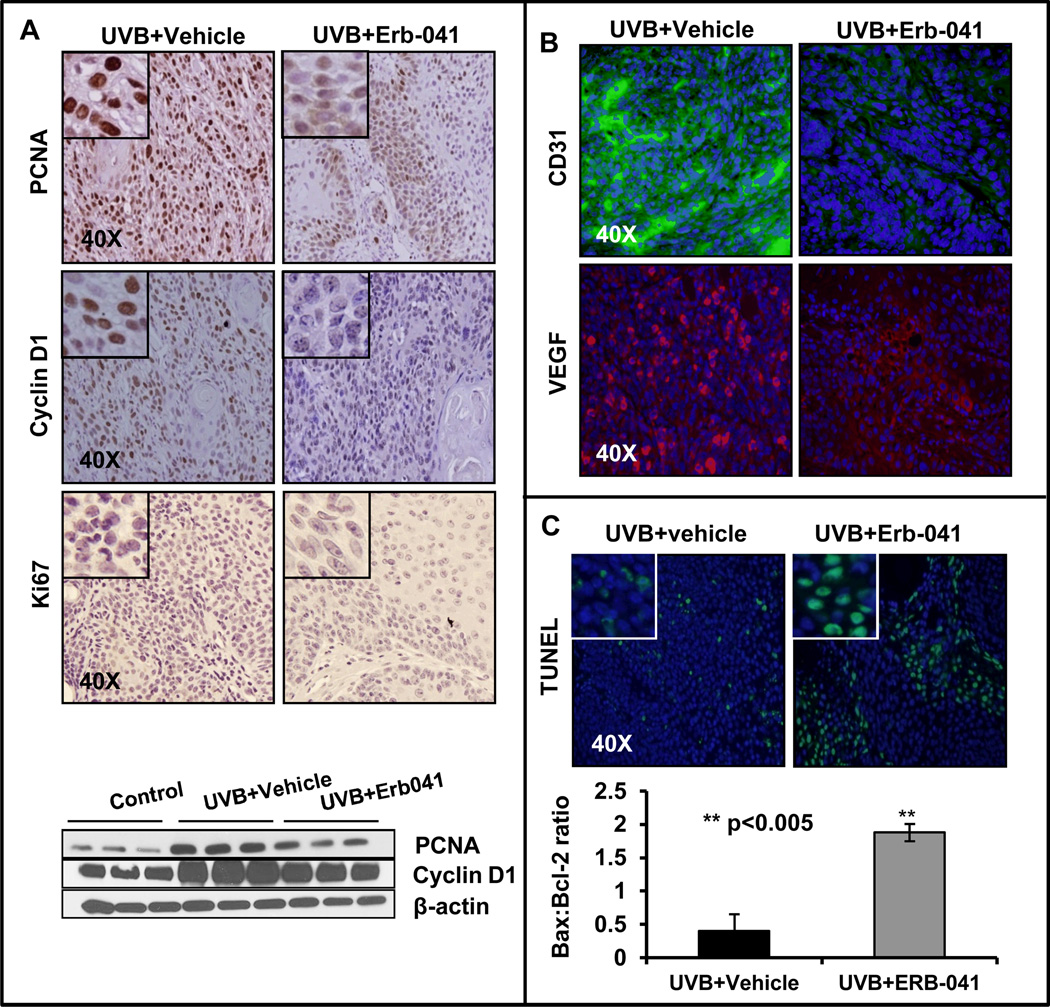
We investigated the effects of Erb-041 treatment on the expression of proliferative biomarkers such as proliferating cell nuclear antigen (PCNA), cyclin D1 and Ki67 in UVB-induced skin tumors. As assessed by immunohistochemistry as well as western blot analysis, Erb-041 treatment significantly (p<0.05) reduced the expression of these proteins (Fig. 2A and S1C). Angiogenesis biomarkers such as CD31/VEGF were assessed in UVB (alone)-irradiated and UVB+Erb-041-treated tumors. As shown in Fig. 2B, the immunostaining for CD31/VEGF was considerably reduced by Erb-041 treatment. The apoptosis in cutaneous tumor tissues was assessed by the presence of TUNEL-positive cells. The number of TUNEL-positive cells was highly increased in Erb-041 treatment group as compared to the UVB (alone) group (Fig. 2C). Since, induction of apoptosis is often correlated with the increased expression of pro-apoptotic Bax and decreased expression of anti-apoptotic Bcl-2, or an increased Bax/Bcl-2 ratio (31), we also assessed these parameters in this study. Erb-041 treatment altered the expression of Bax and Bcl-2 in these tumor lesions (Fig. S1D) in such a way that Bax/Bcl-2 ratio was significantly (p<0.005) increased in tumors (Fig. 2C).
Figure 2. Erb-041 blocks proliferation and angiogenesis, and induces apoptosis in UVB-induced cutaneous lesions in SKH-1 hairless mice.
(A) immunostaining showing expression of PCNA, cyclin D1, Ki67 and western blot analysis showing expression of PCNA and cyclin D1; (B) immunostaining of CD31 and VEGF; (C) TUNEL-staining and Bax:Bcl2 ratio.**p<0.005 when compared to UVB (alone)-treated positive control.
Erb-041 treatment augments the expression of ERβ in murine tumor keratinocytes
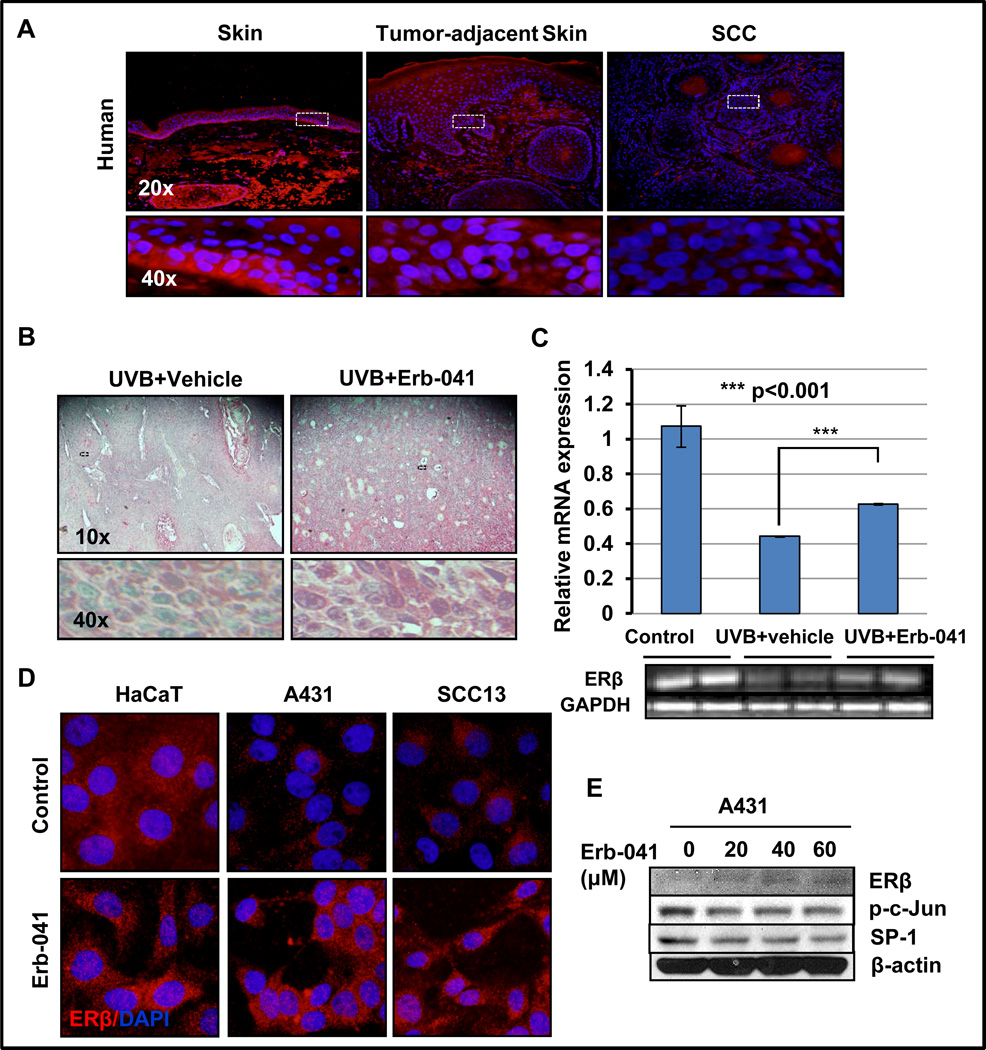
Earlier studies suggested that ERβ is a potent tumor suppressor and plays a crucial role in various cancers (22, 32, 33). Its expression is lost during the pathogenesis of various epithelial neoplasms (33). We, therefore, first assessed its expression in human cutaneous SCCs and tumor cells derived from SCCs. As shown in Fig. 3A, the expression of ERβ in histologically normal human skin was confined to the basal layer of the epidermis. Loss of expression in ERβ was noted in murine SCCs. Interestingly, Erb-041 treatment restored or even enhanced the expression of ERβ not only at protein level but also at transcriptional level in UVB-induced murine SCCs and human SCC cells in culture (Fig. 3B and C). Moreover, its expression was also apparent in the hyperplastic skin adjacent to papilloma and/or SCCs. However, a significant loss of its expression can be seen in human SCCs as well as SCCs-derived A431 and SCC13 cells as compared to immortalized HaCaT keratinocytes (Fig. 3D). Consistent with our in vivo results, Erb-041 treatment induced expression of ERβ in these human cells (Fig. 3E) which was confirmed with immunoblot. Reduced expression of p-c-Jun and SP-1 was also associated with increase in ERβ expression (Fig. 3E).
Figure 3. Erb-041 treatment enhances expression of ERβ.
(A) immunostaining of ERβ in human normal, tumor-adjacent and SCC/skin; (B) immunostaining showing nuclear and cytoplasmic expression of ERβ (C) mRNA expression (semi-quantitative PCR) of ERβ in UVB-(alone)-induced and Erb-041+UVB-induced SCCs; (D) immunocytostaining of ERβ in HaCaT, A431 and SCC13 cells treated with vehicle or Erb-041 for 24h; (E) western blot analysis showing expression of ERβ and its responsive genes p-c-Jun, SP-1 in A431 cells treated with vehicle or Erb-041.
Erb-041 suppresses pro-inflammatory signaling pathway in UVB-induced skin tumors
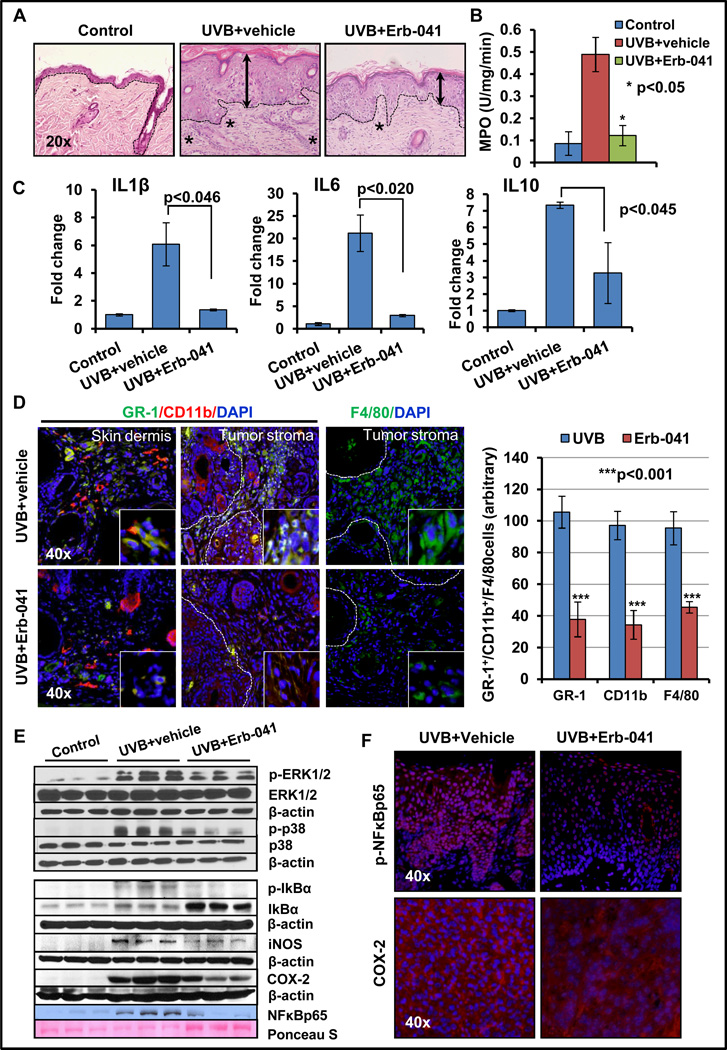
We examined the effects of Erb-041 on UVB-induced inflammation and inflammation-regulating mitogen-activated protein kinase (MAPK) signaling pathways. UVB-induced inflammatory responses in murine skin are characterized by the development of edema and hyperplasia, enhanced leukocyte infiltration in the dermis, leukocytes-secreted inflammatory cytokines, and increased level of COX-2 and prostaglandins (3, 34). Consistently, as shown in Fig. 4A, the chronic exposure of murine skin to UVB induced epidermal hyperplasia and dermal leukocytes infiltration, which was significantly reduced by Erb-041 treatment. MPO activity, a marker of neutrophil infiltration, was also decreased significantly (p<0.05) (Fig. 4B). Tumor micro-environment-associated inflammatory responses which are known to accelerate tumorigenesis (35, 36), were found to be attenuated by Erb-041. Thus a decrease in pro-inflammatory cytokines (IL1β, IL6, and IL10) in tumor-associated skin was noted in Erb-041-treated mice (Fig. 4C). CD11b+/GR1+-myeloid cell population and macrophages in the dermis of UVB-irradiated skin as well as in tumor-stroma contribute to pro-inflammatory skin tumor progression (36, 37). As shown in Fig. 4D, the numbers of CD11b+/GR1+-myeloid cells and F4/80+-macrophages were significantly decreased by Erb-041-treatment. This was also accompanied by a reduction in the phosphorylation-dependent activation of ERK1/2 and p38 MAPKs (Fig. 4E and S2A). Earlier Kim et al. reported that chronic UVB irradiation of the skin induces cytokine production, and activates MAPK signaling pathway (35) which was confirmed this study. UVB-induced inflammation is also known to be associated with NFκB activation (38, 39). NFκB exists as a heterotrimeric complex in cytoplasm which consists of p65, p52/p50 and inhibitory kappa B (IκB) proteins. Phosphorylation of IκB via inhibitor of nuclear factor kappa B kinases (IKKs) leads to release of transcriptionally active p65-p52/p65-p50 complexes and enable them to translocate to the nucleus (38, 39). Transcription activation of NFκB is also evident by the enhanced expression of its target genes including pro-inflammatory cyclooxygenase-2 (COX-2) and iNOS (Fig. 4E and F). The Erb-041-treatment suppressed phosphorylation of IκBα resulting in the accumulation of IκBα as a heterotrimeric complex in the cytoplasm. Concomitantly, by inhibiting the activation of NFκB, Erb-041 also reduced the expression of UVB-induced iNOS and COX-2 in these neoplastic lesions (Fig. 4E, F and S2A). Similarly, nuclear NFκBp65 and phosphorylated-NFκBp65 were reduced drastically in Erb-041-treated tumors as compared to the UVB (alone)-tumors (Fig. 4E and F). These data provide a basis for the anti-inflammatory action of Erb-041 in the skin.
Figure 4. Erb-041 treatment attenuates UVB-induced inflammatory cells infiltration in the skin and tumor stroma.
(A) H&E staining of vehicle- and Erb-041-treated UVB-induced hyperplastic skin showing infiltration of inflammatory cells in the dermis; (B) cutaneous MPO activity; (C) real-time PCR analysis showing expression of IL1β, IL6 and IL10; (D) immunofluorescence analysis of GR-1(green)/CD11b (red)-positive myeloid cells and F4/80 (green)-positive macrophages; (E) western blot analysis of inflammatory signaling regulatory proteins ERK1/2, p38, IκBα(Ser32/36), iNOS & COX-2 and nuclear p-NFκBp65; (F) immunofluorescence staining of p-NFκBp65 and COX-2.
Erb-041 diminished tumor invasiveness via PI3K-AKT pathway and WNT signaling
Epithelial-mesenchymal transition (EMT) is a process by which polarized epithelial cells transform to a mesenchymal fibroblast-like cell phenotype via multiple molecular cascades which result into apoptosis-resistance, enhanced migration, and invasiveness. EMT also increases components of extra cellular matrix (40, 41). In malignant neoplasm, repression of E-cadherin by transcription factors such as Snail and Twist, ultimately leads to up-regulation of mesenchymal marker proteins such as Vimentin, Fibronectin and N-cadherin (41). EMT is known to be regulated by multiple mechanisms including those dependent on PI3K/AKT signaling pathways (7, 41). Therefore, we investigated whether Erb-041 interferes with the EMT progression in UVB-induced tumors. Immunoblot and immunofluorescence analysis confirmed that Erb-041 increased the expression of epithelial biomarker E-cadherin and reduced the expression of mesenchymal markers N-Cadherin, Snail, Slug and Twist (Fig. 5A, B, C and S2B). This is consistent with the observations that Erb-041 reduces the incidence of poorly differentiated invasive SCCs in this study. Additionally, in a wound-healing in vitro assay, we also found that Erb-041 treatment reduced migration potential of SCC cells (Fig. S2C). Erb-041 inhibited about 55% and 71% cell migration when assessed for A431 and SCC13 cells, respectively (Fig. 5D). We also determined the effects of Erb-041 on the phosphorylation-dependent activation of PI3K and AKT in UVB-induced tumor (Fig. 5E and S3A). These proteins are associated with cell survival signaling pathway (41). UVB-induced pathogenesis of cutaneous neoplasm is known to be associated with the activation of this pathway (7, 41). Interestingly, Erb-041 treatment reduced phosoho-PI3K-AKT axis in UVB-induced tumor tissues.
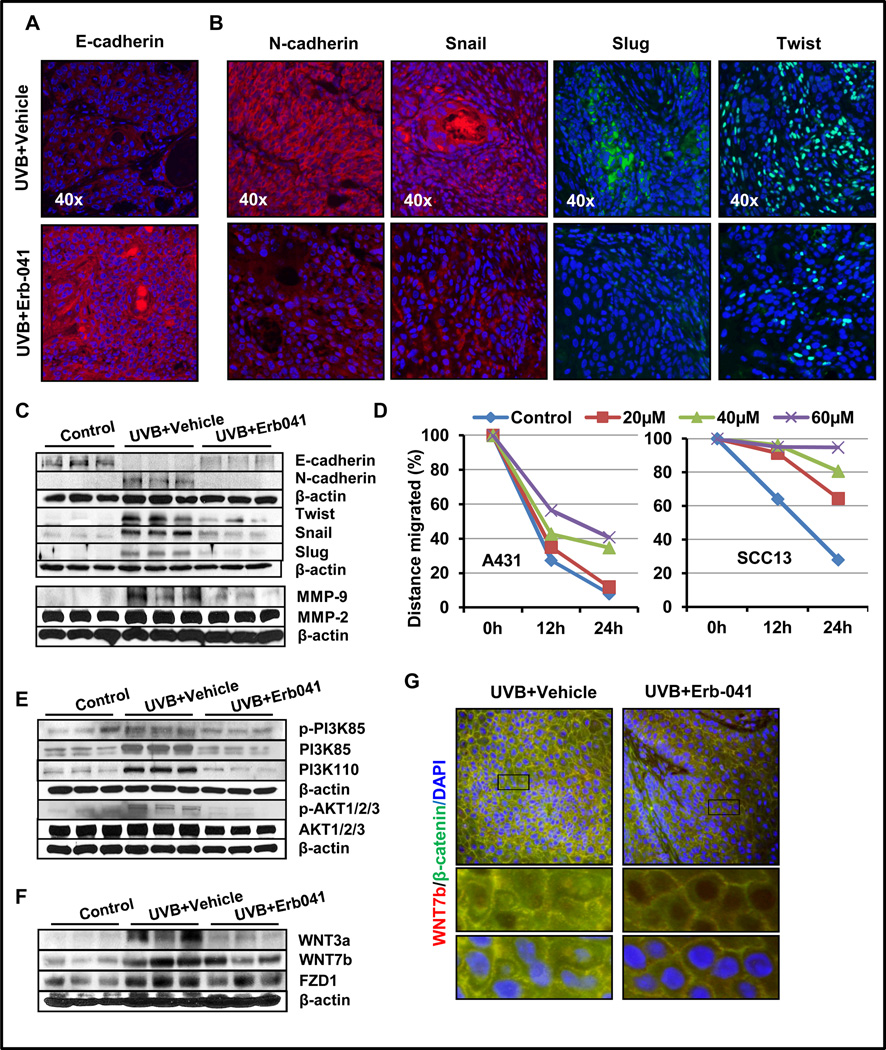
Figure 5. Erb-041 treatment blocks EMT, PI3K/AKT and WNT/β-catenin pathways in UVB-induced tumor lesions.
Immunofluorescence staining showing expression of (A) E-cadherin, (B) N-cadherin, Snail, Slug and Twist; (C) western blot analysis showing the expression of E-cadherin, N-cadherin, Twist, Snail, Slug, MMP-2 and -9; (D) graphs representing the effects of Erb-041 treatment to A431 and SCC13 cells on wound closure in scratch assay; (E) western blot analysis of p-PI3K(85KDa), PI3K(110KDa), p-AKT1/2/3(Ser473); (F) immunofluorescence staining showing co-expression of WNT7b and β-catenin; (G) western blot analysis of WNT3b, WNT7b and FZD1.
Epithelial cell adhesion complex involves binding of E-cadherin/β-catenin/α-catenin complex to F-actin at transmembrane region, and plays a key role in EMT process during tumorigenesis (41, 42). Numerous studies reported that the release of β-catenin in cytoplasm and then its migration to the nucleus are associated with loss of E-cadherin (41, 43). β-catenin-dependent WNT signaling pathway is known to play essential roles in the regulation of cell polarity, proliferation, fate, survival, differentiation, and migration (43). In the presence of WNT ligands, the destruction complex containing proteins adenomatous polyposis coli (APC), glycogen synthase kinase 3β (GSK3β), casein kinase 1 (CK1), β-catenin and Axin gets dissociated. As a consequence, β-catenin releases which leads to activation of transcription factors TCF/LEF, and -dependent target genes (43). In this study, we observed that augmented expression of WNT3a, WNT7b, FZD1 and β-catenin in UVB-induced skin tumors were reduced following Erb-041 treatment (Fig. 5F and S3B). Additionally, in immunofluorescence staining, we noted nuclear localization of β-catenin in UVB (alone)-induced tumor whereas it was considerably reduced in Erb-041-treated UVB-induced tumors (Fig. 5G).
Erb-041 treatment of human SCC cells induced cell differentiation, cell cycle arrest and reduced colony formation in vitro
In an effort to unravel the underlying mechanism of this ERβ agonist, we treated human epidermal immortalized (HaCaT) and A431 and SCC13 cells with various concentration of Erb-041 in vitro. As shown in Fig. S4A and B, Erb-041 treatment induced expression of cytokeratin10, a differentiation marker. We next analyzed its effects on cell cycle progression in these cells. Erb-041 treatment induced G1 phase cell cycle arrest in A431 cells which was associated with the reduction in the expression of G1 cyclins (D1, D2 and D3) and CDK4. A slight but insignificant reduction in the expression of cyclin B1/E, CDC-2 and CDK2 was also noted (Fig. 6A, B and S4C). In a colony formation assay, consistent with its effects on cell cycle progression, Erb-041 dramatically reduced the number and size of A431 and SCC13 colonies (Fig. 6C).
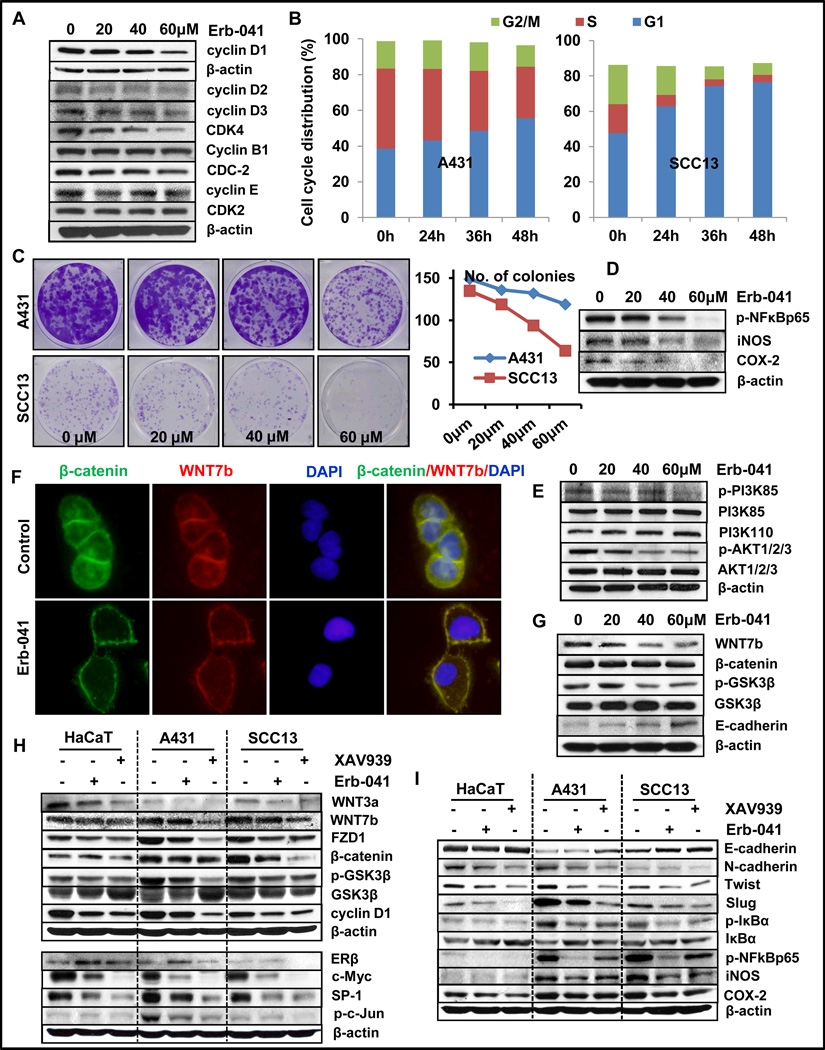
Figure 6. Erb-041 treatment induces G1 cell-cycle arrest, inhibits colony forming potential, inflammation and EMT via WNT/β-catenin pathway.
Cells were seeded in 100mm plates at 1×106 cell density and incubated overnight at 37°C. Thereafter, these cells were treated with Erb-041 (0, 20, 40 and 60µM) for 24h and subjected to western blot analysis. (A) expression of cell cycle regulatory proteins in A431 cells; (B) graphical representation of flow cytometric cell cycle analysis of A431 and SCC13 cells; (C) colony forming potency of A431 and SCC13; (D) western blots showing expression of p-NFκBp65, iNOS and COX-2 proteins in A431 cells; (E) western blots showing expression of p-PI3K(85KDa), PI3K(110KDa) and p-AKT1/2/3(ser473) in A431 cells; (F and G) immunostaining showing co-expression of WNT7b and β-catenin in A431 cells and western blot analysis of WNT7b, β-catenin, p-GSK3β, GSK3β and E-Cadherin proteins; (H and I) western blot analysis showing expression of ERβ and WNT signaling proteins, cMyc, SP-1, p-c-Jun together with expression of proteins involved in EMT and inflammation in HaCaT, A431 and SCC13 cells which were treated with DMSO (vehicle), Erb-041 or XAV939.
Similar to our observations in murine skin, a marked reduction in the expression of inflammation regulatory proteins such as p-NFκBp65, iNOS and COX-2 was observed in A431 cells (Fig. 6D and S4D). Erb-041 treatment diminished phosphorylated-PI3K and -AKT, which was associated with the enhancement in E-cadherin expression and reduction in migration of these cells in an in vitro scratch assay (Fig. 6E).
We also observed that Erb-041 dampened WNT signaling pathway in the murine skin. WNT signaling pathway is known to be associated with the pathogenesis of skin cancer (37). It is known to be involved in the development of invasive SCCs by modulating EMT at least partially (24, 43). We, therefore tested whether Erb-041 manifests similar effects in human-carcinoma cells. Erb-041 treatment reduced expression of WNT7b, β-catenin and p-GSK3β (Fig. 6F and G). These changes were accompanied by the diminished nuclear localization of β-catenin (Fig. 6F). Consistently, we also observed a significant reduction in the expression of its downstream target proteins c-Myc and cyclin D1 (Fig. 6H). The activation of WNT/β-catenin pathway leads to inhibition of axin-mediated β-catenin phosphorylation, leading to the accumulation of nuclear β-catenin and transcription of WNT pathway-responsive genes (43). To confirm that the reduction in WNT signaling pathway in epidermal carcinoma cells may decrease EMT, we employed a small molecule pharmacological inhibitor of WNT signaling pathway, XAV939. XAV939 stabilizes axin through tankyrase inhibition and modulates Wnt-target effectors (44). As shown in Fig. 6H, XAV939 treatment of HaCaT, A431 and SCC13 cells dramatically suppressed the expression of Wnt signaling pathway proteins, WNT3a, WNT7b, FZD1, β-catenin and GSK3β along with cyclin D1. Importantly, XAV939 treatment did not induce ERβ expression, although, it reduced the expression of ERβ’s co-factors SP-1 and p-c-Jun (Fig. 6H, lower panel). Earlier, SP-1 and p-c-Jun were shown to be regulated by WNT signaling pathway (44). XAV939 treatment also ameliorated the expression of EMT regulating proteins. The expression of E-cadherin was increased whereas the expression of N-cadherin, Twist and Slug was decreased (Fig. 6I, upper panel). Interestingly, the expression of inflammatory signaling molecules p-IκBα, p-NFκBp65, iNOS and COX-2 were also reduced in all the cell lines tested in this study (Fig. 6I, lower panel). Many of these effects were similar to those manifested by Erb-041 in these cells (26).
Discussion
Estrogen signaling particularly that regulated by ERβ is considered important in the pathogenesis of various cancers. ERβ expression is often lost during the progression of epithelial cancers (22, 23). This signaling is not only mediated through the estrogen response elements but also impacts cellular growth by modulating various transcription factors AP-1, SP-1, NFκB etc. (16, 17). Consistently, we also observed a decreased in p-c-Jun and SP-1 by Erb-041 in UVB-induced cutaneous tumors. Although the loss of expression of ERβ receptor may occur through multiple mechanisms, promoter methylation of ERβ is considered as an important down-regulator of its expression (25). The importance of up-regulation of ERβ was shown by the studies where valproic acid-mediated demethylation of ERβ which restored its expression in cancer cells, led to anti-proliferative effects (45). Similarly, small molecule antagonists of ERβ, BAG1 and BAG2 resulted in tumor growth arrest and shrinkage (15). However, our results provide additional novel effects of ERβ agonist, Erb-041. Erb-041 not only restored or augmented the expression of ERβ in murine SCCs and in human carcinoma cells but reduced in proliferation and induced differentiation and apoptosis in these models of skin carcinogenesis. Significantly, these effects together led to a profound reduction in the growth of SCCs and the residual SCCs were found to be mostly highly differentiated carcinoma-types.
A link between tumor growth and inflammation is now well-established (37, 38). Inflammatory immune cells are recruited to cancer sites and lead to development of a conducive neoplastic environment which is responsible for facilitating tumor progression (37, 39). These inflammatory hematopoietic cells by virtue of their capabilities to supply soluble growth factor, matrix remodeling enzymes and other bioactive molecules influence cancer cell proliferation, angiogenesis, invasion and metastasis (36, 37, 39). Interestingly, we found that Erb-041 not only reduced cutaneous hyperplasia but also reduced cytokine production including those of IL1β, IL6 and IL10. These changes were associated with a significant decrease in the number of GR1/CD11b-positive myeloid cells, F4/80 macrophages and neutrophils as ascertained by significant decrease in MPO activity. Thus, these results provide evidence that Erb-041 acts by modulating pro-inflammatory tumor microenvironment.
Transcription factor NFkB is a key regulator of many of inflammatory responses. This transcription factor up-regulates the expression of multiple inflammation-linked genes including COX-2, IL1β, IL6, p38, iNOS etc. The observations in this study that these proteins are also down-regulated by Erb-041 treatment in the skin and in residual tumors provide evidence that Erb-041 may act by modulating NFκB-dependent signaling pathway. A significant decrease in the nuclear expression of p65 together with a decrease in its target genes suggest that ERβ and NFκB function in coordination to dampen inflammatory signaling and SCC growth in this mouse model. However, it is also known that immunosurveillance is impaired during the progression of tumorigenesis (36, 37) and ERβ has recently been shown to modulate tumor immunosurveillance (19, 20). Therefore, participation of this additional mechanism in the reduction of cutaneous tumorigenesis by Erb-041 cannot be ruled out at this stage. Inflammation is known to augment invasive tumor growth by promoting epithelial-mesenchymal transition (46, 47). Earlier, we showed that anti-inflammatory agents not only block UVB-induced inflammation but also reduced EMT progression (7, 41). Parallel to these studies, the observations that Erb-041 treatment reduced inflammation and EMT associated with the enhanced expression of E-Cadherin and reduced expression of mesenchymal proteins N-cadherin, Snail, Slug, Twist and MMPs suggest a role of UVB-induced cutaneous inflammation in regulatory EMT in skin SCCs. The reduction in EMT was associated with the diminution of PI3K/AKT signaling provide a molecular basis for the action of Erb-041 for blocking EMT in the malignant cutaneous keratinocytes. Role of PI3K/AKT is already described in EMT (7, 41). Thus, ERβ receptor not only reduced tumorigenesis and inflammation but also diminished progression to an aggressive and invasive tumor phenotype.
The mechanism by which these multi-target effects can occur is not currently well-understood. However, recent studies described a crucial role of WNT signaling in connecting inflammatory and tumor promoting responses (47, 48). Autocrine WNT signaling plays a crucial role in the growth and survival of various cancer cells (43, 49). In this study, we found that WNT3a as well as WNT7b are up-regulated during the UVB-induced carcinogenesis in experimental animals and in humans. This leads to TCF/LEF-dependent transcriptional activation contributing to the promotion of tumor growth (43). Erb-041 treatment decreased both WNT3a and WNT7b expression in immortalized human skin keratinocytes and SCC cells. This decrease in Wnt ligands was also associated with a decrease in overall nuclear β-catenin and its target genes such as cyclin D1, c-Myc, SP-1. Earlier, WNT signaling has been shown to regulate the EMT by balancing the expression of E-cadherin and mesenchymal proteins (41, 43). For example, in various epithelial tumors, activation of WNT signaling drives a transcriptional program reminiscent of EMT which promote cell migration and invasiveness (43). To confirm the role of WNT signaling in regulating ERβ-dependent diminution in EMT and invasive tumor phenotype, we investigated the effects of small molecule XAV939. XAV939 is known to inhibit Wnt signaling (44) and blocks accumulation of β-catenin in colorectal cancer. The mechanism by which this agent acts involves stabilization of axin by inhibiting the poly-ADP-ribosylating enzymes tankyrase 1 and tankyrase 2 (44). In our studies, XAV939 manifested similar results as have been observed by the treatment with Erb-041, suggesting a role of WNT signaling in ERβ receptor-mediated attenuation of EMT in cutaneous SCCs. In summary, our results show that Erb041 is a potent chemopreventive agent which blocks tumorigenesis by inhibiting proliferation and inducing differentiation and apoptosis. The mechanism by which ERβ agonist Erb-041 acts involves diminution of WNT signaling pathway.
Supplementary Material
Acknowledgements
This work has been supported by NIH/NCI N01-CN-43300 274 and R01 CA138998 grants to M.A.
Grant support: This study was supported by Grants NIH/NCI N01-CN-43300 274 and R01 CA138998 from National Cancer Institute to Mohammad Athar.
Footnotes
Conflict of interest: None
References
- 1.Cancer Facts & Figures: American Cancer Society. 2013 http://wwwcancerorg/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845pdf. [Google Scholar]
- 2.Skin Cancer Facts: Skin cancer. http://wwwskincancerorg/skin-cancer-information/skin-cancer-facts. [Google Scholar]
- 3.Bickers DR, Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol. 2006;126:2565–2575. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- 4.Athar M, Elmets CA, Kopelovich L. Pharmacological activation of p53 in cancer cells. Current pharmaceutical design. 2011;17:631–639. doi: 10.2174/138161211795222595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang X, Kim AL, Feith DJ, Pegg AE, Russo J, Zhang H, et al. Ornithine decarboxylase is a target for chemoprevention of basal and squamous cell carcinomas in Ptch1+/− mice. J Clin Invest. 2004;113:867–875. doi: 10.1172/JCI20732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.So PL, Fujimoto MA, Epstein EH., Jr Pharmacologic retinoid signaling and physiologic retinoic acid receptor signaling inhibit basal cell carcinoma tumorigenesis. Mol Cancer Ther. 2008;7:1275–1284. doi: 10.1158/1535-7163.MCT-07-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhary SC, Singh T, Kapur P, Weng Z, Arumugam A, Elmets CA, et al. Nitric oxide-releasing sulindac is a novel skin cancer chemopreventive agent for UVB-induced photocarcinogenesis. Toxicol Appl Pharmacol. 2013;268:249–255. doi: 10.1016/j.taap.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mikulec CD, Rundhaug JE, Simper MS, Lubet RA, Fischer SM. The Chemopreventive Efficacies of Nonsteroidal Anti-inflammatory Drugs: The Relationship of Short-term Biomarkers to Long-term Skin Tumor Outcome. Cancer Prev Res (Phila) 2013;6:675–685. doi: 10.1158/1940-6207.CAPR-13-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyseng-Williamson KA, Keating GM. Vismodegib: a guide to its use in locally advanced or metastatic basal cell carcinoma. American journal of clinical dermatology. 2013;14:61–64. doi: 10.1007/s40257-012-0004-6. [DOI] [PubMed] [Google Scholar]
- 10.Elmets CA, Viner JL, Pentland AP, Cantrell W, Lin HY, Bailey H, et al. Chemoprevention of nonmelanoma skin cancer with celecoxib: a randomized, double-blind, placebo-controlled trial. Journal of the National Cancer Institute. 2010;102:1835–1844. doi: 10.1093/jnci/djq442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao C, Dahlman-Wright K, Gustafsson JA. Estrogen signaling via estrogen receptor {beta} J Biol Chem. 2010;285:39575–39579. doi: 10.1074/jbc.R110.180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung YK, Ho SM. Estrogen receptor beta: switching to a new partner and escaping from estrogen. Science signaling. 2011;4:pe19. doi: 10.1126/scisignal.2001991. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa S, Inoue S, Watanabe T, Orimo A, Hosoi T, Ouchi Y, et al. Molecular cloning and characterization of human estrogen receptor betacx: a potential inhibitor ofestrogen action in human. Nucleic acids research. 1998;26:3505–3512. doi: 10.1093/nar/26.15.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 15.Warner M, Gustafsson JA. The role of estrogen receptor beta (ERbeta) in malignant diseases--a new potential target for antiproliferative drugs in prevention and treatment of cancer. Biochem Biophys Res Commun. 2010;396:63–66. doi: 10.1016/j.bbrc.2010.02.144. [DOI] [PubMed] [Google Scholar]
- 16.Marino M, Galluzzo P, Ascenzi P. Estrogen signaling multiple pathways to impact gene transcription. Current genomics. 2006;7:497–508. doi: 10.2174/138920206779315737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu WF, Tan XJ, Dai YB, Krishnan V, Warner M, Gustafsson JA. Targeting estrogen receptor beta in microglia and T cells to treat experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2013;110:3543–3548. doi: 10.1073/pnas.1300313110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang L, Zhang X, Xie Y, Tu Y, Wang D, Liu Z, et al. Involvement of estrogen receptor variant ER-alpha36, not GPR30, in nongenomic estrogen signaling. Mol Endocrinol. 2010;24:709–721. doi: 10.1210/me.2009-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Islander U, Jochems C, Lagerquist MK, Forsblad-d'Elia H, Carlsten H. Estrogens in rheumatoid arthritis; the immune system and bone. Molecular and cellular endocrinology. 2011;335:14–29. doi: 10.1016/j.mce.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Catley MC, Birrell MA, Hardaker EL, de Alba J, Farrow S, Haj-Yahia S, et al. Estrogen receptor beta: expression profile and possible anti-inflammatory role in disease. J Pharmacol Exp Ther. 2008;326:83–88. doi: 10.1124/jpet.108.136275. [DOI] [PubMed] [Google Scholar]
- 21.Cotrim CZ, Fabris V, Doria ML, Lindberg K, Gustafsson JA, Amado F, et al. Estrogen receptor beta growth-inhibitory effects are repressed through activation of MAPK and PI3K signalling in mammary epithelial and breast cancer cells. Oncogene. 2013;32:2390–2402. doi: 10.1038/onc.2012.261. [DOI] [PubMed] [Google Scholar]
- 22.Rudolph A, Toth C, Hoffmeister M, Roth W, Herpel E, Jansen L, et al. Expression of oestrogen receptor beta and prognosis of colorectal cancer. British journal of cancer. 2012;107:831–839. doi: 10.1038/bjc.2012.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heikkila A, Hagstrom J, Maenpaa H, Louhimo J, Siironen P, Heiskanen I, et al. Loss of estrogen receptor Beta expression in follicular thyroid carcinoma predicts poor outcome. Thyroid : official journal of the American Thyroid Association. 2013;23:456–465. doi: 10.1089/thy.2012.0363. [DOI] [PubMed] [Google Scholar]
- 24.Mak P, Leav I, Pursell B, Bae D, Yang X, Taglienti CA, et al. ERbeta impedes prostate cancer EMT by destabilizing HIF-1alpha and inhibiting VEGF-mediated snail nuclear localization: implications for Gleason grading. Cancer Cell. 2010;17:319–332. doi: 10.1016/j.ccr.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu X, Leav I, Leung YK, Wu M, Liu Q, Gao Y, et al. Dynamic regulation of estrogen receptor-beta expression by DNA methylation during prostate cancer development and metastasis. The American journal of pathology. 2004;164:2003–2012. doi: 10.1016/s0002-9440(10)63760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cvoro A, Tatomer D, Tee MK, Zogovic T, Harris HA, Leitman DC. Selective estrogen receptor-beta agonists repress transcription of proinflammatory genes. Journal of immunology. 2008;180:630–636. doi: 10.4049/jimmunol.180.1.630. [DOI] [PubMed] [Google Scholar]
- 27.Xiu-li W, Wen-jun C, Hui-hua D, Su-ping H, Shi-long F. ERB-041, a selective ER beta agonist, inhibits iNOS production in LPS-activated peritoneal macrophages of endometriosis via suppression of NF-kappaB activation. Molecular immunology. 2009;46:2413–2418. doi: 10.1016/j.molimm.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 28.Bradley PP, Christensen RD, Rothstein G. Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood. 1982;60:618–622. [PubMed] [Google Scholar]
- 29.Kaylani SZ, Xu J, Srivastava RK, Kopelovich L, Pressey JG, Athar M. Rapamycin targeting mTOR and hedgehog signaling pathways blocks human rhabdomyosarcoma growth in xenograft murine model. Biochem Biophys Res Commun. 2013;435:557–561. doi: 10.1016/j.bbrc.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Kurundkar D, Srivastava RK, Chaudhary SC, Ballestas ME, Kopelovich L, Elmets CA, et al. Vorinostat, an HDAC inhibitor attenuates epidermoid squamous cell carcinoma growth by dampening mTOR signaling pathway in a human xenograft murine model. Toxicol Appl Pharmacol. 2013;266:233–244. doi: 10.1016/j.taap.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaudhary SC, Kurundkar D, Elmets CA, Kopelovich L, Athar M. Metformin, an antidiabetic agent reduces growth of cutaneous squamous cell carcinoma by targeting mTOR signaling pathway. Photochem Photobiol. 2012;88:1149–1156. doi: 10.1111/j.1751-1097.2012.01165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mak P, Chang C, Pursell B, Mercurio AM. Estrogen receptor beta sustains epithelial differentiation by regulating prolyl hydroxylase 2 transcription. Proc Natl Acad Sci U S A. 2013;110:4708–4713. doi: 10.1073/pnas.1221654110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mancuso M, Gallo D, Leonardi S, Pierdomenico M, Pasquali E, De Stefano I, et al. Modulation of basal and squamous cell carcinoma by endogenous estrogen in mouse models of skin cancer. Carcinogenesis. 2009;30:340–347. doi: 10.1093/carcin/bgn243. [DOI] [PubMed] [Google Scholar]
- 34.Mukhtar H, Elmets CA. Photocarcinogenesis: mechanisms, models and human health implications. Photochem Photobiol. 1996;63:356–357. doi: 10.1111/j.1751-1097.1996.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 35.Kim AL, Labasi JM, Zhu Y, Tang X, McClure K, Gabel CA, et al. Role of p38 MAPK in UVB-induced inflammatory responses in the skin of SKH-1 hairless mice. J Invest Dermatol. 2005;124:1318–1325. doi: 10.1111/j.0022-202X.2005.23747.x. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan NJ, Tober KL, Burns EM, Schick JS, Riggenbach JA, Mace TA, et al. UV light B-mediated inhibition of skin catalase activity promotes Gr-1+ CD11b+ myeloid cell expansion. J Invest Dermatol. 2012;132:695–702. doi: 10.1038/jid.2011.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Piazza M, Nowell CS, Koch U, Durham AD, Radtke F. Loss of cutaneous TSLP-dependent immune responses skews the balance of inflammation from tumor protective to tumor promoting. Cancer Cell. 2012;22:479–493. doi: 10.1016/j.ccr.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 38.Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 39.DiDonato JA, Mercurio F, Karin M. NF-kappaB and the link between inflammation and cancer. Immunological reviews. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 40.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest. 2009;119:1417–1419. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanchez-Tillo E, de Barrios O, Siles L, Cuatrecasas M, Castells A, Postigo A. beta-catenin/TCF4 complex induces the epithelial-to-mesenchymal transition (EMT)-activator ZEB1 to regulate tumor invasiveness. Proc Natl Acad Sci U S A. 2011;108:19204–19209. doi: 10.1073/pnas.1108977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 44.Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 45.Stettner M, Kaulfuss S, Burfeind P, Schweyer S, Strauss A, Ringert RH, et al. The relevance of estrogen receptor-beta expression to the antiproliferative effects observed with histone deacetylase inhibitors and phytoestrogens in prostate cancer treatment. Mol Cancer Ther. 2007;6:2626–2633. doi: 10.1158/1535-7163.MCT-07-0197. [DOI] [PubMed] [Google Scholar]
- 46.Wong CE, Yu JS, Quigley DA, To MD, Jen KY, Huang PY, et al. Inflammation and Hras signaling control epithelial-mesenchymal transition during skin tumor progression. Genes & development. 2013;27:670–682. doi: 10.1101/gad.210427.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fuxe J, Karlsson MC. TGF-beta-induced epithelial-mesenchymal transition: a link between cancer and inflammation. Seminars in cancer biology. 2012;22:455–461. doi: 10.1016/j.semcancer.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 48.Du Q, Geller DA. Cross-Regulation Between Wnt and NF-kappaB Signaling Pathways. Forum on immunopathological diseases and therapeutics. 2010;1:155–181. doi: 10.1615/ForumImmunDisTher.v1.i3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davidson KC, Adams AM, Goodson JM, McDonald CE, Potter JC, Berndt JD, et al. Wnt/beta-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proc Natl Acad Sci U S A. 2012;109:4485–4490. doi: 10.1073/pnas.1118777109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.