Abstract
Although hoarding disorder (HD) has been historically conceptualized as a subtype or dimension of obsessive-compulsive disorder (OCD), preliminary evidence suggests that these two disorders have distinct neural underpinnings. The aim of the present study was to compare the hemodynamic responses of HD patients, OCD patients, and healthy controls (HC) during response inhibition on a high-conflict Go/NoGo task that has previously proved sensitive to OCD. Participants comprised 24 HD patients, 24 OCD patients, and 24 HCs who completed a Go/NoGo task during functional magnetic resonance imaging (fMRI). Although behavioral data showed no difference among the groups in Go/NoGo task performance, significant differences in hemodynamic activity were noted. During correct rejects (successful response inhibition), HD patients showed greater right precentral gyrus activation, whereas OCD patients exhibited greater right orbitofrontal activation, as assessed using a region of interest approach. During errors of commission (response inhibition failures), OCD patients, but not HD patients, were characterized by excessive activity in left and right orbitofrontal gyrus. The present results lend further support to the biological distinction between HD and OCD, and they are consistent with previous research suggesting frontal hypoactivity in HD patients during hoarding-unrelated tasks.
Keywords: Hoarding, Obsessive-compulsive disorder, Neuroimaging, Functional magnetic resonance imaging (fMRI), Inhibition
1. Introduction
Hoarding disorder (HD), a new diagnosis in DSM-5 (American Psychiatric Association, 2013), is characterized by a pathological inability to discard objects, resulting in debilitating clutter (Frost and Gross, 1993). Historically, hoarding has been conceptualized as a subtype or dimension of obsessive-compulsive disorder (OCD). However, most individuals with HD do not meet other symptom criteria for OCD (Frost et al., 2011), most individuals with OCD do not report significant hoarding behaviors (Samuels et al., 2007), and hoarding demonstrates weak correlations with classic OCD symptoms (Wu and Watson, 2005; Abramowitz et al., 2008). Furthermore, the prevalence of HD may actually be higher than that of OCD (Samuels et al., 2008).
Preliminary evidence suggests that the neural underpinnings of HD and OCD may differ as well. OCD is most robustly characterized by hyperactivity in the orbitofrontal-striatal loop (Whiteside et al., 2004; Rotge et al., 2008), although a dorsolateral prefrontal-striatal loop has also been implicated (Menzies et al., 2008; Rotge et al., 2008). Hyperactivity in these loops may project to other structures such as the hippocampus, anterior cingulate cortex (ACC), and basolateral amygdala (Rauch et al., 1994; Saxena et al., 1998; Adler et al., 2000; Friedlander and Desrocher, 2006; Simon et al., 2010). Neuroimaging studies of HD using positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) suggest a different neural dysfunction. At rest, OCD patients with prominent hoarding symptoms show low baseline glucose metabolism in the ACC (Saxena et al., 2004); during symptom provocation (imagined or real discarding of possessions), excessive hemodynamic activity is seen in the ventromedial prefrontal cortex (VMPFC) in OCD patients with hoarding symptoms (An et al., 2009). Finally, excessive orbitofrontal cortex (OFC) activity is noted in primary HD patients (Tolin et al., 2009).
To date, there have been few direct comparisons between OCD patients and HD patients. Most previous studies sampled OCD patients with and without hoarding symptoms (Saxena et al., 2004; An et al., 2009), which might not be representative of the majority of HD patients who do not have OCD. To our knowledge, only one neuroimaging study to date has compared primary HD patients and OCD patients; compared with OCD patients and healthy control participants, HD patients were characterized by a biphasic abnormality in the insula and ACC during a hoarding-relevant decision-making task (Tolin et al., 2012b). Additional comparison studies are needed to understand the neural similarities and differences between HD and OCD. It is noted that the previous comparison study used a hoarding-specific task, and OCD patients showed an overall lack of activation on that task. It would be helpful, therefore, to compare the neural function of HD and OCD patients using a task that has been shown to selectively activate neural regions of interest (ROIs) in OCD patients.
Neural mechanisms of executive functions are a promising area for comparison between HD and OCD. Executive functions have been identified as the primary area of neuropsychological deficit in OCD patients (Olley et al., 2007; Kashyap et al., 2013). These executive deficits include impairments in behavioral response inhibition (Morein-Zamir et al., 2010). Studies of executive function in HD patients have yielded mixed results (Grisham et al., 2007; Grisham et al., 2010; Tolin et al., 2011), and it is not clear whether that disorder is characterized by the presence of executive deficits, although a problem of motor inhibition seems particularly unlikely. Of note, patterns of neural activity underlying executive functions may differ between OCD patients and healthy controls. During a Go/NoGo task, in which participants must alternatively execute or inhibit a prepotent, planned response (e.g., button press) based on stimulus presentation, on correctly rejected NoGo trials OCD patients (n = 11) showed excessive activation in several frontal and striatal brain regions compared with healthy control subjects. These included rostral and caudal ACC, lateral prefrontal cortex (LPFC), lateral orbitofrontal cortex (LOFC), caudate, and thalamus, as well as portions of the posterior cingulate cortex (PCC) (Maltby et al., 2005). Furthermore, OCD severity was positively correlated with activity in the PCC on these correctly rejected NoGo trials. During errors of commission (button press following a NoGo stimulus), OCD patients showed excessive activation in rostral ACC, LOFC, LPFC, and PCC compared with healthy controls (Maltby et al., 2005). The authors of that study concluded that because of the high conflict from prepotent response tendency created by the task instructions, OCD patients might have selectively activated error-monitoring regions even in the absence of actual errors, which could help explain the repetitive nature of compulsive behaviors. A higher conflict (lower ratio of NoGo to Go trials) study (Page et al., 2009), in which response inhibition was contrasted with response execution (NoGo > Go), also found excessive activity in unmedicated OCD patients (n = 10) vs. healthy controls in the PCC, as well as in the right VMPFC and premotor cortex. However, contrary to the results of Maltby et al. (2005), OCD patients showed attenuated activity in the ventromedial OFC, ACC, caudate, and thalamus. The higher response conflict in that study could have elicited “oddball” effects (Stevens et al., 2000). Furthermore, it is likely that the NoGo > Go contrast more selectively examines mechanisms of response inhibition than of error monitoring. It is also noted that 1 of the 10 OCD subjects in that trial had prominent hoarding symptoms. In a substantially lower conflict task (equal ratio of Go to NoGo trials), during NoGo > Go trials), OCD patients (n = 12) showed diminished activity (compared with healthy controls) in right medial and inferior frontal gyri, precentral and postcentral gyri, superior temporal gyrus, and fusiform gyrus. In addition, OCD severity correlated inversely with NoGo > Go activity in right OFC and ACC, and positively with thalamic and posterior cortical activations. OCD patients showed excessive activity in left insula, lingual gyrus, and head of the caudate (Roth et al., 2007). Neither depressed mood nor medication status appeared to mediate the group differences. As those authors note, due to the equal ratio of NoGo to Go trials, errors were quite infrequent and therefore that study may have shown effects more consistent with response inhibition than with error monitoring.
Thus, across studies, OCD patients exhibit abnormal neural activity during NoGo trials, although methodological differences among the studies preclude many direct comparisons. Results could be related to hyperactive error monitoring (Maltby et al., 2005), exaggerated oddball effects (Page et al., 2009), or underactivation of response-inhibition mechanisms (Roth et al., 2007). The aim of the present study is to compare, using a larger number of participants than in previous trials, the hemodynamic responses of HD patients, OCD patients, and healthy controls during NoGo trials, using a high-conflict Go/NoGo task that has previously proved sensitive to OCD (Maltby et al., 2005). It was predicted that during correct reject trials (successful response inhibition), OCD patients would show excessive activity in ACC, LOFC, caudate, and thalamus compared with the other two groups. During errors of commission (failed response inhibition), OCD patients were expected to show excessive activity in ACC, LOFC, LPFC, and PCC compared with the other two groups. HD participants were expected not to show the same pattern of hyper-activation abnormalities. Rather, based on previous studies not involving hoarding-related decisions (Saxena et al., 2004; Tolin et al., 2012b), it was expected that HD patients would show decreased activity, compared with the other two groups, in ACC, PCC, and insula.
2. Methods
2.1. Participants
Participants comprised 24 patients with HD, 24 patients with OCD, and 24 healthy controls. All provided written, informed consent in accordance with Hartford Hospital IRB procedures. Patients with HD were recruited using advertisements for people with “clutter problems” or “hoarding,” as well as from an existing patient group at a clinic specializing in HD treatment. The patients with OCD were recruited using advertisements seeking people with OCD, and the healthy controls were recruited using advertisements for a brain-imaging study. All assessments were conducted by well-trained postdoctoral fellows or postgraduate research assistants. Participants were classified as having HD if they met diagnostic criteria (Frost and Hartl, 1996; American Psychiatric Association, 2013), hoarding was their primary diagnosis as defined by Clinical Severity Ratings on the Anxiety Disorders Interview Schedule for DSM-IV (Brown et al., 1994), the Clinician's Global Impression (Guy, 1976) rating was “moderately ill” or above, and symptom duration was at least 1 year. One potential participant with comorbid HD and OCD was excluded from the study, given that the primary study aim was to compare HD and OCD. Where there were questions about the severity of hoarding, symptom severity was confirmed via home visit or analysis of current photographs of living space. The patients with OCD met diagnostic criteria for a primary diagnosis of (nonhoarding) OCD, had at least moderate symptom severity as evidenced by a Clinician's Global Impression rating of moderately ill or above, and had a symptom duration of 1 year or more. HD or OCD patients were excluded if they had a history of psychotic disorder, neurological disorder, substance abuse, or serious suicidal ideation. Healthy controls were excluded if they met criteria for a current or past Axis I or Axis II disorder, had a history of neurological disorders, or were taking psychiatric medications. Participants, regardless of diagnostic group, who were unsuitable for MRI scanning (e.g., those with severe claustrophobia, pregnancy, or metal implants) were also excluded.
2.2. Measures
Demographic information, including self-reported race and ethnicity, was collected via a questionnaire. The HD diagnoses were made using the Hoarding Rating Scale-Interview (Tolin et al., 2010b), a semistructured interview that assesses the severity of clutter, acquisition, difficulty discarding, distress, and impairment, each on a 0- to 8-point scale. Other psychiatric diagnoses were ascertained using the Anxiety Disorders Interview Schedule for DSM-IV (Brown et al., 1994). Severity of HD was assessed using the Saving Inventory-Revised (Frost et al., 2004), a 23-item questionnaire. Nonhoarding OCD severity was assessed using the Obsessive Compulsive Inventory-Revised (OCI-R) (Foa et al., 2002), an 18-item self-report measure. For the present purposes, given the intent to contrast OCD and HD, a total OCI-R score was calculated with omission of the three hoarding items. Depression severity was assessed using the Hamilton Rating Scale for Depression (HRSD) (Hamilton, 1960), a 17-item semistructured interview. The Structured Interview Guide for the HRSD (Williams, 1988) was used for administration. Global impressions of illness severity were recorded using the Clinician's Global Impression (Guy, 1976) scale.
2.3. Apparatus
Images were acquired on a Siemens Allegra 3T head-only scanner. A single-shot echo-planar gradient recalled pulse sequence (repetition time/echo time = 1500/28 ms; flip angle = 65°; field of view = 24 cm; matrix = 64; in plane resolution = 3.4 mm; slice thickness (gap) = 4(1) mm) was used to effectively cover the whole brain in 29 slices. Each run consisted of 294 time points, including an initial six time points (9 s) of rest to allow for T1 effects to stabilize. These initial six time points were not included in subsequent analyses. High-resolution T1- and T2-weighted images were also acquired on all participants to ensure that all were free from obvious vascular injury that might otherwise influence both neuropsychological test performance results and interpretation of functional imaging results.
2.4. Procedure
The Go/No-Go task was designed as a rapid, event-related fMRI task. Participants were instructed to make a speeded button press with their right index finger to visual `X' (Go) stimuli and withhold response to pseudo-randomly interspersed `K' (No-Go) stimuli. To establish a prepotent response tendency, 85% of all stimuli were Go events. The task consisted of frequent `X' (P = 0.85) and infrequent `K' (P = 0.15) stimuli presented at 3 × 5 visual degrees for 50 ms each. The minimum interstimulus interval was 1000 ms. Intervals between `K' stimuli were in the range of 10–15 s. Speed was emphasized over accuracy during a practice trial to ensure that participants would produce a sufficient number of commission errors for analysis. Hits and errors were defined as a response (button press) occurring within 1000 ms of an `X' or `K' trial, respectively.
2.5. Image processing
Functional images were reconstructed offline. Each run was corrected for slice-timing errors and separately realigned using INRIalign (Freire and Mangin, 2001; Freire et al., 2002) as implemented in Statistical Parametric Mapping-8 (SPM-8). A mean functional image volume was constructed for each participant for each run from the realigned image volumes and used to determine the parameters for spatial normalization into standardized Montreal Neurological Institute (MNI) space. These normalization parameters were then applied to the corresponding functional image volumes, and the normalized images were smoothed with a 9-mm full width at half-maximum (FWHM) Gaussian kernel. All participants' data were individually inspected to ensure that no subject had translational or rotational head motion greater than the acquired voxel size.
2.6. Data analysis
The conditions in the Go/No-Go experiment were represented using a canonical hemodynamic response model in SPM-8. This model separately represented the hemodynamic responses to hits (successful Go trials), correct rejects (successful No-Go trials), and `false alarm' errors (unsuccessful No-Go trials). These three event classes were determined on a per subject basis depending on whether responses were recorded within 1000 ms post-stimulus, such that the functional imaging data of each participant were modeled individually in SPM-8 and included subject-specific regressors for these three event classes (hits, correct rejects, and false alarms). The standard six motion-corrected parameter estimates (x, y, z displacements and pitch, roll, and yaw rotations) were included as covariates of no interest to statistically control for signal change related to head motion. A high-pass filter (cutoff period = 128 s) was incorporated into the model to remove low-frequency signals. Contrasts corresponding to the main effects of hits, correct rejects, and errors of commission were specified for each participant. All contrast images written by SPM-8 represented brain activity relative to an `implicit baseline' of unmodeled variance. To assess overall between-group differences in brain activity during successful response inhibition (correct rejects) and unsuccessful response inhibition (false alarms), separate three-group one-way analyses of variance (ANOVAs) were conducted in SPM-8 for these two experimental conditions. Initial whole brain analysis revealed no statistically significant areas of activation after correction for searching across the whole brain when employing a standard, stringent correction for whole brain analysis (e.g., family-wise error). As such, we chose to adopt a region-of-interest analytic approach described below.
A single region-of-interest (ROI) analysis was conducted to assess between-group differences in successful and unsuccessful response inhibition, respectively. Regions were chosen based on reviews of three relevant bodies of literature: the Go/No-Go fMRI literature (Kiehl et al., 2000; Liddle et al., 2001; Buchsbaum et al., 2005; Simmonds et al., 2008; Stevens et al., 2009) as well as HD (Volle et al., 2002; Mataix-Cols et al., 2003; Mataix-Cols et al., 2004; Saxena et al., 2004; Anderson et al., 2005; An et al., 2009; Tolin et al., 2009; Tolin et al., 2012a; Tolin et al., 2012b) and OCD neuroimaging literatures (Rauch et al., 1994; Adler et al., 2000; Fitzgerald et al., 2005; Maltby et al., 2005; Nakao et al., 2005; Simon et al., 2010). While regions could have been selected solely based on the current body of Go/No-go neuroimaging literature, the addition of the HD and OCD neuroimaging literatures allowed us to curtail our ROI search area, as not all brain regions related to response inhibition are necessarily of use or of interest in demonstrating that HD is distinct from OCD. For the correct rejects contrast, regions were selected based on the intersection of brain regions consistently reported in Go/No-Go fMRI studies (including two quantitative meta-analyses) with those brain regions consistently shown to have HD and OCD specificity in a wide variety of predominantly symptom-provocation tasks. As most previous Go/No-Go fMRI studies did not consider errors of commission, to compile a set of ROIs for the false alarm contrast, regions were primarily selected from the HD and OCD literatures, noting where these brain regions intersected with activation observed on our group's previously published results with the same Go/No-Go task used in this study (Kiehl et al., 2000; Liddle et al., 2001; Stevens et al., 2009). These two ROI selection processes netted six ROIs for correct rejects (ACC, LOFC, ROFC, right precentral gyrus, LMFG, and precuneus) and eight ROIs for false alarms (ACC, left superior/middle frontal gyrus, LOFC/insula, medial OFC, precuneus, right insula, ROFC, and right superior/middle frontal gyrus). For all 14 ROIs, localization was determined by examining the convergence of the peak stereotactic MNI coordinates reported in the above-cited studies (see Table 2 for a summary of these stereotactic coordinates). For studies where the results were presented in Talairach coordinates, an appropriate transform was applied to convert the results into MNI space (Brett et al., 2002). Spherical ROIs, with a radius of 8 mm, were defined, centered at the peak stereotactic coordinates, to assess hypothesized between-group effects for correct rejects and errors of commission. Using in-house Matlab-based coding, a mean activation value was extracted for each respective ROI from each participant's respective correct reject or false alarm contrast. These mean values were used as input into a single multivariate ANOVA in SPSS to assess the presence of between-group differences across all 14 ROIs. Post hoc univariate tests for each ROI were used to determine which regions most contributed to overall group differences, as well as to depict the nature of regional differences in brain activation. There are practical and theoretical advantages of including all 14 ROIs in a single multivariate ANOVA over performing 14 separate univariate ANOVAs and assessing significance using a Bonferroni-type correction. Practically, multivariate analyses boost statistical power; where there is a significant omnibus (multivariate) F statistic representing between-group differences, no further corrections for multiple comparisons are required to assess statistical significance of those ROIs that drive the overall multivariate effect. Theoretically, multivariate analyses impose the assumption that any observed effects (i.e., between-group differences) are equally likely to occur in any dependent measure (i.e., brain region). Statistical significance of the post hoc univariate tests was determined using a Bonferroni-type correction. Given the obtained group differences in sex and age (see below), these variables were included in the analysis as covariates. As this study represents one of the first to directly compare neural activity in HD and OCD patients in a non-symptom-provocation task, a second, exploratory multivariate ANOVA omitting sex and age as covariates was also conducted to obtain a more complete picture of the results. We note that the results from this exploratory analysis were not appreciably different from the main study results presented in Table 2 and Figs. 1 and 2. The results from this second, exploratory analysis are included as a supplemental table.
Table 2.
Differences in hemodynamic activity between obsessive-compulsive disorder (OCD), hoarding disorder (HD), and healthy control (HC) participants during NoGo trials, corrected for sex and age. (ACC = anterior cingulate cortex; OFC = orbital frontal cortex; MFG = middle frontal gyrus)
| Region | x | y | z | F | p | OCD v HC | HD v HC | OCD v HD |
|---|---|---|---|---|---|---|---|---|
| Correct rejects | ||||||||
| ACC | −6 | 30 | 27 | 2.363 | 0.102 | |||
| L OFC | −39 | 21 | −15 | 1.846 | 0.166 | |||
| Precuneus | 0 | −66 | 15 | 3.023 | 0.055 | |||
| R OFC | 45 | 42 | −15 | 4.705 | 0.012 | 0.007 OCD ↑ | ||
| R Precentral | 45 | 6 | 27 | 6.452 | 0.003 | 0.002 HD ↑ | 0.006 HD ↑ | |
| L MFG | −15 | 48 | 9 | 3.689 | 0.030 | 0.014 HD ↓ | ||
| Errors of commission | ||||||||
| ACC | −12 | 21 | 27 | 2.582 | 0.083 | |||
| L Superior/Middle Frontal | −27 | 39 | 30 | 0.295 | 0.745 | |||
| L OFC/Insula | −39 | 24 | −12 | 5.751 | 0.005 | 0.044 OCD ↑ | 0.004 OCD ↑ | |
| Medial OFC | −12 | 42 | −9 | 2.102 | 0.130 | |||
| Precuneus | −9 | −51 | 48 | 3.048 | 0.054 | |||
| R Insula | 39 | 15 | −3 | 0.892 | 0.414 | |||
| R OFC | 42 | 27 | −15 | 3.547 | 0.034 | 0.022 OCD ↑ | ||
| R Superior/Middle Frontal | 27 | 39 | 30 | 1.815 | 0.171 |
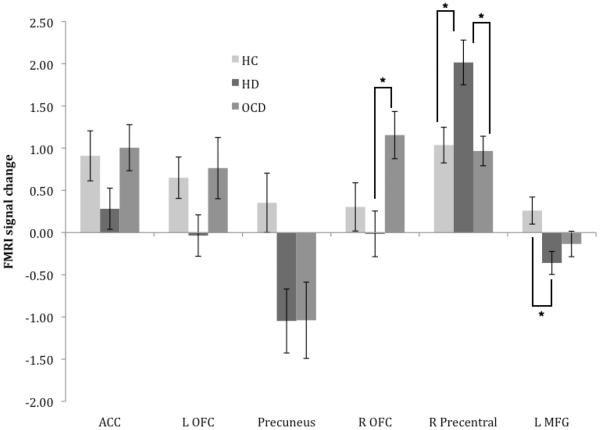
Fig. 1.
Estimated marginal mean (SE) fMRI signal change for healthy control (HC), hoarding disorder (HD), and obsessive-compulsive disorder (OCD) participants during correct rejects, controlling for age and sex. (*p < 0.05, Bonferroni-corrected post-hoc comparison)
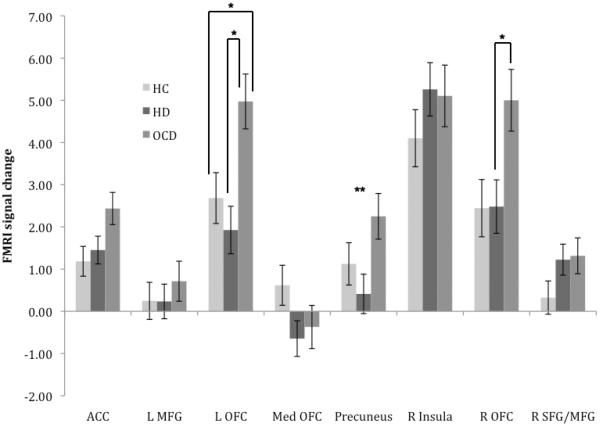
Fig. 2.
Estimated marginal mean (SE) fMRI signal change for healthy control (HC), hoarding disorder (HD), and obsessive-compulsive disorder (OCD) participants during errors of commission, controlling for age and sex. (*p < 0.05, Bonferroni-corrected post hoc comparison; ** indicates significant ANOVA result in the absence of significant between-group post hoc comparison)
3. Results
3.1. Sample description
The HD and HC groups were well matched for age and gender (see Table 1), but the OCD group was significantly younger than and contained more male participants than did the other two groups. As expected, the HD group exhibited significantly greater hoarding severity than did the other two groups, and the OCD group exhibited significantly greater OCD severity than did the other two groups (although some minor but significant elevation was seen in HD participants as well). HD and OCD participants exhibited higher levels of depression than did the HC group and did not differ from each other in rates of comorbid anxiety and depressive disorders.
Table 1.
Sample description
| HD (N = 24) | OCD (N = 24) | HC (N = 24) | F | χ 2 | |
|---|---|---|---|---|---|
| Age | 47.75 (9.91)a | 33.54 (13.03)b | 51.29 (9.88)a | 17.38** | |
| Female [N(%)] | 16 (66.7%)a | 6 (25.0%)b | 20 (83.3%)a | 17.83** | |
| White [N(%)] | 21 (87.5%) | 22 (91.7%) | 23 (95.8%) | 1.09 | |
| SI-R | 62.42 (14.58)a | 10.21 (10.77)b | 4.92 (5.35)b | 203.68** | |
| OCI-R minus hoarding | 9.17 (7.25)a | 18.79 (8.60)b | 1.50 (1.35)c | 42.15** | |
| HRSD | 6.17 (4.31)a | 5.38 (5.60)a | 1.17 (1.79)b | 9.79** | |
| Comorbid anxiety disorder [N(%)] | 12 (50.0%) | 8 (33.3%) | -- | 1.37 | |
| Comorbid depressive disorder [N(%)] | 13 (54.2%) | 8 (33.3%) | -- | 2.12 | |
| On psychiatric medications [N(%)] | 15 (62.5%) | 19 (86.4%) | -- | 3.39 |
Scores are shown as M (SD) unless noted otherwise.
p < 0.05;
p < 0.01.
Within each row, groups with different superscript letters are significantly different (p < 0.05). (SI-R = Saving Inventory - Revised; OCI-R = Obsessive-Compulsive Inventory - Revised; HRSD = Hamilton Rating Scale for Depression; HC = healthy control; HD = hoarding disorder; OCD = obsessive-compulsive disorder).
Rates of psychiatric medication use did not differ between the HD and OCD groups. When specific classes of medications were examined, HD and OCD patients did not differ in use of selective serotonin reuptake inhibitors/selective norepinephrine reuptake inhibitors (37.5% vs. 45.8%, χ2 = 0.343, p = 0.558), other antidepressants (8.3% vs. 4.2%, χ2 = 0.356, p = 0.551), benzodiazepines (8.3% vs. 16.7%, χ2 = 0.762, p = 0.383), antipsychotics (12.5% vs. 8.3%, χ2 = 0.223, p = 0.637), anticonvulsant/mood stabilizers (4.2% vs. 12.5%, χ2 = 1.091, p = 0.296), or stimulant/amphetamines (12.5% vs. 4.2%, χ2 = 1.091, p = 0.296).
3.2. Behavioral data
Commission error rates did not differ significantly among HD (M = 27.91%, SD = 14.66), OCD (M = 24.20%, SD = 15.27), or HC (M = 20.46%, SD = 11.63) participants, F2,71 = 1.71, p = 0.188. Similarly, response time (ms) for hits (correct button press) did not differ significantly among HD (M = 422.99, SD = 35.81), OCD (M = 410.02, SD = 51.57), or HC (M = 427.17, SD = 66.11) participants, F2,71 = 0.69, p = 0.504.
3.3. fMRI data
We noted a significant overall multivariate effect (F28,110 = 2.910, p < 0.001 ) for the primary analysis comparing levels of hemodynamic activity among the three groups across all 14 ROIs, correcting for the effects of age and sex. These results indicated the presence of strong, between-group effects, which are summarized in Table 2 and described below for each contrast of interest. We also noted that this overall multivariate effect remained for our exploratory analysis which omitted corrections for sex and age (F28,114 = 3.238, p < 0.001). These exploratory results are summarized in Supplemental Table 1.
3.3.1. Correct rejects
As shown in Table 2 and Fig. 1, during correct rejects (successful response inhibition), significant between-group differences were observed for our primary analysis in right OFC, right precentral gyrus, and left middle frontal gyrus (MFG; Brodmann areas 9–10). Post hoc tests revealed that the HD group was characterized by greater activity in right precentral gyrus compared with the OCD group and lower activity (greater deactivation) in left MFG compared with the HC group. OCD participants did not significantly differ from the HC participants in any region but were instead characterized by increased activity in right OFC compared with HD participants.
3.3.2. Errors of commission
During errors of commission (failed response inhibition), significant between-group differences were observed in left OFC/insula and right OFC (Table 2, Fig. 2). Post hoc tests revealed that the HD group did not differ significantly from HC participants in any ROI. The OCD group, conversely, was characterized by increased activation in left OFC/insula compared with HC participants and greater activation in both the left and right OFC compared with the HD group.
3.3.3. Correlations between hemodynamic activity and clinical measures
For the HD sample, during correct rejects, HD severity on the SI-R was negatively correlated (controlling for age and sex) with activity in the right precentral gyrus (r = −0.497, p = 0.019). Non-hoarding OCD severity on the OCI-R and did not correlate significantly with activity in any ROI. Depression severity on the HDRS was negatively correlated with activity in the right precentral gyrus (r = −0.489, p = 0.021) and right OFC (r = −0.573, p = 0.005). During errors of commission, HD severity was negatively correlated (controlling for age and sex) with activity in the ACC (r = −0.488, p = 0.021) and right superior/middle frontal gyrus (r = −0.483, p = 0.023). Non-hoarding OCD severity did not correlate significantly with activity in any ROI. Depression severity was negatively correlated with activity in the ACC (r = −0.485, p = 0.022).
4. Discussion
HD is now recognized as a unique diagnostic entity, although DSM-5 includes HD in the category of “obsessive-compulsive and related” disorders (American Psychiatric Association, 2013). To date, there have been few direct neuroimaging comparisons between HD and OCD patients. Two studies found noteworthy differences between OCD patients with and without primary hoarding symptoms (Saxena et al., 2004; An et al., 2009); a more recent study also found significant differences in hemodynamic activity between primary HD patients and primary OCD patients (Tolin et al., 2012b). The present results lend further support to the assertion that HD and OCD are characterized by different neural mechanisms. Unlike the previous comparison that used a hoarding-related symptom provocation (Tolin et al., 2012b), the present study employed a Go/NoGo task that has been used in previous OCD research (Maltby et al., 2005). The results from the present study are consistent with current theories of HD and OCD, and they support our main study hypothesis that the HD and OCD study groups would not exhibit the same patterns of neural activity during task performance. During successful response inhibition, HD patients differed from OCD patients in showing greater right precentral gyrus activation, whereas OCD patients exhibited greater right OFC activation. During errors of commission (response-inhibition failures), OCD patients, but not HD patients, were characterized by excessive activity in the left and right OFC. The latter finding was similarly obtained in correlational analyses of the entire sample, in which OCD severity (but not HD severity) was associated with greater OFC activity.
The findings of elevated OFC activity during correct rejects and errors of commission in OCD patients is consistent with previous findings (Maltby et al., 2005). Decreased OFC activity was reported among OCD patients in another Go/NoGo study (Page et al., 2009). However, the contrast in that study (NoGo > Go) specifically targeted a differential response, whereas the present study focused on absolute response to inhibitory instruction. The OFC hyperactivity is broadly consistent with the orbitofrontal-striatal model of OCD (Rauch et al., 1994; Saxena et al., 1998). The region of the lateral/posterior OFC showing excessive activation among OCD patients in the present study is implicated in neural representations of reward value/desirability (Knutson et al., 2003; Ernst et al., 2004; Rothkirch et al., 2012), possibly indexing the degree to which attention is prioritized to features in the environment or assignment of behavioral significance to the environment, as well as engaged to maintain flexible representations of reward.
In contrast, HD patients were not characterized by the excessive OFC activation seen in OCD patients. Rather, in keeping with other research showing attenuated neural activity during hoarding-unrelated tasks (Tolin et al., 2012b), HD patients showed lower activity in the left MFG during successful response inhibition. The left MFG has been shown to be activated (during both correct rejects and errors of commission) by the Go/NoGo task used in this study in healthy volunteers (Kiehl et al., 2000) and has been implicated in both conflict/error monitoring (van Veen et al., 2001; Fan et al., 2003) and the processing of emotions (Lane et al., 1997; Reiman et al., 1997; Teasdale et al., 1999). HD patients' hypoactivity in this region comports with previous findings of diminished ACC activation in OCD patients with hoarding symptoms (Saxena et al., 2004), and activity in ACC (Tolin et al., 2012b), and MFG (Tolin et al., 2012a) among HD patients during hoarding-incongruent tasks. The ACC and the MFG gyrus are frequently co-activated by tasks requiring cognitive control (Carter et al., 1999; Peterson et al., 1999; Rubia and Smith, 2004; Carter and van Veen, 2007), so the observed HD hypoactivation in the left MFG combined with these previous studies' results regarding both the ACC and MFG suggests a pattern of frontal under-responsiveness may characterize HD. This under-responsiveness may result in attenuated response to salient stimuli and may contribute to the diminished motivation and poor insight frequently observed in HD patients (Frost et al., 2010; Tolin et al., 2010a). The exaggerated right precentral gyrus activity exhibited by HD patients during successful response inhibition was unexpected. The significance of this activity is not immediately clear; although the precentral gyrus is implicated in Go/NoGo response inhibition (Garavan et al., 2002; Buchsbaum et al., 2005), the motor functions typically ascribed to this region have not played a prominent role in recent theories of HD. If replicated, hypotheses about how this abnormality relates to already-conceptualized abnormalities should be generated.
Several study limitations should be noted. Potential participants with comorbid HD and OCD were excluded from the study, and the HD sample was predominantly female and white. We also likely did not include the least insightful HD sufferers, who would not be expected to volunteer for a study. Although alpha levels were corrected for searching within an ROI (i.e., the number of voxels) using small volume correction, the moderately large number of ROIs searched underscores the need for replication. Finally, it should be noted that rates of anxiety and depressive comorbidity were high, and most of the OCD and HD patients were taking psychiatric medications at the time of the study. Additional research is needed to parse the specific effects of HD and OCD symptoms from those of mood and anxiety symptoms, and to examine differences in neural activity in medication-free subjects.
Supplementary Material
Acknowledgements
The authors thank Drs. Godfrey Pearlson, Nicholas Maltby, Kent Kiehl, Pawel Skudlarski, Scott Rauch, Randy Frost, and Gail Steketee for their assistance.
This study was funded by National Institute of Mental Health grant #R01MH074934 to Dr. Tolin. Dr. Tolin has received recent research support from Palo Alto Health Systems, Pfizer, Endo Pharmaceuticals, Merck, and Eli Lilly. Drs. Witt and Stevens have no disclosures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramowitz JS, Wheaton MG, Storch EA. The status of hoarding as a symptom of obsessive-compulsive disorder. Behaviour Research and Therapy. 2008;46:1026–1033. doi: 10.1016/j.brat.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Adler CM, McDonough-Ryan P, Sax KW, Holland SK, Arndt S, Strakowski SM. fMRI of neuronal activation with symptom provocation in unmedicated patients with obsessive compulsive disorder. Journal of Psychiatric Research. 2000;34:317–324. doi: 10.1016/s0022-3956(00)00022-4. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Author; Washington, DC: 2013. [Google Scholar]
- An SK, Mataix-Cols D, Lawrence NS, Wooderson S, Giampietro V, Speckens A, Brammer MJ, Phillips ML. To discard or not to discard: the neural basis of hoarding symptoms in obsessive-compulsive disorder. Molecular Psychiatry. 2009;14:318–331. doi: 10.1038/sj.mp.4002129. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Damasio H, Damasio AR. A neural basis for collecting behaviour in humans. Brain. 2005;128:201–212. doi: 10.1093/brain/awh329. [DOI] [PubMed] [Google Scholar]
- Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nature Reviews Neuroscience. 2002;3:243–249. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- Brown TA, DiNardo P, Barlow DH. Anxiety Disorders Interview Schedule for DSM-IV. The Psychological Corporation; San Antonio, TX: 1994. [Google Scholar]
- Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin Card-sorting Task and component processes. Human Brain Mapping. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Reviews in the Neurosciences. 1999;10:49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cognitive Affective and Behavioral Neuroscience. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, Zarahn E, Leibenluft E, Zametkin A, Towbin K, Blair J, Charney D, Pine DS. Choice selection and reward anticipation: an fMRI study. Neuropsychologia. 2004;42:1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. Cognitive and brain consequences of conflict. Neuroimage. 2003;18:42–57. doi: 10.1006/nimg.2002.1319. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, Taylor SF. Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biological Psychiatry. 2005;57:287–294. doi: 10.1016/j.biopsych.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Foa EB, Huppert JD, Leiberg S, Langner R, Kichic R, Hajcak G, Salkovskis PM. The Obsessive-Compulsive Inventory: development and validation of a short version. Psychological Assessment. 2002;14:485–496. [PubMed] [Google Scholar]
- Freire L, Mangin JF. Motion correction algorithms may create spurious brain activations in the absence of subject motion. Neuroimage. 2001;14:709–722. doi: 10.1006/nimg.2001.0869. [DOI] [PubMed] [Google Scholar]
- Freire L, Roche A, Mangin JF. What is the best similarity measure for motion correction in fMRI time series? IEEE Transactions on Medical Imaging. 2002;21:470–484. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- Friedlander L, Desrocher M. Neuroimaging studies of obsessive-compulsive disorder in adults and children. Clinical Psychology Review. 2006;26:32–49. doi: 10.1016/j.cpr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Frost RO, Gross R. The hoarding of possessions. Behaviour Research and Therapy. 1993;31:367–382. doi: 10.1016/0005-7967(93)90094-b. [DOI] [PubMed] [Google Scholar]
- Frost RO, Hartl TL. A cognitive-behavioral model of compulsive hoarding. Behaviour Research and Therapy. 1996;34:341–350. doi: 10.1016/0005-7967(95)00071-2. [DOI] [PubMed] [Google Scholar]
- Frost RO, Steketee G, Grisham J. Measurement of compulsive hoarding: saving Inventory-Revised. Behaviour Research and Therapy. 2004;42:1163–1182. doi: 10.1016/j.brat.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Frost RO, Steketee G, Tolin DF. Comorbidity in hoarding disorder. Depression and Anxiety. 2011;28:876–884. doi: 10.1002/da.20861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost RO, Tolin DF, Maltby N. Insight-related challenges in the treatment of hoarding. Cognitive and Behavioral Practice. 2010;17:404–413. [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Grisham JR, Brown TA, Savage CR, Steketee G, Barlow DH. Neuropsychological impairment associated with compulsive hoarding. Behaviour Research and Therapy. 2007;45:1471–1483. doi: 10.1016/j.brat.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Grisham JR, Norberg MM, Williams AD, Certoma SP, Kadib R. Categorization and cognitive deficits in compulsive hoarding. Behaviour Research and Therapy. 2010;48:866–872. doi: 10.1016/j.brat.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Psychopharmacology Research Branch, NIMH; Rockville, MD: 1976. [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap H, Kumar JK, Kandavel T, Reddy YC. Neuropsychological functioning in obsessive-compulsive disorder: are executive functions the key deficit? Comprehensive Psychiatry. 2013;54:533–540. doi: 10.1016/j.comppsych.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology. 2000;37:216–223. [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ. Neuroanatomical correlates of happiness, sadness, and disgust. American Journal of Psychiatry. 1997;154:926–933. doi: 10.1176/ajp.154.7.926. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Human Brain Mapping. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltby N, Tolin DF, Worhunsky P, O'Keefe TM, Kiehl KA. Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: an event-related fMRI study. Neuroimage. 2005;24:495–503. doi: 10.1016/j.neuroimage.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Cullen S, Lange K, Zelaya F, Andrew C, Amaro E, Brammer MJ, Williams SC, Speckens A, Phillips ML. Neural correlates of anxiety associated with obsessive-compulsive symptom dimensions in normal volunteers. Biological Psychiatry. 2003;53:482–493. doi: 10.1016/s0006-3223(02)01504-4. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Archives of General Psychiatry. 2004;61:564–576. doi: 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neuroscience and Biobehavioral Reviews. 2008;32:525–549. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morein-Zamir S, Fineberg NA, Robbins TW, Sahakian BJ. Inhibition of thoughts and actions in obsessive-compulsive disorder: extending the endophenotype? Psychological Medicine. 2010;40:263–272. doi: 10.1017/S003329170999033X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, Kudoh A, Tada K, Yoshioka K, Kawamoto M, Togao O, Kanba S. Brain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;57:901–910. doi: 10.1016/j.biopsych.2004.12.039. [DOI] [PubMed] [Google Scholar]
- Olley A, Malhi G, Sachdev P. Memory and executive functioning in obsessive-compulsive disorder: a selective review. Journal of Affective Disorders. 2007;104:15–23. doi: 10.1016/j.jad.2007.02.023. [DOI] [PubMed] [Google Scholar]
- Page LA, Rubia K, Deeley Q, Daly E, Toal F, Mataix-Cols D, Giampietro V, Schmitz N, Murphy DG. A functional magnetic resonance imaging study of inhibitory control in obsessive-compulsive disorder. Psychiatry Research: Neuroimaging. 2009;174:202–209. doi: 10.1016/j.pscychresns.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Gatenby JC, Zhang H, Anderson AW, Gore JC. An fMRI study of Stroop word-color interference: evidence for cingulate subregions subserving multiple distributed attentional systems. Biological Psychiatry. 1999;45:1237–1258. doi: 10.1016/s0006-3223(99)00056-6. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Jenike MA, Alpert NM, Baer L, Breiter HC, Savage CR, Fischman AJ. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Archives of General Psychiatry. 1994;51:62–70. doi: 10.1001/archpsyc.1994.03950010062008. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Lane RD, Ahern GL, Schwartz GE, Davidson RJ, Friston KJ, Yun LS, Chen K. Neuroanatomical correlates of externally and internally generated human emotion. American Journal of Psychiatry. 1997;154:918–925. doi: 10.1176/ajp.154.7.918. [DOI] [PubMed] [Google Scholar]
- Rotge JY, Guehl D, Dilharreguy B, Cuny E, Tignol J, Bioulac B, Allard M, Burbaud P, Aouizerate B. Provocation of obsessive-compulsive symptoms: a quantitative voxel-based meta-analysis of functional neuroimaging studies. Journal of Psychiatry and Neuroscience. 2008;33:405–412. [PMC free article] [PubMed] [Google Scholar]
- Roth RM, Saykin AJ, Flashman LA, Pixley HS, West JD, Mamourian AC. Event-related functional magnetic resonance imaging of response inhibition in obsessive-compulsive disorder. Biological Psychiatry. 2007;62:901–909. doi: 10.1016/j.biopsych.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Rothkirch M, Schmack K, Schlagenhauf F, Sterzer P. Implicit motivational value and salience are processed in distinct areas of orbitofrontal cortex. Neuroimage. 2012;62:1717–1725. doi: 10.1016/j.neuroimage.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith A. The neural correlates of cognitive time management: a review. Acta Neurobiologiae Experimentalis. 2004;64:329–340. doi: 10.55782/ane-2004-1517. [DOI] [PubMed] [Google Scholar]
- Samuels JF, Bienvenu OJ, Grados MA, Cullen B, Riddle MA, Liang KY, Eaton WW, Nestadt G. Prevalence and correlates of hoarding behavior in a community-based sample. Behaviour Research and Therapy. 2008;46:836–844. doi: 10.1016/j.brat.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels JF, Bienvenu OJ, Pinto A, Fyer AJ, McCracken JT, Rauch SL, Murphy DL, Grados MA, Greenberg BD, Knowles JA, Piacentini J, Cannistraro PA, Cullen B, Riddle MA, Rasmussen SA, Pauls DL, Willour VL, Shugart YY, Liang KY, Hoehn-Saric R, Nestadt G. Hoarding in obsessive-compulsive disorder: Results from the OCD Collaborative Genetics Study. Behaviour Research and Therapy. 2007;45:673–686. doi: 10.1016/j.brat.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Saxena S, Brody AL, Maidment KM, Smith EC, Zohrabi N, Katz E, Baker SK, Baxter LR., Jr. Cerebral glucose metabolism in obsessive-compulsive hoarding. American Journal of Psychiatry. 2004;161:1038–1048. doi: 10.1176/appi.ajp.161.6.1038. [DOI] [PubMed] [Google Scholar]
- Saxena S, Brody AL, Schwartz JM, Baxter LR. Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. British Journal of Psychiatry Supplement. 1998;35:26–37. [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D, Kaufmann C, Musch K, Kischkel E, Kathmann N. Fronto-striato-limbic hyperactivation in obsessive-compulsive disorder during individually tailored symptom provocation. Psychophysiology. 2010;47:728–738. doi: 10.1111/j.1469-8986.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- Stevens AA, Skudlarski P, Gatenby JC, Gore JC. Event-related fMRI of auditory and visual oddball tasks. Magnetic Resonance Imaging. 2000;18:495–502. doi: 10.1016/s0730-725x(00)00128-4. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Brain network dynamics during error commission. Human Brain Mapping. 2009;30:24–37. doi: 10.1002/hbm.20478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale JD, Howard RJ, Cox SG, Ha Y, Brammer MJ, Williams SC, Checkley SA. Functional MRI study of the cognitive generation of affect. American Journal of Psychiatry. 1999;156:209–215. doi: 10.1176/ajp.156.2.209. [DOI] [PubMed] [Google Scholar]
- Tolin DF, Fitch KE, Frost RO, Steketee G. Family informants' perceptions of insight in compulsive hoarding. Cognitive Therapy and Research. 2010a;34:69–81. [Google Scholar]
- Tolin DF, Frost RO, Steketee G. A brief interview for assessing compulsive hoarding: the Hoarding Rating Scale-Interview. Psychiatry Research. 2010b;178:147–152. doi: 10.1016/j.psychres.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin DF, Kiehl KA, Worhunsky P, Book GA, Maltby N. An exploratory study of the neural mechanisms of decision making in compulsive hoarding. Psychological Medicine. 2009;39:325–336. doi: 10.1017/S0033291708003371. [DOI] [PubMed] [Google Scholar]
- Tolin DF, Stevens MC, Nave AM, Villavicencio A, Morrison S. Neural mechanisms of cognitive behavioral therapy response in hoarding disorder: a pilot study. Journal of Obsessive-Compulsive and Related Disorders. 2012a;1:180–188. [Google Scholar]
- Tolin DF, Stevens MC, Villavicencio AL, Norberg MM, Calhoun VD, Frost RO, Steketee G, Rauch SL, Pearlson GD. Neural mechanisms of decision making in hoarding disorder. Archives of General Psychiatry. 2012b;69:832–841. doi: 10.1001/archgenpsychiatry.2011.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin DF, Villavicencio A, Umbach A, Kurtz MM. Neuropsychological functioning in hoarding disorder. Psychiatry Research. 2011;189:413–418. doi: 10.1016/j.psychres.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage. 2001;14:1302–1308. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- Volle E, Beato R, Levy R, Dubois B. Forced collectionism after orbitofrontal damage. Neurology. 2002;58:488–490. doi: 10.1212/wnl.58.3.488. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Port JD, Abramowitz JS. A meta-analysis of functional neuroimaging in obsessive-compulsive disorder. Psychiatry Research: Neuroimaging. 2004;132:69–79. doi: 10.1016/j.pscychresns.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Archives of General Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- Wu KD, Watson D. Hoarding and its relation to obsessive-compulsive disorder. Behaviour Research and Therapy. 2005;43:897–921. doi: 10.1016/j.brat.2004.06.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.