Abstract
Background
A novel influenza A(H7N9) virus has emerged in China during the past few months. Inter-species zoonotic transmission appears to be the predominant route of spread. Live poultry markets (LPMs) in the major cities of Shanghai, Hangzhou, Huzhou and Nanjing, where the majority of cases have occurred, were swiftly closed as a precautionary public health measure. Our objective was to quantify the impact of LPM closure in reducing bird-to-human transmission of avian influenza A(H7N9) virus.
Methods
We used data on the illness onset dates and geographical locations of laboratory-confirmed influenza A(H7N9) cases that were officially announced by 7 June 2013. We constructed a statistical model to explain the patterns in incident cases reported in each city based on the assumption of a constant force of infection prior to closure, and a different constant force of infection after closure. We fitted the model using Markov chain Monte Carlo methods.
Findings
There were 85 confirmed influenza A(H7N9) cases in Shanghai, Hangzhou, Huzhou and Nanjing out of a total of 130 confirmed cases in mainland China by 7 June 2013. Closure of LPMs in those four cities reduced the risk of human infections by 97%–99% (range 68%–100%) in each city. Given that LPMs were the predominant source of influenza A(H7N9) exposure in those locations, we estimated the mean incubation period to be 3.3 days.
Interpretation
LPM closures were extremely effective in controlling human risk of influenza A(H7N9). If the influenza A(H7N9) epizootic/epidemic continues, LPM closure should be sustained in at-risk areas and implemented in any urban areas where influenza A(H7N9) reappears in future. In the longer term, evidence-based discussions and deliberations about the role of central slaughtering of all live poultry should be renewed.
Funding
Ministry of Science and Technology, China; Research Fund for the Control of Infectious Disease and University Grants Committee, Hong Kong Special Administrative Region, China; and the US National Institutes of Health.
INTRODUCTION
On 31 March 2013, the first laboratory-confirmed human infection with influenza A(H7N9) was officially announced in Shanghai. The virus has been identified as a novel triple reassortant of avian influenza A(H7N3), A(H7N9) and A(H9N2) viruses with low pathogenicity in poultry 1, 2 and appeared to have spread widely among poultry in the Yangtze river delta.3 As of 7 June 2013, influenza A(H7N9) virus has been associated with typically serious disease in 130 individuals since February 2013,4 and has apparently caused mild human infections.5, 6 Given that there has been no evidence of sustained or efficient human-to-human spread so far,2, 4, 7 the preventive locus remains at the human-animal interface.8–10
Live poultry markets (LPMs) are common in China and many other countries including Thailand, Laos, Singapore, etc, primarily in urban areas. A 2006 survey in Guangzhou, provincial capital of Guangdong that borders Hong Kong in southern China, found that 80% of households reported purchasing poultry at LPMs at least once a year, and more frequent purchases were common.11 LPMs therefore pose an important potential zoonotic risk if the flocks are infected with avian influenza viruses.12 Moreover there is evidence that the prevalence of avian infection can be “amplified” in the closed and dense settings of LPMs, thereby increasing the risk of poultry-to-human infection.13 Hong Kong experienced the first human influenza A(H5N1) outbreak with 18 cases and 6 deaths in 1997.14 The epizootic/epidemic was quickly contained after territory-wide LPM closure and depopulation of all local poultry farms.15 Since 2003, avian influenza A(H5N1) viruses have continued to infect humans, resulting in 43 confirmed cases in mainland China and 628 worldwide, with some infections directly attributable to LPM exposure.4, 16
LPM closure in urban areas of some cities in eastern China has coincided with reductions in human cases of influenza A(H7N9),4 although previous studies only investigated the qualitative impact of LPM closures on reducing bird-to-human transmission phenomenologically without a formal causal inferential framework.10, 17, 18 While the conclusions thereof are apparently persuasive, without robust evidence to define and quantify such impact, policymakers would be hard pressed to justify the continued closure of LPMs in a millennia-old culture of trading live birds, not to mention the tens of billion yuans’ worth of economic value potentially at stake.19 There is imminent urgency in the matter given that conventional wisdom predicts, based on previous observations with influenza viral zoonoses of H5, H7 and H9, that after the “summer lull” in viral activity, autumn will potentially bring a new epidemic wave.4 This issue puts to the test the “one world, one health” concept advocated by the five-year-old joint call by the FAO, OIE, WHO, UN, UNICEF and World Bank to “diminish the risk and minimize the global impact of epidemics and pandemics due to emerging infectious disease”.20 To address this issue, we developed a statistical model to quantify the impact of LPM closure in reducing bird-to-human transmission of influenza A(H7N9) in the major Chinese cities of Shanghai, Hangzhou, Huzhou and Nanjing. Besides being a tool for retrospective analysis, the same model can be used as a risk assessment tool for real-time estimation of LPM closure effectiveness in reducing bird-to-human transmission of avian influenza viruses.
METHODS
Sources of data
China CDC constructed an integrated dataset with detailed demographic, epidemiologic and geographic information on each laboratory-confirmed influenza A(H7N9) case reported to China CDC by 7 June 2013.4 Information used in the present analysis included age, sex, location, residence type (rural or urban area), dates of illness onset. Case definitions, surveillance for identification of influenza A(H7N9) cases, and laboratory test assays are described in previous reports.4, 7
We collected geographic information for wholesale and retail LPMs from the Ministry of Agriculture of China and Agricultural Bureaux of provinces/prefectures affected by influenza A(H7N9), or the official gazetteer issued by National Administration of Surveying, Mapping and Geoinformation. (Appendix)
Ethical Approval
It was determined by the National Health and Family Planning Commission that the collection of data from influenza A(H7N9) cases was part of a continuing public health investigation of an emerging outbreak and was exempt from institutional review board assessment.
Statistical analysis
To assess the impact of LPM closure on the reduction in human cases of influenza A(H7N9), we constructed a Bayesian statistical model to perform a population-based before-after analysis of the time series of illness onset of cases reported in each city. Specifically, we assumed that the force of infection, defined as the daily probability of infection per susceptible individual, was constant at λpre and λpost before and after LPM closures respectively, in each city (more precisely, λpre and λpost corresponded to only infections that would be ascertained; see Appendix). We assumed that the incubation period (i.e. the delay from infection to illness onset) followed a lognormal probability distribution for all individuals. Under these model assumptions, the incidence rate of illness onsets in confirmed influenza A(H7N9) cases before LPM closures would be constant and that after LPM closures would be affected both by (i) infections before closures with longer incubation periods and (ii) infections after closures with shorter incubation periods. Thus, we could use Markov Chain Monte Carlo methods to jointly estimate the force of infection before (λpre) and after (λpost) LPM closure in each city, and the incubation period distribution (more specifically, its mean and variance), based on the observed time series of illness onset of cases in all cities. The ratio λpost/λpre in a specific city reflected the local impact of LPM closure in reducing bird-to-human transmission. This statistical model is methodologically robust and similar to models previously used for assessing the effectiveness of interventions for nosocomial infections and antimicrobial resistance.21, 22 We computed the posterior predictive p-value to assess goodness-of-fit.23 To explore the possibility that incidence patterns could be explained by seasonality, we incorporated seasonal effects (driven by absolute humidity, which is the only common climactic factor that has been shown to have a strong (negative) correlation with the survival and transmission of influenza viruses24, 25) into the model in a sensitivity analysis. See Appendix for details on our statistical methods.
We excluded mild cases from all analyses because mild infections might be substantially underascertained.6 In our main analysis, we also excluded cases in rural residents of surrounding townships and cases that were suspected to be human-to-human transmission because those infections may not have occurred through exposure to LPMs in the urban areas and health-care seeking behaviors may differ in rural vs urban residents.4, 18 We included the rural cases in sensitivity analyses. Because of potential under-ascertainment of recent cases due to onset-to-admission delays, as well as laboratory confirmation and official reporting delays, we only considered influenza A(H7N9) incidence through 7 June 2013. See Appendix for additional technical details.
Role of the funding source
The funding bodies had no role in study design, data collection and analysis, preparation of the manuscript, or the decision to publish. The joint first authors had complete access to the data, and the corresponding authors had final responsibility for the decision to submit the manuscript.
RESULTS
As of 7 June 2013, 130 laboratory-confirmed cases of influenza A(H7N9) infection have been officially announced in mainland China. Almost all (122/130) of these cases suffered serious disease including pneumonia and 40 have died while some others remain in critical condition. The majority (85/130) of the confirmed cases were identified in four major cities in the Yangtze River Delta region, namely Nanjing, Shanghai, Hangzhou and Huzhou. While almost all of the early confirmed cases were identified in this area, later in April and May there was spread away from the epicenter to surrounding provinces.4
In response to the rapid increase in the numbers of confirmed local human cases during late March and early April, local authorities closed all LPMs in Shanghai and Nanjing on April 6 and 8 respectively. Afterwards, most markets in Hangzhou were closed on April 15 and the remainder on April 24, while markets in the five districts of Huzhou were sequentially closed between April 11 and 21 (Figure 1, Figure 2A). In total, 780 LPMs were closed, depopulated and disinfected in the four cities (Figure 1). Pet bird trade was also suspended in those cities.
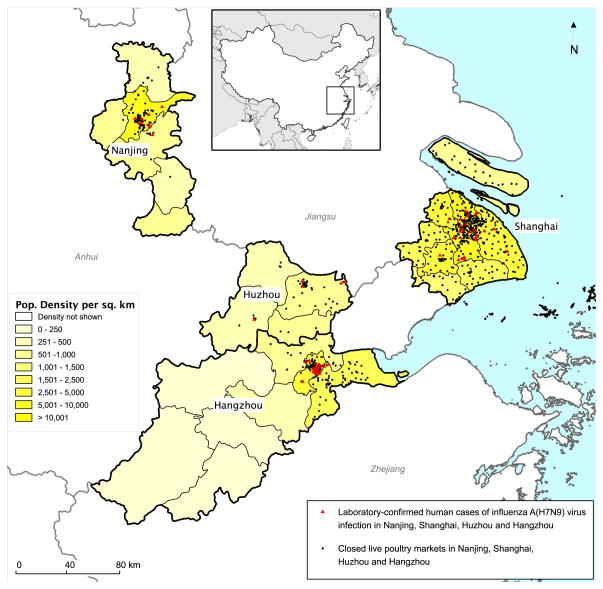
Figure 1. Location of laboratory-confirmed influenza A(H7N9) cases and LPM closures.
The map shows the geographic location of the 60 confirmed A(H7N9) cases (red triangles) in Nanjing, Shanghai, Huzhou and Hangzhou, with illness onset between 19 February 2013 and 16 April 2013. Also shown are the locations of live poultry markets that were closed in the four cities. The small inset shows the location of the enlarged map within China.
Figure 2. Dates of influenza A(H7N9) cases and LPM closures, and parameter estimates.
Panel A: Illness onset dates of 60 influenza A(H7N9) cases in Nanjing, Shanghai, Hangzhou, and Huzhou between 19 February and 16 April. The blue bar for each day indicates the number of laboratory-confirmed cases with onsets on that day. Cyan vertical lines indicate the date of official announcement of the first laboratory-confirmed H7N9 case in each city, red vertical lines indicate the dates of closures of live poultry markets in each city (markets in the five districts in Huzhou were closed on different dates), green vertical lines indicate the last date used in analyses. Panel B: Posterior estimates of the mean daily number of illness onsets of A(H7N9) cases in Nanjing, Shanghai, Hangzhou and Huzhou; darker colors indicate regions with higher posterior density on a given day. Panel C: estimates of the reduction in force of infection associated with closure of live poultry markets in each city. Panel D: Estimated incubation period distribution with the mean and coefficient of variation set at their posterior medians.
Among the 85 confirmed cases in the four cities, 67 were urban residents while 18 were ‘rural’ residents of suburban townships neighboring but outside these cities, who may be nonetheless treated in tertiary referral centers in the provincial capitals. Two confirmed cases in Shanghai apparently contracted the virus by human-to-human transmission.7 Four urban cases had mild illness and we have separately reported that mild cases that did not require hospitalization for medical reasons may have been underascertained.6 One case in Hangzhou resided in a district with late LPM closure (April 24) and no other cases. Excluding the 18 rural cases, the two cluster cases in Shanghai, the 4 mild cases, and the 1 case in Hangzhou mentioned above, we focused in our main analysis on the remaining 60 confirmed and hospitalized cases in the four cities, shown in Figure 1. The dates of illness onset of these 60 confirmed cases are shown in Figure 2A in relation to the dates of LPM closure. Whereas one to three cases were confirmed almost every day in Shanghai and Hangzhou (and one to two cases every few days in Nanjing and very few cases in Huzhou) immediately before markets were ordered to close, very few new cases with onset dates after closure have been officially announced since LPMs were closed (Figure 2A).
The fitted model closely matched the observed incidence patterns with a posterior predictive p-value of 0.9 (Figure 2B). The fitted model (Table 1) suggested that LPM closure substantially reduced the incidence rate of influenza A(H7N9) in all four cities (with posterior probability >0.99). The median of the posterior estimates of the reduction in incidence rate was >97% in all four cities (the lower bounds of the 95% credible intervals [CrI] were 81%, 93%, 92%, and 68% in Nanjing, Shanghai, Hangzhou and Huzhou, respectively; Figure 2C). We estimated that the incubation period distribution had mean 3.3 (95% CrI 1.4, 5.7) days with a coefficient of variation (the ratio of the standard deviation to the mean) of 0.76 (95% CrI: 0.1, 5.5). With the mean and coefficient of variation of the incubation period distribution set at their posterior medians, the incubation period was estimated to be shorter than 8 days for 95% of cases (Figure 2D).
Table 1.
Parameter estimates from Bayesian analysis of A(H7N9) incidence rates before and after live poultry market closures. Posterior means and their 95% credibility intervals are shown.
| Parameter | Nanjing | Shanghai | Hangzhou | Huzhou |
|---|---|---|---|---|
| Expected daily number of infections before LPM closure that would be ascertained | 0.48 (0.19–0.95) | 1.30 (0.79–2.1) | 0.98 (0.62–1.5) | 0.20 (0.07–0.47) |
| Expected daily number of infections after LPM closure that would be ascertained | 0.013 (0.004–0.07) | 0.02 (0.001–0.09) | 0.014 (0.001–0.08) | 0.006 (0–0.042) |
| Reduction in mean daily number of infections associated with complete LPM closure | 97% (81%–100%) | 99% (93%–100%) | 99% (92%–100%) | 97% (68%–100%) |
| Mean incubation period (days) | 3.3 (1.4–5.7) | |||
| Coefficient of variation of incubation period | 0.76 (0.1–5.5) | |||
In sensitivity analysis, the fitted model with the 18 rural cases included suggested that LPM closure reduced incidence by 81%, 93%, 92% and 91% in Nanjing, Shanghai, Hangzhou and Huzhou, respectively (Appendix), i.e. similar to our main results. Similarly, in a separate sensitivity analysis, the fitted model with seasonality (driven by absolute humidity) included did not change the estimates of the impact of LPM closures, i.e. known seasonal factors could not explain the sudden reduction in influenza A(H7N9) cases in each city shortly after LPM closure (Appendix). In addition, this sensitivity analysis indicated that as a real-time risk assessment tool, the model could have robustly concluded by as early as 30 April 2013 that LPM closure was highly effective in reducing incidence (Supplementary Table 1).
DISCUSSION
Our findings demonstrate that LPM closure in the four cities substantially reduced the incidence of human infection with avian influenza A(H7N9) virus. Incidence fell dramatically within 2–3 days of LPM closure (Figure 2A). Two important insights arising from our results follow. First, it confirms that the majority of laboratory-confirmed influenza A(H7N9) cases in these cities were attributable to LPM exposure.26 While 75% of influenza A(H7N9) cases reported recent exposure of varying types and degrees to live poultry directly or to their related environments,4 generally such exposure is fairly common in Chinese urban settings.11 A recent case-control study suggested that the risk of influenza A(H7N9) infection increased with LPM exposure but this result had only borderline statistical significance.27 This exemplifies the challenge of robustly identifying risk factors when the numbers of cases is small and exposure is almost ubiquitous.15, 16 Second, the rapid drop in cases after LPM closure (Figure 2A) allowed us to estimate that the mean incubation period was 3.3 days (Figure 2D) instead of around one week as preliminary epidemiologic investigations had suggested.7 This is consistent with more recent analysis of dates of live poultry exposures reported by the individual influenza A(H7N9) cases which estimated an incubation period with mean 3.1 days.4
Our results are robust to the possibility of a substantial proportion of undetected, and perhaps milder influenza A(H7N9) infections,6, 28 provided that the proportion of influenza A(H7N9) infections that were laboratory -confirmed did not vary during the study period, i.e. temporal (relative) changes in the observed epidemic curve were similar to that in the full epidemic curves. During the influenza A(H7N9) outbreak, only severe respiratory infections were subject to virologic testing. Public awareness and virologic surveillance for influenza A(H7N9) remained heightened throughout the study period. As such, the proportion of infections that were lab-confirmed (i.e. the observed “tip-of-the-iceberg”) should not vary over time. However, if the proportion of infections associated with serious disease were different for infections acquired in LPMs as opposed to other sources, then our estimates of the effectiveness of LPM closure in reducing bird-to-human transmission could be biased.
From a public health standpoint, LPMs should remain closed in all affected areas until the current influenza A(H7N9) epizootic and epidemic are declared definitively over by national authorities, having taken into account the possibility of a potential autumn-winter surge. Given the reports of human cases in other provinces from Beijing to the north, Anhui province to the west and Fujian province to the south, and investigation suggesting that these are indigenous and not imported cases and that the disease remains predominantly a zoonosis,4 local authorities in potentially at-risk areas which operate such markets should consider proactively closing all LPMs based on the precautionary principle. Other biosecurity and surveillance measures in animal husbandry and trading should be stepped up to ensure safety of the entire poultry supply chain, from hatching of day-old chicks through LPMs at retail, and including cross-border (inter-provincial within country as well as overseas) transfer and trade. In the longer term, evidence-based discussions and deliberations about the role of central slaughtering of all live poultry should be renewed.
Evidence on the effectiveness of LPM closures is particularly important because of the serious economic impact that LPM closures have already had on the poultry trade in China. Some have put the economic loss associated with the aforementioned LPM closures at 57 billion yuan (around USD 8 billion), and counting19. In addition there is an ingrained culture of being able to choose and buy live poultry in traditional wet markets. Nevertheless, contingent or permanent LPM closure and the introduction of central slaughtering with additional safeguards for the poultry supply chain from farm to table should be considered based on these findings.
Our study has a number of limitations. First, as an ecologic study our findings could be affected by bias and confounding. Although we have shown that the apparent drop in influenza A(H7N9) incidence after LPM closure could not be attributed to absolute humidity which is the only common climatic factor that has been shown to strongly affect influenza transmission (Appendix), we cannot rule out the possibility that it was caused by some other unknown seasonal factors. During the past decade, there has been a seasonal pattern in incidence of confirmed influenza A(H5N1) virus infections in mainland China, with most cases identified during the winter and none during the summer.4 A complete understanding of the mechanisms underlying such seasonality remains elusive. If seasonality also applied to influenza A(H7N9), changes in environmental conditions other than absolute humidity in April 2013 could explain part of the effect attributed to LPM closures in our analysis. However, LPMs were closed during the first three weeks of April and the specific closure dates differed in each city (Figure 2) although the cities are in close geographic proximity (Figure 1). Incidence of new cases dropped dramatically after the closure dates in each city rather than at a single point in calendar time. In addition, cases continued to appear in other nearby provinces that did not close LPMs (Panel E of Supplementary Figure). This suggests that LPM closure rather than environmental pressure had the predominant role in reducing incidence. Second, our model made the simple assumption that incidence rate in each city was constant before and after closure at different levels (Appendix). We were not able to explore more complex models given the small number of cases, or the potential effect of other preventive measures before LPM closures that would have led to underestimation of the impact of LPM closures in our analyses. Third, we were unable to examine the impact of LPM closure in other Chinese cities that had very small numbers of influenza A(H7N9) cases. Our findings mainly apply to urban areas since LPMs are rarely found in rural areas; policy decisions on LPM closures would mainly be concerned with LPMs in urban areas. Finally, there is the hypothetical potential for detrimental effects of LPM closures, if closures led to an increase in illicit trading or transfers of poultry across wider geographic areas, and caused wider spread of A(H7N9). We believe that such a scenario is unlikely but it would be an interesting area for further research. Alternative potential control measures include the introduction of regular mandatory rest days and/or no keeping of live poultry overnight, both of which have proved successful in Hong Kong.13 Indeed, rest days have recently been introduced or planned in some of the major Chinese cities (e.g. Shanghai, Fujian, Guangdong).29–31
While the A(H7N9) spring outbreak may appear to have passed, we cannot be certain that an autumn surge (which would be in keeping with past seasonal patterns even of novel influenza strains) is not imminent. In fact the medical and public health communities have been reminded of the very real nature of the latter possibility with the most recent sporadic case report from Hebei in Northern China on July 20.32 This sentinel report is of course only the tip of the “clinical iceberg”.6 Such ongoing human case reports, in the absence of efficient human-to-human spread, clearly indicate the widespread endemicity of the virus in the poultry population of China. However most of the previously closed LPMs have now reopened, due to the enormous economic and political pressures otherwise. Yet the current literature still lacks proper scientific evidence on the effectiveness of LPM closure in reducing bird-to-human transmission of avian influenza. If substantiated, this would be the single most effective and efficient intervention to prevent human disease and the associated morbidity and mortality burden. In addition to its marked clinical severity,6 the avian influenza A(H7N9) virus (and other zoonotic influenza viruses in general) has the pandemic potential to become human-to-human transmissible via evolution,12, 33, 34 the probability of which escalates with the number of zoonotic infections35 which is probably 10–100 times that observed in the clinical setting.6 Hence, there is an urgent need for dissemination of the best available scientific evidence on the effectiveness of LPM closure to help policymakers assess the potential benefits and costs of LPM closure in future human outbreaks of influenza A(H7N9) and at the same time to give the necessary evidence to the practising clinician as health advocate to influence this urgent and potentially lifesaving decision, as well as to remain vigilant to the possibility of clinical presentation of human cases at the bedside.
This study is the most comprehensive study to date that quantitatively estimates the impact of LPM closure on reducing bird-to-human transmission by analyzing the most accurate influenza A(H7N9) epidemic data with a novel and robust statistical model. This model takes into account the inherent statistical uncertainty associated with paucity of data, the confounding effect of incubation period (which was not known a priori) on the change in the number of influenza A(H7N9) cases before and after LPM closure, as well as the potential effects of seasonality. Besides being a tool for retrospective analysis, the same model can be used as a risk assessment tool for real-time estimation of LPM closure effectiveness in future human outbreaks of avian influenza viruses. We used this model to show that closure of LPMs, the major exposure source to the causative virus among urban populations, had a definitive effect in reducing risk of human infection of influenza A(H7N9) in major Chinese cities in the Yangtze River Delta, which has been the epicenter of the outbreak. We estimated the mean incubation period for human influenza A(H7N9) infection to be 3.3 days, assuming that LPMs are the predominant source of zoonotic spread in the urban setting. Our findings supported the decision to close LPMs in at-risk areas to prevent further human infections.
Ascertained influenza A(H7N9) patients had high severity and prolonged hospitalization.4, 6 Because of the pandemic potential of the virus12 influenza A(H7N9) patients had and will consume disproportionate amount of healthcare resources. Should influenza A(H7N9) reappear in the fall or winter, which seems plausible in light of the recent new case in Guangdong,36 it will put a substantial burden on local hospitals in affected areas during peak times of other respiratory diseases. LPM closures should be immediately implemented in affected areas in future A(H7N9) outbreaks to minimize the risk of human infection and its effectiveness should be continuously monitored in real-time. Given the potentially huge adverse economic impact of LPM closure, prompt and unanimous support of public health practitioners and clinicians on this necessary intervention will be paramount for protecting human health from the zoonotic and potential pandemic threat of influenza A(H7N9).
Panel: Research in focus
Systematic review
We searched PubMed on 30 July 30 2013, for articles that described LPM closures as control measures for avian influenza. We also searched articles available online at international medical and infectious disease journals. We did not find any articles that quantified the effectiveness of LPM closures in the H7N9 epidemic of 2013. Han et al.10 described the experience in Huzhou, where all 12 confirmed influenza influenza A(H7N9) cases reported recent exposure to poultry, while no new cases were reported after LPM closures. Murhekar et al.18 and Xu et al.17 described the timeline of LPM closures in major Chinese cities and commented on the reduction in incidence after closures, without quantitative analysis. Two studies in affected areas identified relatively high prevalences of influenza A(H7N9) virus in specimens collected from birds and/or the environment of LPMs,2, 10 and linked samples from birds in LPMs with nearby human infections,37 although a large study identified very few infected birds in their intense surveillance efforts (only 52 A(H7N9) positives out of 10,703 samples from poultry markets, poultry farms, wild bird habitats, and poultry and swine slaughterhouses in Shanghai, Anhui, Zhejiang, Jiangsu, Shandong, Hubei, Henan, Jiangxi, Guangdong, Fujian, and Hunan provinces).38 We also found articles discussing the effectiveness of LPM closures39 and rest days13, 40–42 or other measures43 in reducing the risk of human infections with other avian influenza viruses.
Interpretation
Our study showed that LPM closure was associated with a sudden and very strong (>97%) reduction in the incidence of laboratory-confirmed cases of influenza A(H7N9) virus infection in Shanghai, Nanjing, Hangzhou and Huzhou. We used patterns in incidence to estimate a mean incubation period of 3.3 days, which is very similar to the estimated incubation period from individual patient data on poultry exposures with mean 3.1 days.4 In the short-term, our findings suggest that in areas where influenza A(H7N9) is identified in live poultry, LPM closure should be rapidly implemented to protect animal and human health and its effectiveness in incidence reduction could and should be monitored in real-time using robust statistical methods. In the medium to longer term, evidence-based discussions and deliberations about the role of rest days and central slaughtering of all live poultry should be renewed to control the risk posed by avian influenza viruses.
Supplementary Material
Acknowledgments
We thank Jiandong Zheng, Zhaorui Chang, Qian Zhang, Honglong Zhang, Fengfeng Liu, and Lingjia Zeng from the Division of Infectious Disease, Chinese Center for Disease Control and Prevention for data cleaning and preparation. We thank staff members of the Bureau of Disease Control and Prevention, and the Health Emergency Response Office of the National Health and Family Planning Commission and provincial and local departments of health for providing assistance with administration and data collection; staff members at county, prefecture and provincial government offices, and CDCs in Shanghai, Jiangsu, Zhejiang, Anhui, Henan, Beijing, Shandong, Jiangxi, Fujian, and Hunan provinces for providing assistance with field investigation, administration and data collection. We thank Brandford Chan, Angel Li, Tim Tsang, Hoi Wa Wong, and Ying Zhou for technical assistance. The views expressed are those of the authors and do not necessarily represent the policy of the China CDC.
This study was funded by the US National Institutes of Health (Comprehensive International Program for Research on AIDS grant U19 AI51915), the China-U.S. Collaborative Program on Emerging and Re-emerging Infectious Diseases, grants from the Ministry of Science and Technology, China (2012 ZX10004-201), the Harvard Center for Communicable Disease Dynamics from the National Institute of General Medical Sciences (grant no. U54 GM088558), the Research Fund for the Control of Infectious Disease, Food and Health Bureau, Government of the Hong Kong Special Administrative Region, and the Area of Excellence Scheme of the Hong Kong University Grants Committee (grant no. AoE/M-12/06). The funding bodies had no role in study design, data collection and analysis, preparation of the manuscript, or the decision to publish.
Footnotes
Author information:
BJC has received research funding from MedImmune Inc., and consults for Crucell NV. GML has received speaker honoraria from HSBC and CLSA. The authors report no other potential conflicts of interest.
References
- 1.Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368(20):1888–97. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Liang W, Shigui Y, Wu N, Gao H, Sheng J, et al. Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: clinical analysis and characterisation of viral genome. Lancet. 2013;381(9881):1916–25. doi: 10.1016/S0140-6736(13)60903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jonges M, Meijer A, Fouchier R, Koch G, Li J, Pan J, et al. Guiding outbreak management by the use of influenza A(H7Nx) virus sequence analysis. Euro Surveill. 2013;18(16) pii=20460. [PubMed] [Google Scholar]
- 4.Cowling BJ, Jin L, Lau EHY, Liao Q, Wu P, Jiang H, et al. Comparative epidemiology of human infections with avian influenza A H7N9 and H5N1 viruses in China: a population-based study of laboratory-confirmed cases. Lancet. 2013;382(9887):129–37. doi: 10.1016/S0140-6736(13)61171-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ip DK, Liao Q, Wu P, Gao Z, Cao B, Feng L, et al. Detection of mild to moderate influenza A/H7N9 infection by China’s national sentinel surveillance system for influenza-like illness: case series. BMJ. 2013;346:f3693. doi: 10.1136/bmj.f3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu H, Cowling BJ, Feng L, Lau EHY, Liao Q, Tsang TK, et al. Human infection with avian influenza A H7N9 virus: an assessment of clinical severity. Lancet. 2013;382(9887):138–45. doi: 10.1016/S0140-6736(13)61207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q, Zhou L, Zhou M, Chen Z, Li F, Wu H, et al. Preliminary report: epidemiology of the avian influenza A (H7N9) outbreak in China. N Engl J Med. 2013 doi: 10.1056/NEJMoa1304617. [DOI] [Google Scholar]
- 8.Horby P. H7N9 is a virus worth worrying about. Nature. 2013;496(7446):399. doi: 10.1038/496399a. [DOI] [PubMed] [Google Scholar]
- 9.Uyeki TM, Cox NJ. Global Concerns Regarding Novel Influenza A (H7N9) Virus Infections. N Engl J Med. 2013;368(20):1862–4. doi: 10.1056/NEJMp1304661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han J, Jin M, Zhang P, Liu J, Wang L, Wen D, et al. Epidemiological link between exposure to poultry and all influenza A(H7N9) confirmed cases in Huzhou city, China, March to May 2013. Euro Surveill. 2013;18(20):20481. pii. [PubMed] [Google Scholar]
- 11.Liao Q, Lam WT, Leung GM, Jiang C, Fielding R. Live poultry exposure, Guangzhou, China, 2006. Epidemics. 2009;1(4):207–12. doi: 10.1016/j.epidem.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Lam TT, Wang J, Shen Y, Zhou B, Duan L, Cheung CL, et al. The genesis and source of the H7N9 influenza viruses causing human infections in China. Nature. 2013 doi: 10.1038/nature12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung YH, Lau EH, Zhang LJ, Guan Y, Cowling BJ, Peiris JS. Avian influenza and ban on overnight poultry storage in live poultry markets, Hong Kong. Emerg Infect Dis. 2012;18(8):1339–41. doi: 10.3201/eid1808.111879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mounts AW, Kwong H, Izurieta HS, Ho Y, Au T, Lee M, et al. Case-control study of risk factors for avian influenza A (H5N1) disease, Hong Kong, 1997. J Infect Dis. 1999;180(2):505–8. doi: 10.1086/314903. [DOI] [PubMed] [Google Scholar]
- 15.Shortridge KF. Poultry and the influenza H5N1 outbreak in Hong Kong, 1997: abridged chronology and virus isolation. Vaccine. 1999;17 (Suppl 1):S26–S9. doi: 10.1016/s0264-410x(99)00102-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhou L, Liao Q, Dong L, Huai Y, Bai T, Xiang N, et al. Risk factors for human illness with avian influenza A (H5N1) virus infection in China. J Infect Dis. 2009;199(12):1726–34. doi: 10.1086/599206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu J, Lu S, Wang H, Chen C. Reducing exposure to avian influenza H7N9. Lancet. 2013;381(9880):1815–6. doi: 10.1016/S0140-6736(13)60950-2. [DOI] [PubMed] [Google Scholar]
- 18.Murhekar M, Arima Y, Horby P, Vandemaele KA, Vong S, Zijian F, et al. Avian influenza A(H7N9) and the closure of live bird markets. Western Pac Surveill Response J. 2013;4(2) doi: 10.5365/wpsar.2013.4.2.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Y, Gao GF. Lessons learnt from the human infections of avian-origin influenza A H7N9 virus: Live free markets and human health. Sci China Life Sci. 2013;56(6):493–4. doi: 10.1007/s11427-013-4496-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Food and Agriculture Organization, World Organisation for Animal Health, World Health Organization, United Nations Children’s Fund, World Bank, UN System Influenza Coordinator. Contributing to One World, One Health: A strategic framework for reducing risks of infectious diseases at the animal–human–ecosystems interface. 2008. [Google Scholar]
- 21.Gebski V, Ellingson K, Edwards J, Jernigan J, Kleinbaum D. Modelling interrupted time series to evaluate prevention and control of infection in healthcare. Epidemiol Infect. 2012;140(12):2131–41. doi: 10.1017/S0950268812000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shardell M, Harris AD, El-Kamary SS, Furuno JP, Miller RR, Perencevich EN. Statistical analysis and application of quasi experiments to antimicrobial resistance intervention studies. Clin Infect Dis. 2007;45(7):901–7. doi: 10.1086/521255. [DOI] [PubMed] [Google Scholar]
- 23.Gelman A, Meng X-L, Stern H. Posterior predictive assessment of model fitness via realized discrepancies. Stat Sinica. 1996;6:733–807. [Google Scholar]
- 24.Shaman J, Kohn M. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc Natl Acad Sci U S A. 2009;106(9):3243–8. doi: 10.1073/pnas.0806852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaman J, Pitzer VE, Viboud C, Grenfell BT, Lipsitch M. Absolute humidity and the seasonal onset of influenza in the continental United States. PLoS Biol. 2010;8(2):e1000316. doi: 10.1371/journal.pbio.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arima Y, Zu R, Murhekar M, Vong S, Shimadaa T. World Health Organization Regional Office for the Western Pacific Event Management Team. Human infections with avian influenza A(H7N9) virus in China: preliminary assessments of the age and sex distribution. Western Pac Surveill Response J. 2013;4(2) doi: 10.5365/wpsar.2013.4.2.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ai J, Huang Y, Xu K, Ren D, Qi X, Ji H, et al. Case-control study of risk factors for human infection with influenza A(H7N9) virus in Jiangsu Province, China, 2013. Euro Surveill. 2013;18(26) doi: 10.2807/1560-7917.es2013.18.26.20510. [DOI] [PubMed] [Google Scholar]
- 28.Cowling BJ, Freeman G, Wong JY, Wu P, Liao Q, Lau EHY, et al. Preliminary inferences on the age-specific seriousness of human disease caused by avian influenza A(H7N9) infections in China. Euro Surveill. 2013;18(19):20475. pii. [PMC free article] [PubMed] [Google Scholar]
- 29.http://www.gov.cn/gzdt/2013-06/20/content_2429942.htm 30 Jul.
- 30.http://www.gov.cn/gzdt/2013-05/02/content_2394348.htm 30 Jul.
- 31.http://www.gov.cn/gzdt/2013-04/26/content_2390933.htm 30 Jul.
- 32.http://www.chp.gov.hk/en/content/599/30218.html Jul 31.
- 33.Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science. 2012;336(6088):1534–41. doi: 10.1126/science.1213362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imai M, Watanabe T, Hatta M, Das SC, Ozawa M, Shinya K, et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature. 2012;486(7403):420–8. doi: 10.1038/nature10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antia R, Regoes RR, Koella JC, Bergstrom CT. The role of evolution in the emergence of infectious diseases. Nature. 2003;426(6967):658–61. doi: 10.1038/nature02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.http://www.chp.gov.hk/en/content/599/30218.html Aug 31.
- 37.Bao CJ, Cui LB, Zhou MH, Hong L, Gao GF, Wang H. Live-animal markets and influenza A (H7N9) virus infection. N Engl J Med. 2013;368(24):2337–9. doi: 10.1056/NEJMc1306100. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Q, Shi J, Deng G, Guo J, Zeng X, He X, et al. H7N9 influenza viruses are transmissible in ferrets by respiratory droplet. Science. 2013;341(6144):410–4. doi: 10.1126/science.1240532. [DOI] [PubMed] [Google Scholar]
- 39.Mullaney R. Live-bird market closure activities in the northeastern United States. Avian Dis. 2003;47(3 Suppl):1096–8. doi: 10.1637/0005-2086-47.s3.1096. [DOI] [PubMed] [Google Scholar]
- 40.Fournie G, Guitian FJ, Mangtani P, Ghani AC. Impact of the implementation of rest days in live bird markets on the dynamics of H5N1 highly pathogenic avian influenza. J R Soc Interface. 2011;8(61):1079–89. doi: 10.1098/rsif.2010.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lau EH, Leung YH, Zhang LJ, Cowling BJ, Mak SP, Guan Y, et al. Effect of interventions on influenza A (H9N2) isolation in Hong Kong’s live poultry markets, 1999–2005. Emerg Infect Dis. 2007;13(9):1340–7. doi: 10.3201/eid1309.061549. [DOI] [PubMed] [Google Scholar]
- 42.Martin V, Zhou X, Marshall E, Jia B, Fusheng G, FrancoDixon MA, et al. Risk-based surveillance for avian influenza control along poultry market chains in South China: The value of social network analysis. Prev Vet Med. 2011;102(3):196–205. doi: 10.1016/j.prevetmed.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oladokun AT, Meseko CA, Ighodalo E, John B, Ekong PS. Effect of intervention on the control of Highly Pathogenic Avian Influenza in Nigeria. Pan Afr Med J. 2012;13:14. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.