SUMMARY
Contacts between the endoplasmic reticulum and the plasma membrane involve extended synaptotagmins (E-Syts) in mammals or tricalbins in yeast, proteins with multiple C2 domains. One of the tandem C2 domains of E-Syt2 is predicted to bind Ca2+, but no Ca2+-dependent function has been attributed to this protein. We have determined the crystal structures of the tandem C2 domains of E-Syt2 in the absence and presence of Ca2+, and analyzed their Ca2+-binding properties by NMR spectroscopy. Our data reveal an unexpected V-shaped structure with a rigid orientation between the two C2 domains that is not substantially altered by Ca2+. The E-Syt2 C2A domain binds up to four Ca2+ ions, whereas the C2B domain does not bind Ca2+. These results suggest that E-Syt2 performs an as yet unidentified Ca2+-dependent function through its C2A domain, and uncover fundamental differences between the properties of the tandem C2 domains of E-Syts and synaptotagmins.
INTRODUCTION
Membrane contact sites (MCSs) are areas where the membranes from two distinct organelles come into close apposition, facilitating the transfer of signals and molecules between them. Increasing evidence suggests that these sites play important roles in cell physiology (Levine and Loewen, 2006; Toulmay and Prinz, 2011; Elbaz and Schuldiner, 2011). Particularly prominent are contacts between the endoplasmic reticulum (ER) and the plasma membrane, which appear to be a general feature of eukaryotic cells and have been implicated in regulation of cell signaling, metabolism, ER architecture and plasma membrane domain organization (Carrasco and Meyer, 2011; Stefan et al., 2013). However, the molecular machineries that form MCSs and the principles underlying their functions are just beginning to be uncovered.
In yeast, three ER proteins known as tricalbins (Tcb1p, Tcb2p and Tcb3p) were recently shown to play key functions in ER-plasma membrane tethering (Manford et al., 2012). In mammalian cells, such tethering was found to also depend on three proteins called E-Syts [for extended synaptotagmins; (Min et al., 2007)] that are related to tricalbins (Giordano et al., 2013). The term E-Syts reflects their similarity to synaptotagmins (Min et al., 2007), a family of proteins that are involved in Ca2+-regulated secretion and are characterized by an N-terminal transmembrane (TM) sequence and two C-terminal C2 domains (C2A and C2B) (Rizo and Rosenmund, 2008). Tricalbins and E-Syts share similar domain structures comprising multiple C2 domains (5 for E-Syt1, and 3 for E-Syt2 and E-Syt3) and a synaptotagmin-like-mitochondrial-lipid binding protein (SMP) domain that has been implicated in targeting to MCSs (Creutz et al., 2004; Min et al., 2007; Toulmay and Prinz, 2012) (Figure 1A for E-Syt2).
Figure 1.
Crystal structure of the Ca2+-free E-Syt2 C2AB fragment. (A) Domain structure of E-Syt2. The black box represents a hydrophobic region involved in membrane anchoring (Giordano et al., 2013). SMP = synaptotagmin-like-mitochondrial-lipid binding protein domain. (B) Ribbon diagram of the Ca2+-free E-Syt2 C2AB fragment. The C2A domain is red, the C2B domain cyan and the linker between them yellow. The loops at the top, which mediate Ca2+ binding in some C2 domains, are labeled loop 1–4. N and C indicate the N- and C-termini, respectively. (C) Superposition of ribbon diagrams of the E-Syt2 C2A and C2B domains. (D) Superpositions of ribbon diagrams of the C2A (red) and C2B domains (cyan) of E-Syt2 with the C2 domain of intersectin 2 (wheat) and the Munc13–1 C2B domain (pale yellow) (PDB accession codes 3JZY and 3KWT, respectively). See also Figure S1.
C2 domains are widespread modules that commonly bind Ca2+ and phospholipids (Rizo and Sudhof, 1998) but can also be Ca2+-independent and function in protein-protein interactions [e.g. (Lu et al., 2006)]. C2 domains occur in tandem in proteins involved in membrane traffic such as synaptotagmins and also in E-Syts (C2A-C2B in E-Syt2 and E-Syt3; C2A-C2B and C2C-C2D in E-Syt1). The synaptotagmin-1 C2 domains were shown to form β-sandwich structures that bind two or three Ca2+ ions via five aspartate side chains from loops located at the top (Sutton et al., 1995; Shao et al., 1996; Ubach et al., 1998; Fernandez et al., 2001). These loops also mediate Ca2+-dependent phospholipid binding (Zhang et al., 1998; Chapman and Davis, 1998). Among E-Syt C2 domains, the entire set of five aspartate residues is conserved only in the C2A domains of E-Syt2 and E-Syt3, and in the C2A and C2C domains of E-Syt1 (both homologous to the E-Syt2 and E-Syt3 C2A domains), suggesting that these C2 domains but not others bind Ca2+ (Min et al., 2007). However, localization of E-Syt2 to the plasma membrane required its C2C domain (Min et al., 2007), and studies of the function of E-Syts in ER-plasma membrane tethering assigned a Ca2+-dependent role only for E-Syt1 (Giordano et al., 2013). In yeast, no Ca2+-dependent function in ER-plasma membrane tethering was attributed to tricalbins (Manford et al., 2012), although the C2D domains of Tcb1p and Tcb3p were reported to bind Ca2+ while other tricalbin C2 domains contain substitutions in some of the canonical aspartate residues (Schulz and Creutz, 2004). However, predictions of C2 domains properties from sequence analyses need to be interpreted with caution, as distinctive sequences inserted between β-strands often yield unexpected structural features (Ubach et al., 1999; Garcia et al., 2004; Shin et al., 2010), and the presence of a complete or incomplete set of five canonical aspartate residues does not guarantee Ca2+ binding or lack of this activity, respectively (Dai et al., 2004).
While important advances have recently been made in establishing the importance of E-Syts and tricalbins for ER-plasma membrane tethering, much remains to be learned about how the structural and biochemical properties of the C2 domains from these proteins underlie their functions. Particularly important is to unambiguously establish whether the tandem C2 domain-regions of E-Syts bind Ca2+ and contain unique structural features that might be important for their functions. It is also of interest to investigate the similarity between the tandem C2 domains of E-Syts and those of synaptotagmins, which have been extensively studied [e.g. (Sutton et al., 1999; Fuson et al., 2007; Arac et al., 2006; Herrick et al., 2009; Vrljic et al., 2010; Shin et al., 2009)].
To address these questions, we have analyzed the Ca2+-binding properties of a fragment spanning the C2A and C2B domains of E-Syt2 using NMR spectroscopy, and we have determined its crystal structure in the absence and presence of Ca2+. Our data reveal a V-shaped structure with a rigid orientation of the two C2 domains, in contrast to the flexible arrangement of the synaptotagmin C2 domains. The E-Syt2 C2B domain does not bind Ca2+ but the C2A domain binds up to four Ca2+ ions with a wide range of affinities, including a novel Ca2+-binding site that arises from the highly acidic nature of one of the Ca2+-binding loops. These results show that the C2A domain of E-Syt2 shares common features with the synaptotagmin C2A domains but suggest that, overall, the tandem C2 domains of E-Syt2 function by markedly different mechanisms from those characteristic of synaptotagmins. Moreover, our data indicate that the C2A domains of E-Syts perform a general Ca2+-dependent role at ER-plasma membrane contact sites, suggesting that their general function may be dynamically regulated by cell-signaling.
RESULTS
Structure of the Ca2+-free tandem C2 domains of E-Syt2
C2 domains can contain extensions in their termini that are critical for proper folding or optimal solubility [e.g. (Guan et al., 2007; Shin et al., 2010)]. To determine the structure of the E-Syt2 tandem C2 domains, we first tried using a fragment containing the C2A-C2B domains of mouse E-Syt2 with additional N-terminal sequences (residues 283–584), but could not get suitable crystals. We then turned to a shorter fragment spanning the C2A-C2B domains of human E-Syt2 (residues 363–660; below referred to as E-Syt2 C2AB). Crystallization screens and optimizations performed with Ca2+-free protein yielded crystals of E-Syt2 C2AB that had the symmetry of space group P43 and diffracted to 2.1 Å. We were unable to solve the structure by molecular replacement, but we obtained selenomethionine-derivatized crystals and solved the structure of the Ca2+-free E-Syt2 C2AB fragment using phases from single-wavelength anomalous dispersion data. Structural statistics are described in Table 1.
Table 1.
Data collection, phasing and refinement statistics for E-Syt2 C2AB fragment structures.
| Data collection | |||
| Crystal | ESyt | ESyt-Ca2+ | ESyt Seapeak |
| Space group | P43 | C2 | P43 |
| Energy (eV) | 8961.6 | 12,684.9 | 12,684.9 |
| Resolution range (Å) | 41.9 – 2.10 (2.14 – 2.10) | 30.0 – 2.55 (2.59 – 2.55) | 43.3 – 2.33 (2.37 – 2.33) |
| Unique reflections | 48,539 (1,830) | 12,318 (263) | 36,177 (1,608) |
| Multiplicity | 5.5 (3.2) | 3.6 (2.4) | 4.4 (2.9) |
| Data completeness (%) | 97.0 (73.8) | 88.7 (38.0) | 98.9 (87.2) |
| Rmerge (%)b | 6.7 (50.1) | 5.8 (27.6) | 8.0 (44.6) |
| I/σ(I) | 23.4 (1.46) | 33.4 (2.45) | 23.5 (1.38) |
| Wilson B-value (Å2) | 40.3 | 66.6 | 52.4 |
| Phase determination | |||
| Anomalous scatterers | selenium, 10 out of 10 possible sites | ||
| Figure of merit (43.3 – 2.33 Å) | 0.16 | ||
| Refinement statistics | |||
| Crystal | Native | ESyt-Ca2+ | |
| Resolution range (Å) | 41.9 – 2.10 (2.14 – 2.10) | 29.0 – 2.55 (2.81 – 2.55) | |
| No. of reflections RWork/Rfree | 48,422/2.468 (1,979/108) | 12,224/604 (1,782/93) | |
| Data completeness (%) | 96.9 (73.8) | 87.4 (54.0) | |
| Atoms (non-H protein/solvent/metals) | 4,650/146/0 | 2,178/4/3 | |
| Rwork (%) | 20.7 (29.5) | 22.2 (31.5) | |
| Rfree (%) | 24.7 (34.1) | 28.7 (41.4) | |
| R.m.s.d. bond length (Å) | 0.012 | 0.012 | |
| R.m.s.d. bond angle (°) | 1.32 | 1.26 | |
| Mean B-value (Å2) (protein/solvent/metals) | 58.3/46.1/NA | 121.7/105.8/126.38 | |
| Ramachandran plot (%) (favored/additional/disallowed)c | 95.7/4.1/0.2 | 94.7/3.8/1.5 | |
| Maximum likelihood coordinate error | 0.31 | 0.45 | |
| Missing residues | A:552–556, 660 B: 363, 403–409, 550–556, 660 |
363–379, 526–531, 551–555, 660 | |
Data for the outermost shell are given in parentheses.
Bijvoet-pairs were kept separate for data processing.
Rmerge =100 ΣhΣi|Ih, i — 〈Ih〉|/ΣhΣiIh, i, where the outer sum (h) is over the unique reflections and the inner sum (i) is over the set of independent observations of each unique reflection.
As defined by the validation suite MolProbity (Chen et al, 2010).
The asymmetric unit contains two molecules of E-Syt2 C2AB that have very similar structures [0.4 Å root mean square (rms) deviation between 3,362 equivalent atoms] (Figure S1A available online). The N-terminal sequence spanning residues 363–383 forms an extended structure that is not part of the C2A domain (Figures S1A,B) and is involved in extensive crystal contacts. As expected, the E-Syt2 C2A and C2B domains form β-sandwich structures with two four-stranded β-sheets (Figure 1B). The C2A domain spans residues 384–509 while residues 535–656 form the C2B domain (red and cyan, respectively, in Figure 1B). Both C2 domains have a type II topology where the N- and C-termini are located at the bottom of the β-sandwich, opposite to the top loops that mediate Ca2+ binding in C2 domains (Figure 1C) [see (Rizo and Sudhof, 1998)]. This topology is analogous to that found originally in the PLC-δ1 C2 domain (Essen et al., 1996) and differs from the type I topology of the synaptotagmin C2 domains, where the N- and C-termini are located at the top of the β-sandwich (Sutton et al., 1995). As a consequence, the top of the E-Syt2 C2A and C2B domains contain four loops (labeled loop 1–4 in Figures 1B,C), while the bottom side has three loops, one of which forms a short α-helix in both domains. The linker sequence joining the C2A and C2B domains (residues 510–534; yellow in Figures 1B and S1C) forms an α-helix and two loops that pack against both domains, conferring a V-shape to the overall structure.
The structures of the E-Syt2 C2A and C2B domains are very similar, with the main differences observed in the loops joining the β-strands (Figure 1C). Structural superposition using DaliLite (Holm and Park, 2000) yielded an rms deviation of 1.5 Å for 114 equivalent Cα carbons. Searches of the Protein Data Bank (PDB) using DALI (Holm and Sander, 1993) revealed that the known C2 domain structures that are most similar to both the C2A and the C2B domain of E-Syt2 correspond to the C2 domain of intersectin 2 and the C2B domain of Munc13-1, both of which also have topology II (Figure 1D). Pairwise comparisons of the intersectin 2 C2 domain and the Munc13-1 C2B domain with the E-Syt2 C2A and C2B domains yielded Z scores ranging from 16.6 to 19.1 and 1.3–1.5 rms deviations for 116–124 equivalent Cα carbons. A noteworthy feature of the E-Syt2 C2A and C2B domains is that the sequence corresponding to loop 1 is considerably longer than usual. This is more apparent in the structure of the E-Syt2 C2A domain, where loop 1 protrudes at the top of the domain more markedly than in the intersectin 2 C2 domain and the Munc13-1 C2B domain (Figure 1D). The sequence of loop 1 is highly conserved evolutionarily, suggesting that it is functionally significant. No electron density is observed for part of the loop 1 sequence of the C2A domain for the other molecule in the asymmetric unit and for part of the loop 1 sequence of the E-Syt2 C2B domain in both molecules of the asymmetric unit (Figures 1B and S1A), suggesting that loop 1 is flexible in both domains. It is also worth noting that the β-sandwiches of the E-Syt2 C2 domains are also highly similar to many C2 domains with topology I such as those of synaptotagmin-1, but the two α-helices characteristic of the synaptotagmin-1 C2B domain are not observed in the E-Syt2 C2B domain (Figure S1D,E).
Ca2+-binding to the E-Syt2 C2A domain
Because of difficulties in expressing the isolated E-Syt2 C2A and C2B domains in soluble form, we used the E-Syt2 C2AB fragment to study its Ca2+-binding properties. We first used temperature denaturation monitored by circular dichroism (CD), which provided a sensitive tool to test for Ca2+ binding to the synaptotagmin-1 C2A domain (Shao et al., 1997). Ca2+ caused a dramatic decrease in the melting temperature of the wild type (WT) E-Syt2 C2AB fragment, which is unusual in C2 domains (see discussion), but shows that this fragment indeed binds Ca2+ (Figure 2A). Practically no shift in melting temperature was observed for a mutant E-Syt2 C2AB fragment where two of the canonical aspartate residues predicted to form the Ca2+-binding sites of the C2A domain (D401 and D413) were replaced by alanines (Figure 2B), showing that, as expected, Ca2+ binding to the WT E-Syt2 C2AB fragment is mediated by the C2A domain.
Figure 2.
The E-Syt2 C2AB fragment binds Ca2+. (A,B) Thermal denaturation curves monitored by the CD absorption at 215 nM of the WT (A) and D401A,D413A mutant (B) E-Syt2 C2AB fragment in the presence of 0.1 mM EGTA (closed circles) or 1 mM Ca2+ (open circles).
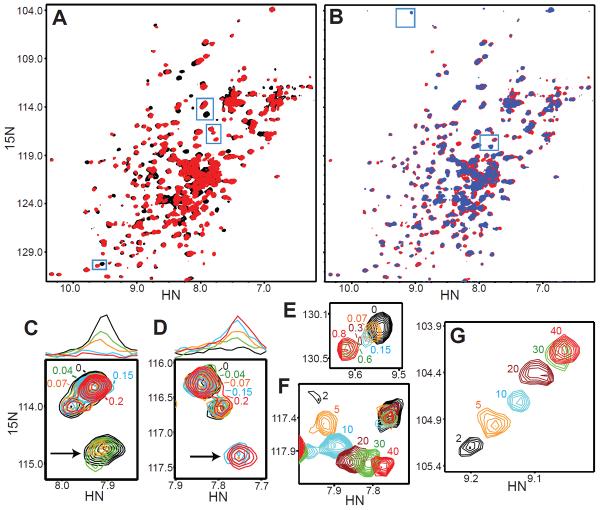
To analyze the Ca2+-binding properties in detail, we used transverse relaxation optimized spectroscopy (TROSY)-enhanced 1H-15N heteronuclear single quantum coherence (HSQC) spectra. In these NMR spectra, Ca2+ binding with slow exchange rates characteristic of high affinity sites leads to gradual disappearance of the NH cross-peaks corresponding to the Ca2+ free state and concomitant appearance of cross-peaks from the Ca2+ bound state, whereas Ca2+ binding with fast exchange rates characteristic of low affinity sites leads to gradual movement of cross-peaks from their positions in the Ca2+-free state to those of the Ca2+-bound state (Rizo et al., 2012). 1H-15N TROSY-HSQC spectra of 0.16 mM 15N-labeled E-Syt2 C2AB fragment exhibited multiple changes upon addition of 1 mM Ca2+ (Figure 3A) and additional changes for a few cross-peaks at 30 mM Ca2+ (Figure 3B), suggesting the presence of at least two Ca2+-binding sites with different affinities. Indeed, Ca2+ titrations showed clearly distinct behaviors in different cross-peaks that define up to four Ca2+-binding sites.
Figure 3.
The E-Syt2 C2AB fragments contains up to four Ca2+-binding sites. (A,B) 1H-15N TROSY-HSQC spectra of 0.15 M E-Syt2 C2AB fragment acquired in the absence of Ca2+ (black contours) or in the presence of 1 mM Ca2+ (red contours) or 30 mM Ca2+ (blue contours). (C–G) Expansions of different regions of 1H-15N TROSY-HSQC spectra of the E-Syt2 C2AB fragment (blue squares in A, B) showing distinct Ca2+-dependent behaviors. The numbers indicate the Ca2+ concentrations used (in mM units) and are color-coded as the cross-peaks from the corresponding spectrum. In panels C and D, the 1D traces shown above the contour plots were taken along the 15N chemical shifts indicated by the arrows and have the same color codes.
Titration from 0 to 0.15 mM Ca2+ led to complete disappearance of a few cross-peaks (Figure 3C) and full appearance of a few new cross-peaks (Figure 3D). This behavior corresponds to a stoichiometric saturation-binding curve characteristic of a Ca2+-binding site with relatively high affinity (KD < 10 μM; we refer to this site as Ca1). Several other cross-peaks moved gradually from 0 to ca. 0.8 mM Ca2+ and exhibited strong broadening at intermediate Ca2+ concentrations (Figure 3E). This behavior must arise from the presence of a second Ca2+ binding site (Ca2) with a lower affinity than Ca1, but the strong broadening suggests that these cross-peaks are also affected by binding to Ca1. This is not unexpected, since the two most common Ca2+-binding sites in C2 domains are very close to each other (Shao et al., 1996). This feature hinders accurate measurement of the affinity of site Ca2 but we estimate the KD to be in the 100–200 μM range based on the mid points observed for cross-peaks with this behavior.
We also observed the appearance of a few new cross-peaks at 2 mM Ca2+, and higher Ca2+ concentrations induced movements of these cross-peaks (Figures 3F,G). For one of the cross-peaks (Figure 3F), the Ca2+-dependent movement was clearly curved and therefore included at least two components arising from two distinct Ca2+-binding sites (Ca3 and Ca4). The two components and the fact that the positions of the cross-peaks below 2 mM Ca2+ were unknown hindered accurate measurement of the corresponding affinities, but we estimate that the KDs of these sites are 2–3 mM and 15–20 mM, respectively. For comparison, the KDs of the three Ca2+-binding sites of the synaptotagmin-1 C2A domain are 60 μM, 0.5 μM and > 2 mM (Ubach et al., 1998; Fernandez-Chacon et al., 2001). Note that phospholipids dramatically enhance these affinities because their headgroups fill empty coordination sites of the Ca2+ ions bound to the synaptotagmin-1 C2A domain (Zhang et al., 1998; Fernandez-Chacon et al., 2001). Correspondingly, Ca2+-dependent phospholipid binding to a fragment spanning the SMP, C2A and C2B domains of E-Syt2 (SMP-C2AB fragment) occurs with an apparent Ca2+ affinity in the low micromolar range, although it is unclear to what extent the SMP domain might contribute to this apparent affinity (Min et al., 2007).
Structure of the Ca2+-bound E-Syt2 C2AB fragment
To define the Ca2+-binding mode of the E-Syt2 C2AB fragment, we performed crystallization screens at different Ca2+ concentrations. We obtained crystals in 2 mM Ca2+ that diffracted to 2.55 Å and had the symmetry of space group C2, with one molecule per asymmetric unit. The structure was solved by molecular replacement using the Ca2+-free structure as search model (Table 1).
The structure of the Ca2+-bound E-Syt2 C2AB fragment also exhibits a V-shape formed by two β-sandwiches and reveals three Ca2+ ions bound to the C2A domain (Figure 4A). Superposition of the Ca2+-bound and Ca2+-free structures showed that the structures are very similar (Figure 4B). The rms deviations between the Ca2+-bound structure and the two structures in the Ca2+-free asymmetric unit are 1.0 Å and 1.1 Å for 3,656 and 3,525 equivalent atoms, respectively. Because the crystals of the Ca2+-free and Ca2+-bound forms were obtained under very different conditions and exhibited symmetries from different space groups, the similarity in the relative orientations of the C2A and C2B domain observed in the Ca2+-free and Ca2+-bound structures strongly suggests that this orientation is rigid and is not affected substantially by Ca2+ binding. The most overt differences between the Ca2+-free and Ca2+-bound structures of the E-Syt2 C2AB fragment are observed in two regions. One region corresponds to the N-terminal sequence, which is disordered in the Ca2+-bound structure but is ordered in the Ca2+-free structures. The second region is in the long loop 1 of the C2A domain, which forms a β-hairpin upon Ca2+ binding. It is unclear to what extent the structural differences observed in this loop occur in solution, since the loop makes different types of crystal contacts in the Ca2+-bound structure and in one of the Ca2+-free structures, whereas this loop is partially disordered in the other Ca2+-free structure (Figure S1A). However, formation of the β-hairpin appears to be necessary to place some of the Ca2+ ligands in the proper orientation for Ca2+ binding (Figure S2). Hence, it seems likely that, in solution, loop 1 of the C2A domain is flexible in the absence of Ca2+ and forms the β-hairpin upon Ca2+ binding.
Figure 4.

Structure of the Ca2+-bound E-Syt2 C2AB fragment. (A) Ribbon diagram of Ca2+-bound E-Syt2 C2AB. The three Ca2+ ions are shown as yellow spheres. The loops at the top region of the β-sandwiches are labeled loop 1–4. N and C indicate the N- and C-termini, respectively. (B) Superposition of the Ca2+-free (orange ribbon) and Ca2+-bound (blue ribbon with yellow spheres) E-Syt2 C2AB fragment. The position of loop 1 of the C2A domain is indicated. (C) Surface representations illustrating the electrostatic potentials of the Ca2+-free and Ca2+-bound E-Syt2 C2AB fragment. The electrostatic potentials were calculated with GRASP (Nicholls et al., 1991). The gradients of electrostatic potential shown ranged from ≥ −7 kBT/e (red) to ≤ 7 kBT/e (blue). See also Figure S2.
Since a Ca2+-induced switch in the electrostatic potential of the synaptotagmin-1 C2 domains is believed to be important for synaptotagmin-1 function (Shao et al., 1997; Arac et al., 2006; Dai et al., 2007), we analyzed the changes caused by Ca2+ binding on the electrostatic properties of the E-Syt2 C2AB fragment. The electrostatic potential of the Ca2+-free C2A domain is highly acidic and becomes somewhat positive around the Ca2+-binding region upon Ca2+ binding (Figure 4C), but to a lesser extent than that observed for the synaptotagmin-1 C2 domains (Fernandez et al., 1998; Fernandez et al., 2001). This finding arises because of the large excess of acidic residues (26) over basic residues (16) in the E-Syt2 C2A domain, and likely underlies the fact that the E-Syt2 SMP-C2AB fragment binds similarly to neutral or acidic phospholipids (Min et al., 2007) whereas the synaptotagmin-1 C2 domains bind preferentially to negatively charged phospholipids (Davletov and Sudhof, 1993; Fernandez et al., 2001). An even more marked distinction is found for the E-Syt2 and synaptotagmin-1 C2B domains, as the E-Syt2 C2B domain is zwitterionic and does not bind Ca2+ (Figure 4A,C) while the synaptotagmin-1 C2B domain is highly basic and becomes even more positively charged upon Ca2+ binding (Fernandez et al., 2001).
The Ca2+-binding mode of the E-Syt2 C2 A domain
Two of the three Ca2+ ions bound to the C2A domain in the crystal structure of the E-Syt2 C2AB fragment are located in a cup-shaped cavity formed by loops 1, 2 and 3 at the top of the β-sandwich, which is common in C2 domains (Rizo and Sudhof, 1998), whereas the other Ca2+ ion binds at a site that is formed by loop 3 outside the cavity (Figure 5A). The two sites within the cup-shaped cavity are the most common in C2 domains (Rizo and Sudhof, 1998) and are found in both synaptotagmin-1 C2 domains. We refer to these two sites as Ca1 and Ca2. Site Ca1 normally has the highest affinity and is critical to position several of the Ca2+-binding ligands in the correct orientations for binding to site Ca2, which in turn has the second highest affinity (Ubach et al., 1998; Fernandez et al., 2001). Hence, it is natural to assume that sites Ca1 and Ca2 correspond to the two sites with the highest affinity observed by NMR spectroscopy (Figure 3). The five canonical aspartate residues are D401, D413, D460, D462 and D467. Site Ca1 is coordinated by seven ligands, including the backbone carbonyl of E461, two oxygens from the D401 side chain, one oxygen each from the D413, D460 and D462 side chains, and on ordered water molecule (Figure 5B). Seven ligands also coordinate site Ca2: the backbone carbonyl of K400, two oxygens from the D462 side chain, one oxygen each from the D401, D460 and D467 side chains, and an ordered water molecule.
Figure 5.

Ca2+-binding mode of the C2A domain in the crystal structure of E-Syt2 C2AB fragment. (A) Ribbon diagram of the C2A domain showing the three bound Ca2+ ions. The top loops are also labeled. N and C indicate the N- and C-termini, respectively. (B) The Ca2+-binding sites of the E-Syt2 C2A domain. The backbone is represented by blue a ribbon diagram, the Ca2+ ligands are labeled and are shown as stick models with carbon atoms colored in cyan, oxygen atoms in red and nitrogen atoms in blue. The bound Ca2+ ions are shown as yellow spheres. Sites Ca1, Ca2 and Ca3 are labeled 1, 2 and 3, respectively. Water molecules involved in Ca2+ coordination are shown as small red spheres and labeled w1–w4. Black lines indicate the coordination of Ca2+ ions by their ligands except when the ligands appear to be in contact with the ions because of the two-dimensional nature of the drawing. See also Figures S2 and S3.
The third Ca2+-binding site in the E-Syt2 C2A domain is formed by only five ligands: the backbone carbonyls of D462 and D464, one oxygen from the D466 side chain, and two ordered water molecules (Figure 5B; see electron density map in Figure S3A). Hence, it is not surprising that this Ca2+-binding site has lower affinity than sites Ca1 and Ca2, and can be attributed to one of the two low-affinity Ca2+-binding sites detected in our NMR data (Figure 3). We thus tentatively assign this site to that with an estimated KD of 2–3 mM (site Ca3). It is noteworthy that, while were able to grow crystals of the E-Syt2 C2AB fragment in 2 mM Ca2+, we barely obtained crystals under similar conditions in the presence of 5 mM Ca2+, perhaps because binding to the fourth site detected by NMR spectroscopy (Ca4) perturbs the electrostatic balance of the crystal lattice. Since the estimated KD of this site is 15–20 mM, it is not surprising that binding to this site is not observed in our crystal structure. However, we note that the backbone carbonyl of K465, two oxygens from the D467 side chain and one oxygen from the D462 side chain appear to be poised to form another Ca2+-binding site that would be analogous to the third site found in the synaptotagmin-1 C2A domain (Ubach et al., 1998) and the synaptotagmin-7 C2B domain (Xue et al., 2010), and might also be coordinated by D464 (Figure S3). Hence, we speculate that this putative site corresponds to Ca4. Overall, the abundance of Ca2+-binding sites in the E-Syt2 C2A domain can be attributed to the highly acidic nature of the sequence that forms loop 3 (DEDPDKDD).
To test the Ca2+-binding site assignments, we performed Ca2+ titrations monitored by 1H-15N TROSY-HSQC spectra with mutant E-Syt2 C2AB fragments containing either a D413N or a D466A substitution (Figures 6,7). In the absence of Ca2+, both mutations caused shifts in only a few cross-peaks compared to WT E-Syt2 C2AB (Figures 6A,7A), showing that the mutations did not disrupt the proper folding of the protein. The D413N mutation in E-Syt2 is analogous to the D178N and D309N mutations that disrupt Ca2+ binding to site Ca1 of the synaptotagmin-1 C2A and C2B domain, respectively, and also impair Ca2+ binding to other sites because of the importance of filling site Ca1 to organize the overall Ca2+-binding mode (Ubach et al., 1998; Fernandez et al., 2001). Correspondingly, 1 mM Ca2+ caused very little perturbations in the 1H-15N TROSY-HSQC spectrum of the E-Syt2 C2AB D413N mutant (Figure 6B), showing that the mutation strongly disrupts overall Ca2+ binding. A few cross-peak changes were observed at Ca2+ concentrations above 1 mM (Figures 6C,D), which can be attributed to residual binding to sites Ca1 or Ca2, and/or to lack of perturbation of sites Ca3 and Ca4. These results strongly support the proposal that sites Ca1 and Ca2 in the crystal structure constitute the two Ca2+-binding sites of the E-Syt2 C2A domain with highest affinity observed in our NMR data.
Figure 6.

The D413N mutation strongly disrupts Ca2+ binding to the E-Syt2 C2A domain. (A)1H-15N TROSY-HSQC spectra of WT (black contours) and D413N mutant (cyan contours) E-Syt2 C2AB in the absence of Ca2+. (B)1H-15N TROSY-HSQC spectra of D413N mutant E-Syt2 C2AB in the absence of Ca2+ (cyan contours) and in the presence of 1 mM Ca2+ (red contours). (C,D) Expansions of different regions of 1H-15N TROSY-HSQC spectra of the D413N mutant E-Syt2 C2AB fragment (indicated by blue squares in panel B) showing distinct Ca2+-dependent behaviors. The numbers indicate the Ca2+ concentrations used (in mM units) and are color-coded as the cross-peaks from the corresponding spectrum.
Figure 7.

The D466A mutation disrupts one of the low-affinity Ca2+ binding sites of the E-Syt2 C A domain. (A) 1H-15N TROSY-HSQC spectra of WT (black contours) and D466A mutant (green contours) E-Syt2 C2AB in the absence of Ca2+. (B)1H-15N TROSY-HSQC spectra of D466A mutant E-Syt2 C2AB in the absence of Ca2+ (green contours) and in the presence of 1 mM Ca2+ (red contours). (C–E) Expansions of different regions of 1H-15N TROSY-HSQC spectra of the D466A mutant E-Syt2 C2AB fragment (indicated by blue squares in panel B) showing distinct Ca2+-dependent behaviors. The numbers indicate the Ca2+ concentrations used (in mM units) and are color-coded as the cross-peaks from the corresponding spectrum. In panel C, the 1D traces shown above the contour plots were taken along the 15N chemical shifts indicated by the arrow and have the same color codes.
The D466A mutation was designed to disrupt binding to site Ca3. As expected, 1 mM Ca2+ caused similar changes in the 1H-15N TROSY-HSQC spectra of this mutant (Figures 7B) and WT E-Syt2 C2AB (Figures 3A). A few cross-peaks of the D466A mutant disappeared almost completely at 0.15 mM Ca2+ and completely at 0.3 mM Ca2+ (Figure 7C), resembling the behavior of cross-peaks that manifest binding to site Ca1 of WT E-Syt2C2AB (Figure 3C). Other cross-peaks of the D466A mutant exhibited gradual movements from 0 to 1 mM Ca2+, and strong broadening around 0.4 mM Ca2+ concentrations (Figure 7D), similar to cross-peaks that reflect binding to sites Ca1 and Ca2 of WT E-Syt2 C2AB (Figure 3E). Thus, the D466A mutation causes little perturbation of sites Ca1 and Ca2, although their affinities appear to be somewhat lower in the mutant, perhaps because the mutation decreases the overall negative charge in the region. Interestingly, the cross-peak that appeared at the top of the spectra of WT E-Syt2 C2AB at 2 mM Ca2+ (Figure 3G) appeared only at 5 mM Ca2+ for the D466A mutant and exhibited much smaller changes than the WT protein at higher Ca2+ concentrations (Figure 7E). These results strongly support the notion that site Ca3 identified by NMR spectroscopy in the WT E-Syt2 C2AB corresponds to the third Ca2+-binding observed in the crystal structure.
DISCUSSION
Investigation of MCSs in general and of ER-plasma membrane contacts in particular has become a subject of intense biological interest because of the identification of diverse functions that occur at these sites and the realization that they may underlie a wide variety of cellular processes. Research in yeast (Manford et al., 2012) and mammalian cells (Giordano et al., 2013) has demonstrated the importance of E-Syts and their yeast homologues, the tricalbins, for formation of ER-plasma membrane contacts. However, the functions of these proteins are poorly understood, in part because no high-resolution structural information on their domains is available. To help bridge this gap in our knowledge, we have elucidated the structure of the E-Syt2 C2AB fragment and analyzed its Ca2+-binding properties. Our data reveal that the E-Syt2 tandem C2AB fragment exhibits an unexpected V-shaped structure with a well-defined orientation of the two C2 domains and show that the E-Syt2 C2A domain binds up to four Ca2+ ions, thus suggesting that E-Syt2 and E-Syt3 physiologically perform a Ca2+-dependent function that remains to be discovered. Overall, our results provide a critical framework to rationalize this and other functions performed by E-Syt tandem C2 domains, and are also important for understanding how C2 domains function in general.
In agreement with the previous observation that the E-Syt2 and E-Syt3 C2C domain mediates plasma membrane localization (Min et al., 2007), recent studies showed that formation of ER-plasma membrane contacts requires the C2C domain of E-Syt2 and E-Syt3, and provided evidence that this function depends on Ca2+-independent interactions with PI(4,5)P2 (Giordano et al., 2013). These studies also indicated that E-Syt1 is recruited to the plasma membrane in a Ca2+-dependent manner, but potential roles of Ca2+ binding to the C2A domains of E-Syt2 and E-Syt3 were not studied. Our results now demonstrate that the E-Syt2 C2A domain binds multiple Ca2+ ions. This observation suggests that the C2A domains from E-Syt2 and the highly similar E-Syt3 must have a Ca2+-dependent function. Moreover, our data indicate that E-Syt1 may have two independent tandem C2 domain modules (C2AB and C2CD) that are also Ca2+-regulated.
Ca2+-dependent phospholipid binding to the C2A domain could enhance the Ca2+-independent ER-plasma membrane tethering activity of the C2C domain (Figure 8). However, since the C2C domain is sufficient for plasma membrane localization, we favor the possibility that Ca2+ binding to the C2A domain plays a role in physiological processes that are Ca2+-regulated, including some form of Ca2+ signaling. For instance, the SMP domain has been implicated in lipid metabolism and lipid transfer between membranes (Kopec et al., 2010; Toulmay and Prinz, 2012), and appears to cooperate with the C2A domain in lipid binding (Min et al., 2007). Thus, Ca2+-dependent phospholipid binding to the C2A domain could favor localization of the SMP domain between the two membranes (Figure 8), facilitating the putative lipid-transfer activity (or other activities) in response to a Ca2+ signal.
Figure 8.

Model of E-Syt function incorporating a Ca2+-dependent function for the C2A domain. The model postulates that, in addition to promoting ER-plasma membrane contact through Ca2+-independent binding of their C2C domain to the plasma membrane, E-Syt2 and E-Syt3 have a Ca2+-dependent function mediated by the C2A domain. This function likely involves Ca2+-induced binding of the C2A domain to the plasma membrane, which could help bring the SMP domain close to the plasma membrane. The C2B domain is not expected to contribute to membrane binding, but could bind to a protein target on the plasma membrane (orange circle labeled X), thus cooperating with the C2A domain.
Our studies reveal similarities but also fundamental differences between the tandem C2 domains of E-Syt2 and synaptotagmin-1. The Ca2+-binding mode of the E-Syt2 C2A domain (Figure 5B) is clearly similar to that of the synaptotagmin-1 C2A domain (Ubach et al., 1998), sharing at least two Ca2+-binding sites with practically identical ligands (sites Ca1 and Ca2) and probably a third site (site Ca4 site of the E-Syt2 C2A domain, called Ca3 for the synaptotagmin-1 C2A domain). The E-Syt2 C2A domain contains an additional site (Ca3) that has not been observed previously. The presence of four Ca2+ binding sites in the E-Syt2 C2A domain is likely facilitated by the highly acidic nature of loop 3 and of the overall domain. This feature probably underlies also the lack of a preference for negatively charged phospholipids (Min et al., 2007) exhibited by the synaptotagmin-1 C2A domain (Davletov and Sudhof, 1993).
More drastic differences are observed between the C2B domains of E-Syt2 and synaptotagmin-1. Thus, the synaptotagmin-1 C2B domain binds Ca2+, which is critical for its function in triggering neurotransmitter release (Mackler et al., 2002), and is characterized by a highly basic nature that is likely also crucial for synaptotagmin-1 function, enabling simultaneous binding to the synaptic vesicle and plasma membranes as well as to the SNARE complex to cooperate in membrane fusion (Arac et al., 2006; Dai et al., 2007; Seven et al., 2013). Clearly, the E-Syt2 C2B domain must perform a different function that is currently unknown. The findings that the E-Syt2 C2B domain does not bind Ca2 and is zwitterionic suggest that it might act as a protein-protein interaction domain. We speculate that binding of the E-Syt2 C2B domain to a receptor on the plasma membrane (labeled 'X' in Figure 8) might be coupled to the activities of the other domains of E-Syt2. Such coupling could be facilitated by the V-shape of the E-Syt2 C2AB fragment resulting from the rigid orientation of the C2A and C2B domains, which constitutes a unique feature of the E-Syt2 C2AB fragment and contrasts with the flexibility in the relative orientation of the synaptotagmin-1 C2 domains (Arac et al., 2006; Herrick et al., 2009; Choi et al., 2010). However, the sequence of the linker between the E-Syt2 C2A and C2B domains is markedly less conserved through evolution than the sequence of the C2 domains (Min et al., 2007) and hence it is unclear whether the V-shape has a critical functional significance.
The decrease in thermal stability of the tandem C2AB fragment (Figure 2) constitutes another unexpected property of E-Syt2 that contrasts with the increased stability normally induced by Ca2+ binding to C2 domains [e.g. (Shao et al., 1997)]. This feature is unlikely to have biological significance but is interesting from the point of view of protein biophysics because ligand binding normally stabilizes proteins. Potential explanations for such a decrease include the possibility that the thermal denaturations of the E-Syt2 C2AB fragment do not reflect protein stability and are primarily governed by kinetic effects, or that Ca2+ binds more tightly to the unfolded protein than the folded protein (Cimmperman et al., 2008).
All these observations suggest that the tandem C2 domains of E-Syt2 and synaptotagmin-1 function by considerably different mechanisms. This is not surprising given the fact that the ESyt2 and synaptotagmin-1 C2 domains have different topologies and have highest structural similarity to C2 domains with topology II such as the intersectin 2 C2 domain and the Munc13-1 C2B domain. Nevertheless, it is also unclear to what extent the E-Syt2 C2 domains share functional properties with these C2 domains, which do not occur in tandem. For instance, the Munc13-1 C2B domain has an unusually long loop 3 that confers in part its phosphoinositide binding properties (Shin et al., 2010) and is not observed in the E-Syt2 C2 domains; the intersectin 2 C2 domain exhibits normal lengths in its top loops; and the E-Syt2 C2A and C2B domains have an unusually long loop 1 that is not shared by the intersectin 2 C2 domain and the Munc13-1 C2B domain. In the E-Syt2 C2A domain, loop 1 appears to undergo a considerable conformational change upon Ca2+ binding (Figures 4B and S2). While the physiological significance of this observation is unknown, it is interesting that part of the sequence of this loop is alternatively spliced (Min et al., 2007) and that splicing of short sequences can dramatically change the Ca2+-depedendent properties of C2 domains (Garcia et al., 2004). Clearly, much research will be required to learn more about E-Syts, about how they function and about their regulation, but the structures of the E-Syt2 C2AB fragment and its Ca2+-binding properties described here provide a foundation to rationalize future studies.
EXPERIMENTAL PROCEDURES
Protein expression and purification
The cDNA sequence of the human E-Syt2 C2AB fragment (residues 363–660) from a previously described construct (Min et al., 2007) was subcloned into a pGEX-KG vector (Guan and Dixon, 1991), to express the protein fused at the N-terminus with glutathione S-transferase (GST) and with a linker containing a TEV cleavage site. All mutants were obtained by site-directed mutagenesis using PCR and custom-designed primers. Proteins were expressed in E. coli BL21 (DE3) cells, inducing with 0.1 mM IPTG at 16 °C for 18 hours. Cells were resuspended in buffer containing 0.15 M NaCl, 0.01 M Tris pH 8.0, 0.1 mM EDTA, 2 mM DTT and 0.5 mM AEBSF. The fusion protein was isolated by affinity chromatography on glutathione-agarose and cleaved with TEV, and the cleaved protein was purified by gel filtration on a HiloadTM 16/60 SuperdexTM 75 column in 0.125 M NaCl, 0.025 M Tris pH 7.4, 0.5 mM TCEP. Uniform 15N labeling was achieved by growing the cells in M9 medium containing 15NH4Cl as nitrogen source. Selenomethionine(SeMet)-derivatized protein was overexpressed in BL21 (DE3) cells grown in M9 medium, adding SeMet and amino acids (Thr, Lys, Phe, Leu, Ile and Val; for inhibition of Met synthesis) 15 minutes before IPTG induction (Van Duyne et al., 1993). Yields were similar for all the proteins (ca. 6 mg of purified protein from one liter of culture). Proteins prepared for crystallization were kept at 4°C or −80°C in gel filtration buffer including 2.5% glycerol, until use.
Circular dichroism
CD spectra were acquired on an Aviv 62DS spectropolarimeter. Thermal denaturations were performed with 15 mM samples of E-Syt2 C2AB fragments in 0.04 M Tris pH7.4, 0.1 M NaCl, 0.1 mM EGTA with or without 1.1 mM CaCl2, monitoring the CD absorption at 215 nM. The fraction of unfolded protein f was calculated as f = (Iobs − If)/(Iu − If), where Iobs is the observed signal intensity, and If and Iu are the intensities corresponding to the folded and unfolded states, respectively. If and Iu were calculated by extrapolation of the linear regions of the denaturation curves.
NMR spectroscopy
All spectra were acquired at 25°C on a Varian INOVA 800 MHz spectrometer equipped with a cold probe. Samples of 0.16 mM E-Syt2 C2AB fragments were dissolved in 25 mM Tris pH 7.4, 125 mM NaCl, 0.5 mM TCEP and 5% D2O. One-dimensional (1D) 1H-NMR spectra of the initial samples were acquired before and after addition of 100 μM EDTA to ensure that there were negligible amounts of Ca2+ at the start of the titrations (Pan et al., 2011). 1H-15N TROSY-HSQC spectra were acquired after successive additions of different Ca2+ concentrations. The data were processed with NMRPipe (Delaglio et al., 1995) and analyzed with NMRView (Johnson and Blevins, 1994).
Crystallization and X-ray diffraction data collection
Crystals of the Ca2+-free E-Syt2 C2AB fragment were grown using the hanging-drop vapor-diffusion method from drops containing 1 μL protein (12.9 mg/ml) and 1 μL of reservoir solution (1.5 M LiSO4, 0.1 M NaAc pH 4.9), and equilibrated over reservoir solution at 20°C. Cryoprotection was performed by transferring the crystals to a final solution of 2.25 M LiCl, 0.125 M NaCl, 0.1 M sodium acetate pH 4.9, 2.5% glycerol and 25% ethylene glycol, and were flash-cooled in liquid nitrogen. E-Syt2 C2AB fragment crystals exhibited the symmetry of space group P43 with cell dimensions of a = 62.30 Å, c = 226.40 Å, contained two molecules per asymmetric unit, and diffracted to a minimum Bragg spacing (dmin) of 2.10 Å when exposed to synchrotron radiation. The SeMet-derivatized E-Syt2 C2AB fragment was crystallized in a similar manner to the native protein, with reservoir solution containing 1.6 M Li2SO4, 0.1 M NaAc pH 5.2, and 0.5 mM TCEP. SeMet E-Syt2 C2AB fragment crystals were cryo-protected with reservoir solution supplemented with 15% ethylene glycol, and were isomorphous to the native crystals but diffracted to a dmin of 2.33 Å.
The Ca2+-bound E-Syt2 C2AB fragment (dissolved in 12.4 mg/ml in 0.125 M NaCl, 0.025 M Tris pH 7.4, 0.5 mM TCEP and 2 mM CaCl2) was crystallized at 20°C using the hanging-drop vapor-diffusion method from drops set up in a 1:1.5 ratio with a reservoir solution containing 4% PEG 8,000, 0.1 M Hepes pH 8.0. The crystals were cryo-protected with solution of 0.1 M Hepes pH8.0, 0.125 M NaCl, 2 mM CaCl2, 0.5 mM TCEP, 6% PEG 8,000 and 40% ethylene glycol. Ca2+-bound E-Syt2 C2AB fragment crystals exhibited the symmetry of space group C2 with cell dimensions of a = 95.84 Å, b= 53.19 Å, c = 85.28 Å, β = 99.958° and contained one molecule per asymmetric unit. The crystals diffracted anisotropically to a minimum Bragg spacing (dmin) of 2.55 Å when exposed to synchrotron radiation. Data were indexed, integrated and scaled using the HKL-3000 program package (Minor et al., 2006). Data collection statistics are provided in Table 1.
Phase determination and structure refinement
Phases for the Ca2+-free E-Syt2 C2AB fragment were obtained from a single-wavelength anomalous dispersion experiment using SeMet-labeled protein with data to a dmin of 2.33 Å. Ten selenium sites were located using the program SHELXD (Schneider and Sheldrick, 2002) and phases were refined with the program Mlphare (Otwinowsky, 1991), resulting in an overall figure-of-merit of 0.16 for data between 43.3 and 2.33 Å. Phases were further improved by density modification with two-fold non-crystallographic symmetry averaging with the program Parrot(4) (Cowtan, 2010) resulting in a figure-of-merit of 0.57. An initial model containing 97% of all the E-Syt2 C2AB fragment residues was automatically generated in the program Buccaneer (Cowtan, 2006).
Additional residues of the E-Syt2 C2AB fragment were manually modeled in the programs O (Jones et al., 1991) and Coot (Emsley et al., 2010). Refinement was performed to a resolution of 2.10 Å using the program Phenix (Afonine et al., 2010) with a random 5% of all data set aside for an Rfree calculation. The current model contains two E-Syt2 C2AB fragment monomers; included are residues 363–551 and 557–659 of monomer A, residues 364–402, 410–549 and 557–659 of monomer B and 146 solvent atoms. The Rwork is 0.207, and the Rfree is 0.247. A Ramachandran plot generated with Molprobity (Chen et al., 2010) indicated that 95.7% of all protein residues are in the most favored regions, and 0.2% (one residue) in the disallowed regions. This residue is located in a surface loop with weak electron density.
Phases for the Ca2+-bound E-Syt2 C2AB fragment were obtained via molecular replacement in the program Phaser (McCoy et al., 2007) using a search model constructed from the coordinates of monomer A of the Ca2+-free E-Syt2 C2AB fragment with the first 20 N-terminal amino acids removed. Model building and refinement was performed as described above, with the following modification: due to the high anisotropy and lower resolution of the data, monomer A of the Ca2+-free E-Syt2 C2AB fragment was used as a reference model to provide an additional source of geometry restraints. The current model contains one E-Syt2 C2AB fragment monomer, three Ca2+ ions and four waters complexed to the calciums. Included are E-Syt2 C2AB fragment residues 380–525, 532–550, 556–659, the Rwork is 0.222, and the Rfree is 0.287. A Ramachandran plot generated with Molprobity indicated that 94.7% of all protein residues are in the most favored regions, and 1.5% (three residues) in the disallowed regions. These residues are located in regions with poorly defined electron density. Phasing and model refinement statistics are provided in Table 1. The structures of the Ca2+-free and Ca2+-bound E-Syt2 C2AB fragment have been deposited in the PDB with accession codes 4NPJ and 4NPK, respectively.
Supplementary Material
Highlights
The crystal structure of the tandem C2AB domains of E-Syt2 has been determined.
The structure has a V-shape with a rigid orientation of the two C2 domains.
The E-Syt2 C2A domain binds four Ca2+ ions while the C2B domain does not bind Ca2+.
E-Syts most likely have an as yet unidentified Ca2+-dependent function.
ACKNOWLEDGMENTS
The structures shown in this report are derived from work performed on beamlines 19-BM and 19-ID at Argonne National Laboratory, Structural Biology Center at the Advanced Photon Source. Argonne is operated by UChicago Argonne, LLC, for the U.S. Department of Energy, Office of Biological and Environmental Research under contract DE-AC02-06CH11357. This work was supported by grant I-1304 from the Welch Foundation (to JR) and NIH grants NS040944 (to JR) and P50-MH086403 (to TCS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Afonine PV, Mustyakimov M, Grosse-Kunstleve RW, Moriarty NW, Langan P, Adams PD. Joint X-ray and neutron refinement with phenix.refine. Acta Crystallogr. D. Biol. Crystallogr. 2010;66:1153–1163. doi: 10.1107/S0907444910026582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arac D, Chen X, Khant HA, Ubach J, Ludtke SJ, Kikkawa M, Johnson AE, Chiu W, Sudhof TC, Rizo J. Close membrane-membrane proximity induced by Ca(2+)-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat. Struct. Mol. Biol. 2006;13:209–217. doi: 10.1038/nsmb1056. [DOI] [PubMed] [Google Scholar]
- Carrasco S, Meyer T. STIM proteins and the endoplasmic reticulum-plasma membrane junctions. Annu. Rev. Biochem. 2011;80:973–1000. doi: 10.1146/annurev-biochem-061609-165311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman ER, Davis AF. Direct interaction of a Ca2+-binding loop of synaptotagmin with lipid bilayers. J. Biol. Chem. 1998;273:13995–14001. doi: 10.1074/jbc.273.22.13995. [DOI] [PubMed] [Google Scholar]
- Chen VB, Arendall WB, III, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D. Biol. Crystallogr. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi UB, Strop P, Vrljic M, Chu S, Brunger AT, Weninger KR. Single-molecule FRET-derived model of the synaptotagmin 1-SNARE fusion complex. Nat. Struct. Mol. Biol. 2010;17:318–324. doi: 10.1038/nsmb.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimmperman P, Baranauskiene L, Jachimoviciute S, Jachno J, Torresan J, Michailoviene V, Matuliene J, Sereikaite J, Bumelis V, Matulis D. A quantitative model of thermal stabilization and destabilization of proteins by ligands. Biophys. J. 2008;95:3222–3231. doi: 10.1529/biophysj.108.134973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowtan K. Recent developments in classical density modification. Acta Crystallogr. D. Biol. Crystallogr. 2010;66:470–478. doi: 10.1107/S090744490903947X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowtan K. The Buccaneer software for automated model building. 1. Tracing protein chains. Acta Crystallogr. D. Biol. Crystallogr. 2006;62:1002–1011. doi: 10.1107/S0907444906022116. [DOI] [PubMed] [Google Scholar]
- Creutz CE, Snyder SL, Schulz TA. Characterization of the yeast tricalbins: membrane-bound multi-C2-domain proteins that form complexes involved in membrane trafficking. Cell Mol. Life Sci. 2004;61:1208–1220. doi: 10.1007/s00018-004-4029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H, Shen N, Arac D, Rizo J. A Quaternary SNARE-Synaptotagmin-Ca(2+)-Phospholipid Complex in Neurotransmitter Release. J. Mol. Biol. 2007;367:848–863. doi: 10.1016/j.jmb.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H, Shin OH, Machius M, Tomchick DR, Sudhof TC, Rizo J. Structural basis for the evolutionary inactivation of Ca2+ binding to synaptotagmin 4. Nat. Struct. Mol. Biol. 2004;11:844–849. doi: 10.1038/nsmb817. [DOI] [PubMed] [Google Scholar]
- Davletov BA, Sudhof TC. A single C2 domain from synaptotagmin I is sufficient for high affinity Ca2+/phospholipid binding. J. Biol. Chem. 1993;268:26386–26390. [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. Nmrpipe - A Multidimensional Spectral Processing System Based on Unix Pipes. Journal of Biomolecular Nmr. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- Elbaz Y, Schuldiner M. Staying in touch: the molecular era of organelle contact sites. Trends Biochem. Sci. 2011;36:616–623. doi: 10.1016/j.tibs.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr. D. Biol. Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essen LO, Perisic O, Cheung R, Katan M, Williams RL. Crystal structure of a mammalian phosphoinositide-specific phospholipase C delta. Nature. 1996;380:595–602. doi: 10.1038/380595a0. [DOI] [PubMed] [Google Scholar]
- Fernandez I, Arac D, Ubach J, Gerber SH, Shin O, Gao Y, Anderson RG, Sudhof TC, Rizo J. Three-dimensional structure of the synaptotagmin 1 c(2)b-domain. Synaptotagmin 1 as a phospholipid binding machine. Neuron. 2001;32:1057–1069. doi: 10.1016/s0896-6273(01)00548-7. [DOI] [PubMed] [Google Scholar]
- Fernandez I, Ubach J, Dulubova I, Zhang X, Sudhof TC, Rizo J. Three-dimensional structure of an evolutionarily conserved N-terminal domain of syntaxin 1A. Cell. 1998;94:841–849. doi: 10.1016/s0092-8674(00)81742-0. [DOI] [PubMed] [Google Scholar]
- Fernandez-Chacon R, Konigstorfer A, Gerber SH, Garcia J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Sudhof TC. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- Fuson KL, Montes M, Robert JJ, Sutton RB. Structure of human synaptotagmin 1 C2AB in the absence of Ca2+ reveals a novel domain association. Biochemistry. 2007;46:13041–13048. doi: 10.1021/bi701651k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J, Gerber SH, Sugita S, Sudhof TC, Rizo J. A conformational switch in the Piccolo C2A domain regulated by alternative splicing. Nat. Struct. Mol. Biol. 2004;11:45–53. doi: 10.1038/nsmb707. [DOI] [PubMed] [Google Scholar]
- Giordano F, Saheki Y, Idevall-Hagren O, Colombo SF, Pirruccello M, Milosevic I, Gracheva EO, Bagriantsev SN, Borgese N, De CP. PI(4,5)P2-Dependent and Ca(2+)-Regulated ER-PM Interactions Mediated by the Extended Synaptotagmins. Cell. 2013;153:1494–1509. doi: 10.1016/j.cell.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan KL, Dixon JE. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal. Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Guan R, Dai H, Tomchick DR, Dulubova I, Machius M, Sudhof TC, Rizo J. Crystal structure of the RIM1alpha C2B domain at 1.7 A resolution. Biochemistry. 2007;46:8988–8998. doi: 10.1021/bi700698a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick DZ, Kuo W, Huang H, Schwieters CD, Ellena JF, Cafiso DS. Solution and membrane-bound conformations of the tandem C2A and C2B domains of synaptotagmin 1: Evidence for bilayer bridging. J. Mol. Biol. 2009;390:913–923. doi: 10.1016/j.jmb.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm L, Park J. DaliLite workbench for protein structure comparison. Bioinformatics. 2000;16:566–567. doi: 10.1093/bioinformatics/16.6.566. [DOI] [PubMed] [Google Scholar]
- Holm L, Sander C. Protein structure comparison by alignment of distance matrices. J. Mol. Biol. 1993;233:123–138. doi: 10.1006/jmbi.1993.1489. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Blevins RA. Nmr View - A Computer-Program for the Visualization and Analysis of Nmr Data. Journal of Biomolecular Nmr. 1994;4:603–614. doi: 10.1007/BF00404272. [DOI] [PubMed] [Google Scholar]
- Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved Methods for Building Protein Models in Electron-Density Maps and the Location of Errors in These Models. Acta Crystallographica Section A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kopec KO, Alva V, Lupas AN. Homology of SMP domains to the TULIP superfamily of lipid-binding proteins provides a structural basis for lipid exchange between ER and mitochondria. Bioinformatics. 2010;26:1927–1931. doi: 10.1093/bioinformatics/btq326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine T, Loewen C. Inter-organelle membrane contact sites: through a glass, darkly. Curr. Opin. Cell Biol. 2006;18:371–378. doi: 10.1016/j.ceb.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Lu J, Machius M, Dulubova I, Dai H, Sudhof TC, Tomchick DR, Rizo J. Structural Basis for a Munc13-1 Homodimer to Munc13-1/RIM Heterodimer Switch. PLoS. Biol. 2006;4:e192. doi: 10.1371/journal.pbio.0040192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackler JM, Drummond JA, Loewen CA, Robinson IM, Reist NE. The C(2)B Ca(2+)-binding motif of synaptotagmin is required for synaptic transmission in vivo. Nature. 2002;418:340–344. doi: 10.1038/nature00846. [DOI] [PubMed] [Google Scholar]
- Manford AG, Stefan CJ, Yuan HL, Macgurn JA, Emr SD. ER-to-plasma membrane tethering proteins regulate cell signaling and ER morphology. Dev. Cell. 2012;23:1129–1140. doi: 10.1016/j.devcel.2012.11.004. [DOI] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J. Appl. Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min SW, Chang WP, Sudhof TC. E-Syts, a family of membranous Ca2+-sensor proteins with multiple C2 domains. Proc. Natl. Acad. Sci. U. S. A. 2007;104:3823–3828. doi: 10.1073/pnas.0611725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor W, Cymborowski M, Otwinowski Z, Chruszcz M. HKL-3000: the integration of data reduction and structure solution--from diffraction images to an initial model in minutes. Acta Crystallogr. D. Biol. Crystallogr. 2006;62:859–866. doi: 10.1107/S0907444906019949. [DOI] [PubMed] [Google Scholar]
- Nicholls A, Sharp KA, Honig B. Protein Folding and Association - Insights from the Interfacial and Thermodynamic Properties of Hydrocarbons. Proteins-Structure Function and Genetics. 1991;11:281–296. doi: 10.1002/prot.340110407. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z. Maximum likelihood refinement of heavy atom paramaters. In: Wolf W, Evans PR, Leslie AGW, editors. Proceedings of the CCP4 Study Weekend. Science & Engineering Research Council; Cambridge, U.K.: 1991. [Google Scholar]
- Pan YR, Lou YC, Seven AB, Rizo J, Chen C. NMR structure and calcium-binding properties of the tellurite resistance protein TerD from Klebsiella pneumoniae. J. Mol. Biol. 2011;405:1188–1201. doi: 10.1016/j.jmb.2010.11.041. [DOI] [PubMed] [Google Scholar]
- Rizo J, Rosen MK, Gardner KH. Enlightening molecular mechanisms through study of protein interactions. J. Mol. Cell Biol. 2012;4:270–283. doi: 10.1093/jmcb/mjs036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizo J, Rosenmund C. Synaptic vesicle fusion. Nat. Struct. Mol. Biol. 2008;15:665–674. doi: 10.1038/nsmb.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizo J, Sudhof TC. C2-domains, structure and function of a universal Ca2+-binding domain. J. Biol. Chem. 1998;273:15879–15882. doi: 10.1074/jbc.273.26.15879. [DOI] [PubMed] [Google Scholar]
- Schneider TR, Sheldrick GM. Substructure solution with SHELXD. Acta Crystallogr. D. Biol. Crystallogr. 2002;58:1772–1779. doi: 10.1107/s0907444902011678. [DOI] [PubMed] [Google Scholar]
- Schulz TA, Creutz CE. The tricalbin C2 domains: lipid-binding properties of a novel, synaptotagmin-like yeast protein family. Biochemistry. 2004;43:3987–3995. doi: 10.1021/bi036082w. [DOI] [PubMed] [Google Scholar]
- Seven AB, Brewer KD, Shi L, Jiang QX, Rizo J. Prevalent mechanism of membrane bridging by synaptotagmin-1. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E3243–E3252. doi: 10.1073/pnas.1310327110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X, Davletov BA, Sutton RB, Sudhof TC, Rizo J. Bipartite Ca2+-binding motif in C2 domains of synaptotagmin and protein kinase C. Science. 1996;273:248–251. doi: 10.1126/science.273.5272.248. [DOI] [PubMed] [Google Scholar]
- Shao X, Li C, Fernandez I, Zhang X, Sudhof TC, Rizo J. Synaptotagmin-syntaxin interaction: the C2 domain as a Ca2+-dependent electrostatic switch. Neuron. 1997;18:133–142. doi: 10.1016/s0896-6273(01)80052-0. [DOI] [PubMed] [Google Scholar]
- Shin OH, Lu J, Rhee JS, Tomchick DR, Pang ZP, Wojcik SM, Camacho-Perez M, Brose N, Machius M, Rizo J, Rosenmund C, Sudhof TC. Munc13 C2B domain is an activity-dependent Ca2+ regulator of synaptic exocytosis. Nat. Struct. Mol. Biol. 2010;17:280–288. doi: 10.1038/nsmb.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin OH, Xu J, Rizo J, Sudhof TC. Differential but convergent functions of Ca2+ binding to synaptotagmin-1 C2 domains mediate neurotransmitter release. Proc. Natl. Acad. Sci. U. S. A. 2009;106:16469–16474. doi: 10.1073/pnas.0908798106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan CJ, Manford AG, Emr SD. ER-PM connections: sites of information transfer and inter-organelle communication. Curr. Opin. Cell Biol. 2013;25:434–442. doi: 10.1016/j.ceb.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RB, Davletov BA, Berghuis AM, Sudhof TC, Sprang SR. Structure of the first C2 domain of synaptotagmin I: a novel Ca2+/phospholipid-binding fold. Cell. 1995;80:929–938. doi: 10.1016/0092-8674(95)90296-1. [DOI] [PubMed] [Google Scholar]
- Sutton RB, Ernst JA, Brunger AT. Crystal structure of the cytosolic C2A-C2B domains of synaptotagmin III. Implications for Ca(+2)-independent snare complex interaction. J. Cell Biol. 1999;147:589–598. doi: 10.1083/jcb.147.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmay A, Prinz WA. Lipid transfer and signaling at organelle contact sites: the tip of the iceberg. Curr. Opin. Cell Biol. 2011;23:458–463. doi: 10.1016/j.ceb.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmay A, Prinz WA. A conserved membrane-binding domain targets proteins to organelle contact sites. J. Cell Sci. 2012;125:49–58. doi: 10.1242/jcs.085118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubach J, Garcia J, Nittler MP, Sudhof TC, Rizo J. Structure of the Janus-faced C2B domain of rabphilin. Nat. Cell Biol. 1999;1:106–112. doi: 10.1038/10076. [DOI] [PubMed] [Google Scholar]
- Ubach J, Zhang X, Shao X, Sudhof TC, Rizo J. Ca2+ binding to synaptotagmin: how many Ca2+ ions bind to the tip of a C2-domain? EMBO J. 1998;17:3921–3930. doi: 10.1093/emboj/17.14.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duyne GD, Standaert RF, Karplus PA, Schreiber SL, Clardy J. Atomic structures of the human immunophilin FKBP-12 complexes with FK506 and rapamycin. J. Mol. Biol. 1993;229:105–124. doi: 10.1006/jmbi.1993.1012. [DOI] [PubMed] [Google Scholar]
- Vrljic M, Strop P, Ernst JA, Sutton RB, Chu S, Brunger AT. Molecular mechanism of the synaptotagmin-SNARE interaction in Ca2+-triggered vesicle fusion. Nat. Struct. Mol. Biol. 2010;17:325–331. doi: 10.1038/nsmb.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Craig TK, Shin OH, Li L, Brautigam CA, Tomchick DR, Sudhof TC, Rosenmund C, Rizo J. Structural and Mutational Analysis of Functional Differentiation between Synaptotagmins-1 and -7. PLoS. ONE. 2010;5 doi: 10.1371/journal.pone.0012544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Rizo J, Sudhof TC. Mechanism of phospholipid binding by the C2A-domain of synaptotagmin I. Biochemistry. 1998;37:12395–12403. doi: 10.1021/bi9807512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.