Abstract
Background
Mutations in LRRK2 are a common cause of familial Parkinson's disease. However, the mechanisms through which LRRK2 mutations contribute to neurodegeneration are poorly understood.
Objective
We investigated the effects of WT, G2019S, R1441C and kinase dead (KD) LRRK2 across multiple different cellular compartments in order to gain insight into the breadth of LRRK2 effects on cellular function.
Methods
Nematodes expressing lgg-1::RFP, hsp1::GFP, hsp4::GFP and hsp6::GFP were crossed to nematode lines expressing WT, G2019S, R1441C or KD LRRK2.
Results
We observed that G2019S and R1441C LRRK2 inhibited autophagy, while WT, G2019S and R1441C LRRK2 increased the response of the mitochondrial hsp6 reporter to stress. The response of the hsp reporters under basal conditions was more nuanced.
Conclusion
These results support a putative role of LRRK2 in the autophagic and mitochondrial systems.
Keywords: Autophagy, Parkinson's disease, heat shock protein, mitochondria, endoplasmic reticulum, stress
Mutations in LRRK2 are the most common cause of familial Parkinsonism, and one the most common genes mutated in sporadic Parkinson's disease. Most cases of LRRK2-associated disease exhibit accumulations of aggregated α-synuclein, forming Lewy bodies and Lewy neurites. Some cases associated with LRRK2 mediated disease exhibit aggregated tau and TDP-43 pathology.
An increasing number of studies suggest that mutant LRRK2 interferes with autophagic function. Chu and colleagues demonstrated that G2019S (GS) LRRK2 interferes with autophagy. Neurons over-expressing GS LRRK2 exhibit abnormally large autophagic vesicles as well as an associated decrease in neurite length that could be reversed by knockdown of essential autophagic genes, including LC3 or Atg7 [1]. Knockout of LRRK2 leads to renal degeneration, which is associated with increases in the autophagic protein, LC3, and the accumulation of aggregated α-synuclein [2]. LRRK2 is also degraded by cell-mediated autophagy, while mutant LRRK2 inhibits cell mediated autophagy, leading to reduced degradation of α-synuclein [3]. Together, these studies point to a strong role for LRRK2 in autophagy.
The actions of LRRK2 appear to extend beyond autophagy. LRRK2 associates with many membranes, and appears to modulate membrane associated function [4]. LRRK2 modulates vesicular uptake at the synapse, in part by modulating EndoA phosphorylation [5, 6]. LRRK2 also associates with a number of GTPases, each of which regulates particular cellular functions. We demonstrated that LRRK2 binds to rac-1, and modulates neurite outgrowth via rac-1 [7]. Other studies show that LRRK2 interacts with rab7L1, which regulates golgi function [8, 9]. Finally, prior work suggests that LRRK2 regulates mitochondrial, endoplasmic reticular (ER) function and autophagic system [10-15]. In an effort to gain a broader understanding of how LRRK2 affects functioning of membrane associated organelles, we investigated the regulation of mitochondria, the endoplasmic reticulum and the autophagosome, using fluorescence reporters.
Methods
C. elegans hsp lines were obtained from CGC. The LRRK2 lines were generated as previously described [10]. C. elegans strains were grown at 20°C unless otherwise indicated. All other methods were performed as described previously [10, 16].
Results
To investigate how LRRK2 affects autophagy, we are in the process of developing a reporter consisting of the nematode homolog of LC3 (lgg-1) coupled to RFP. We generated a lgg-1::RFP construct in N2 nematodes driven by the dopamine specific dat-1 promoter. Validation of the reporter construct is reported in a manuscript that is currently under review elsewhere. The lgg-1::RFP reporter is responsive to known autophagic modulators. Knockdown of ATG-5 reduces fluorescent puncta, while treatment with bafilomycin increase fluorescence from the lgg-1::RFP construct [17]. Together, these data suggest that the fluorescence from the reporter reflects autophagic flux.
Next, we crossed the dat-1::lgg-1::RFP reporter with C. elegans lines expressing WT, G2019S (GS) or R1441C (RC) LRRK2, using the LRRK2 lines that had been previously described by our group [10]. We report here preliminary findings indicating the levels of the lgg-1::RFP report are modulated by LRRK2. In particular, we observe that GS and RC LRRK2 increase levels of the lgg-1::RFP reporter, while WT LRRK2 reduces lgg-1::RFP levels (Fig. 1 A). These results suggest that GS and RC LRRK2 reduces autophagic flux, while WT LRRK2 increases autophagic flux. Further experiments exploring the system are described in a separate paper, which is under review [17].
Fig. 1.
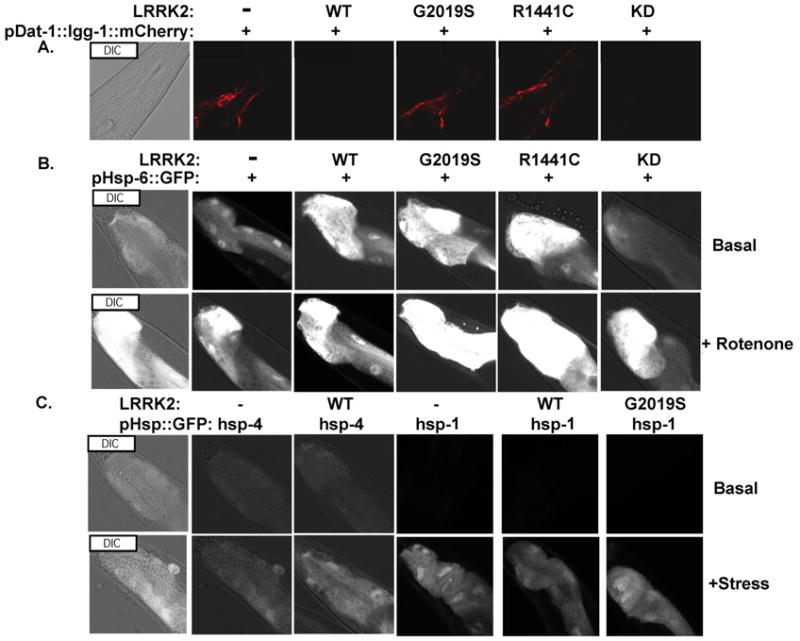
Chromosomally integrated reporters, which reflect autophagic activity in in the CEP dopaminergic neurons and the stress responses elicited in mitochondria, ER and cytoplasm (b, c) with the Distal Tip Cells (DTC) in the posterior part of the nematode a) The pDAT::lgg-1::mCherry reporter: Autophagic fluorescence from day 5 adults. Autophagic puncta are seen in the cell body of CEP neurons and nerve ring in the nematode's head region. WT and KD (kinase dead) LRRK2 reduce mCherry fluorescence, reflecting increased autophagic flux. (b) The pHSP6::GFP reporter: Mitochondrial HSP 70 response in DTC cell with basal and induced (250nM Rotenone, 24 hrs) conditions. WT, G2019S and R1441C LRRK2 increase basal activity. (c) The pHSP4::GFP and pHSP1::GFP reporters: stress responses from ER compartment (hsp-4) and cytoplasmic compartment (hsp-70) from DTC cells with basal and induced conditions with 2.5 mg/ml Tunicamycin treatment and heat shocking at 330C respectively. LRRK2 constructs did not affect fluorescence of these reporters.
Next, we examined the effects of LRRK2 on mitochondrial, endoplasmic reticular (ER) and cytoplasmic stress responses. We used three well-characterized reporters: hsp6::GFP (the nematode homologue of the mammalian mitochondrial hsp-60 protein), hsp4::GFP (the nematode homologue of the mammalian BiP protein), and hsp1::GFP (the nematode homologue of the mammalian cytoplasmic hsp-70) [16]. Each reporter was crossed with nematodes expressing WT, GS or RC LRRK2, and the response of the fluorescent reporter examined under basal or stressed conditions. Under basal growth conditions, WT, GS and RC LRRK2 all increased hsp6::GFP fluorescence. No significant effect was observed on hsp4::GFP fluorescence (Fig. 1B); no fluorescence was observed for the hsp1::GFP or hsp1::GFP/LRRK2 KD lines under basal conditions. As expected, stresses selective for each cell compartment (heat shock, 33 ° C, 45 min; rotenone, 250 nM, 48 hrs; or tunicamycin, 2.5 μg/ml, 48 hrs, each starting at L2) (Fig. 1C & D). However, nematodes expressing WT GS or RC LRRK2 exhibited strongly increased fluorescence for the lines co-expressing hsp-6. LRRK2 did not appear to affect the stress responses for hsp4 or hsp1 (Fig. 1C & D).
Discussion
We used transgenic lines of C. elegans to compare the actions of LRRK2 on four different cellular compartments: the autophagosome, the mitochondria, the endoplasmic reticulum and the cytoplasm. We observed that WT LRRK2 reduced lgg-1::RFP fluorescence, while GS and RC LRRK2 increased lgg-1 fluorescence; these LRRK2-dependent differences suggest that WT LRRK2 increases autophagic flux while GS and RC LRRK2 decrease autophagic flux. The inhibition of autophagy by GS and RC LRRK2 is consistent with observations by others [1, 18]. Cuervo and colleagues also report that GS LRRK2 inhibits autophagy, but they focus on cell mediated autophagy, which is a process that is not present in C. elegans, since nematodes lack a homolog for LAMP2a [3]. Although GS LRRK2 consistently inhibits autophagy in multiple studies, the effects of WT LRRK2 appear to vary depending on the study. We observe that LRRK2 stimulates autophagy. However, other work from our laboratory suggests that the effects of WT LRRK2 vary depending on whether or not α-synuclein is present; co-expressing WT LRRK2 with α-synuclein produces a modest age-dependent inhibition of autophagy [17]. Both Chu and Cuervo's groups observe that WT LRRK2 modestly inhibits autophagy, while Alegre-Abarrategui observe that WT LRRK2 increases autophagy [1, 3, 18]. Thus, the effects of WT LRRK2 on autophagy appear to be modest and depend on the system evaluated. However, GS LRRK2 has been shown to robustly inhibit autophagy in multiple different studies. Autophagic inhibition would interfere with degradation of protein aggregates, which provides a direct mechanism through which the GS mutation might cause neurodegenerative disease.
LRRK2 has also been reported to affect other organelles [4]. Feng and colleagues reported that GS LRRK2 increases vulnerability to ER stress, and multiple groups observe that GS LRRK2 interferes with mitochondrial function [10, 14, 19, 20]. In the current study, we observed that WT, GS and RC LRRK2 increase the response of hsp6 reporters to stress. This points to the importance of LRRK2 to mitochondrial and autophagic functions, which suggests that LRRK2 might exhibit mitochondrial directed actions related to those observed for parkin and PINK1.
Acknowledgments
The work was supported by a grant to BW (NIH NS06082). Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
References
- 1.Plowey ED, et al. Role of autophagy in G2019S-LRRK2-associated neurite shortening in differentiated SH-SY5Y cells. J Neurochem. 2008;105:1048–56. doi: 10.1111/j.1471-4159.2008.05217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tong Y, et al. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of alpha-synuclein, and apoptotic cell death in aged mice. Proc Natl Acad Sci U S A. 2010;107:9879–84. doi: 10.1073/pnas.1004676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orenstein SJ, et al. Interplay of LRRK2 with chaperone-mediated autophagy. Nat Neurosci. 2013 doi: 10.1038/nn.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biskup S, et al. Genes associated with Parkinson syndrome. J Neurol. 2008;255(Suppl 5):8–17. doi: 10.1007/s00415-008-5005-2. [DOI] [PubMed] [Google Scholar]
- 5.Shin N, et al. LRRK2 regulates synaptic vesicle endocytosis. Exp Cell Res. 2008;314:2055–65. doi: 10.1016/j.yexcr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Matta S, et al. LRRK2 controls an EndoA phosphorylation cycle in synaptic endocytosis. Neuron. 2012;75:1008–21. doi: 10.1016/j.neuron.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 7.Chan D, et al. Rac1 protein rescues neurite retraction caused by G2019S leucine-rich repeat kinase 2 (LRRK2) J Biol Chem. 2011;286:16140–9. doi: 10.1074/jbc.M111.234005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacLeod DA, et al. RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson's disease risk. Neuron. 2013;77:425–39. doi: 10.1016/j.neuron.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dodson MW, et al. Roles of the Drosophila LRRK2 homolog in Rab7-dependent lysosomal positioning. Hum Mol Genet. 2012;21:1350–63. doi: 10.1093/hmg/ddr573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saha S, et al. LRRK2 modulates vulnerability to mitochondrial dysfunction in Caenorhabditis elegans. J Neurosci. 2009;29:9210–8. doi: 10.1523/JNEUROSCI.2281-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Domenico F, et al. Redox Proteomics Analyses of the Influence of Co-Expression of Wild-Type or Mutated LRRK2 and Tau on C. elegans Protein Expression and Oxidative Modification: Relevance to Parkinson Disease. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2011.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferree A, et al. Regulation of physiologic actions of LRRK2: focus on autophagy. Neurodegener Dis. 2012;10:238–41. doi: 10.1159/000332599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samann J, et al. Caenorhabditits elegans LRK-1 and PINK-1 act antagonistically in stress response and neurite outgrowth. J Biol Chem. 2009;284:16482–91. doi: 10.1074/jbc.M808255200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan Y, et al. Dysregulated LRRK2 signaling in response to endoplasmic reticulum stress leads to dopaminergic neuron degeneration in C. elegans. PLoS One. 2011;6:e22354. doi: 10.1371/journal.pone.0022354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao C, et al. LRRK2-mediated neurodegeneration and dysfunction of dopaminergic neurons in a Caenorhabditis elegans model of Parkinson's disease. Neurobiol Dis. 2010;40:73–81. doi: 10.1016/j.nbd.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoneda T, et al. Compartment-specific perturbation of protein handling activates genes encoding mitochondrial chaperones. J Cell Sci. 2004;117:4055–66. doi: 10.1242/jcs.01275. [DOI] [PubMed] [Google Scholar]
- 17.Saha S, et al. Mutations in LRRK2 Potentiate Age-Related Impairment of Autophagic Flux. 2013 doi: 10.1186/s13024-015-0022-y. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alegre-Abarrategui J, et al. LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum Mol Genet. 2009;18:4022–34. doi: 10.1093/hmg/ddp346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niu J, et al. Leucine-Rich Repeat Kinase 2 (LRRK2) Disturbs Mitochondrial Dynamics via Dynamin-Like Protein (DLP1) J Neurochem. 2012 doi: 10.1111/j.1471-4159.2012.07809.x. [DOI] [PubMed] [Google Scholar]
- 20.Cherra SJ, 3rd, et al. Mutant LRRK2 elicits calcium imbalance and depletion of dendritic mitochondria in neurons. Am J Pathol. 2012;182:474–84. doi: 10.1016/j.ajpath.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]


