Abstract
Background
Exposure to the fungal allergen Alternaria alternata as well as ryegrass pollen has been implicated in severe asthma symptoms during thunderstorms. We have previously shown that Alternaria extract induces innate type 2 lung inflammation in mice. We hypothesized that the innate eosinophilic response to Alternaria extract may enhance lung inflammation induced by ryegrass.
Methods
Mice were sensitized to ryegrass allergen and administered a single challenge with Alternaria alternata extract before or after final ryegrass challenges. Levels of BAL eosinophils, neutrophils, Th2 cells, innate lymphoid cells (ILC2), IL-5 and IL-13 as well as inflammation and mucus were assessed.
Results
Mice receiving ryegrass sensitization and challenge developed an eosinophilic lung response. A single challenge with Alternaria extract given 3 days before or 3 days after ryegrass challenges resulted in increased eosinophils, peribronchial inflammation and mucus production in the airway compared with ryegrass only challenges. Type 2 innate lymphoid cell (ILC2) and Th2 cell recruitment to the airway was increased after Alternaria extract exposure in ryegrass challenged mice. Innate challenges with Alternaria extract induced BAL eosinophilia, Th2 cell recruitment as well as ILC2 expansion and proliferation.
Conclusions
A single exposure of Alternaria extract in ryegrass sensitized and challenged mice enhances the type-2 lung inflammatory response including airway eosinophilia, peribronchial infiltrate, and mucus production possibly through Th2 cell recruitment and ILC2 expansion. If translated to humans, exposures to both grass pollen and Alternaria may be a potential cause of thunderstorm-related asthma.
Keywords: Alternaria, asthma, thunderstorm asthma, ryegrass
Introduction
The inflammatory response in allergic asthma has largely been characterized by a dysregulated adaptive immune response involving differentiation of Th2 cells that produce IL-4, IL-5, and IL-13 resulting in IgE production, eosinophil infiltration and mucus production in the lungs [1]. Though much has been learned about pathogenic adaptive Th2 cell responses over the past few decades, recent studies have demonstrated that innate type-2 responses also contribute to tissue eosinophilia and mucus production independent of the presence of adaptive immune cells [2-4]. We recently reported that a single administration of the fungal allergen extract Alternaria alternata given to unsensitized mice results in airway eosinophilia and increased IL-5 levels and is present in RAG2 deficient mice that lack B and T cells [5,6]. Further, we have shown that IL-5 producing innate lymphoid cells (ILC2) are rapidly activated after Alternaria extract challenge and contribute to lung eosinophilia [6]. This suggests that the adaptive Th2 paradigm may not completely explain type 2 responses in asthma and that innate immune activation by allergens such as Alternaria may contribute to eosinophilic airway inflammation and mucus production.
Though asthma has many clinical, physiologic, and immunologic phenotypes, the majority of asthmatics have allergic triggers. Exposure to the fungal allergen Alternaria alternata is specifically associated with severe asthma including life-threatening exacerbations as well as thunderstorm-related asthma [7-13]. Thunderstorm asthma is a well-documented clinical phenomenon in which patients develop severe asthma symptoms in epidemics during a thunderstorm outflow in which pollens and fungi are swept up and concentrated at high levels [14,15]. This leads to an abundance of allergen including formation of respirable particles of pollen (<10 μm) and fragmented fungal spores of Alternaria alternata [10]. IgE sensitization to grass pollen allergens including ryegrass is a risk factor for patients to develop asthma exacerbations during thunderstorms suggesting a link between adaptive Th2 responses to grass pollen and thunderstorm asthma [16,17]. Little is known about the underlying immune mechanisms in thunderstorm asthma beyond one report that demonstrated increased sputum eosinophilia and IL-5+ cells in the lungs of symptomatic asthmatics during thunderstorms suggesting a potentiated type 2 response [17]. The clinical associations between Alternaria, grass pollen exposure and epidemics of severe asthma during thunderstorms are intriguing but how these inhaled allergens might lead to increased airway inflammatory responses is unclear.
We hypothesized that an innate type 2 response induced by Alternaria extract may enhance eosinophilic lung inflammation induced by ryegrass pollen. We demonstrate that a single challenge with Alternaria extract enhances lung eosinophilia, peribronchial infiltration, and epithelial mucus production as well as Th2 cell and ILC2 recruitment in ryegrass challenged WT mice. Our data suggests that the combination of innate and adaptive responses to allergens may contribute to severe asthma.
Materials and Methods
Mice
Female BALB/c mice (The Jackson Laboratory, Bar Harbor, ME) were used at 8–10 wk of age. All animal experimental procedures were approved by the University of California, San Diego Animal Subjects Committee.
Ryegrass and Alternaria airway inflammation models
To induce airway inflammation with ryegrass pollen, mice were immunized intraperitoneally with 200 g of ryegrass pollen extract (Lolium perenne, Lot 147138, Greer, Lenoir, NC) adsorbed to 2 mg of an aqueous solution of alum hydroxide (Aldrich) in 500 l PBS or in PBS without alum on days 0 and 7. Intranasal ryegrass pollen (100 g in 40L in PBS, LPS content .71 ng/ml by Lonza limulus assay) was administered for three consecutive days under isoflurane anesthesia. Intranasal Alternaria alternata extract (Lot 177372, Greer) was given at a dose of 25 or 50 g in 40 L of PBS 3 days prior or 3 days after rye grass challenges. In some experiments BAL and lungs from mice were analyzed 3 days after Alternaria extract or PBS challenges. In other experiments, naïve mice were administered 100 μg of ryegrass extract intranasally daily for three days and euthanized one day later. Control challenges consisted of the same volume of PBS alone. All of the mice were euthanized 24 hour after the final allergen challenge.
Lung Processing and BAL Cellular Analysis
BAL and lungs were processed as we have previously described [18]. Briefly, the BAL fluid was obtained by intratracheal insertion of a catheter and lavaged with 0.7 ml of PBS containing 2% filtered bovine serum albumin (Sigma). The BAL fluid was centrifuged at 1,500 rpm for 5 minutes and supernatants collected and frozen until ELISA was performed. Total BAL cells were counted with an Accuri C6 Flow Cytometer (BD). For lung processing, the lungs were fixed in 10% buffered formalin (EMS, PA) before being embedded in paraffin for subsequent sectioning and staining. In some experiments, lungs were placed in RPMI and single cell suspensions for FACS staining were obtained by using a gentleMACS dissociator (Miltenyi) according to manufacturer's protocol.
Flow cytometry
BAL and lung cells were first incubated with a monoclonal antibody to CD16/CD32 (24G.2) for 10 min to block Fc receptors. BAL cells were stained with PE-conjugated Siglec-F, APC-conjugated Gr-1, and FITC-conjugated CD11c (eBioscience). BAL eosinophils were identified as Siglec F-positive CD11c-negative cells as previously reported [19]. Neutrophils were identified as GR-1 positive Siglec-F negative cells. BAL Th2 cells were identified as T1/ST2-positive CD4+ cells after staining with biotin-T1/ST2 (MD biosciences) and CD4 (eBioscience) followed by streptavidin-APC (eBioscience). To identify lung and BAL ILC2, cells were stained with PerCP-conjugated CD45.2 (eBioscience) and lineage staining was performed only with FITC antibodies that included FITC-conjugated lineage cocktail (Biolegend), CD11c (eBioscience), NK1.1 (eBioscience), CD4 (eBioscience), CD5 (BD Biosciences), CD8 (BD Bioscience), FcεR1 (Biolegend), TCRβ (BD Bioscience), TCRγδ (Biolegend), and CD19 (Biolegend). Lung cells were also stained with APC-conjugated Thy1.2 (eBioscience) and ILC2s were identified as lineage-negative Thy1.2-positive lymphocytes as previously described [6,20]. Ki-67 staining was performed using a FoxP3 staining kit (eBioscience) followed by APC-conjugated Ki-67 (eBioscience) [6,20]. Flow cytometry was performed with an Accuri C6 cytometer (BD Biosciences) and sample data were further analyzed with Flow Jo software (Tree Star).
BAL and serum ELISA
BAL supernatant IL-5 and IL-13 were measured by ELISA according to the manufacturer's instructions and read with a BioRad Model 680 microplate reader. All ELISA kits used for BAL studies were from R&D Systems (Minneapolis, MN). For serum ryegrass specific IgE, ELISA plates were coated with ryegrass extract (Greer) dissolved in PBS at a protein concentration of 10 μg/ml. The plate was sealed an incubated overnight at 4C followed by addition of serum samples. The plate was incubated overnight at 4C and 2 μg/ml biotinylated anti-mouse IgE (Biolegend) was added followed by streptavidin-HRP (R&D) diluted 1:200 and developed with TMB A and B (R&D).
Periodic Acid-Schiff (PAS) Analysis
Paraffin-embedded lungs were sectioned at 5 μm onto microscope slides. Periodic Acid-Schiff (PAS) stained airway goblet cells were enumerated under light microscopy examination (X200 magnification). The number of PAS-positive as well as total epithelial cells was assessed in six to ten medium-sized closed circular bronchi (defined by having approximately 100–150 luminal airway epithelial cells) starting with airways having the most mucus. At least 6-10 bronchioles were counted in each slide. Results are expressed as the ratio of PAS positive cells/bronchiole calculated from the number of PAS positive epithelial cells per bronchus divided by the total number of epithelial cells of each bronchiole. All slides were blinded during analysis and repeated by independent observer.
Inflammation Index
Lung sections were stained with Hematoxylin and Eosin (H&E). Peribronchial inflammation was scored as previously reported [18]. Briefly, inflammatory cell infiltrate around the bronchioles was assessed for severity (0, normal; 1, <3 cell diameter thick; 2, 3–10 cells thick; 3, >10 cells thick) and overall extent (0, normal; 1, <10% of sample; 2, 10–25%; 3, >25%) in the visual field. The index was calculated by multiplying severity by extent, with a maximum possible score of 9.
Pulmonary function testing
In some experiments, invasive pulmonary function testing was performed using the Flexivent system (Scireq, Canada) and airway resistance analyzed by Scireq Flexivent 5.1 software as previously described [18]. Briefly, mice were anesthetized, canulated via the trachea, administered increasing doses of methacholine, and airway resistance measured.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism software. P < 0.05 was considered statistically significant.
Results
Development of ryegrass-induced lung inflammation model
To develop an eosinophilic lung inflammation model induced by ryegrass allergen, we sensitized mice via intraperitoneal route twice with ryegrass with or without aluminum hydroxide (alum), a known Th2 adjuvant. Mice were then subsequently challenged by intranasal administration of ryegrass for three days in a row (Fig 1A). Control mice received PBS intraperitoneal injections and/or PBS intranasal instillations. Mice that were sensitized and challenged with rye grass pollen developed significantly increased BAL airway total cell counts, eosinophilia and neutrophilia compared with mice challenged with PBS (Fig 1B & C). Interestingly, ryegrass sensitized and challenged mice developed an eosinophilic airway response even in the absence of the Th2 adjuvant alum, though not the same extent (Fig 1C). Mice receiving intranasal challenges with ryegrass without sensitization were found to have increased neutrophils in the BAL but a modest number of eosinophils suggesting that sensitization was required before significant airway eosinophilia developed. BAL cytokines IL-5 and IL-13 were increased in mice sensitized and challenged with ryegrass compared with controls, but only IL-5 was further increased in the alum/ryegrass sensitization group (Fig 1D). Thus, ryegrass sensitization with alum and subsequent challenges with intranasal ryegrass led to the largest increase in airway eosinophilia and IL-5 levels.
Figure 1.
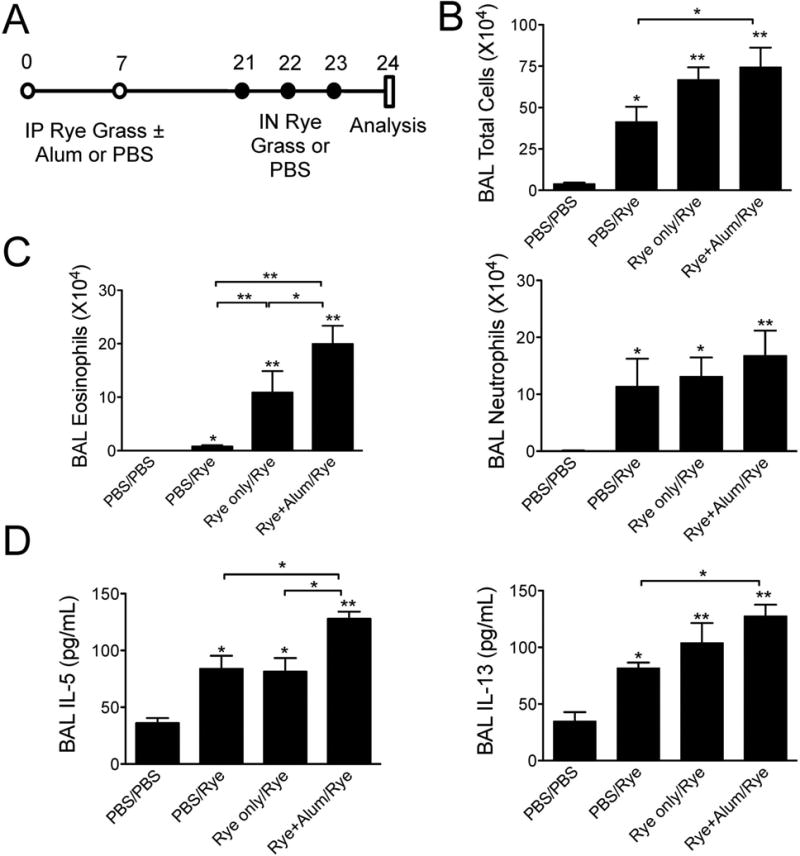
Model of ryegrass-induced eosinophilic lung inflammation. (A) Mouse model protocols. Mice received intraperitoneal injections of ryegrass with or without alum or PBS followed by either PBS or ryegrass intranasal challenges. PBS/Rye groups received intraperitoneal PBS followed by ryegrass challenges. BAL total cells (B), eosinophil and neutrophil counts (C) enumerated one day after last ryegrass challenge. (D) Levels of BAL IL-5 and IL-13. The results are expressed as the mean ± SEM (n = 4-6 mice in each group), *P<0.05, **P < 0.01 compared to PBS/PBS group unless otherwise shown, Mann-whitney.
Two hallmarks of asthma are peribronchial inflammation and epithelial mucus production. We analyzed lung sections stained with hematoxylin and eosin (H & E) to assess peribronchial infiltration as well as Periodic Acid-Schiff (PAS) to quantify mucus production from mice undergoing the protocol in Figure 1A. Lung sections from mice that were sensitized and challenged with ryegrass displayed increased peribronchial infiltration compared to control mice that were not sensitized (Fig 2A). Consistent with the increased airway eosinophilia in Figure 1C, mice receiving alum with ryegrass during sensitization had a further increase in peribronchial inflammation scoring compared with ryegrass sensitization without alum. Lung sections analyzed for numbers of mucus producing (PAS+) cells revealed that only ryegrass-sensitized mice developed epithelial mucus changes and alum administration with ryegrass greatly increased numbers of PAS+ epithelial cells (Fig 2B). Thus, the protocol utilizing ryegrass sensitization with alum led to the most robust airway eosinophilia, BAL IL-5 levels, peribronchial infiltrate, and epithelial mucus production. Importantly, sensitization to ryegrass prior to subsequent intranasal challenges was required for the development of airway eosinophilia and mucus production suggesting that this protocol was an adaptive Th2-driven response.
Figure 2.
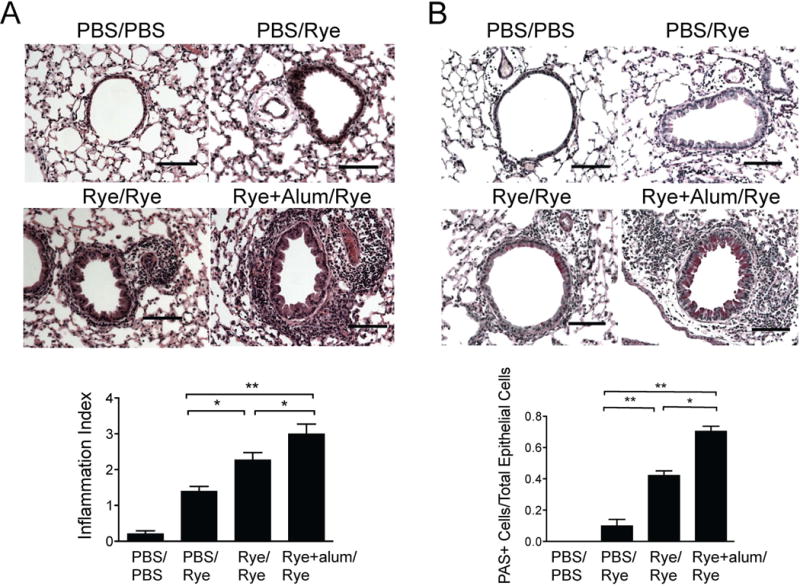
Ryegrass-induced peribronchial inflammation and mucus metaplasia. Lung sections from mice in Figure 1 were stained for H&E (A) and PAS (B) and scored. Scale bars 100μm. Results from at least 8 airways per mouse, 4-6 mice per group, mean± SEM *P<0.05, **P < 0.01, Mann-whitney.
Alternaria extract induces ILC2 proliferation and recruitment of eosinophils and Th2 cells
We have previously demonstrated that a single challenge with Alternaria extract induces an innate airway eosinophilia and type 2 innate lymphoid cell (ILC2) proliferation in naïve mice [5,6]. We have defined the early type 2 response to Alternaria extract as innate because RAG2 deficient mice that lack B and T cells develop Alternaria extract induced eosinophilia [6,21,22]. To confirm that ryegrass sensitized, but not challenged, mice also develop these features, we sensitized mice with ryegrass and alum and subsequently administered one challenged with Alternaria extract or PBS (Fig 3A). Three days later we analyzed BAL for levels of eosinophils and CD4+ T1/ST2+ cells (Figs 3A & B). T1/ST2 is the receptor for IL-33 and is preferentially expressed on murine Th2 cells compared with other CD4+ cells [23,24]. As expected, we detected significantly increased levels of BAL eosinophils after a single challenge with Alternaria extract compared with PBS in ryegrass sensitized mice (Fig 3B). Interestingly, we also found elevated airway Th2 cells (CD4+ T1/ST2+) in mice receiving one Alternaria extract challenge. We further measured the percent of ILC2 of lung lymphocytes as well as ILC2 proliferation in these mice. ILC2 are CD45-positive lineage-negative lymphocytes that do not express CD3, CD4, CD8, Fcεr1, NK1.1, TCR αβ, TCR γδ, or CD19, but do express Thy1.2 [6,20]. Alternaria extract challenged mice displayed an increased % of lung lymphocytes that were ILC2 compared with PBS challenged mice (2.6 vs. 1.7) (Fig 3D). We have previously shown that lung ILC2 have increased proliferation 3 days after one challenge with Alternaria extract in naïve mice [6]. Consistent with these findings, we also found that the %Ki-67 proliferating ILC2 in the lung were approximately double in Alternaria extract versus PBS challenged mice (39.7 vs. 19.8) (Fig 3E). Thus, a single challenge with Alternaria extract induces an innate airway eosinophilia, Th2 cell recruitment, and ILC2 proliferation in rye-grass sensitized mice.
Figure 3.
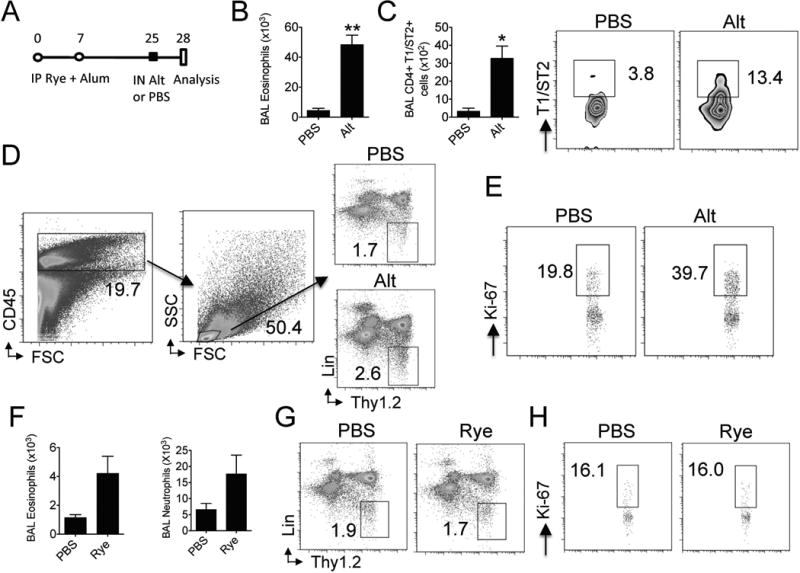
Alternaria extract exposure to ryegrass-sensitized mice induces ILC2 proliferation and recruitment of eosinophils and Th2 cells. (A) Protocol for Alternaria extract challenge in rye-grass sensitized mice. Mice were sensitized with ryegrass and alum on days 0 and 7 and challenged with a single dose of Alternaria extract or PBS on day 25 followed by BAL/lung analysis on day 28. (n= 4 mice per group) (B) BAL eosinophils enumerated on day 28. **P < 0.005, t-test (C) BAL CD4+ T1/ST2+ totals (left) and percent (middle and right). *P < 0.05, t-test (D) Lung ILC2 were identified as CD45+ lymphocytes that are lineage-negative and Thy1.2 positive. Plots from PBS (top right) and Alternaria-challenged mice (bottom right). (E) Lung cells from PBS (left) and Alternaria challenged mice (right) were permeabilized and stained with Ki-67. ILC2 were gated and % Ki-67 ILC2 shown (F) Naïve mice received 3 daily intranasal doses of rye-grass and BAL eosinophils and neutrophils enumerated (n= 4 rye grass challenged and 2 PBS challenged mice). (G) Lung CD45+ lymphocytes from PBS (left) and rye-grass challenged mice (right) were gated and ILC2% shown. (H) Lung ILC2 from PBS (left) and rye-grass challenged mice (right) were gated and analyzed for %Ki-67 positive cells.
Prior to testing the hypothesis that an innate challenge with Alternaria extract may enhance lung inflammation induced by a classic sensitization and challenge ryegrass protocol (Fig 1), we considered the possibility that intranasal ryegrass extract in non-sensitized animals may also result in an innate type 2 lung inflammation. We therefore administered three daily doses of ryegrass to naïve mice and assessed levels of BAL eosinophils, neutrophils, and lung ILC2 proliferation. We detected a modest increase in BAL eosinophils and neutrophils after three rye grass challenges (Fig 3F). Th2 cell recruitment was not different between groups (not shown). Further, there was no difference in ILC2% or proliferation in the lung between groups (Figs 3G & H). Thus, innate challenges with ryegrass extract induce modest levels of airway inflammatory cell infiltration and no detectable ILC2 proliferation compared with Alternaria extract administration.
A single Alternaria extract exposure prior to rye grass challenges enhances lung inflammation
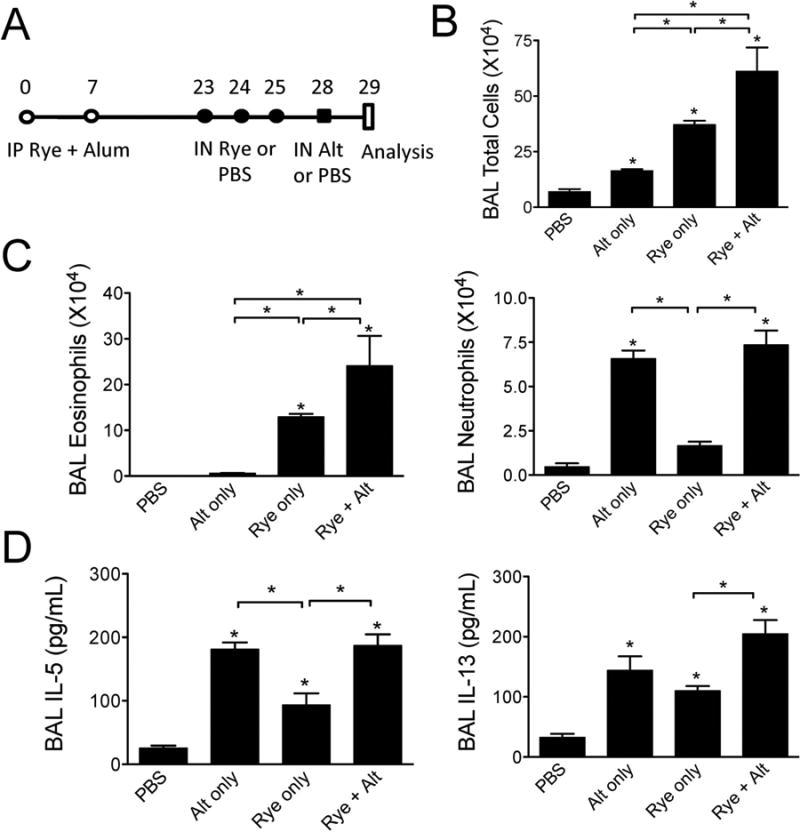
We have previously shown that a single airway challenge with Alternaria extract given to unsensitized naïve mice leads to an influx of airway eosinophils, albeit to levels far lower than an adaptive Th2 response after sensitization and repetitive challenges [5,6]. As the findings in Fig 3 demonstrate induction of innate eosinophilia and ILC2 proliferation after one challenge with Alternaria extract in ryegrass sensitized but unchallenged mice, we next sought to identify a potential effect of a single Alternaria extract challenge given prior to ryegrass exposure. Mice were sensitized with ryegrass and alum (Figs 1 & 2) and given one intranasal challenge with Alternaria given 3 days prior to ryegrass challenges (Fig 4A). Mice challenged with Alternaria extract and ryegrass developed significantly increased levels of BAL eosinophils, neutrophils, and IL-13 compared to ryegrass only challenges (Fig 4 B-D). In contrast, BAL IL-5 levels were not significantly increased above rye grass only challenges when mice received Alternaria extract prior to ryegrass challenges.
Figure 4.
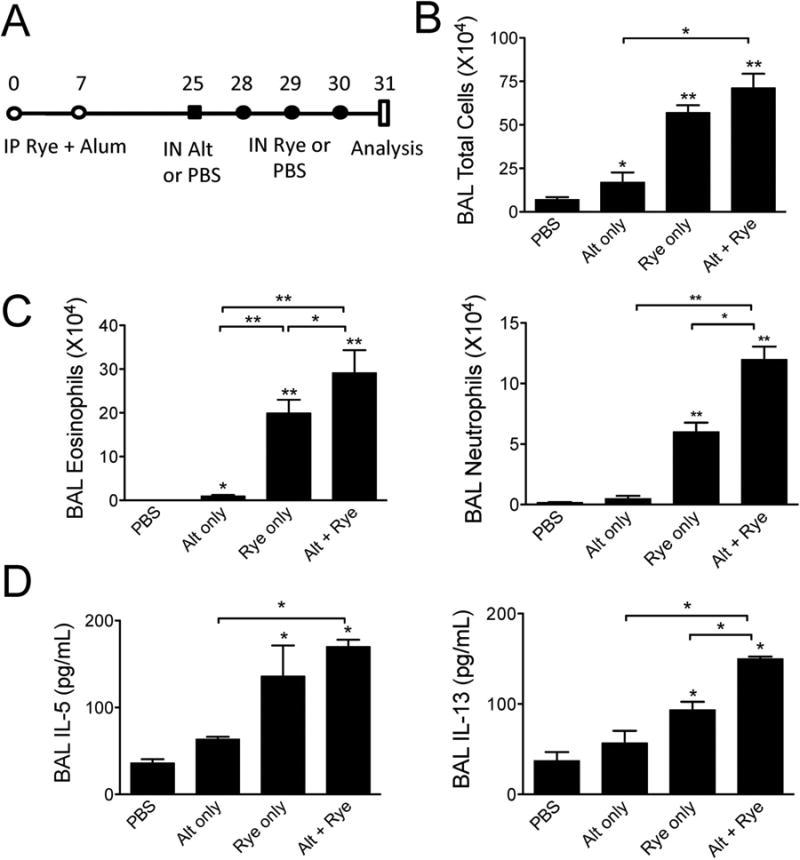
Alternaria extract exposure prior to rye grass challenges enhances lung eosinophilia and IL-13 levels. (A) Protocol showing Alternaria extract (50 μg) administration 3 days prior to ryegrass challenges. BAL total cells (B), eosinophil and neutrophil counts (C) enumerated one day after last ryegrass challenge. (D) Levels of BAL IL-5 and IL-13. The results are expressed as the mean ± SEM (n = 4 mice in each group and repeated once), *P<0.05, **P < 0.01 compared to PBS group unless otherwise shown, Mann-whitney.
We assessed stained lung sections from mice undergoing the protocol in Figure 4 and found that mice receiving one challenge of Alternaria extract after sensitization with ryegrass and alum without further ryegrass intranasal challenges developed modest peribronchial inflammation and mucus production (Fig 5 A & B). Sections from mice receiving ryegrass only sensitization and challenges showed a further increase in airway infiltrate and mucus changes, though the combination of Alternaria extract and ryegrass challenges led to the largest and most significant increases. Thus, a single airway challenge with Alternaria extract given before ryegrass exposure enhances ryegrass-induced lung inflammation and mucus production.
Figure 5.
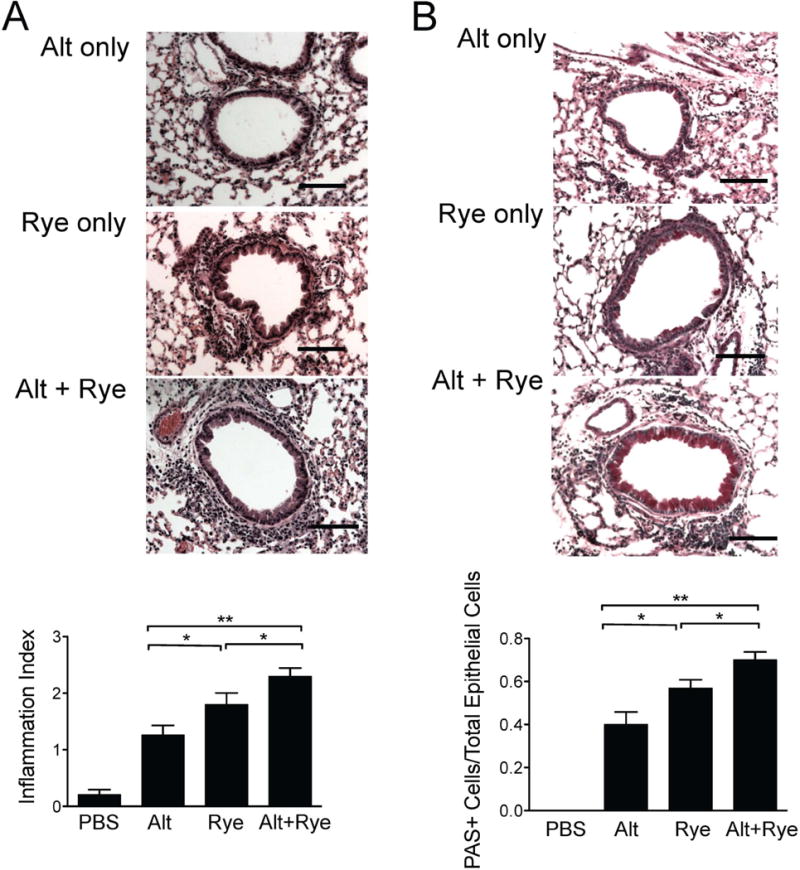
Alternaria extract exposure prior to ryegrass challenges enhances peribronchial inflammation and mucus production. Lung sections from mice in Figure 3 were stained for H&E (A) and PAS (B) and scored (bottom). Scale bars 100μm. Results from at least 8 airways per mouse, 4-6 mice per group, mean± SEM *P<0.05, **P < 0.01, Mann-whitney.
Alternaria extract induces airway eosinophil, Th2 cell, and ILC2 recruitment
To confirm that the effect of one challenge with Alternaria extract in Figs 4 & 5 was not related to the specific Alternaria extract dose utilized (50 μg), we performed the same protocol in Fig 4A with half of the dose of Alternaria extract (25 ug). Despite using a smaller dose of Alternaria extract, we detected an even greater relative increase in BAL total cells and eosinophila (Fig 6A) in ryegrass sensitized and challenged mice receiving Alternaria extract. Levels of neutrophils were not significantly different. As we had detected increased Th2 cell recruitment after a single Alternaria extract challenge in ryegrass sensitized mice (Fig 3C), we further examined Th2 cell recruitment after both Alternaria extract and ryegrass administration in sensitized mice (Fig 6B). We detected an additive effect of Alternaria extract and rye-grass on levels of airway Th2 cells in mice receiving challenges of both extracts. Levels of rye-grass specific serum IgE were elevated in all sensitized groups compared with unsensitized PBS-challenged mice (Fig 6C), though the combination of ryegrass and Alternaria extract challenges did not increase specific IgE above ryegrass alone. However, there was a trend toward specific IgE increases in both ryegrass challenge groups compared with Alternaria extract only suggesting that airway ryegrass challenges (with or without Alternaria extract exposure) further increased serum specific IgE in ryegrass sensitized animals. Next, we determined whether ILC2 were recruited into the BAL in the different groups of mice. ILC2 have been shown to be absent in the BAL of unchallenged mice and are recruited after allergen challenges [25]. Interestingly, we found that the % of BAL lymphocytes that were ILC2 was three-fold higher in Alternaria extract challenged mice (with or without rye-grass challenges) compared to the rye-grass only group (Fig 6D). However, total numbers of BAL ILC2 were significantly higher in the ryegrass challenged mice that received Alternaria extract. Thus, a single exposure of Alternaria extract in ryegrass challenged mice induces recruitment of airway ILC2, eosinophils, and Th2 cells. Finally, we performed invasive airway hyperresponsiveness testing and determined that all three groups (Alt, Rye, and Alt+Rye) showed increased airway resistance compared with naïve mice after administration of 24 mg/ml of methacholine, though the combination of Alternaria extract and ryegrass challenges showed the largest increase (Fig 6E).
Figure 6.
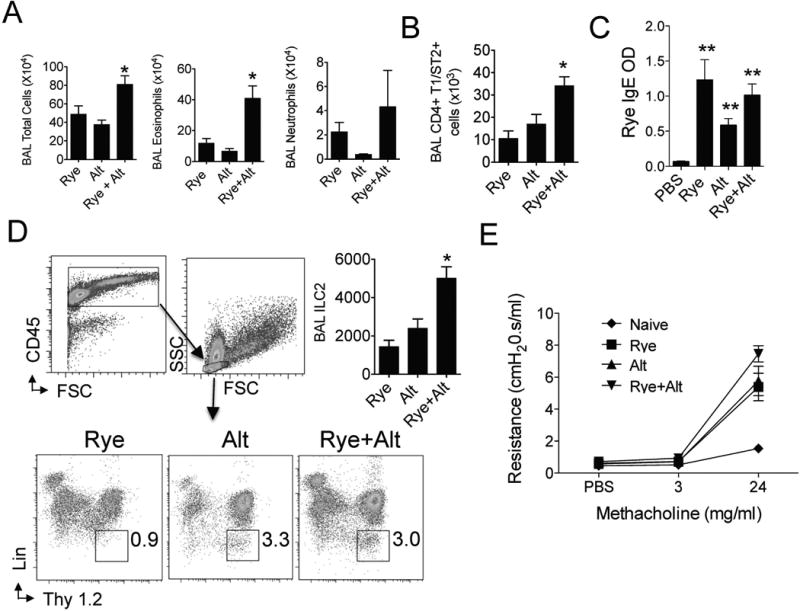
Reduced dose Alternaria extract increases airway eosinophil, Th2 cell, and ILC2 recruitment. Ryegrass sensitized mice were administered either Alternaria extract (25 μg) or PBS followed by either ryegrass or PBS challenges 3 days later. (n= 4 mice per group) (A) BAL total cells, eosinophils, and neutrophils enumerated one day after last challenge. *P < 0.05 compared to ryegrass and Alternaria groups, t-test. (B) BAL CD4+ T1/ST2+ cells enumerated. *P < 0.05 compared to ryegrass and Alternaria groups, t-test. (C) Serum ryegrass IgE levels. **P < 0.01 compared to PBS group, t-test. (D) BAL ILC2 identified as CD45+ lymphocytes (top) and % ILC2 from ryegrass, Alternaria, and rye-grass + Alternaria groups shown (bottom). Total BAL ILC2 enumerated (top right, n=4 mice per group, BAL from 2 mice per group pooled for ILC2 staining). *P < 0.05 compared to rye-grass group, t-test. (E) Lung resistance after instillation of PBS followed by 3 and 24 mg/ml of methacholine (n= 3-4 mice per group).
Alternaria extract exposure after ryegrass challenges increases airway eosinophilia
We considered the timing of a single Alternaria extract challenge might influence the lung inflammation potentiated by Alternaria extract and performed experiments with a single challenge of Alternaria given after ryegrass challenges (Fig 7A). Similar to Alternaria given before ryegrass challenges (Figs 4-6), mice receiving Alternaria extract after ryegrass challenges had significantly increased in BAL eosinophils compared with mice receiving only ryegrass challenges within the same respective protocol (Fig 7 B & C). Alternaria extract given alone to ryegrass sensitized, but not challenged, mice the day before analysis revealed increased neutrophils and a modest number of eosinophils. The numbers of eosinophils in the BAL of mice receiving both Alternaria extract and ryegrass were synergistically increased when compared to the combined means of Alternaria extract only and ryegrass only groups. In contrast, BAL IL-5, IL-13, and neutrophils were increased, but not above Alternaria extract only challenges (Fig 7D). Further, when lung sections from these mice were analyzed for peribronchial infiltration and mucus production, sections from Alternaria extract and ryegrass challenged mice displayed greater levels inflammation and mucus production compared with ryegrass only (Fig 8A & B). The overall magnitude of peribronchial infiltrate was greater when Alternaria extract was given after compared to administration before ryegrass challenges. Taken together, Alternaria extract enhances BAL eosinophilia, airway inflammation, and mucus production in ryegrass sensitized and challenged mice. Importantly, Alternaria extract and ryegrass challenges induced a synergistic effect on BAL eosinophilia when Alternaria extract exposure occurred after ryegrass challenges.
Figure 7.
Alternaria extract exposure after rye grass challenges increases lung eosinophilia. (A) Protocol showing Alternaria extract administration 3 days after to ryegrass challenges. BAL total cells (B), eosinophil and neutrophil counts (C) enumerated one day after PBS or Alternaria extract challenge. (D) Levels of BAL IL-5 and IL-13. The results are expressed as the mean ± SEM (n = 4 mice in each group and repeated once), *P<0.05 compared to PBS challenged mice unless otherwise shown, Mann-whitney.
Figure 8.
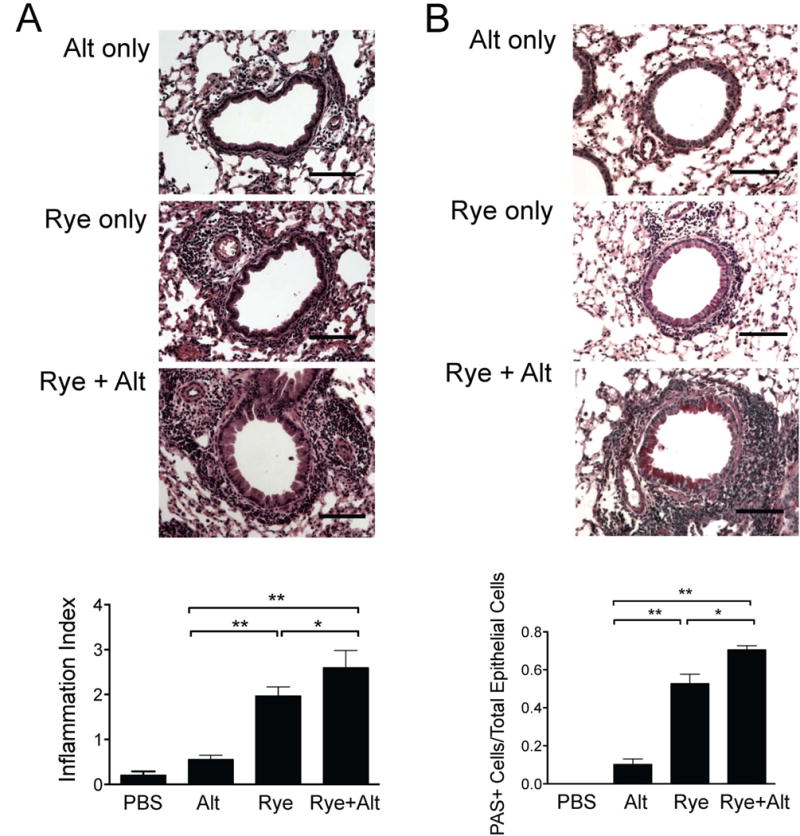
Alternaria extract exposure after ryegrass challenges enhance peribronchial inflammation and mucus production. Lung sections from mice in Figure 5 were stained for H&E (A) and PAS (B) and scored (bottom). Scale bars 100μm. Results from at least 8 airways per mouse, 4-6 mice per group, mean± SEM *P<0.05, **P < 0.01, Mann-whitney.
Discussion
Our studies demonstrate that a single challenge with Alternaria extract given 3 days before or 3 days after rye-grass challenges results in increased airway eosinophils, IL-5, and IL-13 levels as well as peribronchial inflammation and mucus production compared with ryegrass only challenges. Further, a single Alternaria extract given to ryegrass sensitized, but unchallenged, mice induced airway eosinophilia, Th2 cell recruitment, as well as ILC2 expansion and proliferation. Additionally, unsensitized mice receiving three days of ryegrass only developed modest airway eosinophilia and neutrophilia. Finally, we determined that Alternaria extract enhanced recruitment of airway eosinophils, Th2 cells, and ILC2 in ryegrass challenged mice despite not having an effect on serum rye-specific IgE.
Importantly, this is the first study to our knowledge to investigate the effect of an innate type 2 response to an allergen given during a conventional recall lung inflammatory response to another allergen. Innate type 2 responses to allergens have only recently been recognized as possibly contributing to lung inflammatory responses. Studies in mice have demonstrated that allergens can induce eosinophilic lung inflammation, Th2 cytokine production, and mucus metaplasia in the absence of B and T cells [6,21,26,27]. The first report showed that a single administration of purified fungal allergen protease from Aspergillus given to RAG1 deficient mice led to lung eosinophilia within 18 hours [27]. Since then, our group and others have reported that a single airway challenge with Alternaria extract induces an innate type 2 response in mice that is not dependent on adaptive immunity [5,6,21,22]. Further work has identified type 2 innate lymphoid cells (ILC2) as major producers of IL-5 and IL-13 in allergen challenge models including Alternaria extract, papain protease, house dust mite, and OVA models [21,25,26,28]. Our current studies also show that ILC2 proliferate after Alternaria extract challenge in ryegrass sensitized mice and are recruited to the BAL in larger numbers in ryegrass challenged mice. Though the relative contribution of Th2 cytokine production by ILC2 compared to other cells including Th2 cells in our models are unclear, it is conceivable that human asthmatics may have both innate and adaptive Th2 inflammatory responses after allergen inhalation that result in asthma flares.
We chose to develop a model utilizing ryegrass and Alternaria extracts as allergens based on reports of severe asthma symptoms associated with these allergens during thunderstorms [10,16,17]. Though IgE sensitization to these allergens was found to be a risk factor for having asthma exacerbations during thunderstorms, the mechanism leading to asthma symptoms is not clear as has been discussed elsewhere [14,15]. The suggestion that an innate response to Alternaria extract in ryegrass sensitized and challenged mice translates to a mechanism of human thunderstorm asthma is speculative. We have not adequately studied the effect of thunderstorms in allergen challenge animal models or controlled for fungal spore allergens that humans inhale compare with the extracts that we have administered to mice. Further, though doses of allergens used in mouse models are magnitudes higher per weight than humans inhale, insights into potential immune mechanisms may still be gained. Additionally, we chose to study different time points of allergen challenge and endpoint analysis in order to more broadly study the lung response to Alternaria extract under different conditions, and thus direct comparison of results from different protocols may not be possible. Despite these caveats, the timing of symptoms in thunderstorm-related asthma appears to be very rapid in pollen allergic patients and correlates with release of allergen particles including Alternaria, raising the possibility that rapid innate responses could occur in addition to IgE-mediated or other mechanisms [14].
In the current studies, we have detected modestly elevated eosinophils, neutrophils and Th2 cytokines in the BAL of unsensitized mice receiving ryegrass over three days. Recently, ryegrass has been shown to contain immunostimulatory properties including TLR ligands and beta-1,3-glucans [29]. Further, in-vitro studies have previously shown that ryegrass induced detachment of mouse airway epithelial cells in a protease-dependent manner [30]. These reports suggest that ryegrass extract has pro-inflammatory properties that could contribute to an innate type 2 response in addition to that of Alternaria extract in our models. Though we detected a small increase in Th2 cytokines and eosinophils after ryegrass exposue in unsensitized mice, we did not detect a change in ILC2% or proliferation compared with that induced by Alternaria. The possibility exists that the innate response to ryegrass is not ILC2 mediated or that ILC2 cytokine production is induced without a significant effect on proliferation at the time point we measured.
In our studies, we found that a single challenge with Alternaria extract given to ryegrass challenged mice exacerbated the degree of eosinophilic lung inflammation despite not having a further influence on ryegrass specific IgE levels. There are several possible mechanisms that may have contributed to these findings. First, our data supports an increased Th2 cell recruitment to the airway after Alternaria extract exposure and prior to ryegrass challenges. An increased Th2 cell number could more robustly respond to ryegrass antigen resulting in greater IL-5 and IL-13 production and locally increased eosinophilia and mucus production without a significant effect on serum specific IgE production. Second, we have shown that ILC2 proliferate after Alternaria extract exposure and the greater numbers of ILC2 could produce more Th2 cytokines upon exposure to ryegrass in the lung without influence on ryegrass IgE. Finally, other cell types including mast cells, basophils, NKT cells and eosinophils could be induced by Alternaria extract challenge to increase Th2 cytokine production as well as airway epithelial cell chemokine secretion. Though significant complexity likely exists in our model systems, the possibility that a type 2 response in the lung can be enhanced without a significant effect on systemic allergen-specific IgE levels is an interesting subject for further investigation and may lead to an understanding eosinophilic asthma that is discordant with IgE status [31].
Alternaria extract administered after ryegrass challenges led to a more significant increase in the number of BAL eosinophils and a greater degree of lung inflammation compared with Alternaria given before ryegrass. One possibility for this difference may be that eosinophils recruited into the lung by ryegrass challenges become directly activated by subsequent Alternaria extract challenge leading to increased inflammation and eosinophilia. Consistent with this, one report demonstrated that Alternaria extract can directly induce innate activation and degranulation of human eosinophils [32]. As the timing of allergen exposure is likely important in the response seen, we chose to administer Alternaria extract before and after ryegrass to assess for differences in the type 2 responses in the lungs. We did perform experiments with co-administration of Alternaria extract and ryegrass on the same days (not shown), but due to the early additive neutrophilic effect of concomitant ryegrass and Alternaria exposure, it was difficult to identify a significant eosinophilic component at the time points we measured. The Alternaria and ryegrass allergen extracts we utilized are crude complex extracts that likely have the ability to activate many pattern recognition receptors (PRRs) leading to mixed responses including early neutrophilia. Importantly, one study demonstrated that patients with asthma symptoms during thunderstorms developed increased sputum eosinophilia and IL-5+ cells indicative of a type 2 response that is more consistent with the response we detected using the protocols shown in Figures 3 and 7 [17]. Thus, the timing and kinetics of innate and adaptive type 2 responses to allergens may be critical to the type of inflammatory responses that occur in the airway.
Finally, we detected increased mucus production in mice that received one challenge with Alternaria extract in addition to ryegrass challenges. This result was more robust when Alternaria extract was given prior to ryegrass challenges and may be due to sufficient time for mucus accumulation to occur between Alternaria extract challenge and collection of lungs. We also detected an increase in IL-13 levels in the airway in these mice and the relationship between IL-13 and mucus production is strongly supported in the literature [33-35]. Severe asthma symptoms in humans including fatal asthma has been associated with increased airway mucus exudate and could be partially responsible for Alternaria and rye-grass induced symptoms [36].
In summary, we found that a single challenge with Alternaria extract enhances lung eosinophilia, peribronchial infiltration, and mucus production induced by ryegrass challenge. Our data suggests that the combination of innate and adaptive responses to allergens may contribute to severe asthma. Further work to identify mediators of both innate and adaptive responses may lead to identification of predictive biomarkers or therapeutic targets for patients with severe asthma symptoms triggered by allergens.
Footnotes
Grant support: NIH grant 1K08AI080938 and ALA/AAAI Allergic Respiratory Diseases Award to T.A.D.
References
- 1.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 2.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of t(h)2 cytokines by adipose tissue-associated c-kit(+)sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 3.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate il-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doherty TA, Khorram N, Sugimoto K, Sheppard D, Rosenthal P, Cho JY, Pham A, Miller M, Croft M, Broide DH. Alternaria induces stat6-dependent acute airway eosinophilia and epithelial fizz1 expression that promotes airway fibrosis and epithelial thickness. J Immunol. 2012;188:2622–2629. doi: 10.4049/jimmunol.1101632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doherty TA, Khorram N, Chang JE, Kim HK, Rosenthal P, Croft M, Broide DH. Stat6 regulates natural helper cell proliferation during lung inflammation initiated by alternaria. Am J Physiol Lung Cell Mol Physiol. 2012;303:L577–588. doi: 10.1152/ajplung.00174.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Hollaren MT, Yunginger JW, Offord KP, Somers MJ, O'Connell EJ, Ballard DJ, Sachs MI. Exposure to an aeroallergen as a possible precipitating factor in respiratory arrest in young patients with asthma. N Engl J Med. 1991;324:359–363. doi: 10.1056/NEJM199102073240602. [DOI] [PubMed] [Google Scholar]
- 8.Downs SH, Mitakakis TZ, Marks GB, Car NG, Belousova EG, Leuppi JD, Xuan W, Downie SR, Tobias A, Peat JK. Clinical importance of alternaria exposure in children. Am J Respir Crit Care Med. 2001;164:455–459. doi: 10.1164/ajrccm.164.3.2008042. [DOI] [PubMed] [Google Scholar]
- 9.Bush RK, Prochnau JJ. Alternaria-induced asthma. J Allergy Clin Immunol. 2004;113:227–234. doi: 10.1016/j.jaci.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Pulimood TB, Corden JM, Bryden C, Sharples L, Nasser SM. Epidemic asthma and the role of the fungal mold alternaria alternata. J Allergy Clin Immunol. 2007;120:610–617. doi: 10.1016/j.jaci.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 11.Lyons TW, Wakefield DB, Cloutier MM. Mold and alternaria skin test reactivity and asthma in children in connecticut. Ann Allergy Asthma Immunol. 2011;106:301–307. doi: 10.1016/j.anai.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plaza V, Serrano J, Picado C, Cosano J, Ancochea J, de Diego A, Martin JJ, Sanchis J. Clinical characteristics of the fatal and near-fatal asthma in alternaria alternata sensitized patients. Med Clin (Barc) 2003;121:721–724. doi: 10.1016/s0025-7753(03)74076-7. [DOI] [PubMed] [Google Scholar]
- 13.Neukirch C, Henry C, Leynaert B, Liard R, Bousquet J, Neukirch F. Is sensitization to alternaria alternata a risk factor for severe asthma? A population-based study. J Allergy Clin Immunol. 1999;103:709–711. doi: 10.1016/s0091-6749(99)70247-2. [DOI] [PubMed] [Google Scholar]
- 14.D'Amato G, Cecchi L, Annesi-Maesano I. A trans-disciplinary overview of case reports of thunderstorm-related asthma outbreaks and relapse. Eur Respir Rev. 2012;21:82–87. doi: 10.1183/09059180.00001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marks GB, Bush RK. It's blowing in the wind: New insights into thunderstorm-related asthma. J Allergy Clin Immunol. 2007;120:530–532. doi: 10.1016/j.jaci.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Suphioglu C. Thunderstorm asthma due to grass pollen. Int Arch Allergy Immunol. 1998;116:253–260. doi: 10.1159/000023953. [DOI] [PubMed] [Google Scholar]
- 17.Wark PA, Simpson J, Hensley MJ, Gibson PG. Airway inflammation in thunderstorm asthma. Clin Exp Allergy. 2002;32:1750–1756. doi: 10.1046/j.1365-2222.2002.01556.x. [DOI] [PubMed] [Google Scholar]
- 18.Doherty TA, Soroosh P, Khorram N, Fukuyama S, Rosenthal P, Cho JY, Norris PS, Choi H, Scheu S, Pfeffer K, Zuraw BL, Ware CF, Broide DH, Croft M. The tumor necrosis factor family member light is a target for asthmatic airway remodeling. Nat Med. 2011;17:596–603. doi: 10.1038/nm.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens WW, Kim TS, Pujanauski LM, Hao X, Braciale TJ. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J Immunol Methods. 2007;327:63–74. doi: 10.1016/j.jim.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates th2 cytokine production. J Allergy Clin Immunol. 2013;132:205–213. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. Il-33-responsive lineage- cd25+ cd44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188:1503–1513. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kouzaki H, Iijima K, Kobayashi T, O'Grady SM, Kita H. The danger signal, extracellular atp, is a sensor for an airborne allergen and triggers il-33 release and innate th2-type responses. J Immunol. 2011;186:4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lohning M, Grogan JL, Coyle AJ, Yazdanbakhsh M, Meisel C, Gutierrez-Ramos JC, Radbruch A, Kamradt T. T1/st2 expression is enhanced on cd4+ t cells from schistosome egg-induced granulomas: Analysis of th cell cytokine coexpression ex vivo. J Immunol. 1999;162:3882–3889. [PubMed] [Google Scholar]
- 24.Lohning M, Stroehmann A, Coyle AJ, Grogan JL, Lin S, Gutierrez-Ramos JC, Levinson D, Radbruch A, Kamradt T. T1/st2 is preferentially expressed on murine th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for th2 effector function. Proc Natl Acad Sci U S A. 1998;95:6930–6935. doi: 10.1073/pnas.95.12.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, McKenzie AN. Innate il-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol. 2012;129:191–198 e191-194. doi: 10.1016/j.jaci.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 26.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 27.Kiss A, Montes M, Susarla S, Jaensson EA, Drouin SM, Wetsel RA, Yao Z, Martin R, Hamzeh N, Adelagun R, Amar S, Kheradmand F, Corry DB. A new mechanism regulating the initiation of allergic airway inflammation. J Allergy Clin Immunol. 2007;120:334–342. doi: 10.1016/j.jaci.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 28.Klein Wolterink RG, Kleinjan A, van Nimwegen M, Bergen I, de Bruijn M, Levani Y, Hendriks RW. Pulmonary innate lymphoid cells are major producers of il-5 and il-13 in murine models of allergic asthma. Eur J Immunol. 2012;42:1106–1116. doi: 10.1002/eji.201142018. [DOI] [PubMed] [Google Scholar]
- 29.Mittag D, Varese N, Scholzen A, Mansell A, Barker G, Rice G, Rolland JM, O'Hehir RE. Tlr ligands of ryegrass pollen microbial contaminants enhance th1 and th2 responses and decrease induction of foxp3(hi) regulatory t cells. Eur J Immunol. 2013;43:723–733. doi: 10.1002/eji.201242747. [DOI] [PubMed] [Google Scholar]
- 30.Hassim Z, Maronese SE, Kumar RK. Injury to murine airway epithelial cells by pollen enzymes. Thorax. 1998;53:368–371. doi: 10.1136/thx.53.5.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snijders D, Agostini S, Bertuola F, Panizzolo C, Baraldo S, Turato G, Faggian D, Plebani M, Saetta M, Barbato A. Markers of eosinophilic and neutrophilic inflammation in bronchoalveolar lavage of asthmatic and atopic children. Allergy. 2010;65:978–985. doi: 10.1111/j.1398-9995.2009.02282.x. [DOI] [PubMed] [Google Scholar]
- 32.Matsuwaki Y, Wada K, Moriyama H, Kita H. Human eosinophil innate response to alternaria fungus through protease-activated receptor-2. Int Arch Allergy Immunol. 2011;155(Suppl 1):123–128. doi: 10.1159/000327498. [DOI] [PubMed] [Google Scholar]
- 33.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: Central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 34.Tyner JW, Kim EY, Ide K, Pelletier MR, Roswit WT, Morton JD, Battaile JT, Patel AC, Patterson GA, Castro M, Spoor MS, You Y, Brody SL, Holtzman MJ. Blocking airway mucous cell metaplasia by inhibiting egfr antiapoptosis and il-13 transdifferentiation signals. J Clin Invest. 2006;116:309–321. doi: 10.1172/JCI25167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim EY, Battaile JT, Patel AC, You Y, Agapov E, Grayson MH, Benoit LA, Byers DE, Alevy Y, Tucker J, Swanson S, Tidwell R, Tyner JW, Morton JD, Castro M, Polineni D, Patterson GA, Schwendener RA, Allard JD, Peltz G, Holtzman MJ. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med. 2008;14:633–640. doi: 10.1038/nm1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuyper LM, Pare PD, Hogg JC, Lambert RK, Ionescu D, Woods R, Bai TR. Characterization of airway plugging in fatal asthma. Am J Med. 2003;115:6–11. doi: 10.1016/s0002-9343(03)00241-9. [DOI] [PubMed] [Google Scholar]


