Abstract
Neuroinflammation and degeneration of ascending catecholaminergic systems occur early in the neurodegenerative process. Age and the duration of a pro-inflammatory environment induced by continuous intraventricular lipopolysaccharide (LPS) differentially affect the expression profile of pro- and anti-inflammatory genes and proteins as well as the number of activated microglia (express major histocompatibility complex II, MHCII) and the integrity and density of ascending catecholaminergic neural systems originating from the locus coeruleus (LC) and substantia nigra pars compacta (SNpc) in rats. LPS infusion increased gene expression and/or protein levels for both pro- and anti-inflammatory biomarkers. Although LPS infusion stimulated a robust increase in IL-1β gene and protein expression, this increase was blunted with age. LPS infusion also increased the density of activated microglia cells throughout the midbrain and brainstem. Corresponding to the development of a pro-inflammatory environment, LC and SNpc neurons immunopositive for tyrosine-hydroxylase (TH, the rate-limiting synthetic enzyme for dopamine and norepinephrine) decreased in number, along with a decrease in TH gene expression in the midbrain/brainstem region. Our data support the concept that continuous exposure to a pro-inflammatory environment drives exaggerated changes in the production and release of inflammatory mediators that interact with age to impair functional capacity of the SNpc and LC.
Keywords: Alzheimer’s disease, Parkinson’s disease, neuroinflammation, substantia nigra, locus coeruleus, microglia, rat, aging, cytokines
1. Introduction
Activation of the brain’s resident microglia occurs during normal aging, is associated with many neurodegenerative diseases such as Parkinson’s disease (PD) and Alzheimer’s disease (AD), and may drive a self-propagating toxic cycle promoted by the release of pro-inflammatory and loss of protective mediators (Akiyama et al., 2000; Aarsland et al., 2001; Bartels and Leenders, 2005; Block and Hong, 2005; Cribbs et al., 2012; Griffin et al., 1989; Hobson and Meara, 2004; Hughes et al., 2000; Swardfager et al., 2010; Whitton, 2007). When these processes are triggered within vulnerable brain regions, they may lead to the loss of acetylcholinergic neurons in the nucleus basalis magnocellularis (nbM, Willard et al., 1999; Whitton, 2007) as well as dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc), noradrenergic (NE) neurons in the locus coeruleus (LC) and, all regions that show significant early cell loss in the brains of patients with PD and AD (Braak et al., 2003; Grudzien et al., 2007; Halliday et al., 2006; Rudow et al., 2008; Szot et al., 2006).
We and others have speculated that the consequences of neuroinflammation associated with microglial activation, are carefully regulated until, due to normal aging or the deposition of toxic proteins, there is a gradual shift to a non-equilibrium state that is permissive for neurodegenerative processes (Block and Hong, 2005; Colton and Wilcock, 2010; Smith et al., 2012; Wenk and Hauss-Wegrzyniak, 2001). Microglia can assume various phenotypes that are associated either with the release of potentially destructive, pro-inflammatory cytokines and other toxic molecules or the expression of a cytokine profile that sustains repair, recovery and growth. Microglia in various states of activation are detectable many years prior to the onset of neuropathological changes (Cagnin et al., 2006; Gerhard et al., 2006; Imamura et al., 2003). Because vulnerable brain regions are likely exposed for many decades to a complex combination of microglia in various activation states (Bilbo, 2010; Eikelenboom et al., 2010; Heneka et al., 2010; Herrup, 2010). The current study investigated the differential influence of brain age and the duration of the pro-inflammatory environment upon the expression of pro- and anti-inflammatory genes and proteins as well as the number of activated microglia and the integrity and density of ascending catecholaminergic neural systems originating in the LC and SNpc.
2. Methods
2.1. Experimental Design
Young (3 mo), middle-aged (9 mo) and aged (23 mo) male F-344 rats (Harlan Sprague–Dawley) received chronic infusion of lipopolysaccharide (LPS) or its vehicle (artificial cerebral spinal fluid, aCSF) into the IVth ventricle for 21 or 56 days. We believe that this approach best represents the situation present during the early stages of many chronic neurodegenerative diseases. Multiple counter-balanced iterations of this study were performed to produce a total of 132 rats; yielding experimental groups with a minimum of eleven rats that were divided between biochemical (minimum 6 rats/group) and histological (minimum 5 rats/group) analysis. Midbrain/brainstem regions were evaluated for protein and mRNA expression of inflammatory markers and the LPS receptor TLR4 (Toll-Like Receptor 4), as well as the presence of MHC II-IR microglia, which was used to define activated microglia. Changes in these immune factors at three ages and after short (21 days) or long (56 days) of continuous LPS infusion were then evaluated with respect to changes in neurotransmitter systems including expression of genes involved in the regulation of glutamate (glutamate transporter 1, GLT1, and the cystine-glutamate anti-porter, XcT), and gene and histological expression of the enzymes responsible for production of dopamine (tyrosine hydroxylase, TH) and norepinephrine (dopamine-β-hydroxylase, DBH). DBH immunostaining was examined in order to determine specifically the integrity of norepinephrine innervation of the dentate gyrus region of the hippocampus, which does not receive a dense dopaminergic input (Gasbarri et al., 1994). TH immunostaining was used to define specifically the norepinephrine neurons in the LC and dopaminergic neurons in the SNpc.
2.2 Subjects
Rats were maintained on a 12/12-h light–dark cycle with lights off at 09:00 in a temperature-controlled room (22° C) with free access to food and water. All rats were sacrificed during the dark phase of the diurnal cycle. Body weights and general health were closely monitored throughout the study. All rats were allowed at least one week to adapt to their new environment prior to surgery. The experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996, and formal approval to conduct the experiments was obtained from the animal subjects review board from The Ohio State University.
2.3 Surgery
aCSF (140 mM NaCl, 3.0 mM KCl, 2.5 mM CaCl2, 1.0 mM MgCl2, and 1.2 mM Na2HPO4 adjusted to pH 7.4) or LPS (0.25μg/hr, 1.66 mg/ml prepared in aCSF; E. coli, serotype 055:B5, TCA extraction, Sigma) were continuously infused via a cannula implanted into the IVth ventricle (−2.5 mm AP and −7.0 mm DV, relative to lambda) and attached (via Tygon tubing, 0.06 O.D.) to an osmotic minipump (Alzet model #2006, to deliver 0.15μl/hr; Durect Corp., Cupertino, CA) as previously described (Hauss-Wegrzyniak et al., 1998; Marchalant et al., 2007; Rosi et al., 2004). The average fill volume and release rates for the pump allows for an infusion up to 56 days. Post-operative care included lidocaine 1% solution applied to the exposed skin upon closure, 2 ml of isotonic saline by subcutaneous injection to prevent dehydration during recovery and 2% Tylenol in the drinking water for three days prior to and after surgery.
2.4 Tissue collection
Rats used for protein and mRNA analysis were briefly anesthetized and then rapidly decapitated; their midbrain/brainstem (extending from just rostral to the SNpc and just caudal to the LC) and entire hippocampus were quickly dissected on ice and stored at −80 °C until processed. Blood was collected during the rapid decapitation procedure. After centrifugation at 4 °C for 15min at 2500 × g, serum was collected and assayed. Rats used for immunohistochemistry were deeply anesthetized with isoflurane for a transcardiac perfusion with 80 ml of cold 0.9% saline containing 50 U/ml heparin, followed by 120 ml of 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS), pH 7.4, at a rate of 10 ml/min. Brains were post-fixed overnight in the same fixative and then stored in PBS.
2.5 Protein analysis
Brainstem or serum levels of tumor necrosis factor (TNF)-α, interleukin-1 (IL-1)-α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12 IL-13, IL-18 (interferon-γ inducing factor), interferon (IFN)-γ, and granulocyte-macrophage-stimulating factor (GM-CSF) were quantified simultaneously with a magnetic bead-based immunoassay (Bio-Rad, BioPlex Pro Rat Standard, 171-K1002M), according to the manufacturer’s protocol. Briefly, total protein was extracted from frozen midbrain/brainstem with a BioPlex Cell Lysis Kit (BioRad, Richmond, CA). A mixture of distinct capture beads (fluorescently dyed microspheres) each with a specific spectral address and conjugated to an antibody against one of the cytokines listed above were dispensed across a 96-well plate and protected from light. Samples and antigen standards were added in duplicate and the plate was shaken (700 RPM, 1 hr). Then a mixture of biotinylated detection antibodies directed against each of the primary antibodies was added and the plate was shaken (700 RPM, 30 min); unbound materials were washed away (3x) (BioRad, BioPlex Pro wash station). Each well was then incubated with a streptavidin-phycoerythrin conjugate (SA-PE) reporter dye that binds to the detection antibody (700 RPM, 10 min); unbound materials were washed away (3x). Each well was then suspended in assay buffer and shaken (1100 RPM for 30 sec). Finally, the contents were passed through a dual detection multiplexing machine (Bio-Rad MAGPIX Multiplex Reader) with a classification laser that distinguishes each of the proteins by color of its bound antigen-specific bead and a reporter laser that quantifies each molecule based upon the fluorescence of bound antigen-specific SA-PE reporter dye. Values were standardized to protein content of the homogenate obtained with a Bio-Rad protein assay (Bio-Rad), and results are reported as pg/mg protein.
2.6 Real-time polymerase chain reaction mRNA analysis
Brainstems were evaluated for mRNA expression of: TNFα, IL1β, transforming growth factor-β (TGFβ), TLR4, fractalkine receptor (CX3CR1), GLT1, XcT, brain-derived neurotrophic factor (BDNF). TH was evaluated in the midbrain/brainstem as a precursor to LC norepinephrine as well as SNpc dopamine, and DBH was evaluated in the hippocampus because it is a precursor to norepinephrine that can distinguish input from the LC projections. Tissues were homogenized in Trizol (Life Technologies, Carlsbad, CA). Total RNA were extracted with a NucleoSpin RNA II kit (Macherey-Nagel, Düren, Germany); 1μg of total RNA was reverse-transcribed to create a cDNA library using the iScript reverse transcription Supermix for RT-qPCR (Bio-Rad). Primers were designed with PrimerQuest software (Integrated DNA Technologies, Coralville, IA; Table 1), and specificity was ensured by examining primer alignments with the BLAST database. Primers and Sso Advanced SYBR Green Supermix (BioRad) were prepared with RNase-free water. For PCR amplification, mix (19μl) was added to reverse transcription reaction (1 μl) previously diluted (1:20). Assays were run in triplicate on the CFX96, C1000 Thermal Cycler (Bio-Rad). Amplification conditions were: 95 °C for 30 sec and 40 cycles of PCR (denaturation: 95 °C for 5 sec, annealing/extension: 60 °C for 30 sec), followed by melting curves to verify the absence of primer dimers. Two negative controls were performed during each quantitative PCR experiment: reaction without the reverse transcription (-RT) to confirm the absence of genomic DNA contamination, and samples with no added cDNA template (H2O only). The cycle (Ct) at which expression levels crossed threshold was normalized to the Ct of the reference gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH), producing ΔCt with arbitrary units of total gene expression.
Table 1.
Primer sequences and real time polymerase chain reaction conditions.
| Gene (base pairs) | Accession # | Primer Sequences F: Forward; R: Reverse |
Annealing Temp (°C) | Product Length |
|---|---|---|---|---|
| TNFα | X66539.1 | F: CTGGCCAATGGCATGGATCTCAAA | 59.7 | 97 |
| R: AGCCTTGTCCCTTGAAGAGAACCT | 59.8 | |||
| IL1β | NM_031512 | F: ACCTGCTAGTGTGTGATGTTCCCA | 59.9 | 109 |
| R: AGGTGGAGAGCTTTCAGCTCACAT | 59.9 | |||
| TGFβ | NM_021578 | F: TGATACGCCTGAGTGGCTGTCTTT | 60 | 115 |
| R: TTTGCTGTCACAAGAGCAGTGAGC | 59.6 | |||
| TLR4 | NM_019178 | F: TCTGCCCTGCCACCATTTACAGTT | 60.7 | 135 |
| R: TGGTCTCAGGCAGGAAAGGAACAA | 60.1 | |||
| CX3CL1 | NM_134455.1 | F: ACTTCTGTGCTGACCCAAAGGAGA | 59.8 | 105 |
| R: CACGCTTCTCAAACTTGCCACCAT | 60 | |||
| CX3CR1 | NM_133534.1 | F: GTGCAAGCTCACGACTGCTTTCTT | 59.8 | 133 |
| R: GTGTTGCACTGTCCGGTTGTTCAT | 59.9 | |||
| GLT1 | AY_069978 | F: TCTTGCCAGCTTCCTGTTGTCTCA | 60.2 | 116 |
| R: ACACCTTGTGTGGCTTGGTGTTTC | 60 | |||
| GADPH | NM_017008 | F: TGACTCTACCCACGGCAAGTTCAA | 59.9 | 141 |
| R: ACGACATACTCAGCACCAGCATCA | 60 | |||
| XcT | NM_001107673 | F: TGTATGACTGGGAAACCACAGCGA | 60 | 175 |
| R: TACAGAGAAGCAGCTGGAAGCACA | 60 | |||
| BDNF | BC087634 | F: AGCCTGTGTACTTTGTGTCCGAGA | 60.1 | 123 |
| R: TGGACGTTTGCTTCTTTCATGGGC | 60 | |||
| DBH | HS11700 | F: TGGAATCTTGGAGGAGATGTGCGT | 59.9 | 128 |
| R: TGCCGAACCGGTTTACTATGTGGA | 60 |
2.7 Immunocytochemistry
Free-floating coronal sections (40 μm) were obtained with a vibratome (Leica, Model VT1000S) from perfused brains that were kept cold but never frozen. All reactions took place on an agitator at room temperature, except for overnight incubations, and all rinses (3 × 10 min) were in PBS with 0.05% tween 20 (PBSt). Tissue were rinsed, quenched of native peroxidase activity with 0.3% H2O2 in 50% methanol for 1 hr, rinsed, blocked for non-specific binding in 5% normal goat serum (NGS) for 1 hr, and incubated in primary antibody diluted in 5% NGS overnight at 4 °C. Double-immunostaining of all sections was achieved using these antibodies: anti-class II major histocompatibility complex (MHC II, 1: 200, mouse monoclonal, Pharmingen, San Diego, CA, #554926) and anti-TH (1:3000 for the LC and 1:1500 for the SNpc, rabbit polyclonal, Millipore, Billerica, MA, #AB152). Thereafter, sections were incubated in a corresponding biotinylated secondary antibody (1:200, Vector, Burlingame, CA, #BA-1000, BA-2001) for 1.5 hr, rinsed, incubated for 1 hr with avidin-biotinylated horseradish peroxidase (ABC kit, Vector), rinsed, and visualized by incubation with the chromogens 0.05% 3,3-diaminobenzidine tetrahydrochloride (DAB, Vector) or SG Blue (Vector). Double-chromogenic staining continued from this point by placing sections into another primary antibody and repeating these steps. After a final rinse, sections were mounted onto gel-coated slides and air-dried. All slides were then dehydrated with serial dilutions of ethanol and cover-slipped with Cytoseal mounting medium (Allan Scientific, Kalamazoo, MI). No staining was detected in the absence of the primary or secondary antibodies. Confidence was also established for antibodies that produced a band of the correct molecular weight in Western blot analysis.
Immunoreactive (IR) microglia and neurons were examined in the LC or SNpc by light microscopy (Nikon 90i with a DS-5M-L1 digital camera using Elements 3.1; Nikon Instruments, Melville, NY), on an average of ten evenly spaced slices per region. Cells were manually counted with Nikon Elements and expressed as number of IR cells per mm2.
2.8 Statistical analysis
ANOVA were performed followed by Fisher’s PLSD for post hoc comparisons using SigmaPlot (v.12.3 Systat, San Jose, CA). Control aCSF is shown in some graphs as one group collapsed across infusion duration, but aCSF groups were not collapsed for statistical analysis. Results are expressed as means ± SEM, and significant differences are marked between treatment (*), infusion duration (#) and age (†).
3. Results
3.1 Protein analysis
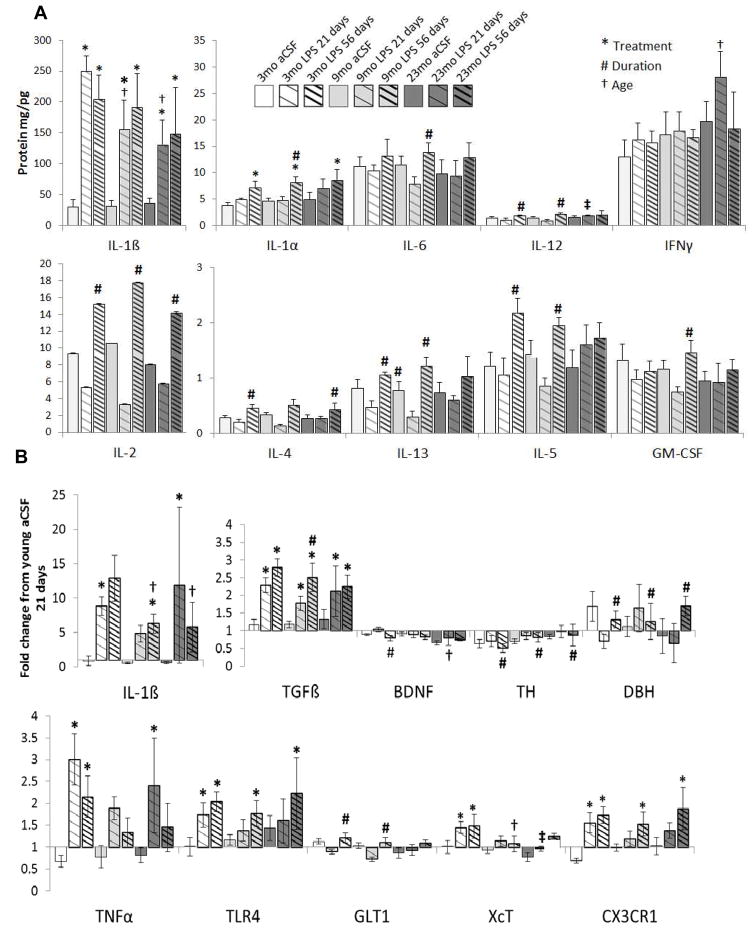
LPS infusion significantly increased the pro-inflammatory cytokines IL-1α (F1,45 = 19.4, p<0.001) and IL-1β (F1,46 = 58.9, p<0.001) levels in the midbrain/brainstem regions that included the SN and LC (Figure 1A). However, the IL-1β response to 21 days of LPS infusion was significantly (p<0.05) blunted in both middle-aged and aged rats. LPS infusion over 56 days significantly (all F>24, p<0.001) increased midbrain/brainstem levels of IL-1α, IL-2, IL-4, IL-5, IL-6, IL-12, IL-13 and GM-CSF compared to 21 days of LPS infusion in rats of the same age. Although aged rats infused with LPS for 21 days had a blunted IL-1β response, this group had elevated pro-inflammatory IFNγ levels compared to 21 day LPS infusion in younger rats (F2,48 = 3.62, p<0.05).
Figure 1.
Brainstem cytokines levels and gene expression. (A) LPS exposure significantly (*p<0.05) increased in IL-1β levels and a far lesser increase in IL-1α levels. The duration of the LPS infusion was independently responsible with a significant (#p<0.001) increase in the brainstem level of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-12, IL-13 and GM-CSF. The age of the rat was independently responsible for an increase in IFNγ levels in the23 mo rats infused with LPS and for a blunting of the LPS effects on IL-1β in the 9 and 23 mo rats (†p<0.05). (B) LPS significantly (*p<0.001) increased the gene expression of the fractalkine receptor, IL1β, TGFβ, TLR4, TNFα and XcT. The duration of the LPS infusion was responsible with a significant (#p<0.01) increase in gene expression of GLT1 and TGFβ. In contrast, the duration of the LPS infusion was responsible with a significant decrease (#p <0.01) in the gene expression of TH, TNFα and BDNF within the brainstem and DBH gene expression within the hippocampus. The age of the rat was independently responsible for a significant (†p<0.001) decrease in the gene expression of BDNF and XcT. Each biomarker represents a minimum of 6 rats/group.
Serum levels of IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL-13, GM-CSF, INFγ and TNFα were unchanged across all age and treatment groups (data not shown), and may indicate that intracranial infusion of LPS did not have a marked response on the peripheral immune response.
3.2 mRNA expression
LPS exposure significantly (all F>32, p<0.001) increased the gene expression of pro-inflammatory IL1β and TNFα, the LPS receptor TLR4, anti-inflammatory TGFβ and CX3CR1 as well as the glutamate-antiporter XcT (Figure 1B). Consistent with the results observed in protein analysis, the IL-1β response to 56 days of LPS infusion was significantly (p<0.05) blunted in both middle-aged and aged rats compared to young rats. Gene expression of TGFβ increases more after 56 days of LPS infusion than 21 days infusion in middle-aged rats (F1,48=6.39, p<0.01) and a similar duration-dependent increase in GLT1 is observed in young and middle-aged rats (F1,48=12.1, p<0.001). TGFβ is anti-inflammatory and GLT1 serves to sequester extracellular glutamate that can be excitotoxic; an increase in these factors after prolonged LPS exposure could a protective mechanism that does not occur in aged rats. Consistent with this, aged rats infused with LPS express less of the trophic factor BDNF than younger rats infused with LPS (F1,48=8.94, p<0.001) and by middle-age rats infused with LPS express less XcT (F1,48=4.13, p<0.05) which could otherwise be used to acquire cysteine for anti-oxidant production. Interestingly, across all ages, 56 days of LPS infusion was responsible for a significant decrease (all F>6, p <0.01) in the gene expression of TH within the midbrain/brainstem and a corresponding elevation in DBH gene expression within the hippocampus; suggesting a loss of midbrain/brainstem catecholamine production capacity and a compensatory increase in hippocampal catecholamine production capacity.
3.3 Immunohistochemistry
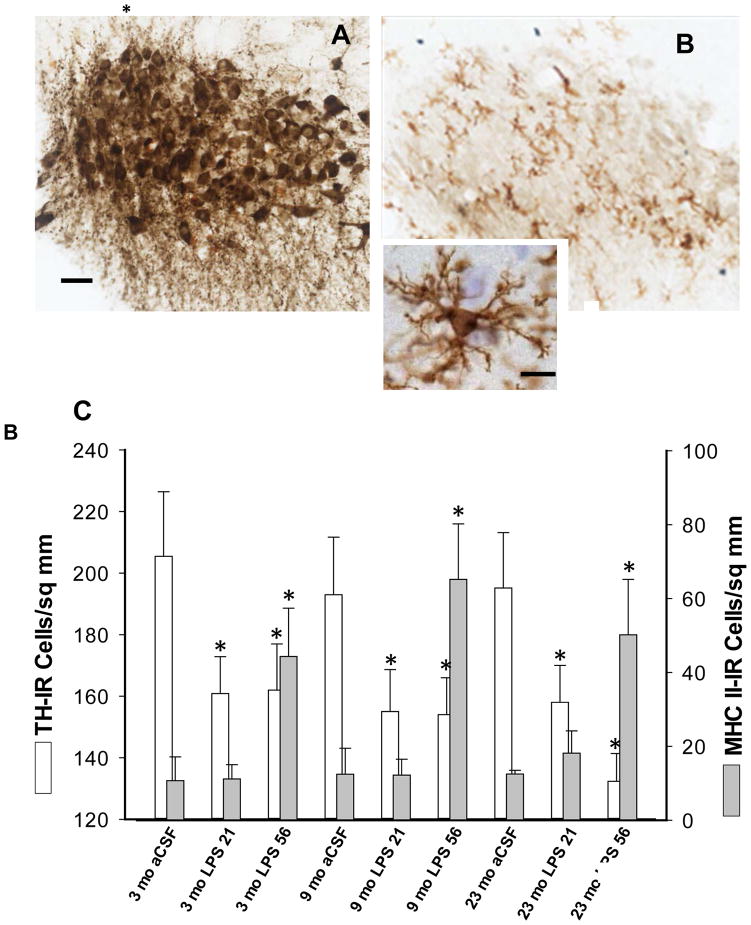
The density of TH-IR cells in the LC, i.e. the number of cell profiles/sq. mm, was significantly decreased by the LPS infusion (F1,41=7.44, p<0.01, Figure 2 A vs. B) and was not dependent upon the age of the rat or the duration of the LPS exposure (p>0.1). The density of MHC II-IR microglia (Figure 2 B, C) throughout the brainstem and particularly within the LC was significantly increased by the LPS infusion (F1,41=14.3, p<0.001) and further increased by the duration of the exposure (F1,41=11.0, p<0.01).
Figure 2.
Photomicrographs of double-immunostained sections showing TH-IR cells and MHC II-IR microglia in the LC. (A) 3 mo aCSF 56 days; (B) 23 mo LPS 56 days. Scale bar for A & B = 200 μm. Inset shows the morphology of a typical microglia (section was counterstained with cresyl violet); scale bar for inset = 2.5 μm. The density of MHC II-IR microglia in the LC was significantly increased by the LPS infusion after 56 days of exposure. Only MHC II-IR microglia with their characteristic bushy morphology and ramified processes are visible in B. The density of darkly-stained TH-IR cells and their fibers (A) in the LC was significantly decreased by the LPS infusion (B, C). The effects of LPS were not influenced by the age of the rat or the duration of the LPS exposure (*p<0.01 vs. 3 mo aCSF 21). The histological analyses in C represent a minimum 5 rats/group.
The density of TH-IR cells in the SNpc (Figure 3) was significantly (F2,42=5.26, p<0.01) decreased due to aging. There was a significant main effect of duration of LPS exposure (F1,42 =6.78, p<0.01); post-hoc tests revealed that the number of TH-IR cells in the SNpc of young and middle-aged rats were elevated after 56 days of LPS infusion compared to 21 days infusion (p<0.05). Our previous stereological analysis did not find evidence for tissue shrinkage (Brothers et al., 2013a). This recovery in the density of TH-IR in the SNpc was not observed in the aged rats. We have previously reported a statistically significant increase in the number of MHC II-IR microglia within the SNpc following LPS exposure that was associated with a decline in the number of phosphorylated (i.e. active) TH-IR SNpc cells (Brothers et al., 2013a).
Figure 3.
Photomicrographs or MHC II-IR and SNpc cells and density quantification of TH-IR cells in the SNpc. (A) 3 mo aCSF 21 days; (B) 3 mo LPS 21 days; (C) 23 mo aCSF 21 days; (D) 23 mo LPS 21 days. Scale bar: 400 μm. Aging significantly decreased the density of TH-IR cells in the SNpc. The number of TH-IR cells in the SNpc of young and middle-aged, but not aged, rats was significantly greater on day 56 than day 21. Many MHC II-IR microglia with their characteristic bushy morphology and ramified processes are visible in B surrounding and within the region of the SNpc; very few darkly stained TH-IR cells are present. (*p<0.01 vs. 3 mo aCSF 21; +p<0.05 vs aCSF and LPS 56). Data from the 21 and 56 day aCSF groups were collapsed for representation. The histological analyses represent a minimum 5 rats/group.
4. Discussion
We induced a pro-inflammatory environment in the brains of young, middle-aged and aged male rats, and then systematically documented an evolving series of biochemical and genetic changes in the midbrain/brainstem induced by the chronic infusion of picomolar levels of LPS into the IVth ventricle. These changes represent a complex interplay between glia and neurons in the presence of continuous stimulation of TLR4 receptors on microglia, and suggest that a prolonged pro-inflammatory environment interacts with age to reduce the capacity of ascending catecholaminergic nuclei in the midbrain and brainstem. These findings relate to the pre-symptomatic inflammatory environment observed in AD and PD and early loss of these ascending systems in disease progression (Akiyama et al., 2000; Aarsland et al., 2001; Bartels and Leenders, 2005; Block and Hong, 2005; Cribbs et al., 2012; Griffin et al., 1989; Hobson and Meara, 2004; Hughes et al., 2000; Swardfager et al., 2010; Whitton, 2007).
LPS was used as a chemical tool to stimulate the TLR4/CD14 complex expressed by microglia; administered as a continuous low dose, LPS was not sufficient to produce any peripheral manifestations of infectious disease processes (such as elevated serum levels of inflammatory cytokines) or down-regulation in microglial TLR4 gene expression in the brain (Bardou et al., 2013; Brothers et al., 2013b). LPS did promote a central pro-inflammatory environment. For example, LPS increased midbrain/brainstem levels of pro-inflammatory IL-1β and IL-1α. IL-1β gene and protein levels correlated significantly with each other (r=0.74, p<0.001). This variance in response to LPS is likely due to the differences in post-translational control of IL-1β protein production (Chen et al., 2006). This distinction may be important, given the role of IL-1β in the degeneration of SNpc DA neurons in PD (Koprich et al., 2008). Interestingly, elevations in IL-1β gene and protein expression due to LPS infusion were blunted in both middle-aged and aged rats. Ordinarily, GM-CSF levels are barely detectable in the middle-aged brain (Dame et al., 1999). However, in the current study, GM-CSF levels increased within the brainstem in response to prolonged (56 days) LPS exposure, but only in the middle-aged rats. GM-CSF is a pro-inflammatory factor released by astrocytes in response to IL-1β or LPS that binds to a specific receptor on microglia leading to up-regulation of MHC II expression (Pierson et al., 2012) and TLR4 gene expression (Parajuli et al., 2012) similar to that seen in the current study. GM-CSF can also enhance the LPS-induced NF-κB nuclear translocation and production of IL-1β (Parajuli et al., 2012), also similar to that seen in the current study. TNFα gene expression was increased by the LPS infusion but this increase was not influenced by the aged of the animal. Typically, with normal aging, microglia release greater amounts of TNFα protein in response to LPS (Bardou et al., 2013; Colton and Wilcock, 2010; Harry, 2013); the discrepancy with our results may be due to age-related changes in post-translational control of TNFα protein production (Chen et al., 2006). Finally, LPS also produced a duration-dependent increase in IL-13. IL-13 induces a class of protein-degrading enzymes, known as matrix metalloproteinases and may also contribute to the development of disorders that involve neuroinflammation such as amyotrophic lateral sclerosis (Shi et al., 2007), PD and multiple sclerosis (Kim and Joh, 2012). Gene expression of the anti-inflammatory cytokine TGFβ was also significantly increased by the LPS infusion. TGFβ can, in turn, induce a persistent up-regulation of genes related to inflammation (Cacheaux et al., 2009). Higher concentrations of TGFβ have been found in the blood and cerebrospinal fluid of patients with PD or AD (Swardfager et al., 2010).
CX3CR1 is neuroprotective and it is involved in terminating the expression of microglia pro-inflammatory biomarkers (Harry, 2013). Normal aging is typically associated with a down-regulation of the production of CX3CR1proteins that may result from cell senescence (Wynne et al., 2010). In the current study, gene expression for this receptor was significantly increased following exposure to LPS; furthermore, this increase was independent of the age of the rat. This apparent disconnection between the influence of aging upon CX3CR1 gene expression and protein production may be due to senescence-induced changes in post-translational control of protein production.
Dysregulation of glutamatergic neurotransmission may underlie the pathology associated with chronic neuroinflammation in the brain (Rosi et al., 2004; Wenk and Hauss-Wegrzyniak, 2001). TNFα can suppress GLT1 transcription (Sitcheran et al., 2005) and induce glutamate release from microglia (Takeuchi et al., 2006). IL-1β can trigger the release of glutamate from neurons (Liu et al., 2011) and inhibit GLT1 expression on astrocytes (Prow and Irani, 2008) and microglia (Takaki et al., 2012). Reactive oxygen species (ROS), such as those produced by activated microglia during neuroinflammation, inhibit glutamate uptake by astrocytes (Trotti et al., 1998). Increased extracellular glutamate also increases microglial release of pro-inflammatory signals (Taylor et al., 2005) which in turn would increase glutamate levels further leading to a positive feedback cycle. When extracellular glutamate levels are elevated, IL-1β can increase system XcT activity on astrocytes, resulting in increased neurotoxicity by glutamate (Jackman et al., 2010).
In the current study, LPS increased gene expression for IL-1β, TNFα and GLT1, as well as IL-1β protein levels. As expected, XcT gene expression increased in parallel to the elevation in IL-1β gene and protein levels. Surprisingly, this compensatory increase in XcT gene expression was not observed in middle-aged and aged rats. XcT is a heteromeric Na+-independent anionic amino acid transport system that specifically facilitates the exchange of anionic amino acids for anionic forms of cystine and glutamate, thereby mediating the formation of glutathione within the neuron. Similarly, there was a duration-dependent increase in GLT1 gene expression in the young and middle-aged rats but not in the aged rats. We have previously shown by flow cytometry a significant increase in the number of CD11b/c-immunopositive microglia expressing GLT1 protein following chronic exposure to LPS (Brothers et al., 2013b). Taken together, our results suggest that with advanced age the brain’s ability to regulate glutamate levels and the production of glutathione are impaired, ultimately leading to increased neuronal vulnerability to the oxidative stress associated with chronic neuroinflammation.
LC degeneration occurs early in the progression of AD (Szot et al., 2006) and coincides with the presence of a pro-inflammatory environment (Cagnin et al., 2006). TH and DBH are precursors in the production of NE, the primary neurotransmitter found in LC projections to the hippocampus. We show a duration-dependent loss in TH gene expression in the midbrain/brainstem and reduced number of TH-IR cells in the LC and SNpc with LPS infusion and age. Overall, the oldest rats who experienced the longest duration of LPS infusion demonstrated the greatest decline in TH-IR cells in LC and SNpc, consistent with previous investigations (Mouton et al., 2012). The current study was designed to investigate for such an age-dependent increase in vulnerability to chronic neuroinflammation and identify a specific inflammatory biomarker underlying the LC cell loss in animal models of AD (Manaye et al., 2013). We anticipated that IL-6 might play a role in the age-related vulnerability (Ye and Johnson, 1999) but IL-6 levels in the current study only increased in response to the duration of the LPS infusion and was not dependent upon the age of the rat. BDNF signaling via trkB neurotrophin receptors is important for the maintenance of the LC innervation of the hippocampus (von Bohlen und Halbach and Minichiello, 2006); in the current study aged rats infused with LPS expressed significantly less of the gene for BDNF than did younger rats infused with LPS.
Interestingly, after 56 days of LPS infusion, DBH is significantly increased in the hippocampus in all age groups compared to a decrease (although not significant) after 21 days of LPS infusion. This suggests a compensatory up-regulation in the DBH gene, consistent with our previous report (Brothers et al., 2013a). A decrease in TH enzyme levels in the midbrain/brainstem suggests a decline in catecholamine function that may underlie aspects of the cognitive impairment seen in AD (Grudzien et al., 2007). Cognitive decline may also be related to the fact that NE acts as an anti-inflammatory molecule within the cortex and hippocampus (Feinstein et al., 2002; Wenk et al., 2003), stimulates BDNF production (Mannari et al., 2008) and supports neurogenesis (Masuda et al., 2012); all functions that could be improved by a compensatory increase in hippocampal DBH. Overall, chronic neuroinflammation leads to impaired LC cellular integrity, reduced hippocampal neurogenesis (Marchalant et al., 2009) and reduced BDNF gene expression (Tong et al., 2008).
SNpc cellular degeneration is influenced by the presence of neuroinflammation early in the progression of PD (Wang et al., 2013; Tome et al., 2013). Previous inflammatory models of PD that injected LPS directly into the SN significantly reduced the number of TH-IR neurons (Herrera et al., 2000; Kim et al., 2000). In contrast, an acute intra-nigral injection of TNF-α (Castano et al., 2002) or an acute peripheral injection of a high dose of LPS both failed to reduce the number of TH SNpc neurons (Mouton et al., 2012). Similarly, we have demonstrated that a single acute injection of LPS into the nbM failed to reduce the number of acetylcholinergic neurons (Willard et al., 1999). In the current study, three weeks of continuous LPS infusion produced a decline in the number of TH-IR cells in young and middle-aged rats, but no additional decline in the number of TH-IR cells in the SNpc of aged rats. After eight weeks of LPS infusion the number of TH-IR cells in the SNpc had completely recovered in young and middle-aged rats. In addition, the density of TH-IR cells in the SNpc was decreased due to normal aging; a decline in SNpc function that is consistent with previous reports (Gozlan et al., 1990; Miguez et al., 1999). Thus, the consequences of pro-inflammatory environment likely depend upon an interaction between the duration of the exposure to specific cytokines (Bardou et al., 2013), the age of the brain and the specific region. For example, the vulnerability of SNpc cells was primarily dependent upon the duration of the pro-inflammatory environment, while the vulnerability of LC cells was not changed by longer exposure to LPS. Overall, the infusion of LPS increased the density of MHC II-IR microglia and expression of pro-inflammatory cytokines (Il-1β and IL-1α) throughout the midbrain/brainstem.
Our data support the concept that continuous exposure to a pro-inflammatory environment drives exaggerated changes in the production and release of inflammatory mediators, decreased production protective factors, altered glutamate regulation and impaired cellular function within the SNpc and LC. Furthermore, our data show that the response to a pro-inflammatory environment changes with age. Overall, these data suggest that early anti-inflammatory intervention is an important therapeutic opportunity in neurodegenerative diseases which have defining pathology of neuroinflammation such as PD and AD.
Acknowledgments
Supported by U.S. Public Health Service, RO1 AG030331, RO1 AG037320 and The Ohio State University Women and Philanthropy Program to GLW.
Footnotes
Disclosure statement
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh-Sorensen P. Risk of dementia in Parkinson’s disease: a community based, prospective study. Neurol. 2001;56:730–736. doi: 10.1212/wnl.56.6.730. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cooper NR, Eikelenboom P, Emmerling M, Fiebich B, Finch CE, Frautschy S, Griffin WS, Hampel H, Landreth G, McGeer PL, Mrak R, MacKenzie I, O’Banion K, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray A. Inflammation in Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardou I, Brothers HM, Kaercher RM, Hopp SC, Wenk GL. Differential effects of duration and age upon the consequences of neuroinflammation in the hippocampus. Neurobiol Aging. 2013;34:2293–2301. doi: 10.1016/j.neurobiolaging.2013.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels AL, Leenders KL. Neuroinflammation in the pathophysiology of Parkinson’s disease: evidence from animal models to human in vivo studies with [11C] PK11195 PET. Mov Disord. 2005;22:1852–1856. doi: 10.1002/mds.21552. [DOI] [PubMed] [Google Scholar]
- Bilbo SD. Early-life infection is a vulnerability factor for aging-related glial alterations and cognitive decline. Neurobiol Learn Mem. 2010;94:57–64. doi: 10.1016/j.nlm.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, DeVos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Brothers HM, Bardou I, Hopp SC, Marchalant Y, Kaercher RM, Turner SM, Mitchem MR, Kigerl K, Wenk GL. Time-dependent compensatory responses to chronic neuroinflammation in hippocampus and brainstem: the potential role of glutamate neurotransmission. J Alz Dis & Parkin. 2013a doi: 10.4172/2161-0460.1000110. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers HM, Bardou I, Hopp SC, Kaercher RM, Wynne-Corona A, Fenn AM, Godbout JP, Wenk GL. Riluzole partially rescues age-associated, but not LPS-induced, loss of glutamate transporters and spatial memory impairment. J Neuroimm Pharmacol. 2013b doi: 10.1007/s11481-013-9476-2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacheaux LP, Ivens S, David Y, Lakhter AJ, Bar-Klein G, Shapira M, Heinemann U, Friedman A, Kaufer D. Transcriptome profiling reveals TGF-beta signaling involvement in epileptogenesis. J Neurosci. 2009;29:8927–8935. doi: 10.1523/JNEUROSCI.0430-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnin A, Kassiou M, Meikle SR, Banati RB. In vivo evidence for microglial activation in neurodegenerative dementia. Acta Neurolog Scand. 2006;114:107–114. doi: 10.1111/j.1600-0404.2006.00694.x. [DOI] [PubMed] [Google Scholar]
- Castano A, Herrera AJ, Cano J, Machado A. The degenerative effect of a single intranigral injection of LPS on the dopaminergic system is prevented by dexamethasone, and not mimicked by rh-TNF-alpha, IL-1beta and IFN-gamma. J Neurochem. 2002;81:150–157. doi: 10.1046/j.1471-4159.2002.00799.x. [DOI] [PubMed] [Google Scholar]
- Chen YL, Huang YL, Lin NY, Chen HC, Chiu WC, Change CJ. Differential regulation of ARE-mediated TNFα and IL-1β mRNA stability by lipopolysaccharide in RAW264.7 cells. Biochem Biophys Res Commun. 2006;346:160–168. doi: 10.1016/j.bbrc.2006.05.093. [DOI] [PubMed] [Google Scholar]
- Colton CA, Wilcock DM. Assessing Activation States in Microglia. CNS and Neurol Disord Drug Targets. 2010;9:174–191. doi: 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- Cribbs DH, Berchtold NC, Perreau V, Coleman PD, Rogers J, Tenner AJ, Cotman CW. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J Neuroinflamm. 2012;9:179. doi: 10.1186/1742-2094-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dame JB, Christensen RD, Juul SE. The distribution of granulocyte-macrophage colony-stimulating factor and its receptor in the developing human fetus. Pediatr Res. 1999;46:358–366. doi: 10.1203/00006450-199910000-00002. [DOI] [PubMed] [Google Scholar]
- Eikelenboom P, van Exel E, Hoozemans JJM, Veerhuis R, Rosemuller AJM, van Gool WA. Neuroinflammation – An early event in both the history and pathogenesis of Alzheimer’s disease. Neurodegen Dis. 2010;7:38–41. doi: 10.1159/000283480. [DOI] [PubMed] [Google Scholar]
- Feinstein DL, Heneka MT, Gavrilyuk V, Dello Russo C, Weinberg G, Galea E. Noradrenergic regulation of inflammatory gene expression in brain. Neurochem Int. 2002;41:357–365. doi: 10.1016/s0197-0186(02)00049-9. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Packard MG, Campana E, Pacitti C. Anterograde and retrograde tracing of projections from the ventral tegmental area to the hippocampal formation in the rat. Brain Res Bull. 1994;33:445–452. doi: 10.1016/0361-9230(94)90288-7. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ. In vivo imaging of microglial activation with [C-11](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol Dis. 2006;21:404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Gozlan H, et al. Aging associated changes in serotoninergic and dopaminergic pre- and postsynaptic neurochemical markers in the rat brain. Neurobiol Aging. 1990;11:437–449. doi: 10.1016/0197-4580(90)90011-n. [DOI] [PubMed] [Google Scholar]
- Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, III, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci, USA. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grudzien A, Shaw P, Weintraub S, Bigio E, Mash DC, Mesulam MM. Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer’s Disease. Neurobiol Aging. 2007;28:327–335. doi: 10.1016/j.neurobiolaging.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Del Tredici K, Braak H. Critical appraisal of brain pathology staging related to presymptomatic and symptomatic cases of sporadic Parkinson’s disease. J Neural Trans Suppl. 2006;70:99–103. doi: 10.1007/978-3-211-45295-0_16. [DOI] [PubMed] [Google Scholar]
- Harry GJ. Microglia during development and aging. Pharmacol & Therap. 2013;139:313–326. doi: 10.1016/j.pharmthera.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Dobrzanski P, Stoehr JD, Wenk GL. Chronic neuroinflammation in rats reproduces components of the neurobiology of Alzheimer’s disease. Brain Res. 1998;780:294–303. doi: 10.1016/s0006-8993(97)01215-8. [DOI] [PubMed] [Google Scholar]
- Heneka MT, O’Banion MK, Terwel D, Kummer MP. Neuroinflammatory processes in Alzheimer’s disease. J Neural Trans. 2010;117:919–947. doi: 10.1007/s00702-010-0438-z. [DOI] [PubMed] [Google Scholar]
- Herrera AJ, Castano A, Venero JL, Cano J, Machado A. The single intranigral injection of LPS as a new model for studying the selective effects of inflammatory reactions on dopaminergic system. Neurobiol Dis. 2000;7:429–447. doi: 10.1006/nbdi.2000.0289. [DOI] [PubMed] [Google Scholar]
- Herrup K. Reimagining Alzheimer’s disease- an age-based hypothesis. J Neurosci. 2010;30:16755–16762. doi: 10.1523/JNEUROSCI.4521-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson P, Meara J. Risk and incidence of dementia in a cohort of older subjects with Parkinson’s disease in the United Kingdom. Mov Disord. 2004;19:1043–1049. doi: 10.1002/mds.20216. [DOI] [PubMed] [Google Scholar]
- Hughes TA, Ross HF, Musa S, Bhattacherjee S, Nathan RN, Mindham RH, Spokes EG. A 10-year study of the incidence of and factors predicting dementia in Parkinson’s disease. Neurol. 2000;54:1596–1602. doi: 10.1212/wnl.54.8.1596. [DOI] [PubMed] [Google Scholar]
- Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropath. 2003;106:518. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- Jackman NA, Uliasz TF, Hewett JA, Hewett SJ. Regulation of system x(c)(−) activity and expression in astrocytes by interleukin-1 beta: implications for hypoxic neuronal injury. Glia. 2010;58:1806–1815. doi: 10.1002/glia.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Joh TH. Matrix metalloproteinases, new insights into the understanding of neurodegenerative disorders. Biomolec Therapeut. 2012;20:133–143. doi: 10.4062/biomolther.2012.20.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J Neurosci. 2000;20:6309–6316. doi: 10.1523/JNEUROSCI.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprich JB, Reske-Nielsen C, Mithal P, Isacson O. Neuroinflammation mediated by IL-1β increases susceptibility of dopamine neurons to degeneration in an animal model of Parkinson’s disease. J Neuroinflamm. 2008;5:8. doi: 10.1186/1742-2094-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Aboud O, Jones RA, Mrak RE, Griffin WST, Barger SW. Apolipoprotein E expression is elevated by interleukin 1 and other interleukin 1-induced factors. J Neuroinflamm. 2011;8:175. doi: 10.1186/1742-2094-8-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaye KF, Mouton PR, Xu G, Drew A, Lei DL, Sharma Y, Rebeck GW, Turner S. Age-related loss of noradrenergic neurons in the brains of triple transgenic mice. Age. 2013;35:139–147. doi: 10.1007/s11357-011-9343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannari C, Origlia N, Scatena A, Del Debbio A, Catena M, Dell’agnello G, Barraco A, Giovannini L, Dell’osso L, Domenici L, Piccinni A. BDNF level in the rat prefrontal cortex increases following chronic but not acute treatment with duloxetine, a dual acting inhibitor of noradrenaline and serotonin re-uptake. Cell Mol Neurobiol. 2008;28:457–468. doi: 10.1007/s10571-007-9254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalant Y, Brothers HM, Norman GJ, Karolina K, DeVries C, Wenk GL. Cannabinoids attenuate the effects of aging upon neuroinflammation and neurogenesis. Neurobiol Dis. 2009;34:300–307. doi: 10.1016/j.nbd.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Marchalant Y, Rosi S, Wenk GL. Anti-inflammatory property of the cannabinoid agonist WIN-55212-2 in a rodent model of chronic brain inflammation. Neurosci. 2007;144:1516–1522. doi: 10.1016/j.neuroscience.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Nakagawa S, Boku S, Nishikawa H, Takamura N, Kato A, Inoue T, Koyama T. Noradrenaline increases neural precursor cells derived from middle-aged rat dentate gyrus through beta2 receptor. Prog Neuropsychopharmacol Biol Psychiat. 2012;36:44–51. doi: 10.1016/j.pnpbp.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Miguez JM, Aldegunde M, Paz-Valinas L, Recio J, Sanchez-Barcelo E. Selective changes in the contents of noradrenaline, dopamine and serotonin in rat brain areas during aging. J Neural Trans. 1999;106:1089–1098. doi: 10.1007/s007020050225. [DOI] [PubMed] [Google Scholar]
- Mouton PR, Kelly-Bell B, Tweedie D, Spangler EL, Perez E, Carlson OD, Short RG, deCabo R, Chang J, Ingram DK, Li Y, Greig NH. The effects of age and lipopolysaccharide (LPS)-mediated peripheral inflammation on numbers of central catecholaminergic neurons. Neurobiol Aging. 2012;33:423–427. doi: 10.1016/j.neurobiolaging.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parajuli B, Sonobe Y, Kawanokuchi J, Doi Y, Noda M, Takeuchi H, Mizuno T, Suzumura A. GM-CSF increases LPS-induced production of proinflammatory mediators via upregulation of TLR4 and CD14 microglia. J Neuroinflam. 2012;9:268. doi: 10.1186/1742-2094-9-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson E, Simmons SB, Castelli L, Goverman JM. Mechanisms regulating regional localization of inflammation during CNS autoimmunity. Immunol Rev. 2012;248:205–215. doi: 10.1111/j.1600-065X.2012.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prow NA, Irani DN. The inflammatory cytokine, interleukin-1 beta, mediates loss of astroglial glutamate transport and drives excitotoxic motor neuron injury in the spinal cord during acute viral encephalomyelitis. J Neurochem. 2008;105:1276–1286. doi: 10.1111/j.1471-4159.2008.05230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Ramirez-Amaya V, Hauss-Wegrzyniak B, Wenk GL. Chronic brain inflammation leads to a decline in hippocampal NMDA R1 receptors. J Neuroinflamm. 2004;1:12–18. doi: 10.1186/1742-2094-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudow G, O’Brien R, Savonenko AV, Resnick SM, Zonderman AB, Pletnikova O, Marsh L, Dawson TM, Crain BJ, West MJ, Troncoso JC. Morphometry of the human substantia nigra in ageing and Parkinson’s disease. Acta Neuropathol. 2008;115:461–470. doi: 10.1007/s00401-008-0352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi N, Kawano Y, Tateishi T, Kikuchi H, Osoegawa M, Ohyagi Y, Kira J. Increased IL-13-producing T cells in ALS: Positive correlations with disease severity and progression rate. J Neuroimmunol. 2007 doi: 10.1016/j.jneuroim.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Sitcheran R, Gupta P, Fisher PB, Baldwin AS. Positive and negative regulation of EAAT2 by NF-kappaB: a role for N-myc in TNFalpha-controlled repression. EMBO. 2005;24:510–520. doi: 10.1038/sj.emboj.7600555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Das A, Ray SK, Banik NL. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull. 2012;87:10–20. doi: 10.1016/j.brainresbull.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swardfager W, Lanctôt K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiat. 2010;68:930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, Raskind MA. Compensatory changes in the noradrenergic nervous system in the locus ceruleus and hippocampus of postmortem subjects with Alzheimer’s disease and dementia with Lewy bodies. J Neurosci. 2006;26:467–478. doi: 10.1523/JNEUROSCI.4265-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki J, Fujimori K, Miura M, Suzuki T, Sekino Y, Sato K. L-glutamate released from activated microglia downregulates astrocytic L-glutamate transporter expression in neuroinflammation: the “collusion” hypothesis for increased extracellular L-glutamate concentration in neuroinflammation. J Neuroinflamm. 2012;9:275. doi: 10.1186/1742-2094-9-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, et al. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem. 2006;281:21362–21368. doi: 10.1074/jbc.M600504200. [DOI] [PubMed] [Google Scholar]
- Taylor DL, Jones F, Kubota ES, Pocock JM. Stimulation of microglial metabotropic glutamate receptor mGlu2 triggers tumor necrosis factor alpha-induced neurotoxicity in concert with microglial-derived Fas ligand. J Neurosci. 2005;25:2952–2964. doi: 10.1523/JNEUROSCI.4456-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tome CML, Tyson T, Rey NL, Grathwohl S, Britschgi M, Brundin P. Inflammation and alpha-Synuclein’s Prion-like Behavior in Parkinson’s Disease-Is There a Link? Molec Neurobiol. 2013;47:561–574. doi: 10.1007/s12035-012-8267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Balazs R, Soiampornkul R, Thangnipon W, Cotman CW. Interleukin-1 beta impairs brain derived neurotrophic factor-induced signal transduction. Neurobiol Aging. 2008;29:1380–1393. doi: 10.1016/j.neurobiolaging.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotti D, Danbolt NC, Volterra A. Glutamate transporters are oxidant-vulnerable: a molecular link between oxidative and excitotoxic neurodegeneration? TIPS. 1998;19:328–334. doi: 10.1016/s0165-6147(98)01230-9. [DOI] [PubMed] [Google Scholar]
- Von Bohlen und Halbach O, Minichiello L. Neurotrophin receptor heterozygosity causes deficits in catecholaminergic innervation of amygdala and hippocampus in aged mice. J Neural Transm. 2006;113:1829–1836. doi: 10.1007/s00702-006-0498-2. [DOI] [PubMed] [Google Scholar]
- Wang J, Song N, Jiang H, Wang J, Xie JX. Pro-inflammatory cytokines modulate iron regulatory protein 1 expression and iron transportation through reactive oxygen/nitrogen species production in ventral mesencephalic neurons. Biochim Biophys Acta Molec Basis Dis. 2013;1832:618–625. doi: 10.1016/j.bbadis.2013.01.021. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Hauss-Wegrzyniak B. Animal models of chronic neuroinflammation as a model of Alzheimer’s Disease. In: Bondy S, Campbell A, editors. Inflammatory Events in Neurodegeneration. Scottsdale, AZ: Prominent Press; 2001. pp. 83–87. [Google Scholar]
- Wenk GL, McGann K, Hauss-Wegrzyniak B, Rosi S. The toxicity of tumor necrosis factor-α upon cholinergic neurons within the nucleus basalis and the role of norepinephrine in the regulation of inflammation: implications for Alzheimer’s disease. Neurosci. 2003;121:719–729. doi: 10.1016/s0306-4522(03)00545-1. [DOI] [PubMed] [Google Scholar]
- Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br J Pharmacol. 2007;150:963–976. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard LB, Hauss-Wegrzyniak B, Wenk GL. The pathological and biochemical consequences of acute and chronic neuroinflammation within the basal forebrain of rats. Neurosci. 1999;88:193–200. doi: 10.1016/s0306-4522(98)00216-4. [DOI] [PubMed] [Google Scholar]
- Wynne AM, Henry CJ, Huang Y, Cleland A, Godbout JP. Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behav Immun. 2010;24:1190–1201. doi: 10.1016/j.bbi.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol. 1999;93:139–148. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]