Abstract
Background
Corticosteroids increase risk for decreased bone mineral density, which can be worsened by vitamin D insufficiency (VDI) or deficiency (VDD).
Procedure
In the Vanderbilt cancer survivorship clinic, we obtained screening total 25-hydroxy vitamin D levels (VDL) in 171 cancer survivors <23 years old who were treated with prolonged corticosteroids for their cancer, and compared this group to a control group of 97 healthy pediatric patients.
Results
VDD was diagnosed in 15.8% and VDI in 34.5% of cancer survivors and VDD/VDI combined was associated with body mass index (BMI) >85th percentile (Odds ratio (OR) = 5.4; p<0.001), older age (OR = 2.2; p=0.012), non-Caucasian or Hispanic race (OR = 4.5 p = 0.008) and summer versus winter season (OR= 0.12, p<0.001). In multivariable analysis, VDI/VDD prevalence did not differ from the control group (VDI/VDD (43.3%). In the combined survivor/control group multivariable analysis, cancer diagnosis did not increase VDI/VDD risk, but significant associations persisted with elevated BMI (p <0.001), age (p=0.004), non-Caucasian or Hispanic race (p<0.001), and seasonality (p<0.001).
Conclusion
VDD/VDI is equally common in pediatric cancer survivors treated with corticosteroids and healthy children. The impact of VDD/VDI in cancer survivors may be greater due to risk for impaired bone health superimposed on that conferred from corticosteroid exposure. Thus, screening VDLs should be obtained in pediatric cancer survivors treated with corticosteroids, particularly in those with elevated BMI, older age, or non-Caucasian race. Prospective studies evaluating the impact of interventions to minimize VDD/VDI on long-term bone health in survivors are required.
Keywords: ALL, Vitamin D sufficiency, Survivorship
Introduction
With excellent survival rates associated with childhood cancer (1), an increasing number of children are long-term survivors and may be at risk for long-term adverse health effects of their primary cancer and its treatments. Therefore, risk-adapted medical follow-up is recommended for childhood cancer survivors (2, 3). To inform such follow-up, the Children’s Oncology Group (COG) has developed risk-adapted, exposure-based long-term follow-up guidelines (3, 4), and such follow-up has identified significant chronic health conditions (4–9).
It is well established that a subset of survivors of childhood cancer are at risk for decreased bone mineral density (BMD) with studies reporting prevalence rates that range widely from 21–65% (4, 6, 10–14). Therefore, the COG guidelines recommend a screening DXA scan or quantitative CT two years after therapy completion in patients treated with corticosteroids, high dose methotrexate, or stem cell transplant (3, 4).
The risk for decreased bone mineral density associated with corticosteroid exposure may be further compounded by vitamin D deficiency (VDD) or insufficiency (VDI), a known risk factor for osteopenia and osteoporosis in the general population (15, 16). VDD is prevalent in survivors of pediatric acute lymphoblastic leukemia (18), where increased adiposity is also reported (5, 7, 9, 17–20) and may result in enhanced sequestration of vitamin D in fat (21).
Therefore, in our institutional cancer survivorship clinic, we initiated screening for VDD in patients treated with corticosteroids as part of their cancer therapy to determine the prevalence of vitamin D abnormalities. Additionally, we compared total 25-hydroxy vitamin D levels (VDL) to a control group of healthy pediatric patients. Furthermore, we examined associations between VDL and known risk factors for VDI/VDD: age, race, season and elevated BMI.
Methods
Construction of the Cohort
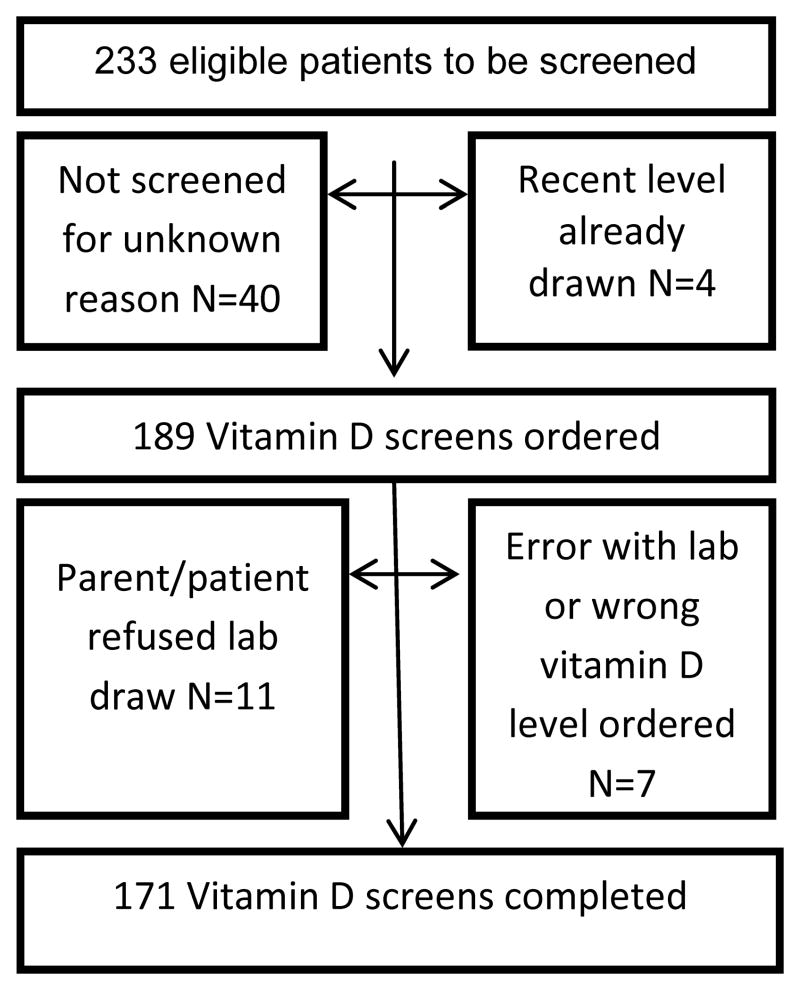
Following Human Subjects Committee approval, a retrospective cohort of all patients with a hematologic malignancy or Langerhans cell histiocytosis (LCH), who were less than 23 years at diagnosis, treated with at least 28 days of total corticosteroids as part of their chemotherapy regimen, and presented to the Vanderbilt REACH for Survivorship Clinic between February 2008 and September 2011 was constructed (N = 233). Of these patients, 171 (73.4%) successfully underwent screening with a total 25-hydroxy (OH) vitamin D level (VDL) (Figure 1). A control group of 97 healthy individuals between the ages of 1–21 years was also constructed from the roster of children in the Vanderbilt pediatric endocrinology clinic or Vanderbilt general pediatrics clinic between June 2010 and January 2013 who had blood work obtained for other reasons and VDL were obtained from that blood sample. Patients were excluded from the control group if they had known osteopenia, osteoporosis, osteogenesis imperfecta, previously diagnosed VDI/VDD, more than two bone fractures during the past year, chronic or current glucocorticoid use, thyrotoxicosis, gastrointestinal disease such as celiac disease causing possible malabsorption, diabetes mellitus, history of or current malignancy, or were of non-weight bearing status.
Figure 1.
Data Collection
Medical Records Abstraction
In the cancer and control groups, retrospective medical record abstraction was conducted for demographics, cancer diagnosis, VDL and season in which VDL was drawn. Cumulative steroid dosing was expressed using prednisone equivalent dosing in mg/m2 and was converted using the following formula: prednisone dosing in mg/m2 + (6.67 * dexamethasone dosing mg/m2). The season during which vitamin D levels were measured was defined as spring (March-May), summer (June-August), fall (September-November), and winter (December-February).
Anthropometric Evaluation
Height and weight were obtained, and body mass index was calculated based on the following formula: weight (kg) / height2 (m2). Z-scores for weight, height and BMI were calculated using age and gender-standardized growth population norms (based on the Centers for Disease Control and Prevention’s Year 2000 growth charts (22). BMI >85th percentile adjusted for age and sex was considered overweight (22).
Laboratory Evaluation
Cancer survivors and control participants provided a blood sample for 25-hydroxyvitamin D, which is the standard indicator of vitamin D status, and levels were measured via liquid chromatography/ tandem mass spectrometry (Mayo Medical Laboratories, Rochester, MN). The total 25-hydroxyvitamin D concentrations were calculated by summing the measured values of 25OHD2 and 25OHD3. Intra- and inter-assay coefficients of variation have previously been reported to be <7% (23). Twenty five-OH vitamin D3 insufficiency (VDI) was defined as a level 20–30 ng /ml and vitamin D deficiency (VDD) as <20 ng /ml (24–26).
DXA scan
To assess impact of VDL on bone health, we evaluated the association of VDI/VDD with decreased bone mineral density as measured by DXA in our cancer survivors.
In a subset of the cancer survivor cohort, total bone mineral density (BMD) and anterior posterior lumbar spine (L1–L4) BMD were determined by dual energy radiographic absorptiometry (DXA, GE Healthcare, Lunar iDXA, Tube model 40782). Whole body (N=91) and lumbar spine (N=88) DXA scans were included for analysis in cancer survivors who met COG guidelines for DXA screening due to corticosteroid exposure and had a VDL measured within two weeks of the DXA scan. Results were expressed as Z-scores for total body BMD and lumbar BMD, which account for age and gender. Osteopenia on DXA scan was defined as Z-score <−1.0 and osteoporosis as a Z-score as a Z-score <−2 (27–29)
Statistical Methods
Patient’s characteristics were summarized by cohort with median and range for continuous variables. Frequencies and percentages were shown for categorical variables. Associations between potential risk factors and both endpoints VDI and VDD were investigated in the following ways. In the univariate analyses, Wilcoxon Rank-Sum tests or Kruskal-Wallis tests were used for continuous variables, and for categorical variables, Fisher exact tests were used to compare between groups. To assess the correlation between two continues variables, the non-parametric spearman rank correlation was used. To assess the effects of potential risk factors including age, sex, overweight (BMI Z-score greater than 85th percentile), cumulative steroid dose, and blood draw season on VDI/VDD, multivariable logistic regression models were employed. Multiple imputation method was used in all regression models to deal with the missing values presented in the study (30). The primary analyses were conducted on the cancer survivor cohort. The secondary analyses further included the patients from the control cohort. All statistical inferences were assessed at a two-sided 5% significant level and all analyses and graphics were conducted or generated using R version 2.15 statistical software (31).
Results
Baseline Characteristics
Baseline characteristics of the cancer and control group are reported in Table I. The median age in both the cancer survivorship group and the control group was similar (median: 12.05 and 10.99 years respectively) although the cancer survivors were more likely to be male (56.1% compared with 36.1%, p=0.002), and Caucasian non-Hispanic (85.4% compared with 69.1%, p<0.001). The median BMI Z-score of the cancer survivorship group was higher (0.96) than the control group (0.51), (p=0.011), and there was a trend toward more overweight subjects in the survivorship group (41.8% compared with 29.9%, p=0.066). Adjustments for these factors were made in the combined multivariable analysis.
Table I.
Characteristics of the Cancer and Control groups
| Characteristics applicable to both groups | Survivorship Group (N=171) | Control Group (N= 97) | P-value |
|---|---|---|---|
| Age in years, median (range) | 12.05 (4.23, 22.4) | 10.99 (1.14, 18.95) | 0.0222 |
| Gender, N (%) | 0.0021 | ||
| Male | 96 (56.1) | 35 (36.1) | |
| Female | 75 (43.9) | 62 (63.9) | |
| Race/Ethnicity, N (%) | <0.0011 | ||
| Caucasian non-Hispanic | 146 (85.4) | 67 (69.1) | |
| African American | 12 (7.0) | 24 (24.7) | |
| Hispanic | 6 (3.5) | 5 (9.7) | |
| Asian | 6 (3.5) | 1 (1.0) | |
| American Indian or Alaska Native | 1 (0.6) | 0 (0) | |
| BMI Z-score, median (range) | 0.96 (−2.91, 3.51) | 0.51 (−3.1, 2.98) | 0.0112 |
| BMI Z-score over 85th percentile, N (%) | 0.0661 | ||
| Overweight | 71 (41.8) | 29 (29.9) | |
| Not overweight | 99 (58.2) | 68 (70.1) | |
| Characteristic applicable to survivorship group only | |||
| Diagnosis, N (%) | |||
| Acute lymphoblastic leukemia | 121 (70.8) | ||
| Lymphoma | 36 (21.1) | ||
| Langerhans cell histiocytosis | 13 (7.6) | ||
| AML | 1 (0.6) | ||
| Time since completing therapy in years, median (range) | 2.68 (0.03, 10.83) | ||
| Past history vitamin D deficiency, N (%) | 3 (1.8) | ||
| Cumulative steroid dosing, (N=165), median (range) | 7057 (600, 17,524) | ||
| Past history of osteonecrosis N (%) | 14 (8.2) | ||
Body mass index is abbreviated as BMI. The p values shown are for the difference between groups and use either
Fisher’s exact test or
Wilcoxon rank sum test. Cumulative steroid dose is expressed as prednisone equivalents in mg/m2
The cancer survivorship group was a median of 2.68 years (range 0.03 to 10.83) off therapy, and the majority of the cohort (70.8%) had a history of leukemia. The median BMI Z-score at the initial survivorship visit was 0.96 (range −2.91 to 3.51) and 41.8% of the cohort was overweight or obese. The median cumulative steroid dose in prednisone equivalents was 7057 mg/m2 (range 600 to 17,524); 8.2% had more limited glucocorticoid exposure due to osteonecrosis during chemotherapy, however this steroid exposure was not statistically different from the group as a whole [median 5256mg/m2 (range 2951 to 12,846)]. Three patients (1.8%) had previously been diagnosed with VDI/VDD while on therapy prior to their survivorship visit. Two of these patients previously received supplementation for low25-hydroxyvitamin D levels which initially corrected the deficiency; however all three again had VDD by the time of the survivorship visit.
Vitamin D levels in Cancer Survivors
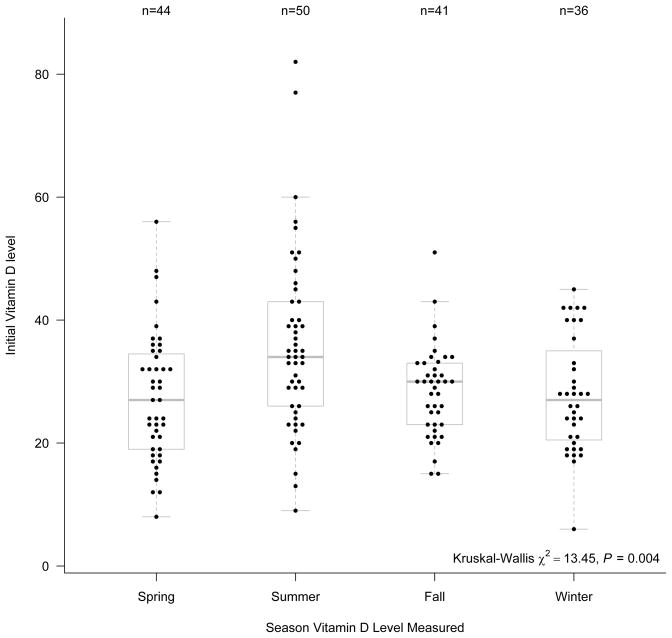
Vitamin D abnormalities were present in 50.3% of the cancer survivor cohort; 34.5% had VDI (59/171) and 15.8% had VDD (27/ 171). The median total 25-hydroxyvitamin D level was 29 ng/ml (range 6 to 82). Total 25-hydroxyvitamin D levels differed significantly depending upon the season of the year they were obtained. The median levels in the spring (27 ng/ml, n=44) and winter (27 ng/ml, n=36) were lower than those in the summer (34 ng/ml, n=50) and fall (30 ng/ml, n=41) (p=0.004) (Figure 2).
Figure 2.
In univariate analysis, VDI/VDD was significantly associated with Non-Caucasian or Hispanic race (p= 0.008). BMI Z-score as well as overweight status were inversely associated with VDI/VDD (p<0.001 for both). The patient’s age was not significantly associated with VDI/VDD (p=0.077) but was inversely correlated with total 25-hydroxyvitamin D levels (ρ= −0.20, p= 0.01). There was no significant correlation between VDD/VDI and cumulative steroid dose in prednisone equivalents, history of osteonecrosis, or days since completion of cancer therapy.
A multivariable regression was performed for patients with VDI/VDD (Table II), which included sex, race, age, season, cumulative steroid dosing, and BMI. An overweight BMI was independently associated with VDI/VDD [Odds ratio (OR) 5.44 95% CI (2.53, 11.67), p<0.001], as was increasing age [OR 2.17, 95% CI (1.18, 3.96), p = 0.012] and non-Caucasian or Hispanic race [OR 4.45, 95% CI 1.49,13.35), p=0.008); total 25-hydroxvitamin D levels obtained in the summer or fall were higher than those drawn in the winter [OR 0.12, 95% CI (0.04, 0.36), p<0.001] for summer and [OR 0.35, 95% CI (0.12, 1.0), p=0.05] for fall, respectively. Neither cumulative steroid dosing nor sex was independently associated with VDI/VDD. A regression model was also performed, which was narrowed to look only for associations for subjects who had VDD and BMI remained of borderline significance [OR 2.29, 95% CI (0.93, 5.45), p=0.062]) as a risk factor for VDD. Race and age were not significantly associated with VDD alone (Table II).
Table II.
Multi-variable logistic regression for associations related to vitamin D insufficiency (20–29 ng/ml) or deficiency (<20 ng/ml) in the cancer group (model 1) and in vitamin D deficiency alone (model 2).
| Characteristic (Model 1) | Odds Ratio | 95% Confidence Intervals | P-value |
|---|---|---|---|
| BMI >85th percentile | 5.44 | (2.53, 11.67) | <0.001 |
| Cumulative steroid dose (8994 vs. 3360) | 1.19 | (0.64, 2.18) | 0.585 |
| Non-Caucasian or Hispanic | 4.45 | (1.49, 13.35) | 0.008 |
| Male sex | 1.08 | (0.52, 2.25) | 0.845 |
| Age in years at survivorship visit (15 vs. 8) | 2.17 | (1.18, 3.96) | 0.012 |
| Vitamin D level drawn Spring vs. Winter | 0.51 | (0.18, 1.44) | 0.204 |
| Vitamin D level drawn Summer vs. Winter | 0.12 | (0.04, 0.36) | <0.001 |
| Vitamin D level drawn Fall vs. Winter | 0.35 | (0.12, 1.00) | 0.050 |
| Characteristic (Model 2) | Odds Ratio | 95% Confidence Intervals | P-value |
| BMI >85th percentile | 2.29 | (0.93, 5.45) | 0.062 |
| Non-Caucasian or Hispanic race | 2.12 | (0.76, 5.92) | 0.151 |
| Age in years at survivorship visit (15 vs. 8) | 1.68 | (0.80, 3.55) | 0.170 |
Cumulative steroid dosing is expressed in prednisone equivalents in mg/m2. Odds ratio comparisons are comparing the 75th vs. 25th percentiles.
Vitamin D levels in the control group
The median total 25-hydroxyvitamin D level in the healthy pediatric control group was 31 (7, 73). Thirty-two percent (31/97) of the patients had VDI and 11.3% (11/97) had VDD, resulting in 43.3% (42/97) with abnormal levels of total 25-hydroxyvitamin D. Non-Caucasian or Hispanic ethnicity was associated with VDI/VDD (p=0.014), and there was a trend to an association with VDI/VDD and increasing age (p=0.051). BMI in the overweight range was significantly associated with VDD/VDD (p=0.024), but a significant association with BMI Z-score was not noted (p=0.17),
Comparison of the cancer survivorship and control cohorts
There was no significant difference in the prevalence of VDI (p=0.309) or VDD (p=0.365) between the survivorship and control groups. A combined logistic regression model evaluating risk factors for VDI/VDD in the combined survivor/control group was performed; overweight BMI [OR 3.78, 95% CI (2.11, 6.77), p<0.001], non-Caucasian or Hispanic ethnicity [OR 4.03, 95% CI (1.92, 8.49), p<0.001], older age [OR 1.95, 95% CI (1.24, 3.06), p=0.004], and a total 25-hydroxyvitamin D level obtained during the summer [OR 0.21, 95% CI (0.1, 0.48), p<0.001] or fall [OR 0.4, 95% CI (0.17, 0.91), p=0.028] compared with winter were all independently associated with VDI/VDD. However, a cancer diagnosis was not independently associated with VDI/VDD [OR 1.3, 95% CI (0.71, 2.37), p=0.392] (Table III).
Table III.
Multi-variable logistic regression for associations related to vitamin D insufficiency (20–29 ng/ml) or deficiency (<20 ng/ml) in the cancer and control groups combined (N=268).
| Characteristic | Odds Ratio | 95% Confidence Intervals | P-value |
|---|---|---|---|
| BMI >85th percentile | 3.78 | (2.11, 6.77) | <0.001 |
| Having cancer vs. non-cancer | 1.30 | (0.71, 2.37) | 0.392 |
| Non-Caucasian or Hispanic | 4.03 | (1.92, 8.49) | <0.001 |
| Male sex | 1.10 | (0.63, 1.92) | 0.744 |
| Age in years at survivorship visit (15 vs. 8) | 1.95 | (1.24, 3.06) | 0.004 |
| Vitamin D level drawn Spring vs. Winter | 0.70 | (0.32, 1.53) | 0.367 |
| Vitamin D level drawn Summer vs. Winter | 0.21 | (0.10, 0.48) | <0.001 |
| Vitamin D level drawn Fall vs. Winter | 0.40 | (0.17, 0.91) | 0.028 |
Odds ratio comparisons are comparing the 75th vs. 25th percentiles.
Association between Vitamin D levels and Bone Mineral Density in Cancer Survivors
There were a total of 91 cancer survivors who had a whole body DXA scan concurrently with a 25-hydroxyvitamin D level. The median whole body DXA scan Z-score was 0.1 (−4.2, 3.6) and was not significantly correlated with 25-hydroxyvitamin D levels (ρ= 0.10, p= 0.374). Several patients met criteria for osteopenia [15/91 (16.5%)] and for osteoporosis [5/91 (5.5%)]; however, neither osteopenia or osteoporosis was significantly associated with 25-hydroxyvitamin D levels (p = 0.32 and p= 0.41respectively). There were also 89 patients who had a lumbar spine DXA scan concordant with a 25-hydroxyvitamin D level. Two of these subjects had a history of vertebral compression fractures; however the history was distant to the timing of the DXA in both cases. The median lumbar spine DXA scan Z-score was 0.0 (−4.2, 3.3) and was not correlated with overall 25-hydroxyvitamin D levels (Spearman ρ= 0.09, p= 0.39). Slightly fewer patients met criteria for osteopenia [14/89 (15.7%) and osteoporosis 5/89 (5.6%)]. In the lumbar spine, again neither osteopenia or osteoporosis was significantly associated with 25-hydroxyvitamin D levels (p = 0.81 and p= 0.16 respectively).
Discussion
Patients exposed to prolonged corticosteroids are at risk for decreased bone mineral density, and VDD/VDI can further increase the risk of osteopenia (15, 16, 32). Therefore, as corticosteroid treatment is integral to some forms of pediatric cancer, it is important to recognize whether pediatric cancer patients with significant corticosteroid treatment have VDI/VDD. Although this has not yet been well-studied, it is generally theorized that Vitamin D sufficiency will benefit bone health (33, 34), which is already potentially impaired in childhood cancer survivors treated with corticosteroids, methotrexate, and/or stem cell transplant (3, 4).
VDI/VDD has been previously reported in pediatric cancer survivors, including those treated with prolonged corticosteroids (35–39). These studies have identified age or pubertal status, race/ethnicity, diagnosis, malignancy, increased BMI, steroid exposure and seasonality as VDI/VDD risk factors (35–38). We have built upon this work by comparing to a population of healthy pediatric patients living in the same geographic area, specifically to demonstrate that VDI/VDD, while highly prevalent among cancer survivors, is not due to cancer history. In contrast, the prevalence of VDI/VDD was similar between cancer survivor and control groups. Furthermore, in our survivor population, interestingly, the VDI/VDD prevalence was slightly lower than that reported in the general US population (61% VDI;9% VDD) (24).
Several studies have recently been published evaluating the risk of VDI or VDD among cancer survivors, with some inconsistencies in results across studies. Sinha et al compared vitamin D levels between 61 pediatric cancer patients/survivors and 60 matched healthy controls, and contrary to our findings, the authors reported a significant difference in VDD prevalence between patients (13/61, 21.3%) and controls 2/60, 3.3%). The cutoff to define VDD for total 25-hydoxy vitamin D levels was <10 ng/ml, compared to 20 ng/dl in our study. If the VDD cutoff of <10 ng/ml was applied to our study, the VDD prevalence still does not differ between the survivors (3/17; 1.8%) and controls (1/97; 1%). However, our population is different from the study reported by Sinha et al., as our study is limited to off-therapy cancer survivors, compared with 80% still on active therapy in the Sinha study. During therapy, sun exposure may be lower as children spend less time outside, and dietary intake of vitamin D may differ due to therapy effects. Further studies are therefore needed to assess whether severe vitamin D deficiency is prevalent in patients on active therapy but then improves off therapy.
BMI in the overweight range was strongly associated with VDD/VDI in both our survivor (OR = 5.4) and control group (OR = 2.4). This finding supports the association between obesity and VDI/VDD reported in the general population (40, 41) and in stem cell transplant survivors (39). However, these findings are not consistent among studies. We previously did not find an association between VDD/ VDI and BMI Z-scores in pediatric ALL survivors, many of whom were pediatric stem cell transplant survivors; this may have been due to a smaller sample size of 78 survivors (37). In a recent study by Choudhary et al, a relationship between BMI and total 25-hydroxy vitamin D levels was also not noted. However, only 10% were obese. In a study by Rosen et al in 201 survivors of multiple cancer diagnoses, 37% were overweight, but a relationship between BMI and vitamin D levels was again not demonstrated (36). In contrast, more than 40% of our cohort was overweight, and BMI was found to be independently associated with VDD/VDI (40, 41); this may be related to our inclusion criteria of prolonged corticosteroid exposure, which is associated with elevated BMI.
Rosen et al. also reported that low 25- hydroxyvitamin D levels are common across different cancer diagnoses, with the lowest levels found in patients treated for osteosarcoma, retinoblastoma, hepatoblastoma, and myeloid leukemias; these are diagnoses in which glucocorticoids are not part of cancer treatment. These subgroups, however, were small (N = 4–13), and there was no comparison with healthy controls. Given our criteria for prolonged corticosteroid exposure for screening, which is unique to hematologic malignancy and LCH, and is most common in ALL, we were unable to demonstrate association with malignancy type. However, we also did not find that history of a cancer diagnosis is a risk factor for VDD/VDI in our combined analysis with healthy controls.
Glucocorticoid use has been linked to the development of vitamin D degradation, although the mechanism for this is not well understood (36). Robien et al. reported that current prednisone exposure was significantly associated with lower VDL in pediatric and adult transplant patients (39). Thus, corticosteroid exposure formed the criteria for our screening. However, among those previously treated with corticosteroids, cumulative dose was not found to be a risk factor for VDI/VDD. This may be due to high corticosteroid exposures in all patients. Further study is needed to evaluate the effects of more diverse corticosteroid exposures on vitamin D levels.
Race and ethnicity were strongly related to serum 25-hydroxyvitamin D levels. Hispanic, Asian, or African American race/ethnicity was associated with low total 25-hydroxyvitamin D levels and being Caucasian non-Hispanic was protective against VDI in both the cancer and control groups. These data confirm previous findings that non-Caucasian race/ethnicity is associated with decreased total 25-hydroxyvitamin D levels in pediatric cancer patients (35, 38). However, race/ethnicity was not significantly associated with risk of VDD in our study. The absence of this effect could be due to a smaller number of patients with VDD; alternatively, race/ethnicity may predispose to mildly low serum 25-hydroxyvitamin D levels, but other factors may be required to cause VDD.
As expected, in our study seasonality also appeared to have an important effect on 25-hydroxyvitamin D levels due to increased UV light availability as well as increased skin exposure in warmer weather. Our findings confirm data reported in pediatric cancer patients (35, 38) and the general pediatric population (42).
We were unable to demonstrate a relationship between osteopenia on DXA scan and 25-hydroxyvitamin D level using either lumbar spine or whole body DXA. However, as this was a cross-sectional analysis of DXA and patients were still relatively close to end of therapy, longer follow-up may be helpful to see if there is a true association.
We report that increasing age is associated with VDI/VDD in both the cancer and the control group. This is consistent with data from other studies from cancer survivors and the general population and may be related to less physical activity and sun exposure in older children and adolescents. (35, 37, 38, 43). Data on these environmental and lifestyle factors, including diet, were not prospectively collected in our cohort.
In summary, our study confirms that race, overweight BMI, and seasonality are risk factors for VDI/VDD, but a history of leukemia, lymphoma or Langerhan’s cell histiocytosis does not appear to increase risk. As our study limited screening to diagnoses with high corticosteroid treatment, it remains unclear whether risk in pediatric cancer survivors treated for solid tumors differs from that of the general population. Another limitation to our study is that we did not systematically collect vitamin D supplementation at time of the survivorship visit. Given that only three patients had previous Vitamin D deficiency, we believe that the lack of significant VDI/VDD in our survivor population was unlikely due to supplementation.
These data support that VDI/VDD is common in the pediatric survivorship population exposed to prolonged corticosteroids, but importantly the prevalence did not differ from a control group of pediatric healthy controls from the same institution and risk factors for VDI/VDD are those seen in the general population. Therefore, one can consider screening as recommended in the general pediatric population where patients with a diagnosis of cancer treated with corticosteroids are not placed in a category of specialized screening (44). However, as cancer survivors treated with corticosteroids are already at risk for osteopenia/osteoporosis, the negative impact of VDI/VDD may be greater in the cancer survivor population. Additional attention to vitamin D abnormalities in pediatric cancer survivors may maximize bone health. While our study suggested that VDI/VDD are not associated with osteopenia/osteoporosis, the association has been well-theorized. It is therefore reasonable to include 25-hydroxyvitamin D screening as part of the recommended screening for those who have had exposure to prolonged corticosteroids, particularly those patients who are overweight, older, or non-Caucasian and therefore at highest risk. The impact of screening upon supplementation, dietary and other lifestyle behavioral practices among these survivors should then be evaluated in future studies. As cutoffs for vitamin D sufficiency remain controversial (a lower limit of 20 ng /ml vs. 30 ng /ml 25-hydroxyvitamin D for vitamin D sufficient), future studies should seek to better define whether these differences are clinically significant in pediatric cancer survivors (21–23,28,34). Additionally, further studies are needed to determine whether vitamin D repletion is associated with improved bone health, as well as the appropriate dosing of vitamin D supplementation required for vitamin D repletion.
Acknowledgments
Grant sponsor: NCRR/NIH; Grant number: 1 UL1RR024975, and K12 CA 90625
References
- 1.Horner MJRL, Krapcho M, et al. Seer cancer statistics review, 1975–2006. Bethesda, MD: National Cancer Institue; 2009. p. 2009. http://seer.cancer.gov/csr/1975_2006/, based on Novemeber 2008 SEER data submission, posted to the SEER web site. [Google Scholar]
- 2.Kremer LC, Mulder RL, Oeffinger KC, et al. A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: A report from the international late effects of childhood cancer guideline harmonization group. Pediatr Blood Cancer. 2013;60:543–549. doi: 10.1002/pbc.24445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landier W, Wallace WH, Hudson MM. Long-term follow-up of pediatric cancer survivors: Education, surveillance, and screening. Pediatr Blood Cancer. 2006;46:149–158. doi: 10.1002/pbc.20612. [DOI] [PubMed] [Google Scholar]
- 4.Landier W, Armenian SH, Lee J, et al. Yield of screening for long-term complications using the children’s oncology group long-term follow-up guidelines. J Clin Oncol. 2012;30:4401–4408. doi: 10.1200/JCO.2012.43.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garmey EG, Liu Q, Sklar CA, et al. Longitudinal changes in obesity and body mass index among adult survivors of childhood acute lymphoblastic leukemia: A report from the childhood cancer survivor study. J Clin Oncol. 2008;26:4639–4645. doi: 10.1200/JCO.2008.16.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. Jama. 2013;309:2371–2381. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meacham LR, Chow EJ, Ness KK, et al. Cardiovascular risk factors in adult survivors of pediatric cancer--a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2010;19:170–181. doi: 10.1158/1055-9965.EPI-09-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oeffinger KC, Adams-Huet B, Victor RG, et al. Insulin resistance and risk factors for cardiovascular disease in young adult survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2009;27:3698–3704. doi: 10.1200/JCO.2008.19.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oeffinger KC, Mertens AC, Sklar CA, et al. Obesity in adult survivors of childhood acute lymphoblastic leukemia: A report from the childhood cancer survivor study. J Clin Oncol. 2003;21:1359–1365. doi: 10.1200/JCO.2003.06.131. [DOI] [PubMed] [Google Scholar]
- 10.Thomas IH, Donohue JE, Ness KK, et al. Bone mineral density in young adult survivors of acute lymphoblastic leukemia. Cancer. 2008;113:3248–3256. doi: 10.1002/cncr.23912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holzer G, Krepler P, Koschat MA, et al. Bone mineral density in long-term survivors of highly malignant osteosarcoma. The Journal of bone and joint surgery British volume. 2003;85:231–237. doi: 10.1302/0301-620x.85b2.13257. [DOI] [PubMed] [Google Scholar]
- 12.Kaste SC, Ahn H, Liu T, et al. Bone mineral density deficits in pediatric patients treated for sarcoma. Pediatr Blood Cancer. 2008;50:1032–1038. doi: 10.1002/pbc.21281. [DOI] [PubMed] [Google Scholar]
- 13.Kaste SC, Jones-Wallace D, Rose SR, et al. Bone mineral decrements in survivors of childhood acute lymphoblastic leukemia: Frequency of occurrence and risk factors for their development. Leukemia. 2001;15:728–734. doi: 10.1038/sj.leu.2402078. [DOI] [PubMed] [Google Scholar]
- 14.Kaste SC, Shidler TJ, Tong X, et al. Bone mineral density and osteonecrosis in survivors of childhood allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004;33:435–441. doi: 10.1038/sj.bmt.1704360. [DOI] [PubMed] [Google Scholar]
- 15.Osteoporosis prevention, diagnosis, and therapy. Jama. 2001;285:785–795. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 16.Vitamin d and adult bone health in australia and new zealand: A position statement. The Medical journal of Australia. 2005;182:281–285. doi: 10.5694/j.1326-5377.2005.tb06701.x. [DOI] [PubMed] [Google Scholar]
- 17.Chow EJ, Pihoker C, Hunt K, et al. Obesity and hypertension among children after treatment for acute lymphoblastic leukemia. Cancer. 2007;110:2313–2320. doi: 10.1002/cncr.23050. [DOI] [PubMed] [Google Scholar]
- 18.Esbenshade AJ, Simmons JH, Koyama T, et al. Body mass index and blood pressure changes over the course of treatment of pediatric acute lymphoblastic leukemia. Pediatr Blood Cancer. 2011;56:372–378. doi: 10.1002/pbc.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurney JG, Ness KK, Sibley SD, et al. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer. 2006;107:1303–1312. doi: 10.1002/cncr.22120. [DOI] [PubMed] [Google Scholar]
- 20.Razzouk BI, Rose SR, Hongeng S, et al. Obesity in survivors of childhood acute lymphoblastic leukemia and lymphoma. J Clin Oncol. 2007;25:1183–1189. doi: 10.1200/JCO.2006.07.8709. [DOI] [PubMed] [Google Scholar]
- 21.Alemzadeh R, Kichler J, Babar G, et al. Hypovitaminosis d in obese children and adolescents: Relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism: clinical and experimental. 2008;57:183–191. doi: 10.1016/j.metabol.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 22.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of high body mass index in us children and adolescents, 2007–2008. Jama. 303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 23.Drake MT, Maurer MJ, Link BK, et al. Vitamin d insufficiency and prognosis in non-hodgkin’s lymphoma. J Clin Oncol. 2010;28:4191–4198. doi: 10.1200/JCO.2010.28.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar J, Muntner P, Kaskel FJ, et al. Prevalence and associations of 25-hydroxyvitamin d deficiency in us children: Nhanes 2001–2004. Pediatrics. 2009;124:e362–370. doi: 10.1542/peds.2009-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin d deficiency: An endocrine society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 26.Vieth R. Why the minimum desirable serum 25-hydroxyvitamin d level should be 75 nmol/l (30 ng/ml) Best practice & research Clinical endocrinology & metabolism. 2011;25:681–691. doi: 10.1016/j.beem.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Diagnosis of osteoporosis in men, premenopausal women, and children. Journal of clinical densitometry: the official journal of the International Society for Clinical Densitometry. 2004;7:17–26. doi: 10.1385/jcd:7:1:17. [DOI] [PubMed] [Google Scholar]
- 28.Carey JJ, Delaney MF, Love TE, et al. Dual-energy x-ray absorptiometry diagnostic discordance between z-scores and t-scores in young adults. Journal of clinical densitometry: the official journal of the International Society for Clinical Densitometry. 2009;12:11–16. doi: 10.1016/j.jocd.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Lim JS, Kim DH, Lee JA, et al. Young age at diagnosis, male sex, and decreased lean mass are risk factors of osteoporosis in long-term survivors of osteosarcoma. J Pediatr Hematol Oncol. 2013;35:54–60. doi: 10.1097/MPH.0b013e318275193b. [DOI] [PubMed] [Google Scholar]
- 30.FEH . Regression modeling strategies: With applications to linear models, logistic regression, and survival analysis. New York, NY: Springer; 2001. [Google Scholar]
- 31.Team RC. R foundation for statistical computing. 2.15. software. Vienna, Austria: 2013. R: A language and environment for statistical computing. [Google Scholar]
- 32.Makitie O, Heikkinen R, Toiviainen-Salo S, et al. Long-term skeletal consequences of childhood acute lymphoblastic leukemia in adult males: A cohort study. Eur J Endocrinol. 2013;168:281–288. doi: 10.1530/EJE-12-0702. [DOI] [PubMed] [Google Scholar]
- 33.Braegger C, Campoy C, Colomb V, et al. Vitamin d in the healthy european paediatric population. Journal of pediatric gastroenterology and nutrition. 2013;56:692–701. doi: 10.1097/MPG.0b013e31828f3c05. [DOI] [PubMed] [Google Scholar]
- 34.Paxton GA, Teale GR, Nowson CA, et al. Vitamin d and health in pregnancy, infants, children and adolescents in australia and new zealand: A position statement. The Medical journal of Australia. 2013;198:142–143. doi: 10.5694/mja11.11592. [DOI] [PubMed] [Google Scholar]
- 35.Choudhary A, Chou J, Heller G, et al. Prevalence of vitamin d insufficiency in survivors of childhood cancer. Pediatr Blood Cancer. 2012 doi: 10.1002/pbc.24403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosen GP, Beebe KL, Shaibi GQ. Vitamin d levels differ by cancer diagnosis and decline over time in survivors of childhood cancer. Pediatr Blood Cancer. 2013;60:949–952. doi: 10.1002/pbc.24349. [DOI] [PubMed] [Google Scholar]
- 37.Simmons JH, Chow EJ, Koehler E, et al. Significant 25-hydroxyvitamin d deficiency in child and adolescent survivors of acute lymphoblastic leukemia: Treatment with chemotherapy compared with allogeneic stem cell transplant. Pediatr Blood Cancer. 2011;56:1114–1119. doi: 10.1002/pbc.22949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sinha A, Avery P, Turner S, et al. Vitamin d status in paediatric patients with cancer. Pediatr Blood Cancer. 2011;57:594–598. doi: 10.1002/pbc.22963. [DOI] [PubMed] [Google Scholar]
- 39.Robien K, Strayer LG, Majhail N, et al. Vitamin d status among long-term survivors of hematopoietic cell transplantation. Bone Marrow Transplant. 2011;46:1472–1479. doi: 10.1038/bmt.2010.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torun E, Gonullu E, Ozgen IT, et al. Vitamin d deficiency and insufficiency in obese children and adolescents and its relationship with insulin resistance. International journal of endocrinology. 2013;2013:631845. doi: 10.1155/2013/631845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turer CB, Lin H, Flores G. Prevalence of vitamin d deficiency among overweight and obese us children. Pediatrics. 2013;131:e152–161. doi: 10.1542/peds.2012-1711. [DOI] [PubMed] [Google Scholar]
- 42.Misra M, Pacaud D, Petryk A, et al. Vitamin d deficiency in children and its management: Review of current knowledge and recommendations. Pediatrics. 2008;122:398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 43.Kimm SY, Barton BA, Obarzanek E, et al. Obesity development during adolescence in a biracial cohort: The nhlbi growth and health study. Pediatrics. 2002;110:e54. doi: 10.1542/peds.110.5.e54. [DOI] [PubMed] [Google Scholar]
- 44.Winzenberg T, Powell S, Shaw KA, et al. Effects of vitamin d supplementation on bone density in healthy children: Systematic review and meta-analysis. BMJ. 2011;342:c7254. doi: 10.1136/bmj.c7254. [DOI] [PMC free article] [PubMed] [Google Scholar]