Abstract
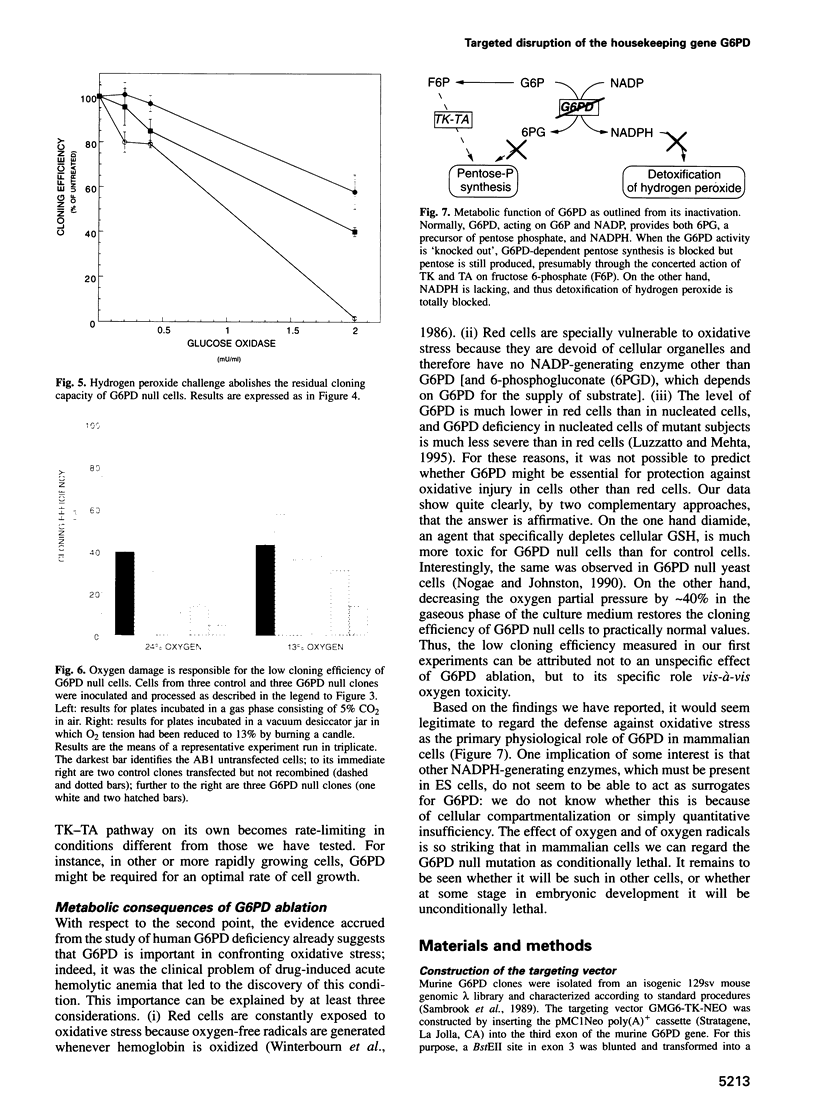
Glucose 6-phosphate dehydrogenase (G6PD) is a housekeeping enzyme encoded in mammals by an X-linked gene. It has important functions in intermediary metabolism because it catalyzes the first step in the pentose phosphate pathway and provides reductive potential in the form of NADPH. In human populations, many mutant G6PD alleles (some present at polymorphic frequencies) cause a partial loss of G6PD activity and a variety of hemolytic anemias, which vary from mild to severe. All these mutants have some residual enzyme activity, and no large deletions in the G6PD gene have ever been found. To test which, if any, function of G6PD is essential, we have disrupted the G6PD gene in male mouse embryonic stem cells by targeted homologous recombination. We have isolated numerous clones, shown to be recombinant by Southern blot analysis, in which G6PD activity is undetectable. We have extensively characterized individual clones and found that they are extremely sensitive to H2O2 and to the sulfydryl group oxidizing agent, diamide. Their markedly impaired cloning efficiency is restored by reducing the oxygen tension. We conclude that G6PD activity is dispensable for pentose synthesis, but is essential to protect cells against even mild oxidative stress.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHEN G., HOCHSTEIN P. GENERATION OF HYDROGEN PEROXIDE IN ERYTHROCYTES BY HEMOLYTIC AGENTS. Biochemistry. 1964 Jul;3:895–900. doi: 10.1021/bi00895a006. [DOI] [PubMed] [Google Scholar]
- D'Urso M., Mareni C., Toniolo D., Piscopo M., Schlessinger D., Luzzatto L. Regulation of glucose 6-phosphate dehydrogenase expression in CHO-human fibroblast somatic cell hybrids. Somatic Cell Genet. 1983 Jul;9(4):429–443. doi: 10.1007/BF01543044. [DOI] [PubMed] [Google Scholar]
- Fraenkel D. G. Selection of Escherichia coli mutants lacking glucose-6-phosphate dehydrogenase or gluconate-6-phosphate dehydrogenase. J Bacteriol. 1968 Apr;95(4):1267–1271. doi: 10.1128/jb.95.4.1267-1271.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorry P., Lufkin T., Dierich A., Rochette-Egly C., Décimo D., Dollé P., Mark M., Durand B., Chambon P. The cellular retinoic acid binding protein I is dispensable. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):9032–9036. doi: 10.1073/pnas.91.19.9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. R., Stamatoyannopoulos G., Naiman S. C., Kliman M. R., Klebanoff S. J., Austin T., Yoshida A., Robinson G. C. Neutrophil dysfunction, chronic granulomatous disease, and non-spherocytic haemolytic anaemia caused by complete deficiency of glucose-6-phosphate dehydrogenase. Lancet. 1973 Sep 8;2(7828):530–534. doi: 10.1016/s0140-6736(73)92350-7. [DOI] [PubMed] [Google Scholar]
- Hooper M., Hardy K., Handyside A., Hunter S., Monk M. HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature. 1987 Mar 19;326(6110):292–295. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
- Kazazian H. H., Jr The thalassemia syndromes: molecular basis and prenatal diagnosis in 1990. Semin Hematol. 1990 Jul;27(3):209–228. [PubMed] [Google Scholar]
- Kletzien R. F., Harris P. K., Foellmi L. A. Glucose-6-phosphate dehydrogenase: a "housekeeping" enzyme subject to tissue-specific regulation by hormones, nutrients, and oxidant stress. FASEB J. 1994 Feb;8(2):174–181. doi: 10.1096/fasebj.8.2.8119488. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M., Wertheim B., Correa W. S. Diamide, a new reagent for the intracellular oxidation of glutathione to the disulfide. Biochem Biophys Res Commun. 1969 Nov 6;37(4):593–596. doi: 10.1016/0006-291x(69)90850-x. [DOI] [PubMed] [Google Scholar]
- Kuehn M. R., Bradley A., Robertson E. J., Evans M. J. A potential animal model for Lesch-Nyhan syndrome through introduction of HPRT mutations into mice. Nature. 1987 Mar 19;326(6110):295–298. doi: 10.1038/326295a0. [DOI] [PubMed] [Google Scholar]
- Laird P. W., Zijderveld A., Linders K., Rudnicki M. A., Jaenisch R., Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991 Aug 11;19(15):4293–4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen F., Gundersen G., Lopez R., Prydz H. CpG islands as gene markers in the human genome. Genomics. 1992 Aug;13(4):1095–1107. doi: 10.1016/0888-7543(92)90024-m. [DOI] [PubMed] [Google Scholar]
- Levy H. R. Glucose-6-phosphate dehydrogenases. Adv Enzymol Relat Areas Mol Biol. 1979;48:97–192. doi: 10.1002/9780470122938.ch3. [DOI] [PubMed] [Google Scholar]
- Lobo Z., Maitra P. K. Pentose phosphate pathway mutants of yeast. Mol Gen Genet. 1982;185(2):367–368. doi: 10.1007/BF00330815. [DOI] [PubMed] [Google Scholar]
- Luzzatto L., Testa U. Human erythrocyte glucose 6-phosphate dehydrogenase: structure and function in normal and mutant subjects. Curr Top Hematol. 1978;1:1–70. [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- McMahon A. P., Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990 Sep 21;62(6):1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- Moguilevsky N., Steens M., Thiriart C., Prieels J. P., Thiry L., Bollen A. Lethal oxidative damage to human immunodeficiency virus by human recombinant myeloperoxidase. FEBS Lett. 1992 May 18;302(3):209–212. doi: 10.1016/0014-5793(92)80442-j. [DOI] [PubMed] [Google Scholar]
- Monaco A. P. Molecular human genetics and the Duchenne/Becker muscular dystrophy gene. Mol Cell Biol Hum Dis Ser. 1993;3:1–11. doi: 10.1007/978-94-011-1528-5_1. [DOI] [PubMed] [Google Scholar]
- Nichols J., Evans E. P., Smith A. G. Establishment of germ-line-competent embryonic stem (ES) cells using differentiation inhibiting activity. Development. 1990 Dec;110(4):1341–1348. doi: 10.1242/dev.110.4.1341. [DOI] [PubMed] [Google Scholar]
- Nogae I., Johnston M. Isolation and characterization of the ZWF1 gene of Saccharomyces cerevisiae, encoding glucose-6-phosphate dehydrogenase. Gene. 1990 Dec 15;96(2):161–169. doi: 10.1016/0378-1119(90)90248-p. [DOI] [PubMed] [Google Scholar]
- Pandolfi P. P., Roth M. E., Karis A., Leonard M. W., Dzierzak E., Grosveld F. G., Engel J. D., Lindenbaum M. H. Targeted disruption of the GATA3 gene causes severe abnormalities in the nervous system and in fetal liver haematopoiesis. Nat Genet. 1995 Sep;11(1):40–44. doi: 10.1038/ng0995-40. [DOI] [PubMed] [Google Scholar]
- Rosenstraus M., Chasin L. A. Isolation of mammalian cell mutants deficient in glucose-6-phosphate dehydrogenase activity: linkage to hypoxanthine phosphoribosyl transferase. Proc Natl Acad Sci U S A. 1975 Feb;72(2):493–497. doi: 10.1073/pnas.72.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki M. A., Braun T., Hinuma S., Jaenisch R. Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell. 1992 Oct 30;71(3):383–390. doi: 10.1016/0092-8674(92)90508-a. [DOI] [PubMed] [Google Scholar]
- Smith A. G., Heath J. K., Donaldson D. D., Wong G. G., Moreau J., Stahl M., Rogers D. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988 Dec 15;336(6200):688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- Thomas D., Cherest H., Surdin-Kerjan Y. Identification of the structural gene for glucose-6-phosphate dehydrogenase in yeast. Inactivation leads to a nutritional requirement for organic sulfur. EMBO J. 1991 Mar;10(3):547–553. doi: 10.1002/j.1460-2075.1991.tb07981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley J. M., Blake D. J., Pearce M., Knight A. E., Kendrick-Jones J., Davies K. E. Dystrophin and related proteins. Curr Opin Genet Dev. 1993 Jun;3(3):484–490. doi: 10.1016/0959-437x(93)90124-8. [DOI] [PubMed] [Google Scholar]
- Tsui L. C. Mutations and sequence variations detected in the cystic fibrosis transmembrane conductance regulator (CFTR) gene: a report from the Cystic Fibrosis Genetic Analysis Consortium. Hum Mutat. 1992;1(3):197–203. doi: 10.1002/humu.1380010304. [DOI] [PubMed] [Google Scholar]
- Vulliamy T., Beutler E., Luzzatto L. Variants of glucose-6-phosphate dehydrogenase are due to missense mutations spread throughout the coding region of the gene. Hum Mutat. 1993;2(3):159–167. doi: 10.1002/humu.1380020302. [DOI] [PubMed] [Google Scholar]
- Vulliamy T., Mason P., Luzzatto L. The molecular basis of glucose-6-phosphate dehydrogenase deficiency. Trends Genet. 1992 Apr;8(4):138–143. doi: 10.1016/0168-9525(92)90372-B. [DOI] [PubMed] [Google Scholar]
- Wajntal A., DeMars R. A tetrazolium method for distinguishing between cultured human fibroblasts having eiter normal or deficient levels of glucose-6-phosphate dehydrogenase. Biochem Genet. 1967 Jun;1(1):61–64. doi: 10.1007/BF00487737. [DOI] [PubMed] [Google Scholar]
- West J. D. A genetically defined animal model of anembryonic pregnancy. Hum Reprod. 1993 Aug;8(8):1316–1323. doi: 10.1093/oxfordjournals.humrep.a138249. [DOI] [PubMed] [Google Scholar]
- Williams R. L., Hilton D. J., Pease S., Willson T. A., Stewart C. L., Gearing D. P., Wagner E. F., Metcalf D., Nicola N. A., Gough N. M. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988 Dec 15;336(6200):684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., Benatti U., De Flora A. Contributions of superoxide, hydrogen peroxide, and transition metal ions to auto-oxidation of the favism-inducing pyrimidine aglycone, divicine, and its reactions with haemoglobin. Biochem Pharmacol. 1986 Jun 15;35(12):2009–2015. doi: 10.1016/0006-2952(86)90734-3. [DOI] [PubMed] [Google Scholar]