Abstract
Gene therapy offers the possibility to treat pancreatic disease in Cystic Fibrosis (CF), caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene; however gene transfer to the pancreas is untested in humans. The pancreatic disease phenotype is very similar between humans and pigs with CF, thus CF pigs create an excellent opportunity to study gene transfer to the pancreas. There are no studies showing efficient transduction of pig pancreas with gene transfer vectors. Our objective is to develop a safe and efficient method to transduce wild-type (WT) porcine pancreatic ducts that express CFTR. We catheterized the umbilical artery of WT newborn pigs and delivered an adeno-associated virus serotype 9 vector expressing green fluorescent protein (AAV9CMV.sceGFP) or vehicle to the celiac artery, the vessel that supplies major branches to the pancreas. This technique resulted in stable and dose-dependent transduction of pancreatic duct epithelial cells that expressed CFTR. Intravenous injection of AAV9CMV.sceGFP did not transduce the pancreas. Our technique offers an opportunity to deliver the CFTR gene to the pancreas of CF pigs. The celiac artery can be accessed via umbilical artery in newborns and via femoral artery at older ages; delivery approaches which can be translated to humans.
Keywords: celiac artery, gene therapy, pancreas, cystic fibrosis, adeno-associated virus serotype 9
INTRODUCTION
Cystic Fibrosis (CF) is a multisystem disease caused by mutations in the gene encoding cystic fibrosis transmembrane conductance regulator (CFTR)1, 2. CFTR is expressed in many epithelial cells, including pancreatic ducts, and functions as an apical membrane anion channel3, 4. Genetic mutations in CFTR determine the exocrine pancreatic function in CF5, 6. In patients with CF who carry two severe mutations that severely affect CFTR function, the pancreatic damage starts in utero7. In these individuals, the damage continues after birth and they become pancreatic insufficient at young ages8. Patients with sufficient pancreatic function carry a mild mutation on at least one allele and have residual CFTR activity (~10% of all CF patients)9, 10. Patients with pancreatic sufficiency are prone to recurrent pancreatitis attacks and progressive decline in the exocrine pancreatic function as a consequence9.
Despite treatment with pancreatic enzymes to prevent severe malnutrition4, 11, 12, exocrine pancreatic insufficiency in CF tracks with delayed growth, accelerated progression of lung disease13–15, and CF-related diabetes (CFRD)16, 17; all associated with increased morbidity and mortality18, 19. Preserving the exocrine pancreatic function in CF may improve disease outcomes. Currently there are no treatments to prevent the pancreatic disease progression in CF.
Designing therapies for CF pancreatic disease has been challenging because the pancreas is not easily accessible in humans, and mice models do not develop pancreatic disease typical of CF20, 21. Newborn CF pigs have pancreatic disease similar to patients with CF and the disease progresses over time, as it does in humans22–27. Therefore the CF pig model creates an opportunity to study gene therapy for pancreatic disease. To date, there are no studies assessing the transduction of pig pancreas.
The available techniques to transduce cells in the pancreas of mice and rats (direct pancreatic injection, retrograde pancreaticobiliary duct delivery, or systemic delivery with temporary clamping of portal vein, hepatic artery, bile duct) are invasive, induce severe pancreatic inflammation and toxicity, and are not desirable for human studies28–34. Other methods are ineffective: intravenous (IV) delivery of adenovirus vectors does not transduce pancreatic cells because the liver rapidly removes the virus from circulation35. In general, adenoviral vector-directed gene transfer to the pancreas has been limited by inflammation and transient expression28–31. AAV vectors are attractive because of their low immunogenicity, excellent safety record36–38, and long-term transgene expression in non-dividing cells, even in the absence of genome integration39. Still, the experience with delivering AAV vectors to the pancreas is limited. Also, the delivery methods are usually invasive and mainly transduce the acinar cells and islets of mice30, 32–34, 40, 41, not the pancreatic duct epithelial cells where CFTR is expressed.
The goal of this work was to express genes in the pancreatic duct epithelial cells of WT pigs and create a framework for future studies for CFTR gene delivery to the pancreatic ducts of CF pigs. AAV9 vector delivery via the celiac artery, the vessel that supplies major branches to the pancreas, efficiently and stably transduced pancreatic duct epithelial cells. This is the first study showing expression of transgenes in pig pancreatic duct cells.
RESULTS
IV Injection of the AAV9 vector does not transduce pancreas in pigs
Our goal was to target the pancreas and primarily CFTR-expressing pancreatic duct epithelial cells, using an efficient and minimally invasive technique. We chose pigs because CF pigs lacking the CFTR function exhibit defective anion transport and replicate the multisystem disease observed in humans with CF, including pancreatic disease22–27.
We delivered AAV9CMV.sceGFP (2.4×1012 viral genome particles (vg) per animal, n=2) intravenously (ear vein) to 1-day-old pigs and we observed no gene transfer to the pancreas, one month after the injection (data not shown). Therefore, systemic venous delivery did not transduce the pancreas in newborn pigs.
Injection of the celiac artery as a novel method to deliver transgenes to the pancreas of newborn pigs
We next assessed vector delivery via the celiac artery, the vessel that supplies major branches to the pancreas in humans and pigs42. Shortly after birth (24–48h), the celiac artery can be easily accessed via the umbilical arteries (Fig. 1). The AAV9 vector was administered to the celiac artery of newborn pigs and all pigs tolerated the procedure well without complications. After the procedure, piglets recovered uneventfully and received standard care.
Figure 1. Celiac artery catheterization via umbilical arteries.
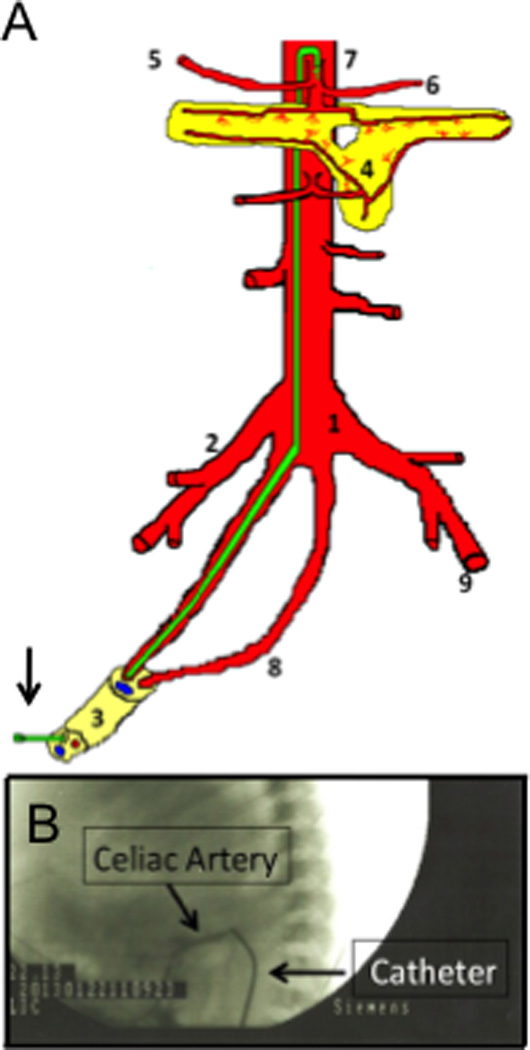
(A) In newborns, celiac artery can be reached by placing a catheter (arrow, green) into the umbilical arteries, which connect to the aorta. The catheter is then advanced to the celiac artery. (1) aorta; (2) right iliac artery; (3) umbilical cord (1 vein and two arteries); (4) pancreas; (5) hepatic artery; (6) splenic artery; (7) celiac artery with catheter; (8) umbilical artery; (9) left femoral artery. (B) Angiography confirming cannulation of the celiac artery (arrows). Vector or vehicle was injected and the catheter was flushed with normal saline.
AAV9 gene delivery via celiac artery did not induce pancreatic inflammation in pigs
Adenoviral vector-directed gene transfer to the pancreas is limited by inflammation and transient expression of the genes in rodents28–31, but AAV vectors typically have low immunogenicity. To determine whether AAV9 caused an immunogenic response in pigs, we monitored their activity level, food intake, and weight gain on a daily basis. We observed no differences between vector and vehicle-treated pigs. One and 3 months after vector delivery, animals were euthanized and pancreata isolated. We examined the pancreatic histology of pigs that received AAV9 at birth and compared this to the control pigs. The pancreas had normal architecture with no infiltrating inflammatory cells after vector delivery (Fig. 2).
Figure 2. AAV9 gene delivery does not induce pancreatic inflammation in pigs.
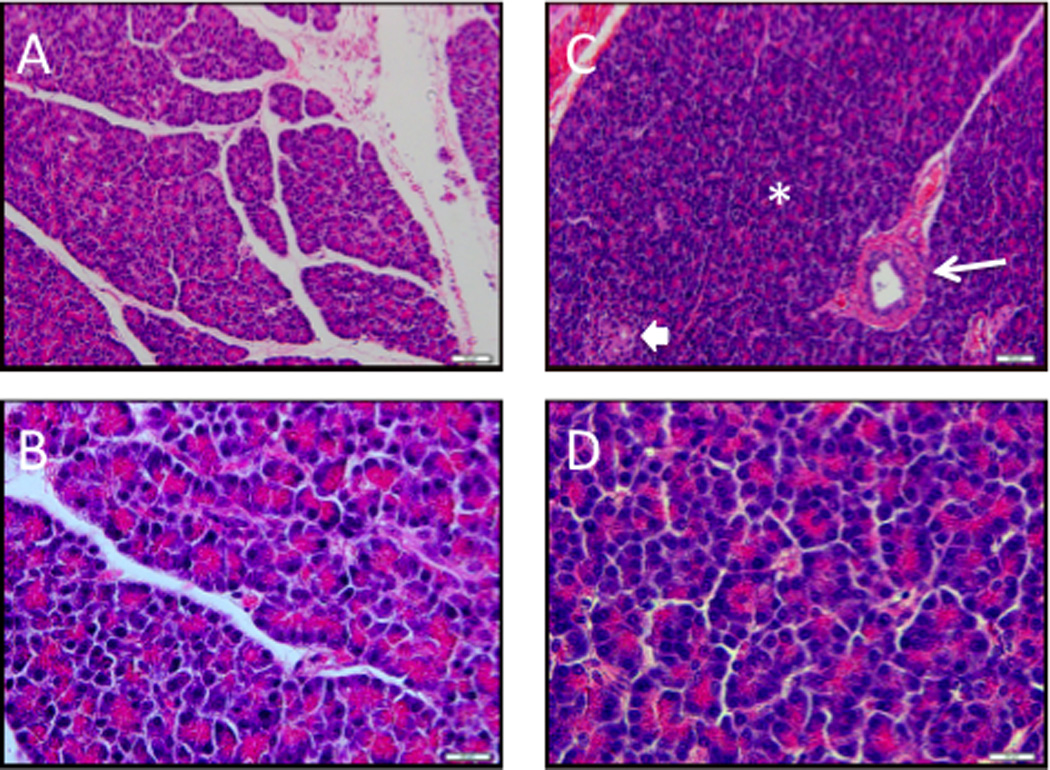
Thirty (A, B) and ninety days (C, D) after the celiac artery injection of AAV9CMV.sceGFP (2.4×1012 vg per animal), pancreas sections were obtained. Pancreas had a lobular architecture, with ducts (arrow), acini (*) and islet cells (block arrow). There were no inflammatory cells (H&E stain). A, C x10 mag, bar = 50 µm; B, D ×60 mag, bar = 20 µm.
GFP is expressed in porcine pancreatic duct epithelial cells following AAV9 vector delivery to the celiac artery
Although AAV vectors have been used to target other organ systems, there is limited information on their delivery to the pancreas. In general, gene transfer to the pancreas has been done in vitro and/or on islet cells of rodents43–46. There are no data reported using AAV vectors in pigs. To determine whether the GFP reporter gene was expressed in pancreatic duct cells following the delivery of AAV9 vector to the celiac artery of newborn pigs, we used immunofluorescence, immunohistochemistry (IHC), and RT-PCR. Fig. 3A–F summarizes our findings in pigs euthanized 1 month after receiving 2.4×1012 vg of AAV9CMV.sceGFP (n=7) or vehicle per animal. Fig. S1 shows immunofluorescence images from pigs that received various doses of the AAV9 vector or vehicle and followed for 1 to 3 months.
Figure 3. AAV9 transduces porcine ductal epithelial cells.
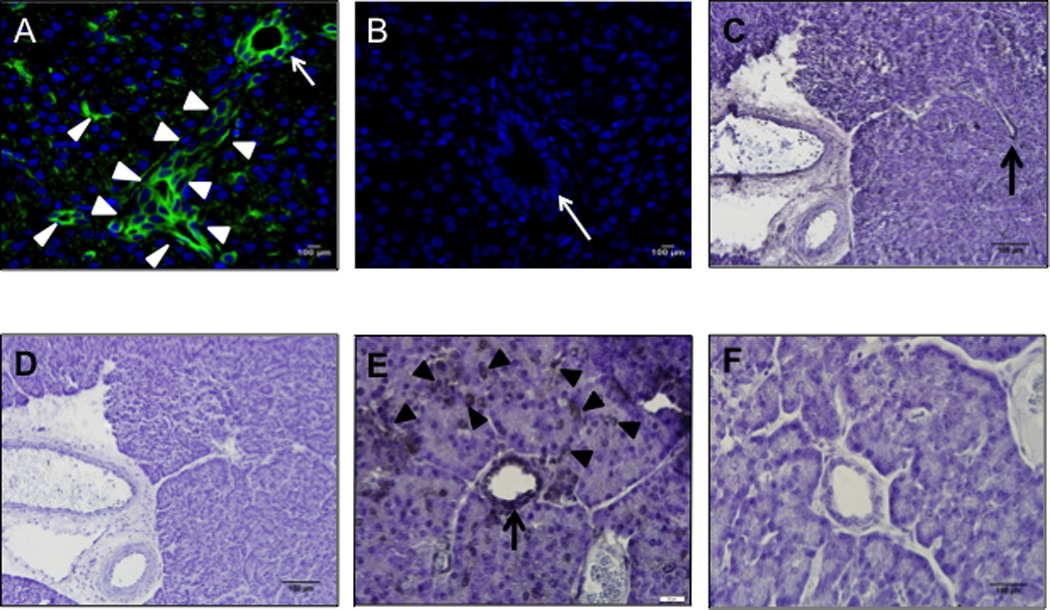
Pancreas sections 30 days after newborn pigs received AAV9CMV.sceGFP (A, C, D, E, F) (2.4×1012 vg per animal) or vehicle (B) into the celiac artery. Immunofluorescence (A, B) and immunohistochemistry (C–F) images are shown. Arrows point to intralobular (larger) ducts, arrowheads point to intercalated (smaller) ducts. C and D; E and F are serial sections from the same animal, primary antibody is omitted in D and F. A, B ×20 mag; C, D ×10 mag, scale bar = 100 µm; E, F ×60 mag, scale bar = 20 µm. Green: GFP, Blue: DAPI nuclei.
One month after delivering the AAV9 vector to the celiac artery, we found GFP expression in pig pancreatic ducts, including the intercalated and intralobular ducts (Fig. 3A, C, E; Fig. S1) that normally have high levels of CFTR47–49. There was no staining detected if the primary antibody was omitted (IHC) (Fig. 3D, F), confirming that the antibody staining was specific to GFP. Vehicle-treated animals were not immunoreactive for GFP (Fig. 3B). Gene expression was dose-dependent and persisted 3 months after treatment (last time point tested) (Fig. 4). GFP expression shown by immunofluorescence and immunohistochemistry in pig pancreas was confirmed with PCR both at 1- and 3-month time points (Fig. 5A). We detected transduction of ~10% of the cells of the pancreas, predominantly ductal epithelial cells, 2 months after delivery of the AAV9CMV.eGFP vector (non-self complimentary form), using 2.4×1012 vg (n=3). Thus, the delivery of AAV9 vector to the celiac artery in newborn pigs effectively transduces the pancreatic duct epithelial cells.
Fig. 4. AAV9 transduces ductal epithelial cells-time and dose response.
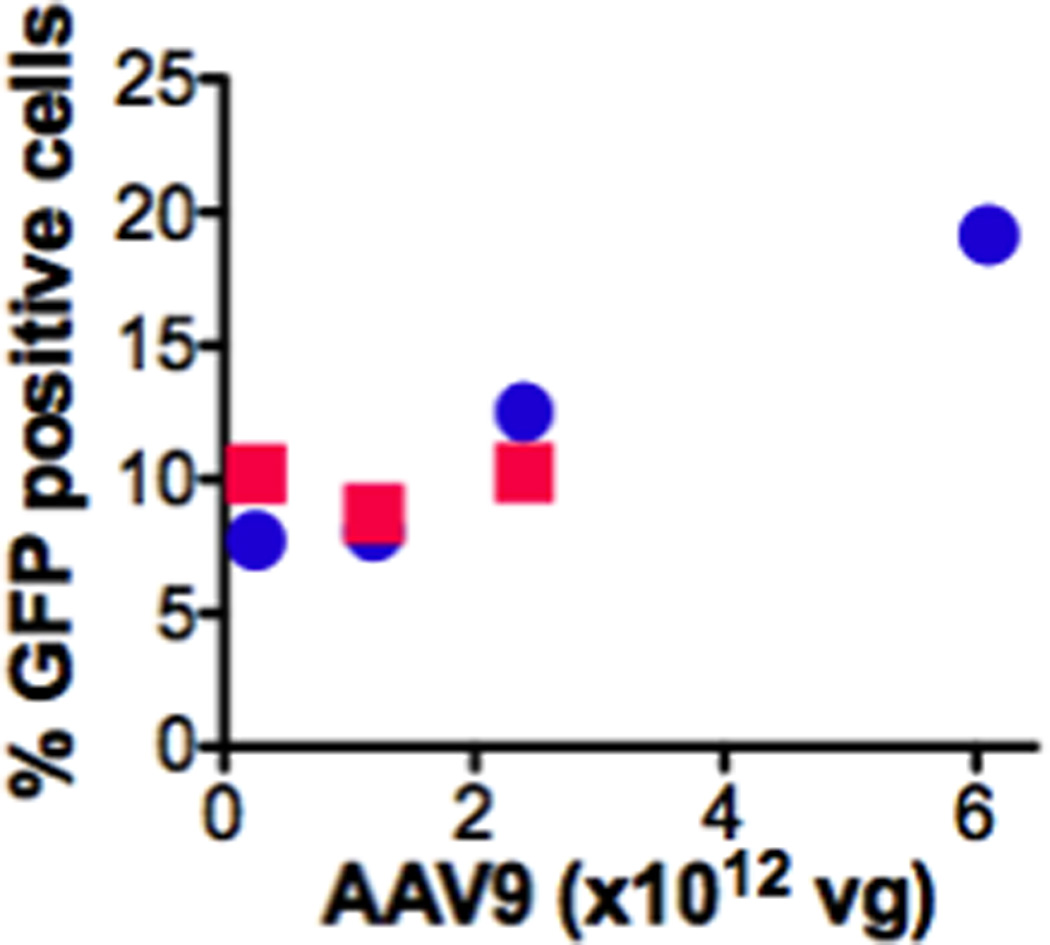
Ten random pancreatic fields (20× mag) were assessed per animal (immunofluorescence). % GFP positive cells were calculated by counting GFP expressing divided by the total number of cells in the field (n=1 for all time points and doses except, n=2 for 6.1×1012 vg at 1 month, and n=7 for 2.4×1012 vg at 1 month). Circles: 1 month; Squares: 3 months.
Fig. 5. Celiac artery delivery of AAV9 vector leads to GFP expression in pancreas and several other tissues.
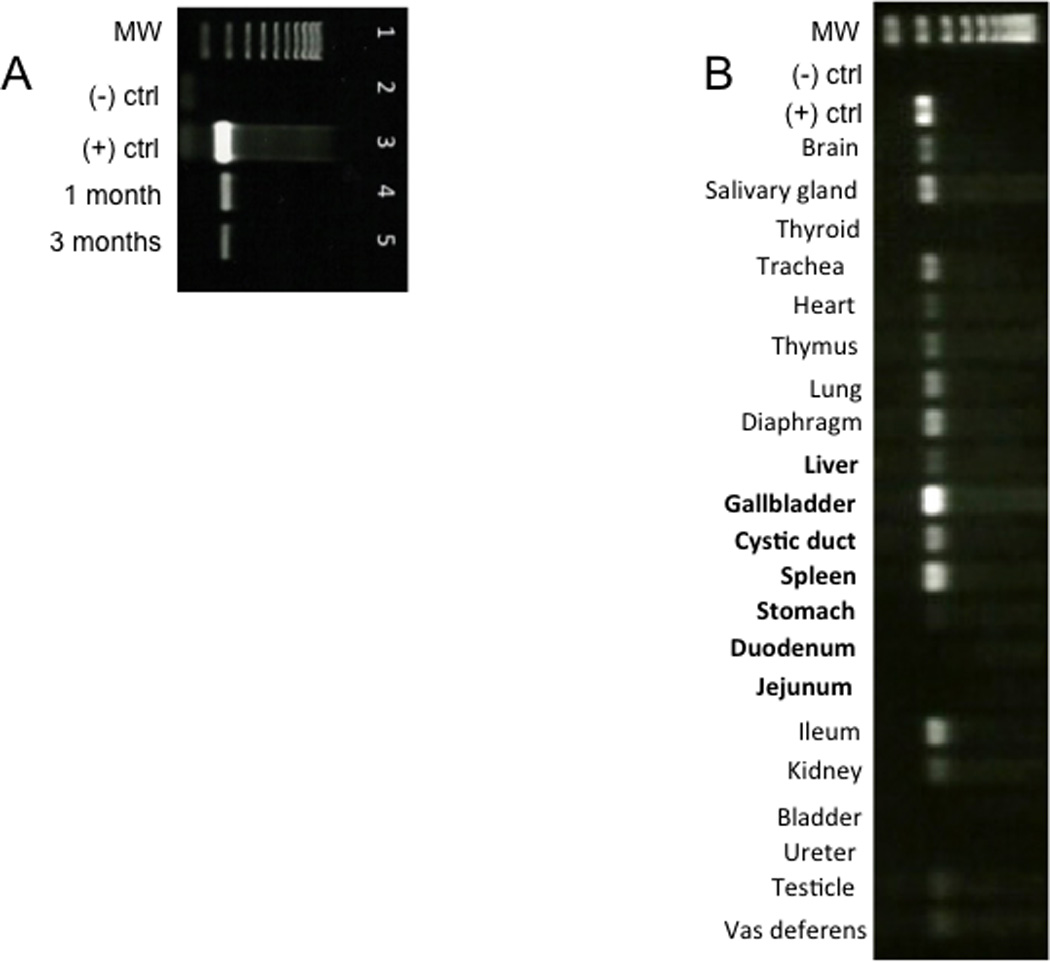
(A) One and three months after delivery of AAV9CMV.sceGFP (2.4×1012 vg per animal) to the celiac artery in the newborn period, RNA was isolated from pancreas and end-point PCR was used to detect GFP mRNA. The results are representative of n=7 for one month exposure and n=1 for three-month exposure. Lane 1=ladder; lane 2=negative control; lane 3=positive control (10 ng GFP plasmid); lane 4=pancreas one month after delivery; lane 5=pancreas three months after delivery. (B). End-point PCR of tissues 30 days after injecting 2.4 × 1012 vg AAV9CMV.sceGFP to the celiac artery of newborn pigs. MW: molecular weight ladder; (−) ctrl: negative control (sham animal); (+) ctrl: positive control (plasmid eGFP). Organs that receive arterial supply from celiac artery are in bold. The stomach and duodenum, two organs that receive blood supply from celiac artery were not transduced.
AAV9 vector delivered to the celiac artery of newborn pigs transduces CFTR-expressing pancreatic duct epithelial cells
Studies in human and pig samples have shown that CFTR is expressed at high levels in the pancreas and localizes to the pancreatic duct epithelia27, 47–49. To determine whether GFP was expressed in CFTR-expressing pancreatic duct epithelial cells following AAV9 delivery, we immunolocalized CFTR in transduced tissues (Fig. 6A). CFTR was expressed on the apical side of duct epithelia and CFTR and GFP co-localized within the same cells. These results confirm that our technique transduces CFTR-expressing duct cells in the pancreas.
Fig. 6. AAV9 vector expression of GFP in CFTR-expressing duct cells.
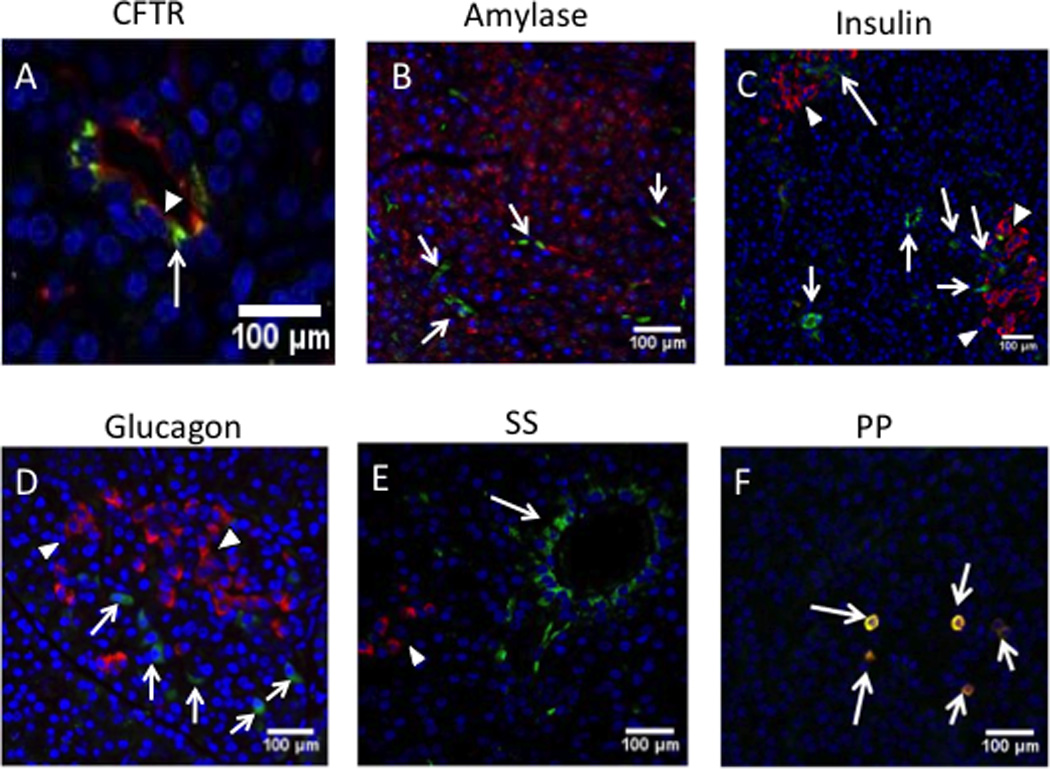
Immunofluorescent images of pancreas from pigs, 30 days after receiving 2.4×1012 vg AAV9CMV.sceGFP in the newborn period. (A) anti-CFTR antibody (red) for pancreatic ducts; (B) anti-amylase (red, arrowheads) for acinar cells; (C) anti-insulin (red, arrowheads) for β cells; (D) anti-glucagon (red, arrowheads) for α cells; (E) anti-somatostatin (SS) (red, arrowheads); (F) anti-pancreatic polypeptide (PP) (red-yellow indicating colocalization with eGFP, arrows); DAPI (blue) for nuclei. AAV9-GFP (green, arrows) was transduced in the cells that were expressing CFTR (red, arrowhead) on the apical side, A ×40 mag; B, C, D, E, F= ×20 mag. A, B, C, D, E= cells expressing GFP are shown with arrows.
AAV9 vector transduces pancreatic polypeptide-secreting cells of the islets
AAV9 transduces β cells and to lesser degree α cells in mice32, but it is not known if porcine pancreatic cells are susceptible to AAV9 transduction. To examine the pancreatic cell subtypes transduced with our technique, we immunostained pancreas sections with antibodies against amylase (acinar cell marker) (Fig. 6B), insulin (β cell marker) (Fig. 6C), glucagon (α cell marker) (Fig. 6D), somatostatin (δ cell marker) (Fig. 6E), and pancreatic polypeptide (PP cell marker) (Fig. 6F). We detected colocalization only with PP cells. These results suggest that the celiac artery injection of AAV9 does not transduce pancreatic acinar cells and only tranduces PP cells of the islets.
Delivering the AAV9 vector to the celiac artery transduces other organs
Because our technique involves a systemic injection of a vector with a CMV promoter, other organs could also be transduced. The celiac artery supplies blood to the stomach, duodenum, spleen, liver, gallbladder, and the vector may also enter the systemic circulation and reach other organs. To determine whether other organs were also transduced following the celiac artery injection of the AAV9CMV.sceGFP vector, we performed end-point RT-PCR for GFP 30 days after the injection. The organs that are transduced by our technique are shown in Fig. 5B. The liver, gallbladder, cystic duct, and spleen receive blood supply from the celiac artery and were transduced by our technique. Interestingly, the organs that receive the blood supply from celiac artery, such as stomach and duodenum were not transduced. The transduction of other organs (salivary gland, trachea, lung, vas deferens, ileum) typically involved in CF may be advantageous for treating this systemic disease.
DISCUSSION
In this study, we describe a novel, safe, and minimally invasive gene delivery technique to efficiently express a reporter gene in the pancreatic duct epithelial cells of pigs, an animal species that has a CF model available. This is the first study showing efficient transduction of pig pancreas with a gene transfer vector.
The pancreas is a retroperitoneal organ and difficult to access. The techniques that deliver genes to the pancreas of mice and rats involve injecting the pancreatic parenchyma or the pancreatic duct or giving it systemically in conjunction with laparotomy and clamping the portal vein, the hepatic artery, or the bile duct28–33. These methods are invasive and are not easily translated to humans. A major advantage of our technique is the ease with which it is performed. Because the umbilical artery is patent in newborn pigs for 24–48 h after birth, it allows easy, noninvasive (no surgical cutdown needed) access to the aorta, celiac artery, and the pancreatic arterial supply. Umbilical artery catheterization is commonly performed in humans; and is well-tolerated by even premature, very low birth weight neonates. Once the umbilical vessels are no longer accessible, the celiac artery can be catheterized via the femoral artery. Therefore, our method has the potential to be translated to humans.
In general, viral vectors delivered to the venous system do not efficiently transduce the pancreas32, 35. This is probably because the vector is removed from the circulation before it reaches the pancreas. Indeed, we have not observed pancreatic gene expression following the IV delivery of the vector. Our technique circumvents this problem by directly delivering the vector to the arterial blood supply of the pancreas, using a minimally invasive approach. The technique is well-tolerated by the animals and leads to efficient transgene expression, 1 and 3 months after delivery.
Inflammation and transient transgene expression have been the major problems with delivering adenoviral vectors to the pancreas28–31. Inflammation has not been observed with AAV vectors, although the experience with delivering AAV vectors to the pancreas is limited,30, 32–34, 40, 41. We observed no pancreatic inflammation in our model 1 and 3 months after gene delivery, confirming that the AAV vectors are suitable for use in pancreatic gene transfer studies.
There is limited information on delivering AAV vectors to the pancreas. In general, gene transfer studies to the pancreas have been done in vitro and/or on islet cells of rodents43–46. Serotypes 1, 2, 5, 6, and 8 have been used in vitro and in vivo in mouse pancreas32–34, 41, 45, with AAV8 and 9 showing most promise30, 32, 34, 40, 41, 50. Transduction of ductal cells has been reported in mouse pancreas with AAV632 and AAV834, but the vectors were delivered via pancreatic duct or direct pancreatic injection. The colocalization of the transgene with CFTR was also not examined. Our studies confirm that AAV serotype 9 is an efficient vector to transduce the pancreas. We have not explored the other serotypes.
Previous studies with AAV delivery to the mouse pancreas reported AAV transduction of acinar cells and islets (mainly β cells)30, 32–34, 40, 41, not the pancreatic duct cells where CFTR is expressed. Delivering transgenes to CFTR-expressing pancreatic ducts is a novel and exciting finding of this study. This method has the potential to transfer CFTR gene to the pancreas of humans with CF. In addition, this approach might be used to target genes that control cell proliferation and survival in humans with pancreatic ductal adenocarcinoma51, or have applications for other genetic or acquired diseases of the pancreas.
Another interesting finding of this study is the expression of transgenes in pancreatic polypeptide-expressing cells. While the exact physiological role of PP is not determined, the plasma levels of this hormone are reduced in humans with CF and in patients who develop diabetes secondary to chronic pancreatitis52–55. The lack of a PP response to hypoglycemia52 or secretin55 confirms the exocrine pancreatic dysfunction in humans with CF. It is not known whether PP plays a role in CF-related diabetes. A future goal is to transduce the CF pig pancreas with a shortened CFTR cDNA56, 57 packaged in the AAV9 capsid and examine whether gene therapy will prevent the progression of pancreatic destruction. It will be interesting to learn whether CFTR gene transfer to the CF pig pancreas will have an effect on PP levels and insulin secretion.
One limitation of our study is the broad expression of transgenes in other organs. The vector likely reaches other organs by entering the systemic venous circulation. Interestingly, while the stomach and duodenum receive their blood supply from the celiac artery, they were not transduced, suggesting that other factors such as AAV9 receptor-mediated uptake may also be involved. Our technique may be advantageous for a systemic disease such as CF that involves multiple organs. For future studies, a promoter that is more specific to the pancreas (i.e. pdx1)58 may achieve more targeted expression.
In summary, we report a novel, efficient and well-tolerated gene delivery technique to the pancreatic duct epithelial cells of a large animal species that has a CF model available. Future studies will explore the utility of this technique to restore CFTR function, anion transport, and prevent pancreatic disease progression in CF pigs. Successful execution of gene therapy in CF pigs would provide an important step towards translational studies in humans with CF.
MATERIALS AND METHODS
Virus preparation
AAV9CMV.sceGFP (self-complementary genome) or AAV9CMV.eGFP were produced by triple-plasmid co-transfection of human HEK 293 cells and purified by Mustang Q membrane cassettes after iodixanol gradient centrifugation. The vectors were dialyzed using 7,000 MWCO Slide-A-Lyzer Mini Dialysis Units (pierce Cat # 69560 (10 µl-100 µl) Rockford, IL, USA), in a 1000:1 buffer (HyClone Cat # RR10417.01) to sample ratio. The dialysis unit was then placed in a flotation device and dialyzed at 4° C for 60 minutes using a low speed setting on a stir plate. The sample was collected and kept on ice until delivery.
Animal Procedures
All studies were approved by the University of Iowa Animal Care and Use Committee. Newborn pigs (Sus scrofa) were obtained during the first 24h of life, when the umbilical cord was still present. The procedure was performed by an interventional pediatric cardiologist (A.D.). He had previously developed a minimally invasive and innovative method for transcatheter intervention of the ductus arteriosus by cannulating the umbilical artery in newborn pigs59. We modified this technique by selectively cannulating the celiac artery, which is the vessel that supplies major branches to the pancreas in humans and pigs42. Shortly after birth (24–48h), the celiac artery can be easily accessed via the umbilical arteries that extend into the umbilical cord (Fig. 1A).
Piglets were anesthetized using spontaneous mask ventilation with isoflurane. Pulse oximetry, breath CO2, heart rate, and body temperature were monitored throughout the procedure. IV hydration was maintained with 10% dextrose infusion through a peripheral vein. Animal was placed in the right lateral decubitus position. The entire procedure was performed under sterile technique. A previously flushed 3.5 Fr. single lumen arterial catheter (Kendall, Argyle, Tyco Healthcare Group, Mansfield, MA, USA) was advanced into the umbilical artery to 20 cm, free flow of arterial blood was obtained and the catheter was flushed with saline. Position in the thoracic aorta was confirmed by fluoroscopy. Under fluoroscopic control the catheter was exchanged over a 0.021” pre-wetted guide wire (Argon Medical Devices, Inc. Athens, TX, USA) for a flushed 4 Fr. Introducer (Cordis, Johnson & Johnson, Miami, FL, USA). The dilator was removed and a 4 Fr. Cobra 1 (C1) Glidecath (Terumo Medical Corporation, New Jersey, USA) was advanced over the wire and placed in the descending aorta. The catheter was flushed with saline after removing the wire. The catheter was slowly withdrawn below the diaphragm and the celiac artery was cannulated. Angiography confirmed the cannulation (Fig. 1B). AAV9CMV.sceGFP (2.4×1011 vg per animal; 1.2×1012 vg per animal; 2.4×1012 vg per animal; 6.1×1012 vg per animal; n=1 for all time points and doses except, n=2 for 6.1×1012 vg at 1 month, n=7 for 2.4×1012 vg at 1 month) or AAV9CMV.eGFP (2.4×1012 vg per animal; n=3 at 2 months) were injected into the celiac artery and the catheter was flushed again with 5 ml normal saline. The vehicle was given to 2 animals as control and they were sacrificed at 1 and 3 months.
After the procedure, piglets recovered uneventfully and received standard care. During the first 24 hours, the piglets were fed colostrum supplement (Manna Pro, Saint Louis, MO, USA) via syringe every 2 hours followed by milk replacer (Multi-species Milk Replacer, Carpentersville, IL, USA) via syringe every 4 hours until competent to feed independently. The piglets were transitioned to pelleted feed at ~ 2 weeks of age. One and 3 months after vector delivery, animals were euthanized using intracardiac Euthasol ® injection (90 mg/kg), followed by bilateral thoracotomy. The animals were not kept beyond 3 months of age, because they become very large (>100 lbs) and challenging to handle in the animal care facility.
Necropsy and tissue harvesting
One or three months after injection, the animals were sedated with intramuscular (IM) injection of Ketamine (20 mg/kg) and Xylazine (0.2–2.2 mg/kg) and euthanized as described above. A full necropsy was performed and tissues were collected. Tissues were placed in 4% paraformaldehyde (PFA) and fixed for 24–48 hours. Following fixation, tissues were either processed and paraffin embedded or placed through a series of sucrose gradients (10%, 20%, and 30%) for cryoprotection and snap frozen.
Immunohistochemistry (IHC) staining
Frozen tissue sections were cut (10 µm) and fixed in 10% ice-cold zinc formalin for 5’ and washed with dH2O. Sections were then immersed in Phosphate Buffer Solution (PBS) for 5’ and transferred into 0.2% Triton-X for 10 minutes for permeabilization. Sections were washed in PBSx3 for 5 minutes each. Endogenous peroxidase activity was quenched in 3% hydrogen peroxide (H2O2) at for 8’ and washed in PBSx3 for 5’ each. Sections were blocked in 5% normal goat serum for 30’ at room temperature (RT) and incubated at RT with primary (rabbit polyclonal anti-GFP, 1:400) for 1h, followed by secondary antibody (Envision plus Rabbit) for 30’. Signal development was performed using a chromogen diaminobenzidine (DAB) solution for 10 minutes and washed in running tap water for 10’. Tissues were counterstained in Harris Hematoxylin for 20’’, transferred back under the running tap water for 5 minutes, dehydrated through graded alcohols, cleared in xylenes, and mounted.
Immunofluorescence (IF) staining
Frozen tissue sections were cut (10 µm) and fixed in 10% cold Z-fix for 5’. Sections were washed in tap water and then placed in three washes of PBS for 5’ each. Tissues were permeabilized in 0.2% Triton X-100 for 10’ and washed in PBSx3. Nonspecific background staining was blocked using a 5% normal goat serum for 30’. Sections were incubated with primary antibody 1:400 anti-GFP (Abcam GR8 722-1, Cambridge, MA, USA) at 4° C overnight, followed with secondary antibody (Alexa-flour 488) for 30’ at RT. Slides were washed with PBSx3, mounted with Vectashield and DAPI. Ten random pancreatic fields (20× mag) were assessed per animal and % GFP positive cells were calculated by counting GFP expressing divided by the total number of cells in the field.
End-Point RT-PCR
End-point RT-PCR was performed as a confirmation of GFP presence from tissues collected during necropsy, snap frozen in liquid nitrogen, and stored at −80° C. The tissues were homogenized (no. 03-392-106 grinder, 0.5 mL pestle size; Fisher Scientific, Pittsburgh, PA, USA) and RNA was extracted using Qiagen RNeasy Lipid Tissue Kit (no.74084; Qiagen) with the optional DNase digestion step performed to prevent genomic DNA contamination. Following RNA extraction, all samples were measured for RNA concentration using NanoDrop 1000 (Thermo Scientific, Rockford, IL, USA). Samples were randomly selected to obtain RNA integrity numbers (RIN) using Agilent 2100 bioanalyzer system (Agilent Technologies, Santa Clara, CA, USA). RIN numbers ranged from 7.4–9.2, indicating minimally degraded RNA suitable for downstream applications. Reverse-Transcriptase RT-PCR was performed using SuperScript® VILO™ Master Mix (Cat. No. 11755050, Invitrogen, Grand Island, NY, USA), 1000 ng starting RNA concentration, and UltraPure™ RNase/DNase-Free distilled water (Cat. No. 10977015, Invitrogen, Grand Island, NY, USA). The thermal cycler (Product No. PTC-1148C, Bio-Rad, Hercules, CA, USA) settings were 25° C for 10 minutes, 42° C for one hour, and 85° C for 5 minutes. End-point RT-PCR was then performed on the cDNA synthesized using HotStartTaq Master Mix Kit (Cat. No. 203446, Qiagen, Valencia, CA, USA), 10 mM eGFP forward primer 5’-ACG TAA ACG GCC ACA AGT TC-3’, 10mM eGFP reverse primer 5’-AAG TAG TGC TGC TTC ATG TG-3’ (Integrated DNA Technologies, Coralville, IA, USA). A 1.5% agarose gel was prepared and samples run at 120v for 30 minutes.
Statistics
To measure transduction efficiency, ten random pancreatic fields (20× mag) were assessed for all time points and concentrations (immunofluorescence) (n=1 for all time points and doses except, n=2 for 6.1×1012 vg at 1 month, and n=7 for 2.4×1012 vg at 1 month). % GFP positive cells were calculated by counting GFP-expressing cells divided by the total number of cells in the field. Data were presented as the average of individual data points.
Supplementary Material
Pancreas sections after newborn pigs received AAV9CMV.sceGFP (B, C, E, F) or vehicle (A, D) into the celiac artery. Immunofluorescence images are shown. (B, E) 1.2×1012 vg; (F) 2.4×1012 vg; (C) 6.1×1012 vg; A–C euthanized after 1 month; D–F euthanized after 3 months. Arrows point to ducts, arrowheads point to intercalated ducts, scale bar = 100 µm.
ACKNOWLEDGMENTS
We would like to thank Joseph Zabner for his helpful input on the manuscript. We would like to thank Maisam Abu-El-Haija, Shelby Burdette, Leah Reznikov, Hyder Chowdhry, Michael Rector, John Morgan, Benjamin Steines for their technical assistance with handling tissues, animal care/feedings, necropsies, and the Gene Transfer Vector Core for virus production.
This work was supported by National institute of Health (NIH) DK084049 (AU); University of Iowa Biological Sciences Funding Program (AU), NIH/University of Iowa Center for Gene Therapy of Cystic Fibrosis and Other Genetic Diseases Pilot Grant (AU); NIH DK54759 (BD); NIH P01 HL51670 (PBM); Cystic Fibrosis Foundation Student Traineeship Grant (EB).
Footnotes
CONFLICTS OF INTERESTS
Authors have no conflicts of interests.
Supplementary information is available at GT's website.
REFERENCES
- 1.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 2.Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245(4922):1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 3.Welsh MJ, Ramsey BW, Accurso FJ, Cutting GR. Cystic Fibrosis. In: Scriver CRBAL, Sly WS, Valle D, editors. The Metabolic and Molecular Basis of Inherited Disease. New York: McGraw-Hill; 2001. p. 5121. [Google Scholar]
- 4.Borowitz D, Durie PR, Clarke LL, Werlin SL, Taylor CJ, Semler J, et al. Gastrointestinal outcomes and confounders in cystic fibrosis. J.Pediatr.Gastroenterol.Nutr. 2005;41(3):273–285. doi: 10.1097/01.mpg.0000178439.64675.8d. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed N, Corey M, Forstner G, Zielenski J, Tsui LC, Ellis L, et al. Molecular consequences of cystic fibrosis transmembrane regulator (CFTR) gene mutations in the exocrine pancreas. Gut. 2003;52(8):1159–1164. doi: 10.1136/gut.52.8.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kristidis P, Bozon D, Corey M, Markiewicz D, Rommens J, Tsui LC, et al. Genetic determination of exocrine pancreatic function in cystic fibrosis. Am.J.Hum.Genet. 1992;50(6):1178–1184. [PMC free article] [PubMed] [Google Scholar]
- 7.Imrie JR, Fagan DG, Sturgess JM. Quantitative evaluation of the development of the exocrine pancreas in cystic fibrosis and control infants. Am.J.Pathol. 1979;95(3):697–707. [PMC free article] [PubMed] [Google Scholar]
- 8.Couper RT, Corey M, Durie PR, Forstner GG, Moore DJ. Longitudinal evaluation of serum trypsinogen measurement in pancreatic-insufficient and pancreatic-sufficient patients with cystic fibrosis. J.Pediatr. 1995;127(3):408–413. doi: 10.1016/s0022-3476(95)70072-2. [DOI] [PubMed] [Google Scholar]
- 9.Ooi CY, Dorfman R, Cipolli M, Gonska T, Castellani C, Keenan K, et al. Type of CFTR mutation determines risk of pancreatitis in patients with cystic fibrosis. Gastroenterology. 2011;140(1):153–161. doi: 10.1053/j.gastro.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 10.Augarten A, Ben TA, Madgar I, Barak A, Akons H, Laufer J, et al. The changing face of the exocrine pancreas in cystic fibrosis: the correlation between pancreatic status, pancreatitis and cystic fibrosis genotype. Eur.J Gastroenterol.Hepatol. 2008;20(3):164–168. doi: 10.1097/MEG.0b013e3282f36d04. [DOI] [PubMed] [Google Scholar]
- 11.Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease: Clinical and pathological study. Am J Dis Child. 1938;56:344–399. [Google Scholar]
- 12.Oppenheimer EH, Esterly JR. Pathology of cystic fibrosis review of the literature and comparison with 146 autopsied cases. Perspect.Pediatr.Pathol. 1975;2:241–278. [PubMed] [Google Scholar]
- 13.Corey M, Edwards L, Levison H, Knowles M. Longitudinal analysis of pulmonary function decline in patients with cystic fibrosis. J.Pediatr. 1997;131(6):809–814. doi: 10.1016/s0022-3476(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 14.Gaskin K, Gurwitz D, Durie P, Corey M, Levison H, Forstner G. Improved respiratory prognosis in patients with cystic fibrosis with normal fat absorption. J.Pediatr. 1982;100(6):857–862. doi: 10.1016/s0022-3476(82)80501-5. [DOI] [PubMed] [Google Scholar]
- 15.Kraemer R, Rudeberg A, Hadorn B, Rossi E. Relative underweight in cystic fibrosis and its prognostic value. Acta Paediatr.Scand. 1978;67(1):33–37. doi: 10.1111/j.1651-2227.1978.tb16273.x. [DOI] [PubMed] [Google Scholar]
- 16.Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W. Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care. 2009;32(9):1626–1631. doi: 10.2337/dc09-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stecenko AA, Moran A. Update on cystic fibrosis-related diabetes. Curr Opin Pulm Med. 2010;16(6):611–615. doi: 10.1097/MCP.0b013e32833e8700. [DOI] [PubMed] [Google Scholar]
- 18.Milla CE, Warwick WJ, Moran A. Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline. Am.J.Respir.Crit Care Med. 2000;162(3 Pt 1):891–895. doi: 10.1164/ajrccm.162.3.9904075. [DOI] [PubMed] [Google Scholar]
- 19.Chamnan P, Shine BS, Haworth CS, Bilton D, Adler AI. Diabetes as a determinant of mortality in cystic fibrosis. Diabetes Care. 2010;33(2):311–316. doi: 10.2337/dc09-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, et al. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257(5073):1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 21.Clarke LL, Grubb BR, Gabriel SE, Smithies O, Koller BH, Boucher RC. Defective epithelial chloride transport in a gene-targeted mouse model of cystic fibrosis. Science. 1992;257(5073):1125–1128. doi: 10.1126/science.257.5073.1125. [DOI] [PubMed] [Google Scholar]
- 22.Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321(5897):1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci.Transl.Med. 2010;2(29):29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostedgaard LS, Meyerholz DK, Chen JH, Pezzulo AA, Karp PH, Rokhlina T, et al. The DeltaF508 mutation causes CFTR misprocessing and cystic fibrosis-like disease in pigs. Sci Transl Med. 2011;3(74):74ra24. doi: 10.1126/scitranslmed.3001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abu-El-Haija M, Ramachandran S, Meyerholz DK, Griffin M, Giriyappa RL, Stoltz DA, et al. Pancreatic damage in fetal and newborn cystic fibrosis pigs involves the activation of inflammatory and remodeling pathways. Am J Pathol. 2012;181(2):499–507. doi: 10.1016/j.ajpath.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyerholz DK, Stoltz DA, Pezzulo AA, Welsh MJ. Pathology of gastrointestinal organs in a porcine model of cystic fibrosis. Am J Pathol. 2010;176(3):1377–1389. doi: 10.2353/ajpath.2010.090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uc A, Giriyappa R, Meyerholz DK, Griffin M, Ostedgaard LS, Tang XX, et al. Pancreatic and biliary secretion are both altered in cystic fibrosis pigs. Am J Physiol Gastrointest Liver Physiol. 2012;303(8):G961–G968. doi: 10.1152/ajpgi.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raper SE, DeMatteo RP. Adenovirus-mediated in vivo gene transfer and expression in normal rat pancreas. Pancreas. 1996;12(4):401–410. doi: 10.1097/00006676-199605000-00013. [DOI] [PubMed] [Google Scholar]
- 29.McClane SJ, Hamilton TE, Burke CV, Raper SE. Functional consequences of adenovirus-mediated murine pancreatic gene transfer. Hum Gene Ther. 1997;8(6):739–746. doi: 10.1089/hum.1997.8.6-739. [DOI] [PubMed] [Google Scholar]
- 30.Wang AY, Peng PD, Ehrhardt A, Storm TA, Kay MA. Comparison of adenoviral and adeno-associated viral vectors for pancreatic gene delivery in vivo. Hum Gene Ther. 2004;15(4):405–413. doi: 10.1089/104303404322959551. [DOI] [PubMed] [Google Scholar]
- 31.Ayuso E, Chillon M, Agudo J, Haurigot V, Bosch A, Carretero A, et al. In vivo gene transfer to pancreatic beta cells by systemic delivery of adenoviral vectors. Hum Gene Ther. 2004;15(8):805–812. doi: 10.1089/1043034041648426. [DOI] [PubMed] [Google Scholar]
- 32.Jimenez V, Ayuso E, Mallol C, Agudo J, Casellas A, Obach M, et al. In vivo genetic engineering of murine pancreatic beta cells mediated by single-stranded adeno-associated viral vectors of serotypes 6, 8 and 9. Diabetologia. 2011;54(5):1075–1086. doi: 10.1007/s00125-011-2070-3. [DOI] [PubMed] [Google Scholar]
- 33.Loiler SA, Tang Q, Clarke T, Campbell-Thompson ML, Chiodo V, Hauswirth W, et al. Localized gene expression following administration of adeno-associated viral vectors via pancreatic ducts. Mol Ther. 2005;12(3):519–527. doi: 10.1016/j.ymthe.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Cheng H, Wolfe SH, Valencia V, Qian K, Shen L, Phillips MI, et al. Efficient and persistent transduction of exocrine and endocrine pancreas by adeno-associated virus type 8. J Biomed Sci. 2007;14(5):585–594. doi: 10.1007/s11373-007-9159-1. [DOI] [PubMed] [Google Scholar]
- 35.Ye X, Jerebtsova M, Ray PE. Liver bypass significantly increases the transduction efficiency of recombinant adenoviral vectors in the lung, intestine, and kidney. Hum Gene Ther. 2000;11(4):621–627. doi: 10.1089/10430340050015806. [DOI] [PubMed] [Google Scholar]
- 36.Aitken ML, Moss RB, Waltz DA, Dovey ME, Tonelli MR, McNamara SC, et al. A phase I study of aerosolized administration of tgAAVCF to cystic fibrosis subjects with mild lung disease. Hum.Gene Ther. 2001;12(15):1907–1916. doi: 10.1089/104303401753153956. [DOI] [PubMed] [Google Scholar]
- 37.Wagner JA, Nepomuceno IB, Messner AH, Moran ML, Batson EP, Dimiceli S, et al. A phase II, double-blind, randomized, placebo-controlled clinical trial of tgAAVCF using maxillary sinus delivery in patients with cystic fibrosis with antrostomies. Hum.Gene Ther. 2002;13(11):1349–1359. doi: 10.1089/104303402760128577. [DOI] [PubMed] [Google Scholar]
- 38.Wagner JA, Messner AH, Moran ML, Daifuku R, Kouyama K, Desch JK, et al. Safety and biological efficacy of an adeno-associated virus vector-cystic fibrosis transmembrane regulator (AAV-CFTR) in the cystic fibrosis maxillary sinus. Laryngoscope. 1999;109(2 Pt 1):266–274. doi: 10.1097/00005537-199902000-00017. [DOI] [PubMed] [Google Scholar]
- 39.Flotte TR, Carter BJ. Adeno-associated virus vectors for gene therapy. Gene Ther. 1995;2(6):357–362. [PubMed] [Google Scholar]
- 40.Inagaki K, Fuess S, Storm TA, Gibson GA, McTiernan CF, Kay MA, et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther. 2006;14(1):45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Z, Zhu T, Rehman KK, Bertera S, Zhang J, Chen C, et al. Widespread and stable pancreatic gene transfer by adeno-associated virus vectors via different routes. Diabetes. 2006;55(4):875–884. doi: 10.2337/diabetes.55.04.06.db05-0927. [DOI] [PubMed] [Google Scholar]
- 42.Ferrer J, Scott WE, 3rd, Weegman BP, Suszynski TM, Sutherland DE, Hering BJ, et al. Pig pancreas anatomy: implications for pancreas procurement, preservation, and islet isolation. Transplantation. 2008;86(11):1503–1510. doi: 10.1097/TP.0b013e31818bfda1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prasad KM, Yang Z, Bleich D, Nadler JL. Adeno-associated virus vector mediated gene transfer to pancreatic beta cells. Gene Ther. 2000;7(18):1553–1561. doi: 10.1038/sj.gt.3301279. [DOI] [PubMed] [Google Scholar]
- 44.Yang YW, Kotin RM. Glucose-responsive gene delivery in pancreatic Islet cells via recombinant adeno-associated viral vectors. Pharm Res. 2000;17(9):1056–1061. doi: 10.1023/a:1026445426982. [DOI] [PubMed] [Google Scholar]
- 45.Loiler SA, Conlon TJ, Song S, Tang Q, Warrington KH, Agarwal A, et al. Targeting recombinant adeno-associated virus vectors to enhance gene transfer to pancreatic islets and liver. Gene Ther. 2003;10(18):1551–1558. doi: 10.1038/sj.gt.3302046. [DOI] [PubMed] [Google Scholar]
- 46.Rehman KK, Wang Z, Bottino R, Balamurugan AN, Trucco M, Li J, et al. Efficient gene delivery to human and rodent islets with double-stranded (ds) AAV-based vectors. Gene Ther. 2005;12(17):1313–1323. doi: 10.1038/sj.gt.3302530. [DOI] [PubMed] [Google Scholar]
- 47.Burghardt B, Elkaer ML, Kwon TH, Racz GZ, Varga G, Steward MC, et al. Distribution of aquaporin water channels AQP1 and AQP5 in the ductal system of the human pancreas. Gut. 2003;52(7):1008–1016. doi: 10.1136/gut.52.7.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marino CR, Matovcik LM, Gorelick FS, Cohn JA. Localization of the cystic fibrosis transmembrane conductance regulator in pancreas. J.Clin.Invest. 1991;88(2):712–716. doi: 10.1172/JCI115358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strong TV, Boehm K, Collins FS. Localization of cystic fibrosis transmembrane conductance regulator mRNA in the human gastrointestinal tract by in situ hybridization. J.Clin.Invest. 1994;93(1):347–354. doi: 10.1172/JCI116966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rehman KK, Trucco M, Wang Z, Xiao X, Robbins PD. AAV8-mediated gene transfer of interleukin-4 to endogenous beta-cells prevents the onset of diabetes in NOD mice. Mol Ther. 2008;16(8):1409–1416. doi: 10.1038/mt.2008.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michl P, Gress TM. Current concepts and novel targets in advanced pancreatic cancer. Gut. 2013;62(2):317–326. doi: 10.1136/gutjnl-2012-303588. [DOI] [PubMed] [Google Scholar]
- 52.Moran A, Diem P, Klein DJ, Levitt MD, Robertson RP. Pancreatic endocrine function in cystic fibrosis. J.Pediatr. 1991;118(5):715–723. doi: 10.1016/s0022-3476(05)80032-0. [DOI] [PubMed] [Google Scholar]
- 53.Larsen S, Hilsted J, Tronier B, Worning H. Pancreatic hormone secretion in chronic pancreatitis without residual beta-cell function. Acta Endocrinol (Copenh) 1988;118(3):357–364. doi: 10.1530/acta.0.1180357. [DOI] [PubMed] [Google Scholar]
- 54.Nousia-Arvanitakis S, Tomita T, Desai N, Kimmel JR. Pancreatic polypeptide in cystic fibrosis. Arch.Pathol.Lab Med. 1985;109(8):722–726. [PubMed] [Google Scholar]
- 55.Stern A, Davidson GP, Kirubakaran CP, Deutsch J, Smith A, Hansky J. Pancreatic polypeptide secretion. A marker for disturbed pancreatic function in cystic fibrosis. Dig.Dis.Sci. 1983;28(10):870–873. doi: 10.1007/BF01317035. [DOI] [PubMed] [Google Scholar]
- 56.Ostedgaard LS, Rokhlina T, Karp PH, Lashmit P, Afione S, Schmidt M, et al. A shortened adeno-associated virus expression cassette for CFTR gene transfer to cystic fibrosis airway epithelia. Proc.Natl.Acad.Sci.U.S.A. 2005;102(8):2952–2957. doi: 10.1073/pnas.0409845102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ostedgaard LS, Zabner J, Vermeer DW, Rokhlina T, Karp PH, Stecenko AA, et al. CFTR with a partially deleted R domain corrects the cystic fibrosis chloride transport defect in human airway epithelia in vitro and in mouse nasal mucosa in vivo. Proc.Natl.Acad.Sci.U.S.A. 2002;99(5):3093–3098. doi: 10.1073/pnas.261714599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahlgren U, Jonsson J, Edlund H. The morphogenesis of the pancreatic mesenchyme is uncoupled from that of the pancreatic epithelium in IPF1/PDX1-deficient mice. Development. 1996;122(5):1409–1416. doi: 10.1242/dev.122.5.1409. [DOI] [PubMed] [Google Scholar]
- 59.Divekar A, Gutsol A, Dakshinamurti S. Transumbilical catheter intervention of ductus arteriosus in neonatal swine. J Invest Surg. 2007;20(5):313–317. doi: 10.1080/08941930701598842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pancreas sections after newborn pigs received AAV9CMV.sceGFP (B, C, E, F) or vehicle (A, D) into the celiac artery. Immunofluorescence images are shown. (B, E) 1.2×1012 vg; (F) 2.4×1012 vg; (C) 6.1×1012 vg; A–C euthanized after 1 month; D–F euthanized after 3 months. Arrows point to ducts, arrowheads point to intercalated ducts, scale bar = 100 µm.


