Abstract
Objective
Depression has been associated with increased risk of heart failure (HF). Since anxiety is highly comorbid with depression, we sought to establish if anxiety, depression, or their co-occurrence are associated with incident HF.
Methods
A retrospective cohort (n=236,079) including VA patients (ages 50–80), free of cardiovascular disease (CVD) at baseline, was followed between 2001 and 2007. Cox proportional hazards models were computed to estimate the association between anxiety disorders alone, major depressive disorder (MDD) alone, and the combination of anxiety and MDD, with incident HF before and after adjusting for sociodemographics, CVD risk factors (type 2 diabetes, hypertension, hyperlipidemia, obesity), nicotine dependence/personal history of tobacco use, substance use disorders (alcohol and illicit drug abuse/dependence) and psychotropic medication.
Results
Compared to unaffected patients, those with anxiety only, MDD only, and both disorders were at increased risk of incident HF in age-adjusted models (HR=1.19, 95% C.I., 1.10–1.28; HR=1.21 95%C.I., 1.13–1.28; HR=1.24, 95%C.I., 1.17–1.32, respectively). After controlling for psychotropics in a full model the association between anxiety only, MDD only, and both disorders and incident HF increased (HRs: 1.46, 1.56 and 1.74, respectively).
Conclusions
Anxiety disorders, MDD, and co-occurring anxiety and MDD are associated with incident HF in this large cohort of VA patients free of CVD at baseline. This risk of HF is greater after accounting for protective effects of psychotropic medications. Prospective studies are needed to clarify the role of depression and anxiety and their pharmacological treatment in the etiology of HF.
Keywords: anxiety, depression, heart failure, veteran, psychotropic medication, medical records, administrative data
Introduction
Depression is prevalent in patients with established heart failure HF (1–3) and it contributes to increased health care utilization (4), greater likelihood of hospitalization (5–8) poor health status (9, 10), increased rates of clinical events (8, 11), and mortality (12–17). There have been but two studies of the effect of depression on incident HF. Williams et al. (18) found that elderly women but not men with depression, as defined by a CES-D score >20, were at nearly a two-fold increased risk of incident HF. The Systolic Hypertension in the Elderly Program (SHEP) study showed that depression, defined as CES-D>15, was significantly associated with incident HF (HR=2.8) (19).
Anxiety disorders, commonly comorbid with depression (20), are also associated with adverse outcomes in patients with established HF including hospital readmission (21), poorer health-related quality of life (21), and greater health services utilization (22). Anxiety disorders have been shown to increase the risk of myocardial infarction (23), but whether they increase the risk for incident HF and whether that effect occurs independent of the effects of depression are unknown. The aim of the present study was to determine if the risk of HF was greater in patients with: 1) a diagnosis of one or more common anxiety disorders in the present cohort (anxiety disorder not otherwise specified (NOS), generalized anxiety disorder (GAD) and posttraumatic stress disorder (PTSD)) but free of MDD, 2) MDD but free of anxiety disorders and 3) co-occurring anxiety and depressive disorders.
Methods
Administrative data for this retrospective cohort study were obtained from electronic files maintained by the Veterans Health Administration and included inpatient and outpatient diagnoses (ICD-9-CM codes), current procedural terminology (CPT) codes, pharmacy benefits management (PBM) records, and vital status. Data were available from fiscal year (FY) 1999, the first year in which national VA data are considered complete, to FY2007. The datasets are maintained by the VHA Office of Information at the Austin Information Technology Center (www.virec.research.va.gov/DataSourcesName/Medical-SAS-Datasets/SAS.htm).
The cohort was restricted to patients between 18 and 80 years old at baseline. All patients with an ICD-9-CM code for cardiovascular or cerebrovascular disease in FY1999 or FY2000 (ICD-9-CM codes 402–405, 410–417, 420–429) were excluded from the cohort, as were those with a diagnosis of bipolar disorder or affective psychosis. Among the remaining patients, 236,681 had a depression diagnosis (ICD–9–CM: 296.2, 296.3, or 311). We henceforth refer to depression diagnoses as ‘MDD’. A primary diagnosis is the providers’ record of the primary purpose for the clinic visit. As a comparison group, 299,734 CVD-free non-depressed patients were randomly selected from all VA patients (n=1,380,433) enrolled in 1999 and 2000. Additional details of cohort construction have been previously reported (23, 24).
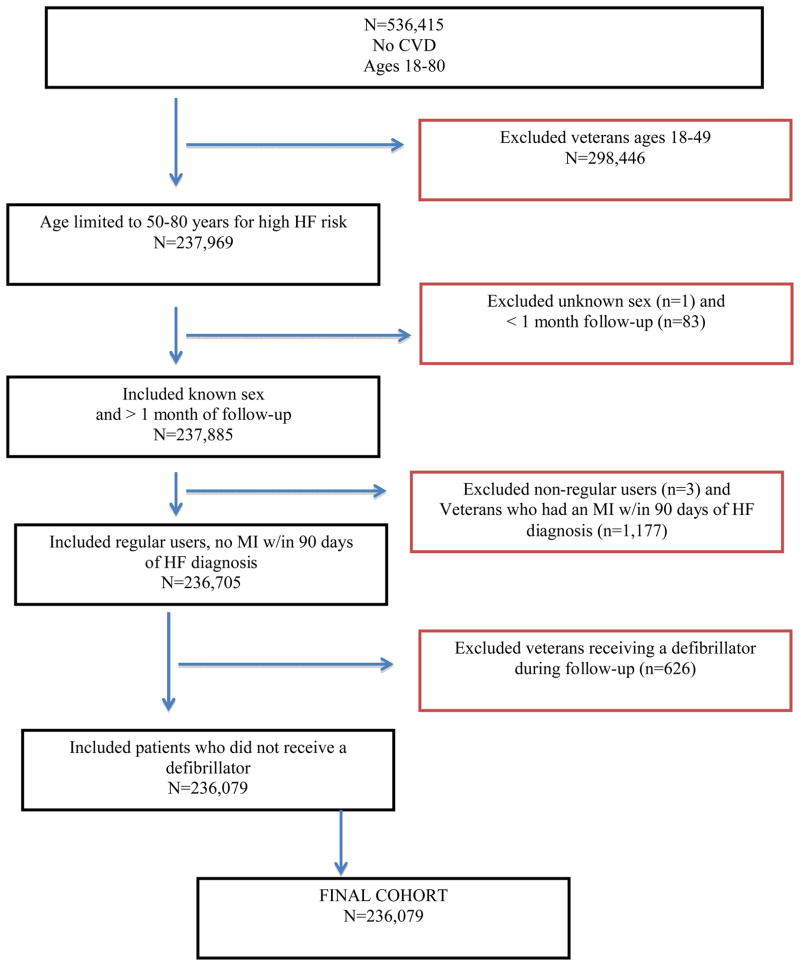
Inclusion and exclusion criteria for the present analysis are shown in Figure 1. For the purposes of this study, the cohort was limited to patients who were between 50 and 80 years of age at entry (n=237,969) because this is the period of highest risk for incident HF. Patients of unknown sex were excluded (n=1) as were patients with less than 1 month of follow-up (n=83). The study cohort was also limited to regular VA users, as defined by having at least 1 visit in both FY1999 and FY2000 (n=3 excluded). Patients who experienced an MI within 90 days prior to receiving an index HF diagnosis were excluded (n=1,177) in order to avoid temporally confounding MI diagnosis and sequelae with incident HF. Patients who received a defibrillator before the end of follow-up (either by censoring or HF diagnosis) were excluded because these devices have been associated with anxiety and this might confound the main association of interest (25) (n=626). The final cohort included 236,079 patients.
Figure 1.
Flow chart of cohort inclusion and exclusion criteria
Sensitivity analysis testing the effect of the 90-day MI and defibrillator exclusion criteria were conducted. We found that there were no significant differences in the rate of incident HF in age-adjusted or full models when these patients were included in the cohort. However, we continued to exclude them because of the underlying theoretical assumptions. Allowing at least 90-days between an MI and diagnosis of HF strengthens the validity of the HF diagnosis. Patients are at increased risk of developing HF within 30 days post MI. We took a conservative approach to the 30-day period and expanded this high-risk period to 90 days, knowing that we may have excluded patients who had HF due to anxiety. Post-hoc analysis without this exclusion are discussed in the limitations section. Given the existing evidence that defibrillators are associated with incident anxiety (25), including patients with these devices introduces a potential confounder into the exposure. Additionally, anxiety resulting from the introduction of a device does not meet the DSM-IV criteria for an anxiety disorder and we are unable to make this determination in administrative data.
Predictor variable: MDD and Anxiety Disorders
All psychiatric disorders were defined by the presence of either 1 inpatient or 2 outpatient ICD-9-CM codes within a 12-month period. In VA patients, the presence in the medical record of two or more MDD diagnoses has an 88% positive predictive value (PPV) and 71% negative predictive value (NPV) compared to self-reported lifetime history of MDD (26). MDD was defined by ICD-9-CM codes (296.2, 296.3, 300.4, 311), and anxiety disorders included the most common diagnoses in the VA population (GAD, anxiety disorder NOS, and PTSD), and were defined by the following ICD-9-CM codes, respectively: 300.02, 300.0, 309.81. The prevalence of other anxiety disorders (social phobia, panic disorder and obsessive compulsive disorder) was less than 2% in the overall cohort and so were not included in this analysis.
We modeled anxiety disorders as a composite variable indicating a diagnosis of one or more conditions. The composite variable was preferred to modeling individual disorders for several reasons. Results of fitting age-adjusted survival models that included each specific anxiety disorder revealed that the 95% confidence intervals were overlapping, suggesting that there were no significant differences in the magnitude of rate of incident HF by type of anxiety disorder. Second, preliminary data analysis indicated there was no evidence of an additive effect of multiple anxiety disorders on risk of HF. Third, administrative data precluded determining which anxiety disorder is the primary diagnosis when patients have multiple diagnoses. Fourth, diagnostic practices and accuracy of anxiety disorder diagnosis is likely to vary by provider type, with greater diagnostic accuracy when the diagnosis is made by mental health specialists as compared to other provider specialties. Finally, the composite variable overcomes the potential for multicollinearity in models that contain multiple types of anxiety disorder. Therefore, the independent variable contained the following 4 mutually exclusive categories: 1) anxiety disorder and MDD, 2) anxiety disorder alone, 3) MDD alone and 4) unaffected. Co-occurring anxiety and MDD diagnoses must have been recorded prior to incident HF but were not required to occur on the same date.
Outcome: incident HF
Incident HF from 10/1/2000 to 9/30/2007 was identified via ICD-9-CM codes (398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 425.4x, 425.5x, 425.7x, 425.8x, 425.9x, 428.xx) from inpatient and outpatient records. This combination of ICD-9-CM codes has 88% PPV and 97% NPV for in-hospital discharge records (27). This is one of the most accurate algorithms for identifying heart failure in administrative data (28).
Covariates
Covariates were selected a priori if they were likely to be associated with anxiety, MDD, and HF. Socio-demographic covariates included age, gender, race, marital status, and having only VA health care benefits vs. co-insurance (a proxy of lower income and method to account for access to non-VA care), because younger age, white race, non-married status, and lower income are each positively associated with MDD and correlated with HF. CVD risk factors associated with both MDD and risk of HF included: hypertension (ICD-9-CM 401–401.9), hyperlipidemia (ICD-9-CM 272–272.2), type 2 diabetes (ICD-9-CM 250.x; (also identified by antidiabetic medications and insulin) and obesity (ICD-9-CM 278.0–278.02; or BMI>30 calculated from medical record of height and weight). Because anxiety disorders and MDD are strongly associated with substance use disorders which in turn contribute to heart disease, we adjusted for any medical record indicating smoking history (either nicotine dependence (ICD–9–CM code 305.1) or V15.82 indicating personal history of tobacco use), illicit drug abuse or dependence and alcohol abuse or dependence (303.0, 304.0–305.9). Only conditions that were present before HF or censoring were counted. Variables for prescriptions of any selective serotonin reuptake inhibitor (SSRI), any tricyclic antidepressant (TCA), any serotonin norepinephrine reuptake inhibitor (SNRI), any other antidepressant and any benzodiazepine (benzo) received at any time during the study period were included to control for pharmacological treatment of depression and anxiety disorders and the potential effect of treatment on risk for incident HF (24). A list of these drugs can be found in Supplemental Table 1 (SDC 1).
Analytic Design
Chi square statistics were used in age-stratified bivariate analyses to determine whether anxiety and depressive disorders are associated with incident HF. Chi square and t-tests were used to compare covariates by heart failure status and to compare covariates by anxiety-MDD status. Cox proportional hazards models with time-dependent covariates were computed to determine the effects of anxiety disorder and MDD, anxiety disorder alone and MDD alone, relative to unaffected, on incident HF. Separate models were computed sequentially adjusting for age, sociodemographics (VA insurance status, gender, race, marital status), then for CVD risk factors (type 2 diabetes, hypertension, hyperlipidemia, obesity) and finally for nicotine dependence/personal history of tobacco use, substance use disorders (alcohol abuse/dependence, illicit drug abuse/dependence) and finally psychotropic use. Demographics were modeled from their baseline value and all other covariates were treated as time-dependent. Specifically, the survival models treated depression and anxiety disorders as time dependent therefore a patient who started follow-up without depression or anxiety may contribute time to depression or anxiety if they received a diagnosis between baseline and incident heart failure or before end of follow-up.
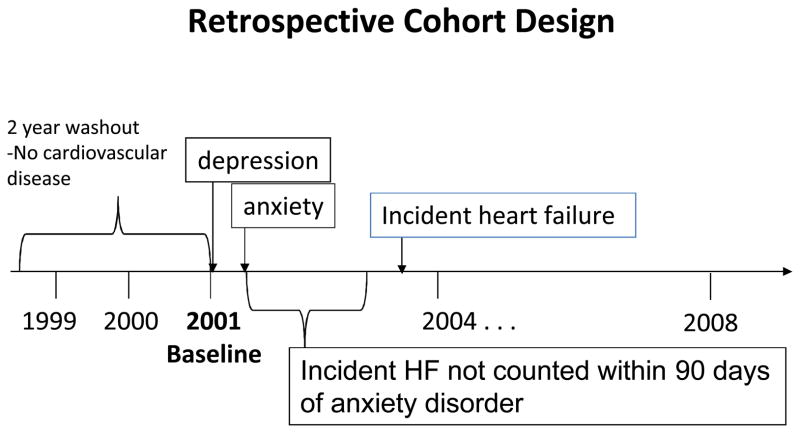
Time-dependent variables were fixed to occur at least 90 days before the outcome. The purpose of this timing was to preserve the temporality of the exposure-outcome relationship and the biological plausibility of anxiety and MDD leading to incident HF. With this method, only psychiatric diagnoses occurring at least 90 days prior to a HF diagnosis were considered, which was central to the fundamental biological plausibility -- that these diagnoses were contributing to the development of HF, either directly or indirectly. Sensitivity analysis showed that removing the 90-day time dependence from the model did not result in significant change in the HRs for anxiety, depression or both disorders, however we retained this condition to preserve the underlying temporality in the model. An illustration of the study design is shown in Figure 2.
Figure 2.
Study design.
The unit of follow-up time was in months, and the duration of follow-up was calculated as time from 10/1/2000 until diagnosis of incident HF, or, if no diagnosis of HF was present, follow-up duration ended with the patient’s death or last documented contact with the VA system. SAS version 9.2 software (SAS Institute, Cary, NC) was used for all of the analyses, with α levels set at .05, using two tailed tests to allow for both risk and protective effects. The PROC PHREG procedure was used to compute Cox proportional hazards models.
This study was approved by the institutional review boards at Washington University, Saint Louis University and the St. Louis VAMC. An exemption from informed consent was received due to impractibility of obtaining informed consent in administrative data.
Results
The proportion of patients with MDD was 22.9%, PTSD 14.4%, anxiety NOS 8.9% and GAD 4.4%. During the follow-up period, 4.7% of the patients developed HF. Prior to adjusting for age, there was not a statistically significant association between anxiety only and depression only with incident HF. In those with both anxiety and depression, these diagnoses were associated with a small but significant protective effect. The incidence of heart disease varied by age. Table 1 shows the incidence rate of HF by the anxiety disorder-MDD exposure variable stratified by age group. Overall, incidence of HF during the observation period was 8.2/1000 PY and increased from 4.7/1000 PY in 51–55 year old patients to 15.4/1000 PY in 76–80 year old patients. Patients with MDD only had a higher incidence rate of HF across age groups compared to unaffected patients. Higher incidence of HF in patients with anxiety was observed only in patients 66–70 years of age and those 71–75 years of age.
Table 1.
Incidence per 1000 Person Years and (number of events) of HF by psychiatric diagnosis and age-group.
| Total Cohort (n=236,079) | 50–55 years (n=66,314) | 56–60 years (n=38,047) | 61–65 years (n=28,204) | 66–70 years (n=35,962) | 71–75 years (n=31,417) | 76–80 years (n=35,775) | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| total | 8.2 (10,994) | 4.7 (1,882) | 6.4 (1,445) | 7.2 (1,148) | 9.3 (1,877) | 11.3 (1,908) | 15.4 (2,734) |
| Neither Anxiety or MDD (n=159,482) | 8.6 (7,652) | 4.4 (858) | 6.2 (827) | 6.8 (794) | 9.0 (1,451) | 11.0 (1,529) | 15.4 (2,193) |
| Anxiety only (n=22,457) | 6.9 (824) | 4.5 (280) | 5.7 (120) | 6.2 (46) | 11.1 (102) | 13.4 (101) | 15.3 (175) |
| MDD only (n=24,721) | 9.0 (1,253) | 5.9 (289) | 8.1 (250) | 10.2 (193) | 9.8 (169) | 14.5 (168) | 17.1 (184) |
| Anxiety and MDD (n=29,419) | 6.5 (1,265) | 4.6 (455) | 6.1 (248) | 7.3 (115) | 10.7 (155) | 10.6 (110) | 13.9 (182) |
The association of covariates by incident heart failure is shown in Table 2. Compared to those without HF, patients with heart failure were significantly older and more frequently male, non-white, unmarried, holders of supplemental insurance policies, and significantly more likely to have diagnoses of hypertension, type 2 diabetes, and obesity (Table 2). As well, patients with incident HF were less likely to have substance abuse/dependence, hyperlipidemia, and nicotine dependence/personal history of tobacco use in these bivariate analyses. Substance abuse/dependence and nicotine dependence/personal history of tobacco use may be less common in HF due to these patients’ older age. Patients with HF were less likely to have a prescription for any SSRI, SNRI, other antidepressant or benzodiazepine. There was no difference in TCA prescriptions between those who had incident HF and those who did not.
Table 2.
Distribution of covariates by incident heart failure in a cohort of 236,079 Veterans Administration patients.
| Incident Heart Failure | |||||
|---|---|---|---|---|---|
|
| |||||
| Covariates | Total Cohort (n=236,079) | Yes (n=10,994) | No (n=225,085) | OR (95%CI) | p-value* |
| Age at Baseline (mean yrs, STD, 95% CI age of pts with HF) | 62.8 (9.3) | 66.1 (9.3) | 62.6 (9.3) | (65.9–66.3) | <.0011 |
| Gender: | |||||
| Female (REF) | 15,550 (6.6%) | 286 (2.6%) | 15,264 (6.8%) | .37 (.33–.41) | <.001 |
| Male | 220,529 (93.4%) | 10,708 (97.4%) | 209,821 (93.2%) | ||
| Race: | |||||
| White (REF) | 185,776 (78.7%) | 8,662 (78.8%) | 177,114 (78.7%) | .86 (.83–.90) | <.001 |
| Non-white | 41,028 (17.4%) | 2,274 (20.7%) | 38,754 (17.2%) | ||
| Unknown | 9,275 (3.9%) | 58 (0.5%) | 9,217 (4.1%) | ||
| Marital Status: | |||||
| Married (REF) | 130,598 (55.3%) | 5,914 (53.8%) | 124,684 (55.4%) | .89 (.87–.91) | <.001 |
| Unmarried2 | 95,755 (40.6%) | 4,952 (45.0%) | 90,803 (40.3%) | ||
| Unknown | 9,726 (4.1%) | 128 (1.2%) | 9,598 (4.3%) | ||
| Insurance: | |||||
| VA Insurance only | 128,463 (54.4%) | 5,528 (50.3%) | 122,935 (54.6%) | .84 (.81–.87) | <.001 |
| Other Insurance (REF) | 107,598 (45.6%) | 5,465 (49.7%) | 102,133 (45.4%) | ||
| Substance Abuse/dependence | 44,838 (19.0%) | 1,814 (16.5%) | 43,024 (19.1%) | .84 (.79–.88) | <.001 |
| Nicotine dependence/personal hist tobacco use | 74,304 (31.5%) | 3,323 (30.2%) | 70,981 (31.5%) | .94 (.90–.98) | .004 |
| Hypertension | 170,868 (72.4%) | 8,946 (81.4%) | 161,922 (71.9%) | 1.70 (1.62–1.79) | <.001 |
| Hyperlipidemia | 141,978 (60.1%) | 5,705 (51.9%) | 136,273 (60.5%) | .70 (.68–.73) | <.001 |
| Diabetes II | 74,429 (31.5%) | 4,908 (44.6%) | 69,521 (30.9%) | 1.81 (1.74–1.88) | <.001 |
| Obesity | 114,658 (48.6%) | 6,284 (56.8%) | 108,410 (48.2%) | 1.42 (1.36–1.47) | <.001 |
|
| |||||
| Psychotropics
| |||||
| Any SSRI | 72,944 (30.9%) | 3,034 (27.6%) | 69,910 (31.1%) | .85 (.81–.88) | <.001 |
|
| |||||
| Any TCA | 33,250 (14.1%) | 1,549 (14.1%) | 31,701 (14.1%) | 1.00 (.95–1.06) | 1.00 |
|
| |||||
| Any SNRI | 23,902 (10.1%) | 747 (6.8%) | 23,155 (10.3%) | .64 (.59–.69) | <.001 |
|
| |||||
| Any Other antidepressant | 56,055 (23.7%) | 2,202 (20.0%) | 53,853 (23.9%) | .80 (.76.84) | <.001 |
|
| |||||
| Any Benzo | 65,543 (27.8%) | 2,750 (25.0%) | 62,793 (27.9%) | .86 (.83–.90) | <.001 |
t-test
divorced, widowed, separated or never married
when not indicated, p-values are from binary logit models
Table 3 shows the distribution of covariates by the anxiety-MDD variable. Patients with both anxiety and MDD were more likely to have diagnoses of substance abuse or dependence and nicotine/personal history of tobacco use and to receive a prescription for all of the classes of psychotropics.
Table 3.
Distribution of covariates by anxiety and depression diagnoses.
| Covariate %(n) | No anxiety or depression n=159,482 |
Anxiety only n=22,457 |
Depression only n=24,721 |
Anxiety and Depression n=29,419 |
Chi-square p-value |
|---|---|---|---|---|---|
| Age (mean (95%CI)) | 64.5 (64.5–64.6) | 59.0 (58.9–59.1) | 60.5 (60.4–60.7) | 58.1 (58.0–58.2) | <.0001* |
| Gender | |||||
| Male | 93.0 (148,359) | 97.4 (21,868) | 92.2 (22,790) | 93.5 (27,512) | <.0001 |
| Female | 7.0 (11,123) | 2.6 (589) | 7.8 (1,931) | 6.5 (1,907) | |
| Race | |||||
| White | 77.3 (123,194) | 77.1 (17,316) | 84.3 (20,844) | 83.0 (24,422) | <.0001 |
| Non-White | 17.9 (28,576) | 20.4 (4,581) | 13.5 (3,336) | 15.4 (4,535) | |
| Unknown | 4.8 (7,712) | 2.5 (560) | 2.2 (541) | 1.6 (462) | |
| Marital | |||||
| Married | 58.2(92,777) | 56.6 (12,700) | 45.6 (11,260) | 47.1 (13,861) | <.0001 |
| Unmarried | 36.4 (58,088) | 41.4 (9,290) | 53.2 (13,157) | 51.7 (15,220) | |
| Unknown | 5.4 (8,617) | 2.1 (467) | 1.2 (304) | 1.2 (338) | |
| Insurance | |||||
| Additional | 48.0 (76,485) | 41.3 (9,281) | 41.6 (10,284) | 39.3 (11,548) | <.0001 |
| VA only | 52.0 (82,981) | 58.7 (13,174) | 58.4 (14,437) | 60.8 (17,871) | |
| Substance abuse | 10.8 (17,252) | 31.5 (7,062) | 30.3 (7,482) | 44.3 (13,042) | <.0001 |
| Nicotine/ personal history of tobacco use | 26.1 (41,606) | 41.2 (9,244) | 39.0 (9,645) | 46.9 (13,009) | <.0001 |
| Hypertension | 71.6 (114,228) | 76.0 (17,074) | 71.3 (17,627) | 74.6 (21,939) | <.0001 |
| Hyperlipidemia | 57.5 (91,759) | 67.0 (15,043) | 62.0 (15,328) | 67.5 (19,848) | <.0001 |
| Type 2 DM | 31.0 (49,380) | 33.3 (7,487) | 31.8 (7,866) | 33.0 (9,696) | <.0001 |
| Obesity | 44.9 (71,663) | 54.8 (12,295) | 54.2 (13,388) | 58.9 (17,312) | <.0001 |
| SSRI | 11.8 (18,879) | 61.3 (13,771) | 69.1 (17,071) | 78.9 (23,223) | <.0001 |
| TCA | 9.2 (14,671) | 21.6 (4,851) | 21.8 (5,386) | 28.4 (8,342) | <.0001 |
| SNRI | 2.1 (3,353) | 18.9 (4,249) | 21.9 (5,401) | 37.1 (10,899) | <.0001 |
| Other | 8.9 (14,153) | 45.2 (10,147) | 49.5 (12,229) | 66.4 (19,526) | <.0001 |
| Benzodiazepine | 14.0 (22,240) | 56.1 (12,590) | 41.9 (10,369) | 69.2 (20,344) | <.0001 |
All statistical tests are Chi-square tests unless indicated.
F-test (ANOVA)
The results of the Cox proportional hazard models are shown in Table 4. When adjusting only for age (model 1), anxiety disorders alone, MDD alone and co-occurring anxiety and MDD were all significantly associated with incident HF (HR=1.19, 95%C.I., 1.10–1.20; HR=1.21, 95%C.I., 1.13–1.28; and HR=1.24, 95%C.I., 1.17–1.32, respectively). There was little attenuation of these hazard ratios after adjusting for the effects of sociodemographics (model 2) and after addition of CVD risk factors (model 3). When substance abuse/dependence and nicotine dependence/personal history of tobacco use were added (model 4), the magnitude of the effect of anxiety and MDD on incident HF was reduced considerably. In this model, the association between anxiety disorders alone and incident HF was 1.10 (95%C.I., 1.02–1.13), for MDD the HR was 1.11 (95%C.I., 1.04–1.18) and for co-occurring anxiety and MDD, the fully adjusted HR was 1.08 (95%C.I., 1.02–1.15). This latter point estimate was significantly less than that observed in the age only adjusted model (i.e. HR=1.24, 95%C.I., 1.17–1.32 (model 1)). As well, female gender was associated with significantly reduced hazard of HF. Non-white race, being unmarried at baseline, and having additional health insurance coverage were all positively associated with incident HF. Hypertension and obesity were significant risk factors for HF, and hyperlipidemia had a protective effect.
Table 4.
Association (HR (95%CI)) between anxiety disorder, MDD and incident heart failure in a cohort of 236,079 Veterans Administration patients
| Model 1 Age Adjusted |
Model 2 Sociodemographics |
Model 3 CVD Risk Factors |
Model 4 Substance Abuse |
Model 5 Psychotropics |
|
|---|---|---|---|---|---|
|
| |||||
| Anxiety only | 1.19 (1.10–1.28) | 1.15 (1.07–1.24) | 1.15 (1.07–1.24) | 1.10 (1.02–1.19) | 1.46 (1.35–1.58) |
| MDD only | 1.21 (1.13–1.28) | 1.17 (1.10–1.24) | 1.16 (1.09–1.23) | 1.11 (1.04–1.18) | 1.56 (1.45–1.67) |
| Anxiety and MDD | 1.24 (1.17–1.32) | 1.19 (1.12–1.27) | 1.17 (1.10–1.25) | 1.08 (1.02–1.15) | 1.74 (1.61–1.88) |
| Neither anxiety or MDD | 1.0 | 1.0 | 1.0 | 1.0 | |
| Gender (Female) | 0.55 (0.49–0.62) | 0.59 (0.52–0.66) | 0.62 (0.55–0.70) | .64 (.57–.72) | |
| Race: | 1.0 | 1.0 | 1.0 | 1.0 | |
| White | 1.27 (1.22–1.34) | 1.13 (1.08–1.19) | 1.13 (1.08–1.19) | 1.10 (1.05–1.15) | |
| Non-white | 0.30 (0.23–0.39) | 0.34 (0.26–0.44) | 0.36 (0.27–0.46) | .33 (.25–.43) | |
| Unknown | |||||
| Marital status: | 1.0 | 1.0 | 1.0 | 1.0 | |
| Married | 1.32 (1.27–1.37) | 1.32 (1.27–1.38) | 1.26 (1.22–1.32) | 1.25 (1.20–1.30) | |
| Unmarried1 | 0.59 (0.49–0.70) | 0.75 (0.63–0.89) | 0.77 (0.64–0.92) | .74 (.62–.88) | |
| Unknown | |||||
| VA Insurance | 1.10 (1.05–1.14) | 1.13 (1.08–1.18) | 1.11 (1.07–1.16) | 1.11 (1.10–1.15) | |
| Type 2 diabetes | 1.86 (1.79–1.94) | 1.88 (1.81–1.96) | 1.90 (1.82–1.97) | ||
| Hypertension | 1.68 (1.60–1.77) | 1.65 (1.57–1.74) | 1.68 (1.59–1.76) | ||
| Hyperlipidemia | 0.76 (0.73–0.79) | 0.76 (0.73–0.79) | .76 (.73–.80) | ||
| Obesity | 1.52 (1.46–1.59) | 1.56 (1.50–1.63) | 1.57 (1.51–1.64) | ||
| Nicotine dependence/ personal history tobacco use | 1.48 (1.42–1.55) | 1.53 (1.47–1.60) | |||
| Substance use disorders2 | 1.18 (1.12–1.25) | 1.24 (1.17–1.31) | |||
|
| |||||
| Psychotropics | |||||
| Any SSRI | .77 (.89–.95) | ||||
| Any TCA | .94 (.89–1.00) | ||||
| Any SNRI | .64 (.59–.69) | ||||
| Any Other antidepressant | .80 (.75–.84) | ||||
| Any benzo | .86 (.82–.91) | ||||
divorced, widowed, separated or never married,
Alcohol and or illicit drug abuse/dependence
Cox proportional hazards models
In the final, full model (model 5), in which psychotropic use was added, having at least one prescription for any SSRI, SNRI, other antidepressant or benzodiazepine was associated with significantly reduced hazard for incident HF (table 4). In the same model, the magnitude of the association between anxiety, MDD and anxiety and MDD increased to 1.46, 1.56 and 1.74, respectively.
We modeled anxiety as an aggregate of several anxiety disorders commonly diagnosed in the VA population. To determine if any one anxiety disorder accounts for greater risk of HF, in post hoc analyses we dissected this variable into its component anxiety disorders and looked for any differential risk posed by the individual disorders. We found that Anxiety Disorder Unspecified, GAD and PTSD all imparted similar hazard for incident HF (HR=1.65 (95%CI 1.48–1.84); HR=1.38 (95%CI: 1.13–1.67) and HR=1.33 (95%CI: 1.20–1.46), respectively).
Discussion
This is the first study to investigate the association of anxiety disorders, MDD and their co-occurrence with incident HF in a population of patients without a known, pre-existing diagnosis of heart disease, including HF. Because mood disorders are more common among younger persons at lower risk of HF, unadjusted results indicated a protective effect of anxiety and depression. In the model that corrected only for age, patients with anxiety disorders, MDD, or both were each about 20% more likely to develop HF compared to those without these conditions. There was no evidence that risk increased when patients had both anxiety and MDD as compared to either disorder alone. This effect remained significant after additional adjustment for other HF risk factors including sociodemographics, CVD risk factors and nicotine dependence/personal history of tobacco use and substance use disorders. The magnitude of the HRs for anxiety, MDD, and both disorders actually increased after adjusting for psychotropic prescriptions, suggesting that psychotropic prescriptions may be confounding the observed association between anxiety and MDD and incident HF.
The overarching goal of this investigation was to determine if anxiety disorders, MDD and the combination of these diagnoses were associated with differential risk for incident HF. Based on our results, these disorders and their combination are associated with a similar level of risk for incident HF (approximately 10–20%) and are attenuated to a similar degree after adjusting for covariates until prescription of psychotropics is considered. After adjusting for presence of any prescription of a psychotropic, differential risk for incident HF emerged and those with both MDD and anxiety had greater risk than those with one disorder, and those with MDD alone had greater risk than those with anxiety alone. Correspondingly, psychotropic prescriptions were associated with reduced risk of incident HF, besides TCAs. This finding of reduced risk of incident HF after adjustment for psychotropic prescriptions is consistent with similar work in depressed VA patients. Scherrer et al. (2011) found that 12 weeks of prescriptions of any class of antidepressants was associated with decreased risk of incident MI in this population of depressed VA patients (24).
In the current study we found a similar reduction in risk of incident CVD with a less rigorous definition of psychotropic use/prescriptions than in previous work (24). We only required at least 1 prescription of a drug at any time during the study period compared to the 12-weeks of continuous prescriptions in the Scherrer et al. (2011) study and found a similar reduction in risk. This suggests that the reduction in risk is more likely due to some common characteristic of patients who receive prescriptions for psychotropics than of the drugs themselves. For example, patients with psychiatric diagnoses who are able to follow-up with a physician and receive a psychotropic prescription may be more frequent attenders of other clinics and more adherent to all medical regimens, giving them an overall health advantage. Additionally, while in administrative claims data we cannot be certain that a prescription of a drug in the medical record equates with compliant use of medications, we can conclude that exposure to these drugs is a confounder and an accurate estimate of the association between anxiety and depression and risk of HF is needed to account for treatment effects.
This study may be the first published report to look at the how the combination of depression and anxiety affect risk for incident HF. In our sub-analysis of individual anxiety disorders, we found that when modeled individually, anxiety disorders were similar enough in magnitude to model as a combined variable. This variable combined diagnoses of Anxiety Disorder Unspecified, GAD and PTSD with MDD. The clinical significance of minor differences in hazard between patients with different anxiety diagnoses is difficult to interpret because some participants may have more than one disorder. In summary we conclude that anxiety disorders, regardless of type, increase risk of HF.
The biological mechanisms underlying the anxiety, depression and HF association is uncertain. In addition to lifestyle factors, anxiety, depression and cardiovascular disease may have a shared genetic pathway. As reported in twin studies, depression and heart disease share common genetic factors (29). The genetic overlap between these phenotypes has been found to be especially likely in women (30). Other potential physiological mechanisms include serotonin-mediated elevated platelet activity, increased inflammation, hypothalamic-pituitary-adrenal axis (HPA) dysfunction, decreased heart rate variability, elevated catecholamines, endothelial dysfunction and low brain-derived neurotrophic factor (BDNF) (31).
Strengths
This study was based on a large, nationally representative sample of VA patients with sufficient follow-up time to detect incident cases of HF. The retrospective cohort design with a 2-year CVD-free period at baseline is a unique resource for investigating incident HF. Replication is needed in private payer records and national record systems as maintained in Canada and Europe. The use of time-dependent covariates and the requirement that anxiety and depression diagnoses had to occur at least 90 days prior to the outcome also ensured that the temporal relationship between psychiatric disorders and HF was correctly specified.
Limitations
Administrative medical record data have inherent limitations as compared to prospective clinical assessments. Evaluation of more sensitive markers of disease severity and information regarding the relationship between depression, anxiety and severity of HF at time of diagnosis would require large cohort follow-ups. Our results may not generalize beyond the VA population and additional research in other populations is needed to determine if the effect of MDD and anxiety on risk of HF is similar to that observed in the present VA patient population. Misclassification may occur if mild or transient cases of MDD and of HF are undiagnosed. Because the VA has excellent mental health screening in place, this may be less of a concern than would be the case in the private sector. Because of the standardized method of detection in the VA, we do not believe our results are systematically biased by non-mental health professional diagnoses. To the degree that non-mental health providers make the diagnosis and make the wrong diagnosis, we would expect this to occur at random and not be systematically associated with subsequent risk of heart failure. Desai et al. (32)found that 85% of patients without a depression diagnosis had documentation of receiving a depression screen and 8.8% screened positive. Of those who screened positive, 54% received a follow-up evaluation and only 1.1% were confirmed as having depression. Despite routine screening in the VA, it is possible that un-diagnosed cases of depression and/or anxiety were misclassified as unaffected, leading to bias of our estimate toward the null. We are not aware of similar studies of anxiety, but screening for PTSD is routine in VA primary care. It is also possible that patients with early stages of HF may not seek treatment as often as those with more advanced illness.
Covariate misclassification may bias results if covariates were systematically under- or over-diagnosed by anxiety, MDD, or HF status. It is possible that amount of screening and accuracy of detecting tobacco and other substance use disorders is poorer in patients without MDD and/or anxiety due to less contact with healthcare. In this scenario, the true prevalence of these disorders would be higher in patients free of anxiety and MDD and our estimate of the effect of nicotine dependence/personal history of tobacco use-substance abuse/dependence would be more similar between groups, making substance abuse and nicotine dependence/personal history of tobacco use a less likely explanation for the increase in incident HF.
Our theoretical decision to exclude patients who had a HF diagnosis within 90 days following an MI may have reduced the generalizability of findings to all HF patients. However, we conducted post-hoc analysis by allowing these patients into the cohort and adjusting for MI. Results of the full model with MI included as a covariate indicated there was little change in the association between anxiety, MDD and incident HF. In this model, the association between anxiety disorders alone and incident HF was 1.12 (95%C.I., 1.05–1.20), for MDD the HR= 1.09 (95%C.I., 1.02–1.15) and for co-occurring anxiety and MDD, the fully adjusted HR was 1.09 (95%C.I., 1.03–1.16).
Conclusions
Our findings suggest that diagnosed anxiety disorders and MDD may be associated with risk for future HF. Each disorder imparts similar magnitude of risk and there is no evidence of increasing risk with psychiatric comorbidity until psychotropic prescriptions are considered. With adjustment for psychotropic prescriptions, risk for incident HF is greatest in those with both anxiety and depression and those with MDD alone have greater risk than those with anxiety alone. Further research in other populations is necessary to determine if the effect of MDD and anxiety on risk of HF is similar to that observed in the present VA patient population and to determine if these findings translate into clinically significant outcomes such as preventing or delaying onset of HF through adequate pharmacotherapy and psychotherapy for anxiety and MDD.
Supplementary Material
Acknowledgments
Funding Sources:
This study was supported by a Career Development-2 (CDA-2) grant from Veterans Administration Health Services Research & Development (VA HSR&D) to Dr. Scherrer. Dr. Garfield was supported by an assistantship from the Graduate School at Saint Louis University. Dr. Garfield is now supported by a T32 postdoctoral fellowship from NHLBI.
Abbreviations
- Anxiety NOS
Anxiety Not Otherwise Specified
- Benzo
Benzodiazepine
- BMI
Body Mass Index
- CVD
Cardiovascular Disease
- CPT
Current Procedural Terminology
- FY
Fiscal Year
- GAD
Generalized Anxiety Disorder
- HF
Heart Failure
- HR
Hazard Ratio
- ICD-9-CM
International Classification of Disease – 9th Edition – Clinical Modification
- MDD
Major Depressive Disorder
- MI
Myocardial Infarction
- NHANES
National Health and Nutrition Examination Survey
- NPV
Negative Predictive Value
- PBM
Prescription Benefits Management
- PPV
Positive Predictive Value
- PTSD
Post-Traumatic Stress Disorder
- SNRI
Serotonin norepinephrine reuptake inhibitor
- SSRI
Selective serotonin reuptake inhibitor
- TCA
Tricyclic antidepressant
- VA/VHA
Veteran’s Administration/Veteran’s Health Administration
Footnotes
Disclosures: The authors do not report any conflicts of interest.
References
- 1.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure: a meta-analytic review of prevalence, intervention effects and association with clinical outcomes. Journal of the American College of Cardiology. 2006;48:1527–37. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 2.Freedland KE, Rich MW, Skala JA, Carney RM, Davilá-Román VG, Jaffe AS. Prevalence of depression in hospitalized patients with congestive heart failure. Psychosomatic Medicine. 2003;65:119–28. doi: 10.1097/01.psy.0000038938.67401.85. [DOI] [PubMed] [Google Scholar]
- 3.Cully JA, Phillips LL, Kunik ME, Stanley MA, Deswal A. Predicting quality of life in veterans with heart failure: The role of disease severity, depression, and comorbid anxiety. Behavioral Medicine. 2010;36:70–6. doi: 10.1080/08964280903521297. [DOI] [PubMed] [Google Scholar]
- 4.Himelhoch S, Weller WE, Wu AW, Anderson GF, Cooper LA. Chronic medical illness, depression, and the use of acute medical services among Medicare beneficiaries. Medical Care. 2004;42:512–21. doi: 10.1097/01.mlr.0000127998.89246.ef. [DOI] [PubMed] [Google Scholar]
- 5.Fulop G, Strain JJ, Stettin G. Congestive heart failure and depression in older adults: clinical course and health services use at 6 months after hospitalization. Psychosomatics. 2003;44:367–73. doi: 10.1176/appi.psy.44.5.367. [DOI] [PubMed] [Google Scholar]
- 6.Faris R, Purcell H, Henein MY, Coats AJ. Clinical depression is common and significantly associated with reduced survival in patients with non-ischaemic heart failure. European Journal of Heart Failure. 2002;4:541–51. doi: 10.1016/s1388-9842(02)00101-0. [DOI] [PubMed] [Google Scholar]
- 7.Johnson TJ, Basu S, Pisani BA, Avery EF, Mendez JC, Calvin JE, Jr, Powell LH. Depression predicts repeated heart failure hospitalizations. Journal of Cardiac Failure. 2012;18:246–52. doi: 10.1016/j.cardfail.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 8.de Denus S, Spinler SA, Jessup M, Kao A. History of depressionas a predictor of adverse outcomes in patients hospitalized for decompensated heart failure. Pharmacotherapy. 2004;24:1306–10. doi: 10.1592/phco.24.14.1306.43146. [DOI] [PubMed] [Google Scholar]
- 9.Rumsfeld JS, Havranek E, Masoudi FA, Peterson ED, Jones P, Tooley JF, Krumholz HM, Spertus JA. Depressive symptoms are the strongest predictor of short-term declines in health status in patients with heart failure. Journal of the American College of Cardiology. 2003;42:1811–7. doi: 10.1016/j.jacc.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan M, Levy WC, Russo JE, Spertus JA. Depression and health status in patients with advanced heart failure: a preospective study in tertiary care. Journal of Cardiac Failure. 2004:10. doi: 10.1016/j.cardfail.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Jiang W, Krishnan R, Kuchibhalta M, Cuffe MS, Martsberger C, Arias RM, O’Connor CM, Investigators S-C. Characteristics of depression remission and its relation with cardiovascular outcome among patients with chronic heart failure (from the SADHART-CHF Study) American Journal of Cardiology. 2011;107:545–51. doi: 10.1016/j.amjcard.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murberg TA, Furze G. Depressive symptoms and mortality in patients with congestive heart failure: a six-year follow-up study. Medical Science Monitor. 2004;10:643–8. [PubMed] [Google Scholar]
- 13.Jünger J, Schellberg D, Müller-Tasch T, Raupp G, Zugck C, Haunstetter A, Zipfel S, Herzog W, Hauss M. Depression increasingly predicts mortality in the course of congestive heart failure. European Journal of Heart Failure. 2005;7:261–7. doi: 10.1016/j.ejheart.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 14.O’Connor CM, Jiang W, Kuchibhalta M, Mehta RH, Clary GL, Cuffe MS, Christopher EJ, Alexander JD, Califf RM, Krishnan RR. Antidepressant use, depression, and survival in patients with heart failure. Archives of Internal Medicine. 2008;168:2232–7. doi: 10.1001/archinte.168.20.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albert NM, Fonarow GC, Abraham WT, Georghiade M, Greenberg BH, Nunez E, O’Connor CM, Stough WH, Yancy CW, Young JB. Depression and clinical outcomes in heart failure: an OPTIMIZE-HF analysis. American Journal of Medicine. 2009;122:366–73. doi: 10.1016/j.amjmed.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 16.Frasure-Smith N, Lespérance F, Habra M, Talajic M, Khairy P, Dorian P, Roy D. Elevated depression symptoms predict long-term cardiovascular mortality in patients with atrial fibrillation and heart failure. Circulation. 2009;120:134–40. doi: 10.1161/CIRCULATIONAHA.109.851675. [DOI] [PubMed] [Google Scholar]
- 17.Freedland KE, Carney RM, Rich MW. Effect of depression on prognosis in heart failure. Heart Failure Clinics. 2011;7:11–21. doi: 10.1016/j.hfc.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams SA, Kasl SV, Heiat A, Abramson JL, Krumholz HM, Vaccarino V. Depression and risk of heart failure among the elderly: a prospective community-based study. Psychosomatic Medicine. 2002;64:6–12. doi: 10.1097/00006842-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Abramson J, Berger A, Krumholz HM, Vaccarino V. Depression and risk of heart failure among older persons with isolated systolic hypertension. Archives of Internal Medicine. 2001;161:1725–30. doi: 10.1001/archinte.161.14.1725. [DOI] [PubMed] [Google Scholar]
- 20.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of majore depressive disorder. Results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 21.Volz A, Schmid JP, Zwahlen M, Kohls S, Saner H, Barth J. Predictors of readmission and health related quality of life in patients with chronic heart failure: a comparison of different psychosocial aspects. Journal of Behavioral Medicine. 2011;34:13–22. doi: 10.1007/s10865-010-9282-8. [DOI] [PubMed] [Google Scholar]
- 22.Cully JA, Jimenez DE, Ledoux TA, Deswal A. Recognition and treatment of depression and anxiety symptoms in heart failure. Primary Care Companion to the Journal of Clinical Psychiatry. 2009;11:103–9. doi: 10.4088/pcc.08m00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scherrer JF, Chrusciel T, Zeringue A, Garfield LD, Hauptman PJ, Lustman PJ, Freedland KE, Carney RM, Bucholz KK, Owen R, True WR. Anxiety disorders increase risk for incident myocardial infarction in depressed and nondepressed Veterans Administration patients. American Heart Journal. 2010;159:772–9. doi: 10.1016/j.ahj.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 24.Scherrer JF, Garfield LD, Lustman PJ, Hauptman PJ, Chrusciel T, Zeringue A, Carney RM, Freedland KE, Bucholz KK, Owen R, Newcomer JW, True WR. Antidepressant drug compliance: reduced risk of MI and mortality in depressed patients. American Journal of Medicine. 2011;124:318–24. doi: 10.1016/j.amjmed.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 25.Freedenberg V, Thomas SA, Friedmann E. Anxiety and depression in implanted cardioverter-definrillator recipients and heart failure: a review. Heart Failure Clinics. 2011;7:59–68. doi: 10.1016/j.hfc.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Frayne SM, MIller DR, Sharkansky EJ, Jackson VW, Wang F, Halanych JH, Berlowitz DR, Kader B, Keane TM. Using administrative data to identify mental illness: What approach is best? American Journal of Medical Quality. 2010;25:42–50. doi: 10.1177/1062860609346347. [DOI] [PubMed] [Google Scholar]
- 27.Quan H, Li B, Saunders LD, Parsons GA, CIN, Allibhai A, Ghali WA. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Services Research. 2008;43:1424–41. doi: 10.1111/j.1475-6773.2007.00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quach S, Blais C, Quan H. Administartive data have high variation in validity for recording heart failure. Canadian Journal of Cardiology. 2010;26:e306–e12. doi: 10.1016/s0828-282x(10)70438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scherrer JF, Xian H, Bucholz KK, Eisen SA, Lyons MJ, Goldberg J, Tsuang M, True WR. A twin study of depression symptoms, hypertension, and heart disease in middle-aged men. Psychosom Med. [Research Support, U.S. Gov’t, Non-P.H.S. Research Support, U.S. Gov’t, P.H.S. Twin Study] 2003;65:548–57. doi: 10.1097/01.psy.0000077507.29863.cb. [DOI] [PubMed] [Google Scholar]
- 30.Mulle JG, Vaccarino V. Cardiovascular disease, psychosocial factors, and genetics: the case for depression. Progress in Cardiovascular Disease. 2013;55:557–62. doi: 10.1016/j.pcad.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huffman JC, Celano CM, Beach SR, Motiwala SR, Januzzi JL. Depression and cardiac disease: Epidemiology, mechanisms and diganosis. Cardiovascular Psychiatry and Neurology. 2013;2013 doi: 10.1155/2013/695925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desai MM, Rosenheck RA, Craig TJ. Case-finding for depression among medical outpatients in the Veterans Health Administration. Medical Care. 2006;44:175–81. doi: 10.1097/01.mlr.0000196962.97345.21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.