Abstract
To investigate the role of Insulin-like growth factor-1 (IGF-1), in localized osteosarcoma, serum levels of IGF-1, IGFBP-2, and IGFBP-3 were measured in 224 similarly treated, newly diagnosed patients. We demonstrated that younger patients had lower concentrations of IGF-1 and IGFBP-3 compared to older (p < 0.001) along with lower IGFBP-3:IGF-1 and IGFBP-2:IGF-1 ratios (p < 0.001). IGFBP-2 did not correlate with age (p = 0.16), yet IGFBP-2:IGF-1 ratios were higher in the younger population (p<0.001). These findings show that older patients have higher concentrations of free IGF-1. None of IGF-1, IGFBP-2, nor IGFBP-3 concentrations correlated with event-free nor overall survival.
INTRODUCTION
The insulin growth factor-1 (IGF-1) signaling pathway, hypothesized to play an important role in the development of clinical osteosarcoma, plays a significant role in laboratory models of osteosarcoma (1). Osteosarcoma primary tumors express both IGF-1 and the IGF-1 receptor (IGF-1R), and supplementation of osteosarcoma cell lines with IGF-1 increases their growth (2–4). IGF-1R antibody treatment or receptor level reduction by siRNA results in decreased invasiveness and slows growth of osteosarcoma xenografts (3, 5–7).
Regulation of IGF-1 signaling is controlled in part by limiting the amount of free IGF-1 through interaction with IGF-1 binding proteins. Two of the better characterized binding proteins are IGFBP-3 and IGFBP-2, both of which have been shown to sequester IGF-1, thus decreasing the amount of free IGF-1 ligand signaling. However, the roles of IGFBP-3 and IGFBP-2 are unclear in osteosarcoma. To prospectively investigate the IGF-1 pathway in newly diagnosed, similarly treated, localized osteosarcoma, serum levels of IGF-1, IGFBP-2, and IGFBP-3 were measured and the analysis compared these biomarker levels to patient age and correlated with patient outcomes, measured by both event-free survival (EFS) and overall survival (OS).
MATERIALS AND METHODS
Patients were eligible if they were enrolled on the Pediatric Oncology Group (POG) and Children’s Cancer Group (CCG) therapeutic clinical trial P9754 and/or the tumor banking study P9851 (8) and received neo-adjuvant and adjuvant chemotherapy per clinical trial guidelines or institutional preference. Patients diagnosed with secondary osteosarcoma or metastatic disease were excluded from this analysis. Both of these studies were approved by both local and central institutional review boards and informed consent and assent was obtained from the patient or parent/legal guardian for patients less than 18 years old and minors. Serum samples were collected at diagnosis, prior to staring treatment and levels of IGF-1, IGFBP-2, and IGFBP-3 were measured, stratified into quartiles (Supplemental Table 1), and analyzed as described (9). Event-free survival (EFS) and overall survival (OS) were defined as described and calculated using the method of Kapan and Meier (9, 10). Equality of risk of event across groups defined by patient characteristics was assessed using the logrank test (10). Data received by the COG as of noon PST on March 21, 2011 were used for analysis. The measured variables were checked for association with each other and with the demographic characteristics of the patients within each of the analytic populations by the exact conditional test of proportions (11).
RESULTS
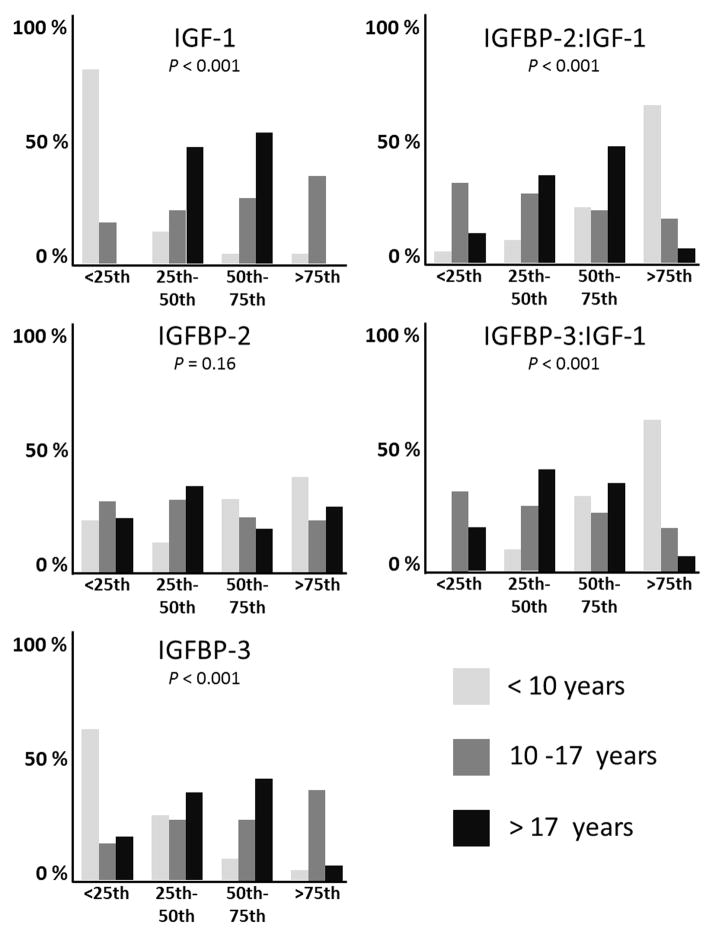
There were 224 samples available for analysis from eligible patients with localized osteosarcoma (Supplemental Fig. 1). Nine patients did not have follow up data, four patients had only IGF-1 and IGFBP-3 measurements, and sixty patients had only IGFBP-2 measurements. Therefore, IGF-1 and IGFBP-3 levels were determined in 142 patients and IGFBP-2 was measured in 198 patients. Since thirteen samples were replicates (obtained at the same point), a total of 202 unique patient samples were available for analysis. When multiple samples were obtained from the same patient, the values were averaged. IGF-1, IGFBP-2, and IGFBP-3 concentrations were correlated to patient age at diagnosis. Patients were stratified into three groups: Age ≤ 10 years, 10–17 years, and greater than 18 years. Younger patients had lower concentrations of IGF-1 and IGFBP-3 compared to older patients (p < 0.001), shown in Supplemental Table 2 and Fig. 1. Older patients had lower IGFBP-3:IGF-1 and IGFBP-2:IGF-1 ratios (p < 0.001), Interestingly, IGFBP-2 did not correlate with age (p = 0.16) but IGFBP-2:IGF-1 ratios were higher in the younger population (p<0.001). These findings demonstrate that older patients have higher concentrations of free IGF-1.
Fig. 1.
IGF-1, IGFBP-2, IGFBP-3, IGFBP-2:IGF-1, and IGFBP-3:IGF-1 ratios compared to age. Serum levels and ratios were stratified into quartiles and compared. P values are demonstrated.
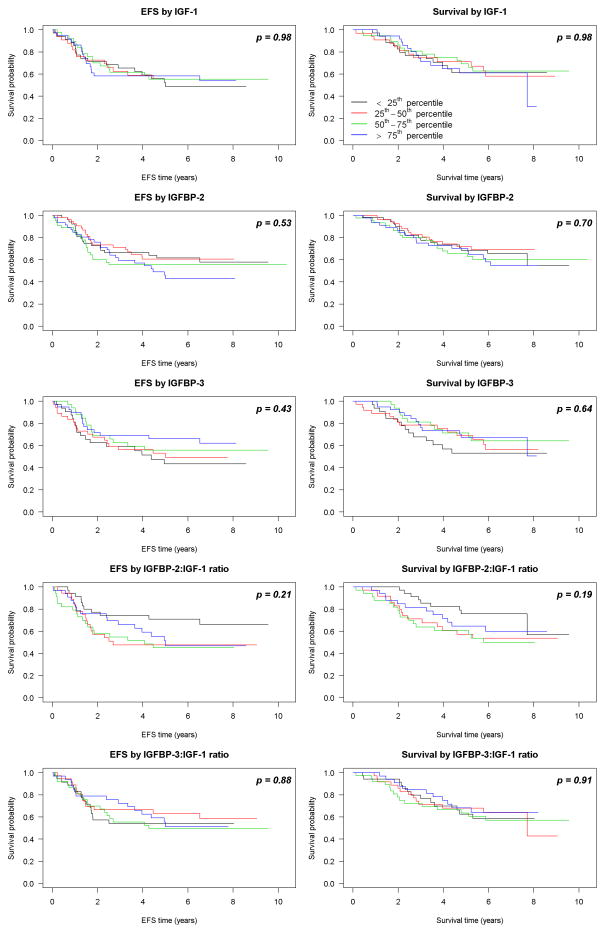
As shown in Fig. 2, IGF-1 concentration did not correlate with EFS or OS (p = 0.98 and 0.98, respectively). Similar findings were observed for IGFBP-2 (p= 0.54 and 0.70), IGFBP-3 (p = 0.43 and 0.63), and IGFBP-2:IGF-1 (p = 0.88 and 0.91). Patients with lower IGFBP-3:IGF-1 ratios were associated with reduced risk for EFS-event and death, although these were not considered statistically different from no relationship (p= 0.21 and 0.12, respectively).
Fig. 2.
EFS and OS as demonstrated by Kaplan-Meier Analysis compared to IGF-1, IGFBP-2, IGFBP-3, IGFBP-2:IGF-1, and IGFBP-3:IGF-1 ratios. Serum levels and ratios were stratified into quartiles and compared. P values are demonstrated.
DISCUSSION
In this study, we investigated IGF-1 concentrations in patients with newly diagnosed non-metastatic osteosarcoma. Serum samples from 202 newly diagnosed patients with localized osteosarcoma enrolled on prospective clinical trials were analyzed to determine concentrations of IGF-1, IGFBP-3, and IGFBP-2. A major strength of this study is that serum samples were collected prospectively from a large number of newly diagnosed patients, without the knowledge of IGF status. We demonstrated that older patients had increased serum concentrations of IGF-1 and decreased IGFBP-3:IGF-1 ratios, showing that older patients have increased free IGF-1. IGF-1 concentrations are low at birth and slowly rise through childhood and peak during puberty, and remain elevated until the fourth decade of life, when they begin to decrease (12). Increased IGF-1 levels have been observed previously in older patients newly diagnosed with Ewing Sarcoma and is more likely due to normal physiology than malignancy (9, 13). Furthermore, when IGF-1, IGFBP-2, and IGFBP-3 levels were evaluated based upon patient outcome, there were no significant correlations (EFS and OS).
The determination of how free IGF-1 levels respond to treatment could provide insight on how to best use agents that target the IGF-1 signaling pathway. In addition, IGFBP-3 has IGF-1 independent functions that contribute to cancer pathogenesis. Several studies have shown that IGFBP-3 promotes apoptosis and suppresses metastasis in prostate cancer cell lines and can inhibit cell cycle progression in skin, kidney, and breast cancers (14, 15). The IGF-1 independent effects of IGFBP-3 in osteosarcoma are poorly understood, but may have anti-proliferative effects and warrant further investigation. While IGFBP-1, -4, -5, and -6 are found in the circulation, they generally each represent less than 10% of circulating IGFBP, thus were not assayed for in this project (16).
Despite very promising preclinical data suggesting efficacy of IGF-1 receptor antagonists, these agents have not demonstrated robust clinical activity (17, 18). Prior to directly targeting the IGF-1R, the somatostatin analog Oncolar (octreotide pamoate long-acting release) was used to lower ligand levels in the circulation. Mansky et al demonstrated the ability of Oncolar to lower serum concentrations of IGF-1, but there was no clinical response to this agent (19). Furthermore, a recent report by Schwartz et al revealed that only 3/24 patients with relapsed osteosarcoma treated with cixutumumab and temsirolimus had a partial response to therapy (20). To enhance the likelihood of success in future clinical trials that target the IGF-1 pathway in osteosarcoma, patients should be stratified based upon novel patient or tumor biomarkers.
Supplementary Material
Supplemental Figure 1. Patient Tree. Red Box depicts unique patient samples.
Acknowledgments
Research is supported by the Chair’s Grant U10 CA98543 and Human Specimen Banking Grant U24 CA114766 of the COG from the NCI, NIH, Bethesda, MD, USA. Additional support for research is provided by the QuadW Foundaton, AFLAC, Children’s Cancer Foundation (Baltimore MD), Go4theGoal, Dani’s Foundation, Alex’s Lemonade Stand Foundation, Liddy Shriver Sarcoma Initiative, Burroughs Wellcome Clinical Scientist Award in Translational Research (JT), and the NIH R01CA88004 (JT), R01CA133662 (JT), R01CA138212 (JT), and RC4CA156509 (JT).
References
- 1.Shevah OLaron Z. Patients with congenital deficiency of IGF-1 seem protected from the development of malignancies: A preliminary report. Growth hormone & IGF research : official journal of the Growth Hormone Research Society and the International IGF Research Society. 2007;17:54–57. doi: 10.1016/j.ghir.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Kappel CC, Velez-Yanguas MC, Hirschfeld S, et al. Human osteosarcoma cell lines are dependent on insulin-like growth factor i for in vitro growth. Cancer Res. 1994;54:2803–2807. [PubMed] [Google Scholar]
- 3.Burrow S, Andrulis IL, Pollak M, et al. Expression of insulin-like growth factor receptor, IGF-1, and IGF-2 in primary and metastatic osteosarcoma. J Surg Oncol. 1998;69:21–27. doi: 10.1002/(sici)1096-9098(199809)69:1<21::aid-jso5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 4.Abdeen A, Chou AJ, Healey JH, et al. Correlation between clinical outcome and growth factor pathway expression in osteogenic sarcoma. Cancer. 2009;115:5243–5250. doi: 10.1002/cncr.24562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SY, Toretsky JA, Scher D, et al. The role of IGF-1R in pediatric malignancies. Oncologist. 2009;14:83–91. doi: 10.1634/theoncologist.2008-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolb EA, Gorlick R, Houghton PJ, et al. Initial testing (stage 1) of a monoclonal antibody (sch 717454) against the IGF-1 receptor by the pediatric preclinical testing program. Pediatric blood & cancer. 2008;50:1190–1197. doi: 10.1002/pbc.21450. [DOI] [PubMed] [Google Scholar]
- 7.Wang YH, Han XD, Qiu Y, et al. Increased expression of insulin-like growth factor-1 receptor is correlated with tumor metastasis and prognosis in patients with osteosarcoma. J Surg Oncol. 2012;105:235–243. doi: 10.1002/jso.22077. [DOI] [PubMed] [Google Scholar]
- 8.Janeway KA, Barkauskas DA, Krailo MD, et al. Outcome for adolescent and young adult patients with osteosarcoma: A report from the children’s oncology group. Cancer. 2012;118:4597–4605. doi: 10.1002/cncr.27414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borinstein SC, Barkauskas DA, Krailo M, et al. Investigation of the insulin-like growth factor-1 signaling pathway in localized ewing sarcoma: A report from the children’s oncology group. Cancer. 2011;117:4966–4976. doi: 10.1002/cncr.26112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalbfleisch JDaPRL. The statistical analysis of failure time data. New York: John Wiley and Sons; 2002. [Google Scholar]
- 11.Bishop SFSE, Holland YMM. Discrete multivariate analysis. Cambridge, MA: MIT Press; 1975. [Google Scholar]
- 12.Sperling M. Pediatric endocrinology. Philadelphia, PA: Saunders/Elsevier; 2008. p. xv.p. 889. [Google Scholar]
- 13.Lofqvist C, Andersson E, Gelander L, et al. Reference values for IGF-I throughout childhood and adolescence: A model that accounts simultaneously for the effect of gender, age, and puberty. J Clin Endocrinol Metab. 2001;86:5870–5876. doi: 10.1210/jcem.86.12.8117. [DOI] [PubMed] [Google Scholar]
- 14.Mehta HH, Gao Q, Galet C, et al. IGFBP-3 is a metastasis suppression gene in prostate cancer. Cancer Res. 2011;71:5154–5163. doi: 10.1158/0008-5472.CAN-10-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu C, Liu X, Wang Y, et al. Insulin-like factor binding protein-3 promotes the G1 cell cycle arrest in several cancer cell lines. Gene. 2013;512:127–133. doi: 10.1016/j.gene.2012.09.080. [DOI] [PubMed] [Google Scholar]
- 16.Jehle PM, Schulten K, Schulz W, et al. Serum levels of insulin-like growth factor (IGF)-I and IGF binding protein (IGFBP)-1 to -6 and their relationship to bone metabolism in osteoporosis patients. European journal of internal medicine. 2003;14:32–38. doi: 10.1016/s0953-6205(02)00183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asmane I, Watkin E, Alberti L, et al. Insulin-like growth factor type 1 receptor (IGF-1R) exclusive nuclear staining: A predictive biomarker for IGF-1R monoclonal antibody (ab) therapy in sarcomas. Eur J Cancer. 2012;48:3027–3035. doi: 10.1016/j.ejca.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Malempati S, Weigel B, Ingle AM, et al. Phase I/II trial and pharmacokinetic study of cixutumumab in pediatric patients with refractory solid tumors and ewing sarcoma: A report from the children’s oncology group. J Clin Oncol. 2012;30:256–262. doi: 10.1200/JCO.2011.37.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mansky PJ, Liewehr DJ, Steinberg SM, et al. Treatment of metastatic osteosarcoma with the somatostatin analog oncolar: Significant reduction of insulin-like growth factor-1 serum levels. J Pediatr Hematol Oncol. 2002;24:440–446. doi: 10.1097/00043426-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz GK, Tap WD, Qin L-X, et al. A phase ii multicenter study of the IGF-1 receptor antibody cixutumumab (a12) and the mtor inhibitor temsirolimus (tem) in patients (pts) with refractory IGF-1R positive (+) and negative (−) bone and soft tissue sarcomas (sts) ASCO Meeting Abstracts. 2012;30:10003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Patient Tree. Red Box depicts unique patient samples.