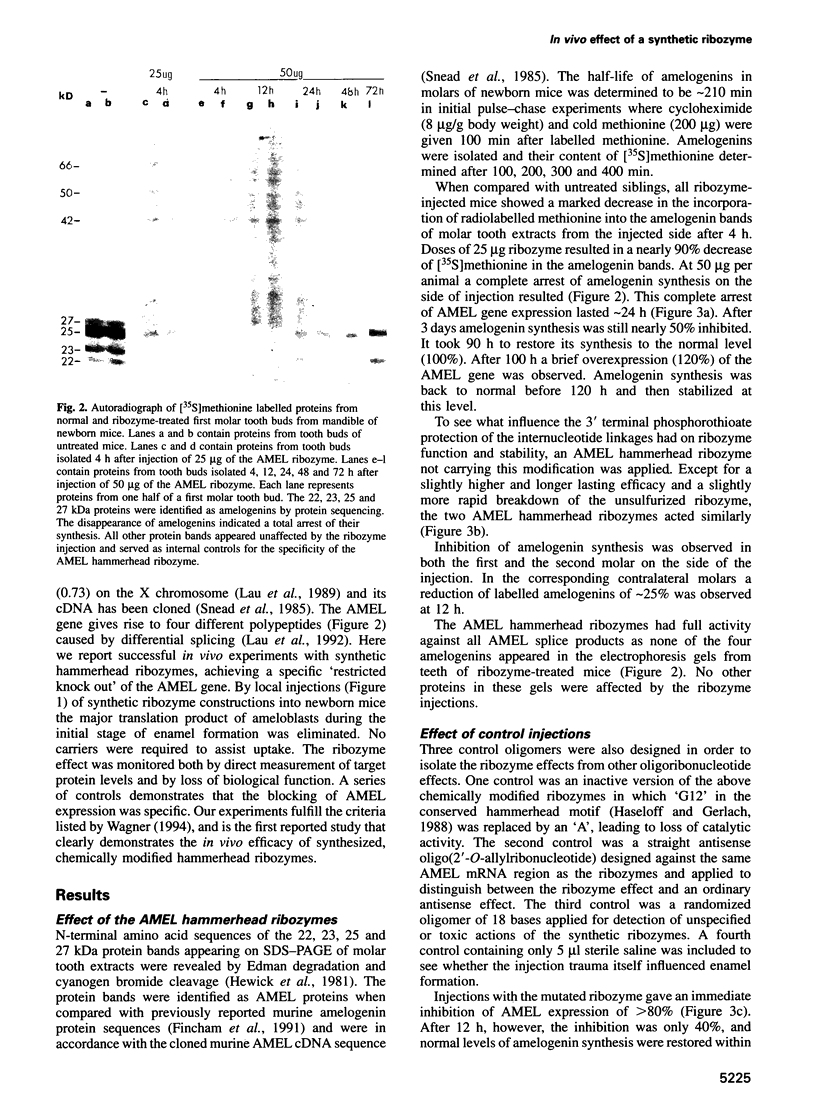
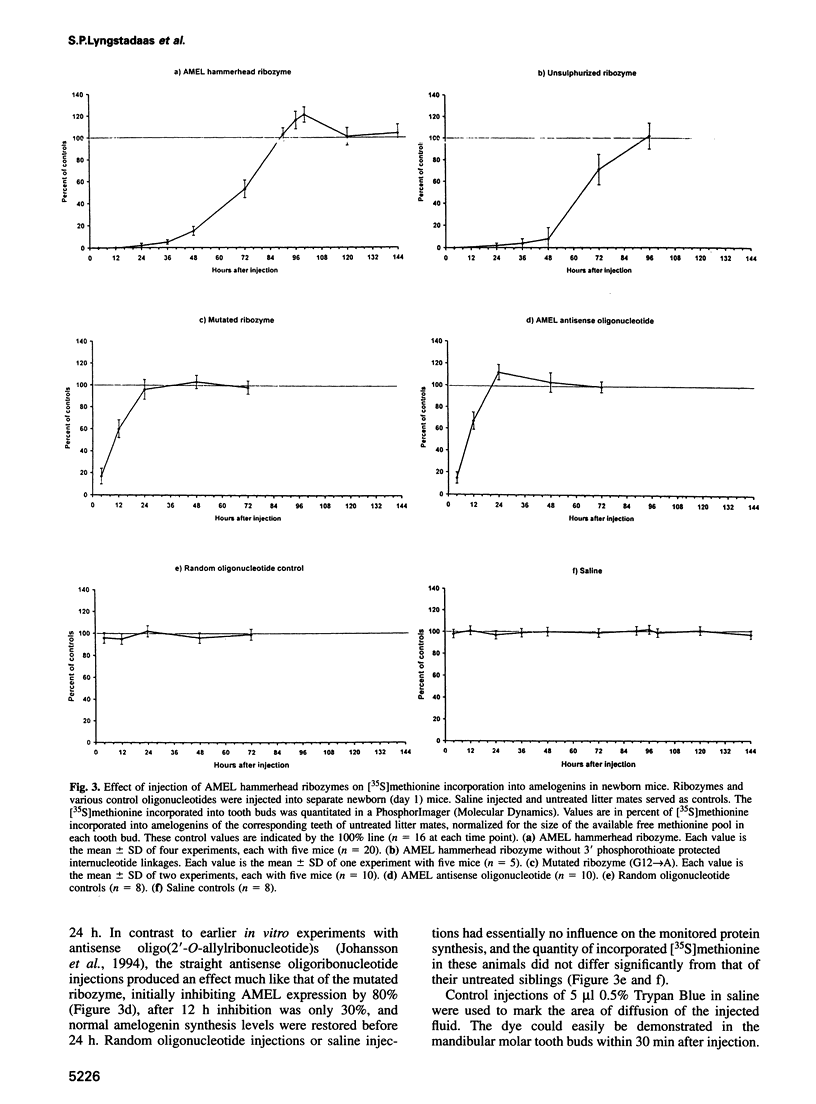
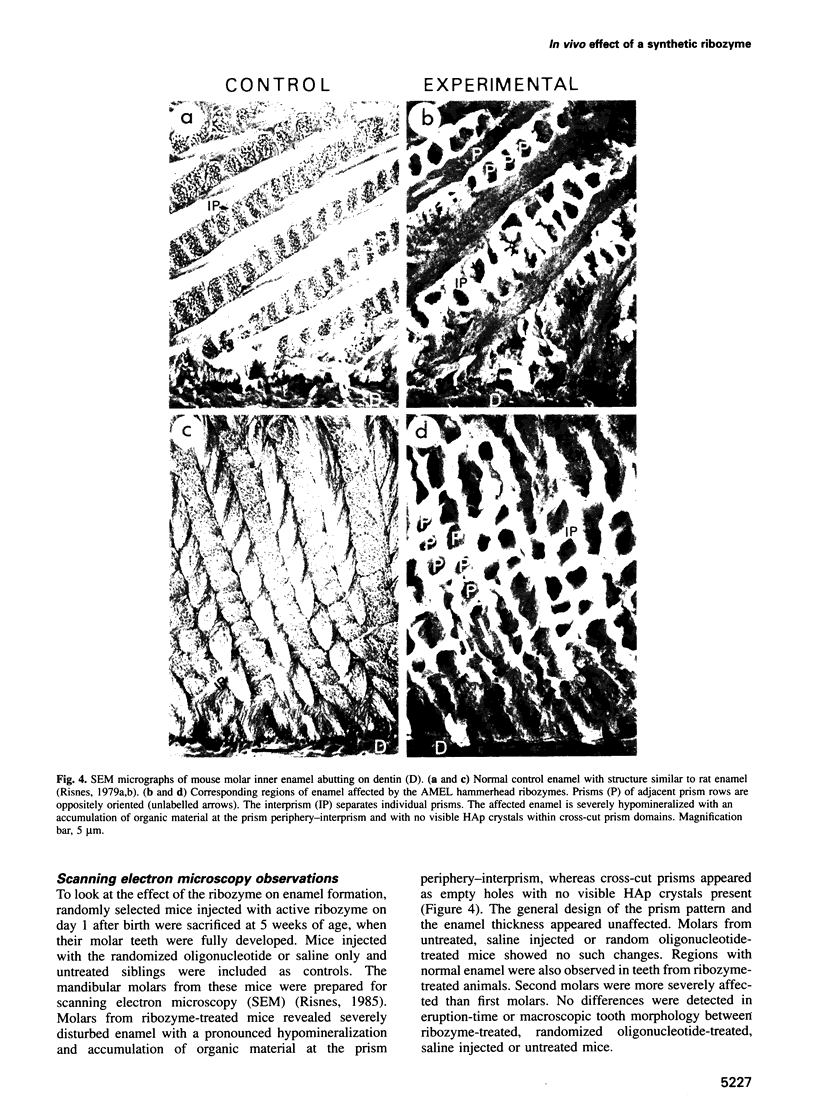
Abstract
Ribozymes are small RNA structures capable of cleaving RNA target molecules in a catalytic fashion. Designed ribozymes can be targeted to specific mRNAs, blocking their expression without affecting normal functions of other genes. Because of their specific and catalytic mode of action ribozymes are ideal agents for therapeutic interventions against malfunctioning or foreign gene products. Here we report successful experiments to 'knock out' a major translation product in vivo using synthesized, chemically modified ribozymes. The ribozymes, designed to cleave amelogenin mRNA, were injected close to developing mandibular molar teeth in newborn mice, resulting in a prolonged and specific arrest of amelogenin synthesis not caused by general toxicity. No carriers were required to assist cellular uptake. Amelogenins are highly conserved tissue-specific proteins that play a central role in mammalian enamel biomineralization. Ultrastructural analyses of in vivo ribozyme-treated teeth demonstrated their failure to develop normally mineralized enamel. These results demonstrate that synthesized ribozymes can be highly effective in achieving both timed and localized 'knock-out' of important gene products in vivo, and suggest new possibilities for suppression of gene expression for research and therapeutic purposes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brookes S. J., Bonass W. A., Kirkham J., Robinson C. The human amelogenin C-terminal sequence is completely homologous to the C-terminal sequence of amelogenin in all species so far studied. J Dent Res. 1994 Apr;73(4):716–717. doi: 10.1177/00220345940730040401. [DOI] [PubMed] [Google Scholar]
- COHN S. A. Development of the molar teeth in the albino mouse. Am J Anat. 1957 Sep;101(2):295–319. doi: 10.1002/aja.1001010205. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Uhlenbeck O. C. Ribozymes. Hammerhead nailed down. Nature. 1994 Nov 3;372(6501):39–40. doi: 10.1038/372039a0. [DOI] [PubMed] [Google Scholar]
- Chen E., Piddington R., Decker S., Park J., Yuan Z. A., Abrams W. R., Rosenbloom J., Feldman G., Gibson C. W. Regulation of amelogenin gene expression during tooth development. Dev Dyn. 1994 Mar;199(3):189–198. doi: 10.1002/aja.1001990304. [DOI] [PubMed] [Google Scholar]
- Diekwisch T., David S., Bringas P., Jr, Santos V., Slavkin H. C. Antisense inhibition of AMEL translation demonstrates supramolecular controls for enamel HAP crystal growth during embryonic mouse molar development. Development. 1993 Feb;117(2):471–482. doi: 10.1242/dev.117.2.471. [DOI] [PubMed] [Google Scholar]
- Fincham A. G., Hu Y., Lau E. C., Slavkin H. C., Snead M. L. Amelogenin post-secretory processing during biomineralization in the postnatal mouse molar tooth. Arch Oral Biol. 1991;36(4):305–317. doi: 10.1016/0003-9969(91)90101-y. [DOI] [PubMed] [Google Scholar]
- Fincham A. G., Moradian-Oldak J., Simmer J. P., Sarte P., Lau E. C., Diekwisch T., Slavkin H. C. Self-assembly of a recombinant amelogenin protein generates supramolecular structures. J Struct Biol. 1994 Mar-Apr;112(2):103–109. doi: 10.1006/jsbi.1994.1011. [DOI] [PubMed] [Google Scholar]
- GAUNT W. A. THE DEVELOPMENT OF THE TEETH AND JAWS OF THE ALBINO MOUSE. Acta Anat (Basel) 1964;57:115–151. doi: 10.1159/000142542. [DOI] [PubMed] [Google Scholar]
- Gasparutto D., Livache T., Bazin H., Duplaa A. M., Guy A., Khorlin A., Molko D., Roget A., Téoule R. Chemical synthesis of a biologically active natural tRNA with its minor bases. Nucleic Acids Res. 1992 Oct 11;20(19):5159–5166. doi: 10.1093/nar/20.19.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild J., Kohli V. Ribozymes that cleave an RNA sequence from human immunodeficiency virus: the effect of flanking sequence on rate. Arch Biochem Biophys. 1991 Feb 1;284(2):386–391. doi: 10.1016/0003-9861(91)90313-8. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Gerlach W. L. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988 Aug 18;334(6183):585–591. doi: 10.1038/334585a0. [DOI] [PubMed] [Google Scholar]
- Hertel K. J., Pardi A., Uhlenbeck O. C., Koizumi M., Ohtsuka E., Uesugi S., Cedergren R., Eckstein F., Gerlach W. L., Hodgson R. Numbering system for the hammerhead. Nucleic Acids Res. 1992 Jun 25;20(12):3252–3252. doi: 10.1093/nar/20.12.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Iribarren A. M., Sproat B. S., Neuner P., Sulston I., Ryder U., Lamond A. I. 2'-O-alkyl oligoribonucleotides as antisense probes. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7747–7751. doi: 10.1073/pnas.87.19.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson H. E., Belsham G. J., Sproat B. S., Hentze M. W. Target-specific arrest of mRNA translation by antisense 2'-O-alkyloligoribonucleotides. Nucleic Acids Res. 1994 Nov 11;22(22):4591–4598. doi: 10.1093/nar/22.22.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamond A. I., Sproat B. S. Antisense oligonucleotides made of 2'-O-alkylRNA: their properties and applications in RNA biochemistry. FEBS Lett. 1993 Jun 28;325(1-2):123–127. doi: 10.1016/0014-5793(93)81427-2. [DOI] [PubMed] [Google Scholar]
- Lau E. C., Mohandas T. K., Shapiro L. J., Slavkin H. C., Snead M. L. Human and mouse amelogenin gene loci are on the sex chromosomes. Genomics. 1989 Feb;4(2):162–168. doi: 10.1016/0888-7543(89)90295-4. [DOI] [PubMed] [Google Scholar]
- Lau E. C., Simmer J. P., Bringas P., Jr, Hsu D. D., Hu C. C., Zeichner-David M., Thiemann F., Snead M. L., Slavkin H. C., Fincham A. G. Alternative splicing of the mouse amelogenin primary RNA transcript contributes to amelogenin heterogeneity. Biochem Biophys Res Commun. 1992 Nov 16;188(3):1253–1260. doi: 10.1016/0006-291x(92)91366-x. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Paolella G., Sproat B. S., Lamond A. I. Nuclease resistant ribozymes with high catalytic activity. EMBO J. 1992 May;11(5):1913–1919. doi: 10.1002/j.1460-2075.1992.tb05244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pley H. W., Flaherty K. M., McKay D. B. Three-dimensional structure of a hammerhead ribozyme. Nature. 1994 Nov 3;372(6501):68–74. doi: 10.1038/372068a0. [DOI] [PubMed] [Google Scholar]
- Pyle A. M. Ribozymes: a distinct class of metalloenzymes. Science. 1993 Aug 6;261(5122):709–714. doi: 10.1126/science.7688142. [DOI] [PubMed] [Google Scholar]
- Risnes S. A scanning electron microscope study of aberrations in the prism pattern of rat incisor inner enamel. Am J Anat. 1979 Mar;154(3):419–436. doi: 10.1002/aja.1001540307. [DOI] [PubMed] [Google Scholar]
- Risnes S. Multiangular viewing of dental enamel in the SEM: an apparatus for controlled mechanical specimen preparation. Scand J Dent Res. 1985 Apr;93(2):135–138. doi: 10.1111/j.1600-0722.1985.tb01321.x. [DOI] [PubMed] [Google Scholar]
- Risnes S. The prism pattern of rat molar enamel: a scanning electron microscope study. Am J Anat. 1979 Jun;155(2):245–257. doi: 10.1002/aja.1001550207. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Goldberg M., Takuma S., Garant P. R. Cell biology of tooth enamel formation. Functional electron microscopic monographs. Monogr Oral Sci. 1990;14:1–199. [PubMed] [Google Scholar]
- Snead M. L., Lau E. C., Zeichner-David M., Fincham A. G., Woo S. L., Slavkin H. C. DNA sequence for cloned cDNA for murine amelogenin reveal the amino acid sequence for enamel-specific protein. Biochem Biophys Res Commun. 1985 Jun 28;129(3):812–818. doi: 10.1016/0006-291x(85)91964-3. [DOI] [PubMed] [Google Scholar]
- Termine J. D., Belcourt A. B., Christner P. J., Conn K. M., Nylen M. U. Properties of dissociatively extracted fetal tooth matrix proteins. I. Principal molecular species in developing bovine enamel. J Biol Chem. 1980 Oct 25;255(20):9760–9768. [PubMed] [Google Scholar]
- Tuschl T., Gohlke C., Jovin T. M., Westhof E., Eckstein F. A three-dimensional model for the hammerhead ribozyme based on fluorescence measurements. Science. 1994 Nov 4;266(5186):785–789. doi: 10.1126/science.7973630. [DOI] [PubMed] [Google Scholar]
- Uhlenbeck O. C. A small catalytic oligoribonucleotide. Nature. 1987 Aug 13;328(6131):596–600. doi: 10.1038/328596a0. [DOI] [PubMed] [Google Scholar]
- Wagner R. W. Gene inhibition using antisense oligodeoxynucleotides. Nature. 1994 Nov 24;372(6504):333–335. doi: 10.1038/372333a0. [DOI] [PubMed] [Google Scholar]