Abstract
Background
Previous studies have indicated U-shaped associations between blood pressure (BP) and mortality in dialysis patients. We hypothesized that a similar association exists between pre-transplant BP and post-transplant outcomes in dialysis patients who undergo successful kidney transplantation.
Methods
Data from the Scientific Registry of Transplant Recipients were linked to the 5-year cohort of a large dialysis organization in the United States. We identified all dialysis patients who received a kidney transplant during this period. Unadjusted and multivariate adjusted predictors of transplant outcomes were examined.
Results
The 13,881 patients included in our study were 47±14 years old and included 42% women. There was no association between pre-transplant systolic BP and post-transplant mortality, although a decreased risk trend was observed in those with low post-dialysis systolic BP. Compared to patients with pre-dialysis diastolic BP 70–<80 mmHg, patients with pre-dialysis diastolic BP<50 mmHg experienced lower risk of post-transplant death (HR:0.74, 95%CI:0.55–0.99). However, compared to patients with post-dialysis diastolic BP 70–<80 mmHg, patients with post-dialysis diastolic BP≥100 mmHg experienced higher risk of death (HR: 3.50, 95%CI: 1.57–7.84). In addition, very low (<mmHg for diastolic BP and <110 mmHg for systolic BP) pre-transplant BP was associated with lower risk of graft loss.
Conclusions
Low post-dialysis systolic and low pre-dialysis diastolic BP are associated with lower post-transplant risk of death, whereas very high post-dialysis diastolic BP is associated with higher mortality in kidney transplant recipients. BP variations of dialysis patients prior to kidney transplantation may have a bearing on post-transplant outcome, which warrants additional studies.
Keywords: blood pressure, delayed graft function, graft loss, kidney transplantation, mortality
Introduction
More than eighty percent of the 400,000 patients on maintenance hemodialysis (MHD) in the United States have systolic hypertension.(1) The Kidney Disease Outcomes Quality Initiative (K/DOQI) recommended blood pressure (BP) targets to be achieved by means of anti-hypertensive therapy or other interventions in MHD patients are <140 / 90 mmHg pre-hemodialysis and <130 / mmHg post-hemodialysis.(2) Whereas a few studies have indicated that, similar to the general population,(3) high systolic or diastolic BP is associated with increased death risk in dialysis patients,(4–6) a number of large epidemiologic studies have paradoxically indicated inverse(5, 7–17) or U-shaped(9–12, 18–21) associations between BP and mortality in dialysis patients.
Arterial hypertension is common in kidney transplant recipients.(1) More than 80% of these patients have hypertension during the first year after renal transplantation.(1) It is well-known that arterial hypertension has adverse effects on kidney graft function and survival,(22–24) and treatment of hypertension may have positive effects on the kidney grafts’ and patients’ survival.(25, 26) However, it is not clear whether a history of hypertension or the level of BP during the dialysis period has any effect on post-transplant outcomes. Aull-Watschinger et al. examined the predictors of cerebrovascular events after kidney transplantation in more than 1600 kidney transplant recipients.(27) Atrial fibrillation and presence of diabetes mellitus, but not hypertension were predictors of cerebrovascular events.(27) Nevertheless, arterial hypertension is associated with left ventricular hypertrophy(28) and stroke,(29) which could affect post-transplant survival. In addition, low BP during the dialysis period has been associated with higher mortality.(30, 31)
The impact of pre-transplant recipients’ systolic and diastolic BP level on transplant outcomes is still unclear. We sought to examine the association of systolic and diastolic BP levels with all-cause mortality, graft failure, and delayed graft function (DGF) in a US-based renal transplant population. We hypothesized that high and low systolic and diastolic BP were associated with poor graft and patient outcomes.
Patients and Methods
Subjects
We linked data, using patients’ social security numbers, on all kidney transplant recipients listed in the Scientific Registry of Transplant Recipients (SRTR) up to June 2007 to a list of individuals with CKD who underwent maintenance hemodialysis treatment from July 2001 to June 2006 in one of the outpatient dialysis facilities of a US-based large dialysis organization (LDO). The study was approved by the Institutional Review Boards of both Los Angeles Biomedical Research Institute at Harbor-UCLA and DaVita Clinical Research.
Clinical and Demographic Measures
The creation of the national LDO dialysis patient cohort has been described previously.(32–38) To minimize measurement variability, all repeated measures for each patient during any given calendar quarter, i.e., over a 13-week interval, were averaged and the summary estimate was used in all models. Average values for each patient were obtained from up to 20 calendar quarters (q1 through q20) for each laboratory and clinical measure, resulting in up to six years of follow-up. The first (baseline) studied quarter for each patient was the calendar quarter in which the patient’s dialysis vintage was >90 days. Demographic data and details of medical history were collected, with information on age, gender, race, type of insurance, marital status, presence of diabetes, height, post-hemodialysis dry weight (to calculate averaged body mass index [BMI]) and dialysis vintage. Dialysis vintage was defined as the duration of time between the first day of dialysis treatment and the day of kidney transplantation.
Blood Pressure Measures
Seated pre- and post-hemodialysis BP values were measured during every single hemodialysis treatment session by means of automatically inflated cuffs using a digital monitor attached to each hemodialysis machine according to standard dialysis unit protocols. All available BP values of the same sort were averaged within each of the 20 calendar quarters of the 5-year cohort. For instance, if an MHD patient attended all 39 thrice-weekly hemodialysis treatment sessions over 13 weeks, all 39 pre-hemodialysis systolic BP (SBP) values were added and divided by 39 to obtain the averaged pre-hemodialysis SBP value for that calendar quarter of that given patient. BP values of <5 mmHg or >300 mmHg were deemed implausible and excluded from analyses. Patients were classified in BP groups according to their averaged BP values at all quarters in the cohort. We divided the quarterly averaged pre- and post-hemodialysis BP values into 9 a priori selected categories of SBP (<110 mmHg, 110 to 119 mmHg, 120 to 129 mmHg, 130 to 139 mmHg, 140 to 149 mmHg, 150 to 159 mmHg, 160 to 169 mmHg, 170 to 179 mmHg, and ≥180 mmHg) and 7 a priori selected categories of diastolic BP (DBP) (<50 mmHg, 50 to 59 mmHg, 60 to 69 mmHg, 70 to 79 mmHg, 80 to 89 mmHg, 90 to 99 mmHg, and ≥100 mmHg).
Laboratory Measures
Blood samples were drawn using uniform techniques in all of the LDO dialysis clinics and were transported to the LDO Laboratory in Deland, Florida, typically within 24 hours. All laboratory values were measured by automated and standardized methods in the LDO laboratory. Most laboratory values were measured monthly, including serum urea, creatinine, albumin, calcium, phosphorus, bicarbonate, and total iron binding capacity (TIBC). Serum ferritin and intact PTH were measured at least quarterly. Hemoglobin was measured at least monthly in essentially all patients and weekly to biweekly in most patients. Most blood samples were collected pre-dialysis with the exception of post-dialysis serum urea nitrogen to calculate urea kinetics. Kt/V (single pool) was calculated using urea kinetic modeling equations as described elsewhere.(39)
Statistical Methods
Data were summarized using proportions and means (± standard deviation [SD]). We examined p-values for trends across pre-transplant BP categories. Time-to-event survival analyses were done to determine the association of pre-transplant recipients’ systolic and diastolic BP level with all-cause mortality and graft failure (defined as re-initiation of dialysis treatment or re-transplantation). Survival analyses to calculate hazard ratios (HR) and 95% confidence interval (95% CI) of death or graft failure employed Cox proportional hazards regression. In mortality analyses, patients were followed until event (death) or censoring (graft failure or end of follow-up period), whichever happened first. In graft failure analyses, patients were followed until event (graft failure) or censoring (death or end of follow-up period), whichever happened first. Proportional hazard assumption was tested using log (−log) against survival plots. Post-transplant delayed graft function (DGF) was defined as the need for any dialysis therapy in the first week after transplantation.(40) Logistic regression models were employed to estimate the odds ratio (OR) and 95% CI of post-transplant DGF.
For each regression analysis, four levels of multivariate adjustment were examined: (I) an unadjusted model that included pre-transplant recipients’ systolic and diastolic BP level as the predictor; (II) Case-mix adjusted models that included the above plus age, gender, recipient race-ethnicity (African Americans and other self-categorized Blacks, Non-Hispanic Whites, Asians, Hispanics and others), diabetes mellitus, dialysis vintage (<6 month, 6 month to 2 years, 2–<5 years and ≥5 years), primary insurance (Medicare, Medicaid, private and others), marital status (married, single, divorced, widowed and other or unknown), dialysis dose as indicated by Kt/V (single pool), presence or absence of a hemodialysis catheter, and residual renal function during the entry quarter and 8 co-morbidities (atherosclerotic heart disease, congestive heart failure, cancer, chronic obstructive pulmonary disease, cerebrovascular disease, hypertension, peripheral vascular disease, tobacco use); (III) Case-Mix and Malnutrition-inflammation-complex syndrome (MICS) adjusted models which included all of the above covariates plus 9 surrogates of nutritional status and inflammation measured during the last calendar quarter before transplantation included: body mass index (BMI) and 8 laboratory variables, i.e. nPCR as an indicator of daily protein intake (also known as the normalized protein nitrogen appearance (nPNA)),(41) and serum or blood concentrations of TIBC, ferritin, calcium, phosphorous, bicarbonate, peripheral white blood cell count (WBC), lymphocyte percentage and albumin; and (IV) Case-mix, MICS and transplant data adjusted models included all of the above plus 8 transplant-related variables: (1) donor type (deceased or living), (2) donor age, (3) donor gender, (4) panel reactive antibody (PRA) titer (last value prior to transplant), (5) number of HLA mismatches, (6) cold ischemia time, (7) DGF (except when DGF was a dependent variable in logistic regression models), and (8) extended donor criteria (EDC) using standard definition (donor history of hypertension and/or serum creatinine of donor > 1.5 mg/dL and/or cause of death in donor is cerebrovascular event). All analyses were carried out with SAS version 9.3 (SAS Institute Inc., Cary, North Carolina).
Results
The original 5-year (7/2001–6/2006) LDO national database included 164,789 adult subjects. After merging this LDO database with the SRTR database 65,386 patients were identified, of which 17,629 had undergone one or more kidney transplantation during their lifetime, but only 14,508 dialysis patients had undergone kidney transplantation for the first time. From these 14,508 dialyzed patients, we excluded patients who did not have BP measurements (n=627). The analytic cohort consisted of the remaining 13,881 patients who underwent first kidney transplantation during the observation period and who were followed until death, graft failure, loss of follow up, or survival until June 30th 2007 (Figure S1). There were 991 deaths (7.1%) and 2,669 graft failures (20.6 %) irrespective of subsequent death. The median cohort follow up was 722 days (interquartile range was: 359–1,209 days). The baseline characteristics of waitlisted but non-transplanted patients have been described elsewhere.(42) Table 1 shows the clinical, demographic and laboratory data of the 13,881 transplanted patients across nine pre-transplant pre-dialysis systolic BP categories.
Table 1.
Baseline characteristics of 13,881 dialysis patients who underwent renal transplantation between 7/2001 and 6/2006
| Pre-transplant pre-dialysis systolic BP (mmHg) |
<110 | 110– <120 |
120– <130 |
130– <140 |
140– <150 |
150– <160 |
160– <170 |
170– <180 |
>=180 | p for trend |
|---|---|---|---|---|---|---|---|---|---|---|
| N [%] | 3900 [28] | 362 [3] | 798 [6] | 1508 [11] | 2127 [15] | 2144 [15] | 1540 [11] | 832 [6] | 670 [5] | N/A |
| Age (years) | 47 | 47 | 47 | 47 | 48 | 48 | 50 | 50 | 50 | <0.001 |
| Gender (% women) | 42 | 42 | 43 | 40 | 37 | 37 | 36 | 38 | 39 | <0.001 |
| Race (% African-American) | 22 | 20 | 23 | 25 | 28 | 29 | 31 | 29 | 33 | <0.001 |
| Diabetes mellitus (%) | 31 | 26 | 25 | 29 | 32 | 36 | 45 | 52 | 52 | <0.001 |
| BMI (kg/m2) | 26.3 | 26.1 | 26.6 | 26.5 | 26.6 | 26.7 | 26.8 | 27.4 | 27.5 | <0.001 |
| Comorbidities (%): | ||||||||||
| Presence of atherosclerotic heart disease (%) | 8 | 12 | 10 | 8 | 9 | 9 | 9 | 9 | 9 | 0.07 |
| Presence of congestive heart failure (%) | 8 | 8 | 8 | 8 | 11 | 10 | 12 | 14 | 17 | <0.001 |
| Presence of hypertension (%) | 75 | 70 | 72 | 74 | 78 | 79 | 80 | 80 | 82 | <0.001 |
| Presence of cerebrovascular events (%) | 2 | 2 | 2 | 2 | 3 | 3 | 3 | 3 | 2 | 0.30 |
| Presence of peripheral vascular disease (%) | 4 | 4 | 3 | 3 | 4 | 4 | 5 | 4 | 5 | 0.26 |
| Presence of chronic obstructive pulmonary disease (%) | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 0.33 |
| Presence of cancer (%) | 2 | 3 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 0.21 |
| Tobacco use (%) | 3 | 4 | 3 | 3 | 3 | 4 | 4 | 4 | 5 | 0.29 |
| Dialysis Vintage (%): | ||||||||||
| 0–6 months | 15 | 15 | 16 | 12 | 10 | 0 | 8 | 11 | 12 | <0.001 |
| 6–24 months | 32 | 29 | 32 | 29 | 28 | 27 | 27 | 23 | 31 | <0.001 |
| 2–5 years | 35 | 33 | 30 | 35 | 37 | 38 | 40 | 40 | 36 | <0.001 |
| >5 years | 18 | 23 | 22 | 24 | 25 | 25 | 25 | 26 | 22 | <0.001 |
| Other variables: | ||||||||||
| Kt/V | 1.53 | 1.58 | 1.59 | 1.6 | 1.59 | 1.57 | 1.56 | 1.53 | 1.53 | <0.001 |
| nPCR (g/kg/day) | 1.02 | 0.97 | 1.01 | 1.02 | 1.03 | 1.03 | 1.03 | 1.03 | 1.02 | <0.001 |
| Laboratory variables: | ||||||||||
| Serum creatinine (mg/dL) | 10.6 | 9.7 | 9.6 | 10.2 | 10.4 | 10.5 | 10.5 | 10.5 | 10.3 | <0.001 |
| Serum albumin (mg/dL) | 3.88 | 3.87 | 3.93 | 3.98 | 3.99 | 3.97 | 3.95 | 3.90 | 3.88 | <0.001 |
| Serum calcium (mg/dL) | 9.4 | 9.3 | 9.4 | 9.4 | 9.4 | 9.4 | 9.4 | 9.4 | 9.3 | 0.18 |
| Blood hemoglobin (g/dL) | 12.0 | 12.0 | 12.3 | 12.2 | 12.2 | 12.2 | 12.1 | 12.0 | 11.9 | <0.001 |
| iPTH (pg/ml) | 452 | 447 | 344 | 417 | 386 | 370 | 420 | 383 | 463 | 0.08 |
| WBC (× 103/l) | 7.1 | 7.0 | 6.8 | 6.9 | 6.9 | 7.0 | 7.0 | 7.2 | 7.3 | <0.001 |
| Number of HLA mismatch | 3.5 | 3.5 | 3.6 | 3.6 | 3.6 | 3.7 | 3.7 | 3.6 | 3.6 | <0.001 |
| PRA (%) | 9 | 13 | 10 | 11 | 11 | 10 | 10 | 11 | 10 | 0.01 |
| PRA >20% (%) | 13 | 19 | 14 | 16 | 16 | 15 | 15 | 15 | 16 | 0.05 |
| Donor age (years) | 38 | 40 | 39 | 39 | 39 | 39 | 40 | 40 | 40 | 0.005 |
| Donor gender (% women) | 47 | 53 | 46 | 48 | 48 | 46 | 48 | 48 | 44 | 0.29 |
| Donor Type (% Living) | 34 | 39 | 38 | 33 | 30 | 32 | 30 | 29 | 33 | <0.001 |
| ECD kidney (%)* | 16 | 15 | 16 | 18 | 20 | 20 | 21 | 21 | 22 | <0.001 |
| Cold Ischemia time (hours)* | 14.0 | 12.7 | 13.7 | 13.6 | 14.6 | 13.9 | 14.6 | 15.3 | 14.0 | 0.002 |
Data are presented as means or percent.
HLA: Human Leucocyte Antibody. PRA: panel reactive antibody (last value prior to transplant). WBC: White Blood Cell count. BMI: Body Mass Index. iPTH: intact parathyroid hormone, EDC: Extended Donor Criteria. nPCR: normalized protein catabolic rate
in recipients who received kidney from deceased donors
Mortality Outcomes
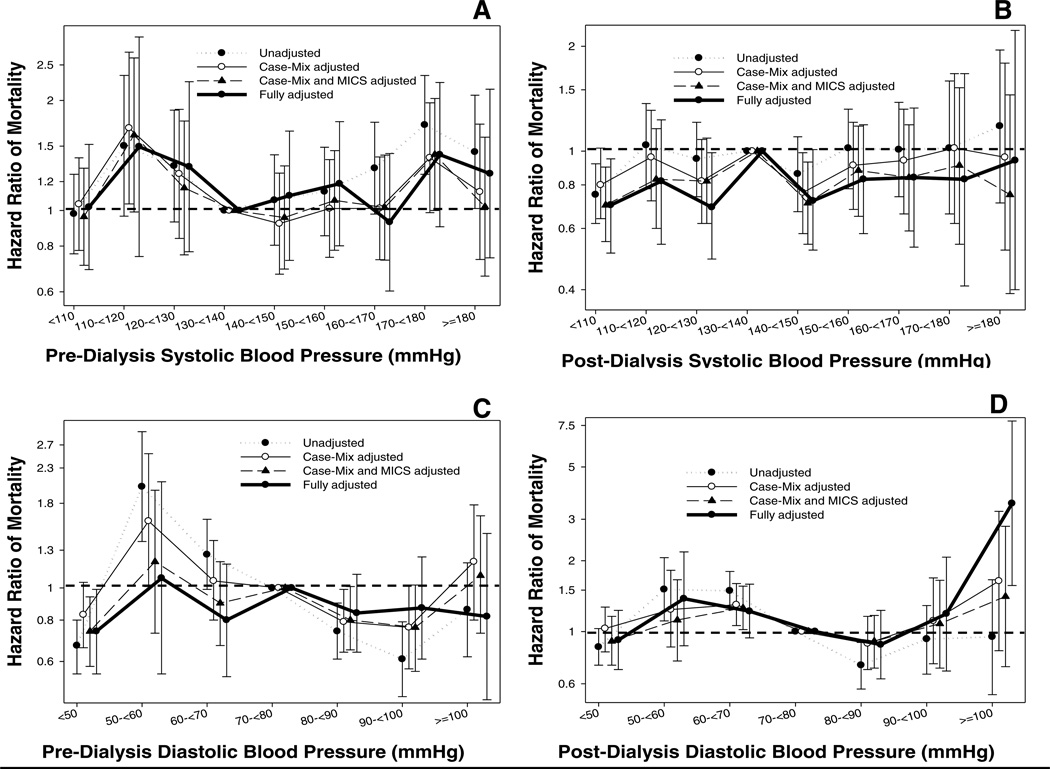
Figure 1 (and Table S1) shows the association between pre-transplant BP categories and post-transplant graft loss-censored mortality. There was no association between both pre-dialysis and post-dialysis pre-transplant systolic BP and mortality, except for patients with low post-dialysis systolic BP of < 110 mmHg who had a lower risk of death when compared to patients with post-dialysis systolic BP 130–<140 mmHg (HR: 0.70, 95% CI: 0.51–0.95). A similar flat association was observed for both pre-dialysis and post-dialysis diastolic BP and mortality; however, low (< 50 mmHg) pre-dialysis diastolic BP was associated with a lower risk of death (HR: 0.74, 95%CI: 0.55–0.99), and very high post-dialysis diastolic BP (≥ 100 mmHg) was associated with a higher risk of death (HR: 3.50, 95%CI: 1.57–7.84).
Figure 1.
Hazard ratio (95% confidence intervals) of post-transplant mortality (Panel A: pre-dialysis systolic BP; Panel B: post-dialysis systolic BP, Panel C: pre-dialysis diastolic BP, Panel D: post-dialysis diastolic BP) across different categories of blood pressure level using Cox regression analyses in 13,881 long-term dialysis patients who underwent renal transplantation and who were observed over a 6-year study period (7/2001–6/2007).
Case-mix models adjusted for age, gender, recipient race-ethnicity, diabetes mellitus, dialysis vintage, primary insurance, marital status, dialysis dose as indicated by Kt/V (single pool), presence or absence of a hemodialysis catheter, and residual renal function during the entry quarter and 8 co-morbidities; Malnutrition-inflammation-complex syndrome (MICS) models adjusted for all of the above covariates plus body mass index, nPCR, serum or blood concentrations of TIBC, ferritin, calcium, phosphorous, bicarbonate, peripheral white blood cell count, lymphocyte percentage and albumin; Case-mix, MICS and transplant models adjusted for all of the above plus donor type, donor age, donor gender, panel reactive antibody (PRA) titer (last value prior to transplant), number of HLA mismatches, cold ischemia time, DGF (except when DGF was a dependent variable in logistic regression models), and extended donor criteria.
Graft Survival
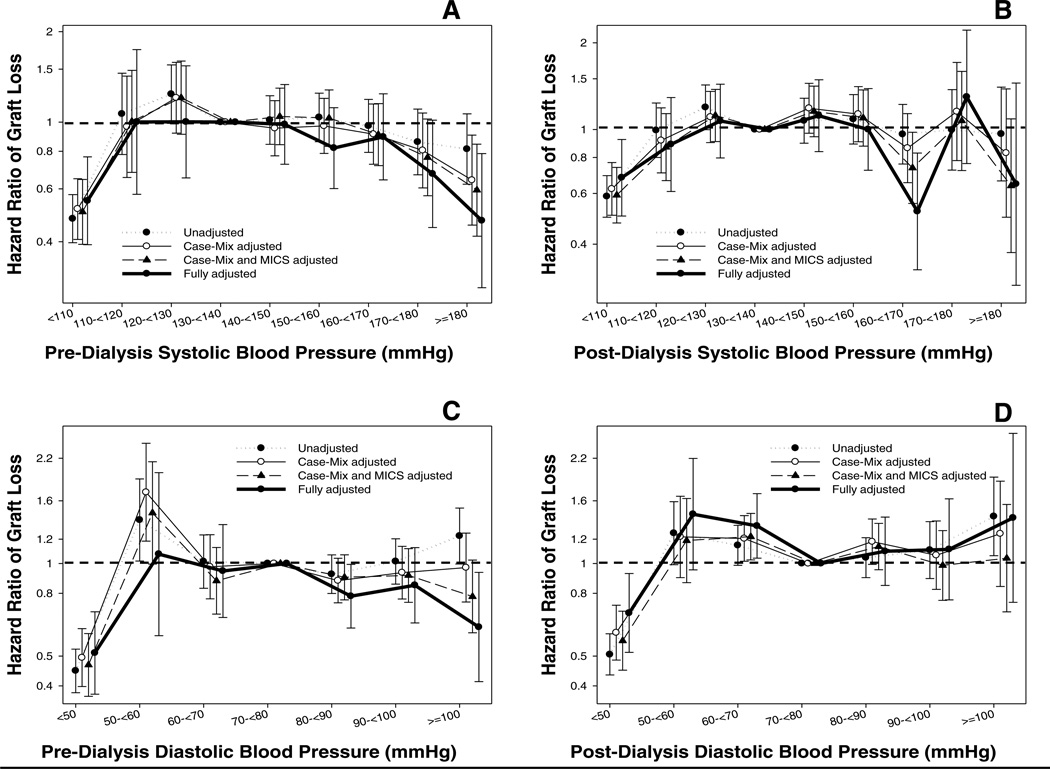
Figure 2 (and Table S2) shows the association between pre-transplant BP categories and post-transplant death-censored graft loss. In our fully adjusted model, low pre- and post-dialysis systolic and diastolic pre-transplant BP were all associated with a lower risk of death-censored graft loss. Interestingly, high pre-dialysis systolic (≥ 180 mmHg) (HR: 0.47, 95%CI: 0.28–0.79) and high pre-dialysis diastolic (≥ 100 mmHg) (HR: 0.62, 95%CI: 0.41–0.94) BPs were also associated with a lower risk of death-censored graft loss.
Figure 2.
Hazard ratio (95% confidence intervals) of post-transplant graft loss (Panel A: pre-dialysis systolic BP; Panel B: post-dialysis systolic BP, Panel C: pre-dialysis diastolic BP, Panel D: post-dialysis diastolic BP) across different categories of blood pressure level using Cox regression analyses in 13,881 long-term dialysis patients who underwent renal transplantation and who were observed over a 6-year study period (7/2001–6/2007).
Case-mix models adjusted for age, gender, recipient race-ethnicity, diabetes mellitus, dialysis vintage, primary insurance, marital status, dialysis dose as indicated by Kt/V (single pool), presence or absence of a hemodialysis catheter, and residual renal function during the entry quarter and 8 co-morbidities; Malnutrition-inflammation-complex syndrome (MICS) models adjusted for all of the above covariates plus body mass index, nPCR, serum or blood concentrations of TIBC, ferritin, calcium, phosphorous, bicarbonate, peripheral white blood cell count, lymphocyte percentage and albumin; Case-mix, MICS and transplant models adjusted for all of the above plus donor type, donor age, donor gender, panel reactive antibody (PRA) titer (last value prior to transplant), number of HLA mismatches, cold ischemia time, DGF (except when DGF was a dependent variable in logistic regression models), and extended donor criteria.
Delayed Graft Function
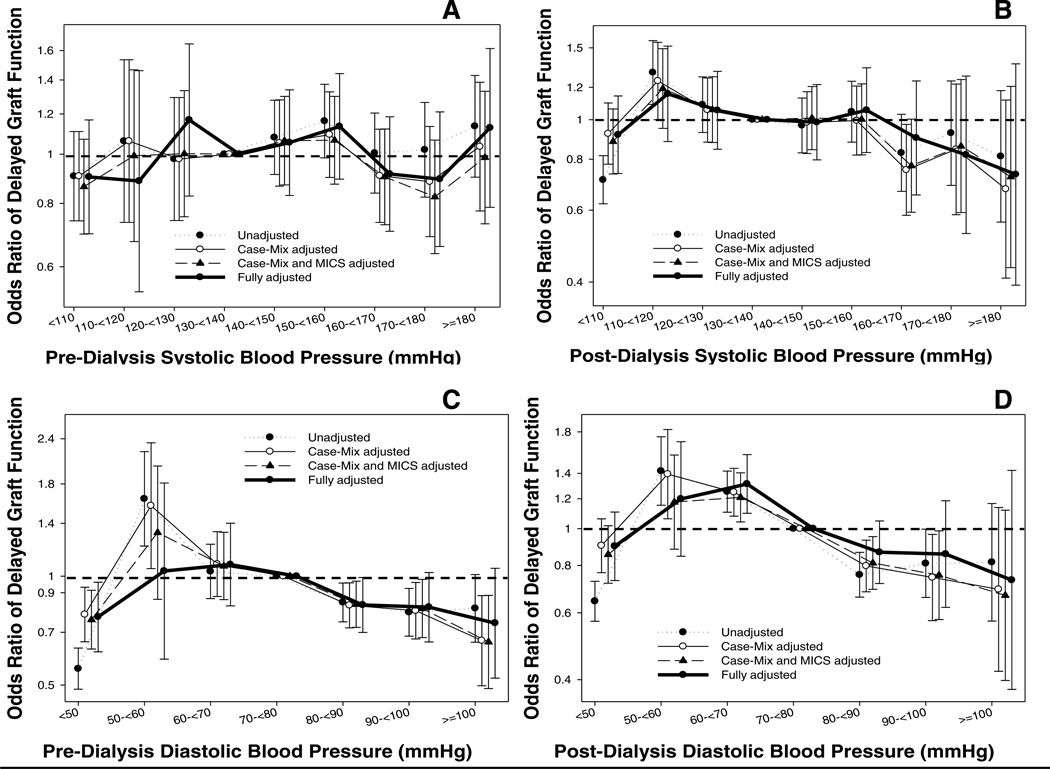
Figure 3 (and Table S3) shows the association between pre-transplant BP categories and post-transplant DGF. There was no association between both pre-dialysis and post-dialysis pre-transplant systolic BP and DGF, except for a lower odds of DGF associated with low (<50 mmHg) pre-dialysis diastolic BP (OR: 0.77, 95% CI: 0.62–0.96).
Figure 3.
Odds ratio (95% confidence intervals) of post-transplant delayed graft function (Panel A: pre-dialysis systolic BP; Panel B: post-dialysis systolic BP, Panel C: pre-dialysis diastolic BP, Panel D: post-dialysis diastolic BP) across different categories of blood pressure level using logistic regression analyses in 13,881 long-term dialysis patients who underwent renal transplantation and who were observed over a 6-year study period (7/2001–6/2007).
Case-mix models adjusted for age, gender, recipient race-ethnicity, diabetes mellitus, dialysis vintage, primary insurance, marital status, dialysis dose as indicated by Kt/V (single pool), presence or absence of a hemodialysis catheter, and residual renal function during the entry quarter and 8 co-morbidities; Malnutrition-inflammation-complex syndrome (MICS) models adjusted for all of the above covariates plus body mass index, nPCR, serum or blood concentrations of TIBC, ferritin, calcium, phosphorous, bicarbonate, peripheral white blood cell count, lymphocyte percentage and albumin; Case-mix, MICS and transplant models adjusted for all of the above plus donor type, donor age, donor gender, panel reactive antibody (PRA) titer (last value prior to transplant), number of HLA mismatches, cold ischemia time, DGF (except when DGF was a dependent variable in logistic regression models), and extended donor criteria.
Discussion
In this retrospective analysis of 13,881 primary kidney transplant recipients, we assessed the association between pre-transplant recipients’ systolic and diastolic BP levels and risk of death-censored graft failure and graft loss-censored death. Only low (< 110 mmHg) post-dialysis systolic and low (< 50 mmHg) pre-dialysis diastolic BP groups were associated with better survival, while high post-dialysis diastolic (≥100 mmHg) BP was associated with increased mortality. In addition, low pre-transplant BP was associated with lower risk of death-censored graft loss.
Several important pre-transplant risk factors have been identified in the last few years,(32, 35, 36, 38, 43, 44) but the association of pre-transplant BP and post-transplant mortality had not been previously assessed. Due to lack of data, most of the guidelines on the evaluation of kidney transplant candidates have not included any recommendations about the ideal BP of waitlisted patients.(45–49) The recent recommendation from the American Society of Transplantation mentioned that hypertension (BP ≥140/90 mm Hg or taking antihypertensive medications) is a risk factor of ischemic heart disease and other atherosclerotic disease, but did not provide an ideal range of BP during the waitlisted period.(47) Neither the European Best Practice Guideline nor the evaluation guideline from the United Kingdom mentioned anything about what the preferred BP might be in waitlisted dialysis patients.(48, 49) Based on our results, only very high diastolic BP was associated with higher post-transplant mortality risk.
The current observational study indicates that both very low pre-transplant systolic and diastolic BPs were associated with lower risk of graft loss. Other retrospective studies have suggested that hypertension before transplantation is also associated with poor outcomes after renal transplantation.(24, 47, 50) Poorly controlled BP appears to be a strong risk factor for allograft failure in African American transplant recipients, and the level of pre-transplant BP identifies those patients as being at the highest risk for renal allograft failure.(24, 51) In addition, pre-transplant hypertension has been shown to be associated with post-transplant hypertension.(52) In a study by Kasiske et al., each 10 mmHg higher post-transplant systolic BP was associated with 17% and 18% increased risk of transplant failure and death, respectively.(53) In a second study of nearly 25,000 primary deceased donor kidney recipients, when compared to patients with sustained increases in systolic BP, improved long-term transplant outcomes were observed in recipients whose systolic BP decreased from >150 mmHg at 1 year post-transplant to <140 mmHg at 3 years post-transplant. (54)
There was no association between both pre-dialysis and post-dialysis pre-transplant systolic BP and delayed graft function in our study. Ozdemir et al. examined 142 renal transplant recipients and found that pre-transplant systolic BP level of <120 mmHg was associated with a more than 3 times higher risk of delayed graft function.(55) In addition, when only living-related recipients were considered, the systolic BP level demonstrated a significant positive association with DGF. (55) Potential explanations for the different outcomes seen in our study include the larger size and the lower proportion of living donors in our cohort.
Our study results should be viewed in light of several potential limitations. Inherent to the retrospective observational nature of our study, we cannot establish causality. Furthermore, we did not have data on the BP post transplantation, which may not necessarily have correlated with pre-transplant values. Repeated post-transplant measures of BP and laboratory variables and immunosuppressive, anti-hypertensive and other medical regimens were not available in the SRTR database, but in the full model we did adjust for a number of transplant-related variables. Data on anti-hypertensive medication usage and treatment may have further affected the BP and outcomes relationship. While we had comprehensive data on numerous BP values for each subject analyzed, we did not evaluate pulse pressure as a variable. Pulse pressure has been shown to be a biomarker and strong predictor of clinical outcomes particularly in elderly populations.(56) Generalizability to the US chronic kidney disease population may be limited due to the lower proportion of diabetics and the lack of re-transplanted patients in our cohort. Additionally, the mean age of our study population was significantly younger than that of the United States Renal Data System population and may not be representative of the end stage renal disease population in general. Thus, our findings contrast somewhat with previous observational studies,(17) where higher pre- and post-HD BP’s correlated with better survival. The transplant population studied here likely represented younger and healthier subjects where HTN worked as a more traditional risk factor on vascular outcomes. Another potential limitation is we did not have access to data on deaths after graft loss.
To our knowledge this is the first study examining the association between pre-transplant systolic and diastolic BP levels and post-transplant short- and long-term outcomes in kidney allograft recipients. Strengths of this study include the racially and ethnically heterogeneous patient population with repeated BP measurement values and relatively long follow-up period. Though we acknowledge many confounders that may contribute to the relationship studied, we performed multi-level adjustments, which included several important pre-transplant measures.
Conclusion
In this retrospective analysis of 13,881 primary kidney transplant recipients, the lowest post-dialysis systolic and low pre-dialysis diastolic BPs were associated with decreased risk of death, while the highest post-dialysis diastolic BP was associated with increased risk of death. Low pre-transplant BP was also associated with lower risk of graft loss. Though the current observational study is limited by unaccounted for confounders, our study suggests that low BP in waitlisted patients may be a biomarker for improved post transplant outcomes.
Table 2.
Post-transplant outcomes of 13,881 dialysis patients who underwent renal transplantation between 7/2001 and 6/2006
| Pre-transplant pre-dialysis systolic BP (mmHg) |
<110 | 110– <120 |
120– <130 |
130– <140 |
140– <150 |
150– <160 |
160– <170 |
170– <180 |
>=180 | p for trend |
|---|---|---|---|---|---|---|---|---|---|---|
| N [%] | 3900 [28] | 362 [3] | 798 [6] | 1508 [11] | 2127 [15] | 2144 [15] | 1540 [11] | 832 [6] | 670 [5] | N/A |
| Deaths (n) [Crude Death Rate %] | 349 [8.9] | 26 [7.2] | 51 [6.4] | 80 [5.3] | 117 [5.5] | 131 [6.1] | 109 [7.1] | 79 [9.5] | 49 [7.3] | <.001 |
| Crude all-cause mortality rate /1000 patient-years) [95% CI] | 28 [25–31] | 41 [28–60] | 36 [27–47] | 28 [22–34] | 30 [25–36] | 32 [27–38] | 37 [31–45] | 49 [39–61] | 39 [30–52] | N/A |
| Cardiovascular Deaths (n) [Crude CV Death Rate %] | 62 [1.6] | 5 [1.4] | 12 [1.5] | 15 [1.0] | 28 [1.3] | 29 [1.4] | 23 [1.5] | 15 [1.8] | 5 [1.0] | 0.63 |
| Crude CV mortality rate (/1000 patient-years) [95% CI] | 5 [4–6] | 8 [3–19] | 8 [5–15] | 5 [3–9] | 7 [5–10] | 7 [5–10] | 8 [5–12] | 9 [6–15] | 4 [2–10] | N/A |
| Graft failure (n) [Crude Graft Failure Rate %] | 339 [8.7] | 50 [13.8] | 128 [16.0] | 193 [12.8] | 262 [12.3] | 266 [12.4] | 182 [11.8] | 89 [10.7] | 73 [10.9] | <0.001 |
| Crude graft failure rate (/1000 patient-years) [95% CI] | 28 [25–30] | 78 [59–103] | 89 [75–106] | 67 [58–77] | 66 [59–75] | 65 [57–73] | 62 [53–71] | 55 [45–68] | 58 [46–73] | N/A |
| DGF (n) [Crude DFG %] | 652 [17.5] | 73 [21.9] | 152 [20.8] | 287 [20.5] | 442 [22.1] | 474 [23.6] | 309 [21.1] | 171 [21.4] | 139 [21.9] | <0.001 |
| History of acute rejection (%) | 4.2 | 4.2 | 4.1 | 4.2 | 4.7 | 3.2 | 3.7 | 4.8 | 4.4 | 0.71 |
Values in brackets indicate the crude death and cardiovascular death rate, crude graft failure rate and crude DGF rate in the indicated group during the 6 years of observation.
DGF: Delayed Graft Function. CI: Confidence Interval
Acknowledgement
We thank DaVita Clinical Research (DCR) for providing the clinical data and review for this research project.
Funding Source:
The study was supported by KKZ’s research grant from the American Heart Association grant (0655776Y). KKZ’s other funding sources include the National Institute of Diabetes, Digestive and Kidney Disease of the National Institute of Health (R01 DK078106); a research grant from DaVita Clinical Research and a philanthropic grant from Mr. Harold Simmons. MMZ’s funding sources include National Institute On Aging of the National Institutes of Health (R21AG047036). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Relevant Potential Conflict of Interest:
Dr. Krishnan is employee of DaVita. Dr. Kalantar-Zadeh was the medical director of DaVita Harbor-UCLA/MFI in Long Beach, CA. Other authors have not declared any conflict of interest.
References
- 1.National Institute of Health Volume3. Betheda, MD: National Institute of Health, National Institute of Diabetes and Digestive and Kidney Disease; 2009. US Renal Data System: USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the Untied States. [Google Scholar]
- 2.National Kidney Foundation: K/ DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45:S1. [PubMed] [Google Scholar]
- 3.Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, Kannel WB, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. The New England journal of medicine. 2001;345(18):1291. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 4.Tomita J, Kimura G, Inoue T, Inenaga T, Sanai T, Kawano Y, et al. Role of systolic blood pressure in determining prognosis of hemodialyzed patients. Am J Kidney Dis. 1995;25(3):405. doi: 10.1016/0272-6386(95)90101-9. [DOI] [PubMed] [Google Scholar]
- 5.Foley RN, Herzog CA, Collins AJ. Blood pressure and long-term mortality in United States hemodialysis patients: USRDS Waves 3 and 4 Study. Kidney international. 2002;62(5):1784. doi: 10.1046/j.1523-1755.2002.00636.x. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez JM, Carbonell ME, Mazzuchi N, Petruccelli D. Simultaneous analysis of morbidity and mortality factors in chronic hemodialysis patients. Kidney international. 1992;41(4):1029. doi: 10.1038/ki.1992.156. [DOI] [PubMed] [Google Scholar]
- 7.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. Impact of hypertension on cardiomyopathy, morbidity and mortality in end-stage renal disease. Kidney international. 1996;49(5):1379. doi: 10.1038/ki.1996.194. [DOI] [PubMed] [Google Scholar]
- 8.Klassen PS, Lowrie EG, Reddan DN, DeLong ER, Coladonato JA, Szczech LA, et al. Association between pulse pressure and mortality in patients undergoing maintenance hemodialysis. JAMA : the journal of the American Medical Association. 2002;287(12):1548. doi: 10.1001/jama.287.12.1548. [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Lacson E, Jr, Lowrie EG, Ofsthun NJ, Kuhlmann MK, Lazarus JM, et al. The epidemiology of systolic blood pressure and death risk in hemodialysis patients. Am J Kidney Dis. 2006;48(4):606. doi: 10.1053/j.ajkd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Stidley CA, Hunt WC, Tentori F, Schmidt D, Rohrscheib M, Paine S, et al. Changing relationship of blood pressure with mortality over time among hemodialysis patients. J Am Soc Nephrol. 2006;17(2):513. doi: 10.1681/ASN.2004110921. [DOI] [PubMed] [Google Scholar]
- 11.Port FK, Hulbert-Shearon TE, Wolfe RA, Bloembergen WE, Golper TA, Agodoa LY, et al. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33(3):507. doi: 10.1016/s0272-6386(99)70188-5. [DOI] [PubMed] [Google Scholar]
- 12.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, Greenland S, Kopple JD. Reverse epidemiology of hypertension and cardiovascular death in the hemodialysis population: the 58th annual fall conference and scientific sessions. Hypertension. 2005;45(4):811. doi: 10.1161/01.HYP.0000154895.18269.67. [DOI] [PubMed] [Google Scholar]
- 13.Iseki K, Miyasato F, Tokuyama K, Nishime K, Uehara H, Shiohira Y, et al. Low diastolic blood pressure, hypoalbuminemia, and risk of death in a cohort of chronic hemodialysis patients. Kidney international. 1997;51(4):1212. doi: 10.1038/ki.1997.165. [DOI] [PubMed] [Google Scholar]
- 14.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99(18):2434. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 15.Salem MM. Hypertension in the haemodialysis population: any relationship to 2-years survival? Nephrol Dial Transplant. 1999;14(1):125. doi: 10.1093/ndt/14.1.125. [DOI] [PubMed] [Google Scholar]
- 16.Goldfarb-Rumyantzev AS, Baird BC, Leypoldt JK, Cheung AK. The association between BP and mortality in patients on chronic peritoneal dialysis. Nephrol Dial Transplant. 2005;20(8):1693. doi: 10.1093/ndt/gfh856. [DOI] [PubMed] [Google Scholar]
- 17.Molnar MZ, Lukowsky LR, Streja E, Dukkipati R, Jing J, Nissenson AR, et al. Blood pressure and survival in long-term hemodialysis patients with and without polycystic kidney disease. J Hypertens. 2010;28(12):2475. doi: 10.1097/HJH.0b013e32833e4fd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zager PG, Nikolic J, Brown RH, Campbell MA, Hunt WC, Peterson D, et al. "U" curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney international. 1998;54(2):561. doi: 10.1046/j.1523-1755.1998.00005.x. [DOI] [PubMed] [Google Scholar]
- 19.Lynn KL, McGregor DO, Moesbergen T, Buttimore AL, Inkster JA, Wells JE. Hypertension as a determinant of survival for patients treated with home dialysis. Kidney international. 2002;62(6):2281. doi: 10.1046/j.1523-1755.2002.00685.x. [DOI] [PubMed] [Google Scholar]
- 20.Mazzuchi N, Carbonell E, Fernandez-Cean J. Importance of blood pressure control in hemodialysis patient survival. Kidney international. 2000;58(5):2147. doi: 10.1111/j.1523-1755.2000.00388.x. [DOI] [PubMed] [Google Scholar]
- 21.Degoulet P, Legrain M, Reach I, Aime F, Devries C, Rojas P, et al. Mortality risk factors in patients treated by chronic hemodialysis. Report of the Diaphane collaborative study. Nephron. 1982;31(2):103. doi: 10.1159/000182627. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Fresnedo G, Palomar R, Escallada R, Martin de Francisco AL, Cotorruelo JG, Zubimendi JA, et al. Hypertension and long-term renal allograft survival: effect of early glomerular filtration rate. Nephrol Dial Transplant. 2001;16(Suppl 1):105. doi: 10.1093/ndt/16.suppl_1.105. [DOI] [PubMed] [Google Scholar]
- 23.Opelz G, Wujciak T, Ritz E. Association of chronic kidney graft failure with recipient blood pressure. Collaborative Transplant Study. Kidney international. 1998;53(1):217. doi: 10.1046/j.1523-1755.1998.00744.x. [DOI] [PubMed] [Google Scholar]
- 24.Cosio FG, Falkenhain ME, Pesavento TE, Henry ML, Elkhammas EA, Davies EA, et al. Relationships between arterial hypertension and renal allograft survival in African-American patients. Am J Kidney Dis. 1997;29(3):419. doi: 10.1016/s0272-6386(97)90204-3. [DOI] [PubMed] [Google Scholar]
- 25.Hillebrand U, Suwelack BM, Loley K, Lang D, Reuter S, Amler S, et al. Blood pressure, antihypertensive treatment, and graft survival in kidney transplant patients. Transpl Int. 2009;22(11):1073. doi: 10.1111/j.1432-2277.2009.00922.x. [DOI] [PubMed] [Google Scholar]
- 26.Tutone VK, Mark PB, Stewart GA, Tan CC, Rodger RS, Geddes CC, et al. Hypertension, antihypertensive agents and outcomes following renal transplantation. Clin Transplant. 2005;19(2):181. doi: 10.1111/j.1399-0012.2004.00315.x. [DOI] [PubMed] [Google Scholar]
- 27.Aull-Watschinger S, Konstantin H, Demetriou D, Schillinger M, Habicht A, Horl WH, et al. Pre-transplant predictors of cerebrovascular events after kidney transplantation. Nephrol Dial Transplant. 2008;23(4):1429. doi: 10.1093/ndt/gfm766. [DOI] [PubMed] [Google Scholar]
- 28.Elias MF, Sullivan LM, Elias PK, D'Agostino RB, Sr, Wolf PA, Seshadri S, et al. Left ventricular mass, blood pressure, and lowered cognitive performance in the Framingham offspring. Hypertension. 2007;49(3):439. doi: 10.1161/01.HYP.0000256361.68158.24. [DOI] [PubMed] [Google Scholar]
- 29.Thrift AG, McNeil JJ, Forbes A, Donnan GA. Risk factors for cerebral hemorrhage in the era of well-controlled hypertension. Melbourne Risk Factor Study (MERFS) Group. Stroke; a journal of cerebral circulation. 1996;27(11):2020. doi: 10.1161/01.str.27.11.2020. [DOI] [PubMed] [Google Scholar]
- 30.Messerli FH, Mancia G, Conti CR, Hewkin AC, Kupfer S, Champion A, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Annals of internal medicine. 2006;144(12):884. doi: 10.7326/0003-4819-144-12-200606200-00005. [DOI] [PubMed] [Google Scholar]
- 31.D'Agostino RB, Belanger AJ, Kannel WB, Cruickshank JM. Relation of low diastolic blood pressure to coronary heart disease death in presence of myocardial infarction: the Framingham Study. BMJ. 1991;303(6799):385. doi: 10.1136/bmj.303.6799.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molnar MZ, Mehrotra R, Duong U, Bunnapradist S, Lukowsky LR, Krishnan M, et al. Dialysis modality and outcomes in kidney transplant recipients. Clin J Am Soc Nephrol. 2012;7(2):332. doi: 10.2215/CJN.07110711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duong U, Kalantar-Zadeh K, Molnar MZ, Zaritsky JJ, Teitelbaum I, Kovesdy CP, et al. Mortality Associated with Dose Response of Erythropoiesis-Stimulating Agents in Hemodialysis versus Peritoneal Dialysis Patients. American journal of nephrology. 2012;35(2):198. doi: 10.1159/000335685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lukowsky LR, Molnar MZ, Zaritsky JJ, Sim JJ, Mucsi I, Kovesdy CP, et al. Mineral and bone disorders and survival in hemodialysis patients with and without polycystic kidney disease. Nephrol Dial Transplant. 2012;27(7):2899. doi: 10.1093/ndt/gfr747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molnar MZ, Huang E, Hoshino J, Krishnan M, Nissenson AR, Kovesdy CP, et al. Association of pretransplant glycemic control with posttransplant outcomes in diabetic kidney transplant recipients. Diabetes care. 2011;34(12):2536. doi: 10.2337/dc11-0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sampaio MS, Molnar MZ, Kovesdy CP, Mehrotra R, Mucsi I, Sim JJ, et al. Association of pretransplant serum phosphorus with posttransplant outcomes. Clin J Am Soc Nephrol. 2011;6(11):2712. doi: 10.2215/CJN.06190611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molnar MZ, Streja E, Kovesdy CP, Budoff MJ, Nissenson AR, Krishnan M, et al. High platelet count as a link between renal cachexia and cardiovascular mortality in end-stage renal disease patients. Am J Clin Nutr. 2011;94(3):945. doi: 10.3945/ajcn.111.014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molnar MZ, Kovesdy CP, Bunnapradist S, Streja E, Mehrotra R, Krishnan M, et al. Associations of pretransplant serum albumin with post-transplant outcomes in kidney transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(5):1006. doi: 10.1111/j.1600-6143.2011.03480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller JE, Kovesdy CP, Nissenson AR, Mehrotra R, Streja E, Van Wyck D, et al. Association of hemodialysis treatment time and dose with mortality and the role of race and sex. Am J Kidney Dis. 2010;55(1):100. doi: 10.1053/j.ajkd.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yarlagadda SG, Coca SG, Garg AX, Doshi M, Poggio E, Marcus RJ, et al. Marked variation in the definition and diagnosis of delayed graft function: a systematic review. Nephrol Dial Transplant. 2008;23(9):2995. doi: 10.1093/ndt/gfn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shinaberger CS, Greenland S, Kopple JD, Van Wyck D, Mehrotra R, Kovesdy CP, et al. Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am J Clin Nutr. 2008;88(6):1511. doi: 10.3945/ajcn.2008.26665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molnar MZ, Streja E, Kovesdy CP, Bunnapradist S, Sampaio MS, Jing J, et al. Associations of body mass index and weight loss with mortality in transplant-waitlisted maintenance hemodialysis patients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(4):725. doi: 10.1111/j.1600-6143.2011.03468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Streja E, Molnar MZ, Kovesdy CP, Bunnapradist S, Jing J, Nissenson AR, et al. Associations of pretransplant weight and muscle mass with mortality in renal transplant recipients. Clin J Am Soc Nephrol. 2011;6(6):1463. doi: 10.2215/CJN.09131010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molnar MZ, Kovesdy CP, Mucsi I, Bunnapradist S, Streja E, Krishnan M, et al. Higher recipient body mass index is associated with post-transplant delayed kidney graft function. Kidney international. 2011;80(2):218. doi: 10.1038/ki.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kasiske BL, Ramos EL, Gaston RS, Bia MJ, Danovitch GM, Bowen PA, et al. The evaluation of renal transplant candidates: clinical practice guidelines. Patient Care and Education Committee of the American Society of Transplant Physicians. J Am Soc Nephrol. 1995;6(1):1. doi: 10.1681/ASN.V611. [DOI] [PubMed] [Google Scholar]
- 46.Pilmore H. Cardiac assessment for renal transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(4):659. doi: 10.1111/j.1600-6143.2006.01253.x. [DOI] [PubMed] [Google Scholar]
- 47.Kasiske BL, Cangro CB, Hariharan S, Hricik DE, Kerman RH, Roth D, et al. The evaluation of renal transplantation candidates: clinical practice guidelines. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2001;1(Suppl 2):3. [PubMed] [Google Scholar]
- 48.Dudley C, Harden P. Assessment of the Potential Kidney Transplant Recipient. 5th Edition ed. UK Renal Association; 2010. [DOI] [PubMed] [Google Scholar]
- 49.European Best Practice Guidelines for Renal Transplantation (part 1) Nephrol Dial Transplant. 2000;15(Suppl 7):1. [PubMed] [Google Scholar]
- 50.Frei U, Schindler R, Wieters D, Grouven U, Brunkhorst R, Koch KM. Pre-transplant hypertension: a major risk factor for chronic progressive renal allograft dysfunction? Nephrol Dial Transplant. 1995;10(7):1206. [PubMed] [Google Scholar]
- 51.Cosio FG, Dillon JJ, Falkenhain ME, Tesi RJ, Henry ML, Elkhammas EA, et al. Racial differences in renal allograft survival: the role of systemic hypertension. Kidney international. 1995;47(4):1136. doi: 10.1038/ki.1995.162. [DOI] [PubMed] [Google Scholar]
- 52.Perez Fontan M, Rodriguez-Carmona A, Garcia Falcon T, Fernandez Rivera C, Valdes F. Early immunologic and nonimmunologic predictors of arterial hypertension after renal transplantation. Am J Kidney Dis. 1999;33(1):21. doi: 10.1016/s0272-6386(99)70253-2. [DOI] [PubMed] [Google Scholar]
- 53.Kasiske BL, Anjum S, Shah R, Skogen J, Kandaswamy C, Danielson B, et al. Hypertension after kidney transplantation. Am J Kidney Dis. 2004;43(6):1071. doi: 10.1053/j.ajkd.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 54.Opelz G, Dohler B. Improved long-term outcomes after renal transplantation associated with blood pressure control. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5(11):2725. doi: 10.1111/j.1600-6143.2005.01093.x. [DOI] [PubMed] [Google Scholar]
- 55.Ozdemir FN, Ibis A, Altunoglu A, Usluogullari A, Arat Z, Haberal M. Pretransplantation systolic blood pressure and the risk of delayed graft function in young living-related renal allograft recipients. Transplant Proc. 2007;39(4):842. doi: 10.1016/j.transproceed.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 56.Glynn RJ, Chae CU, Guralnik JM, Taylor JO, Hennekens CH. Pulse pressure and mortality in older people. Archives of internal medicine. 2000;160(18):2765. doi: 10.1001/archinte.160.18.2765. [DOI] [PubMed] [Google Scholar]