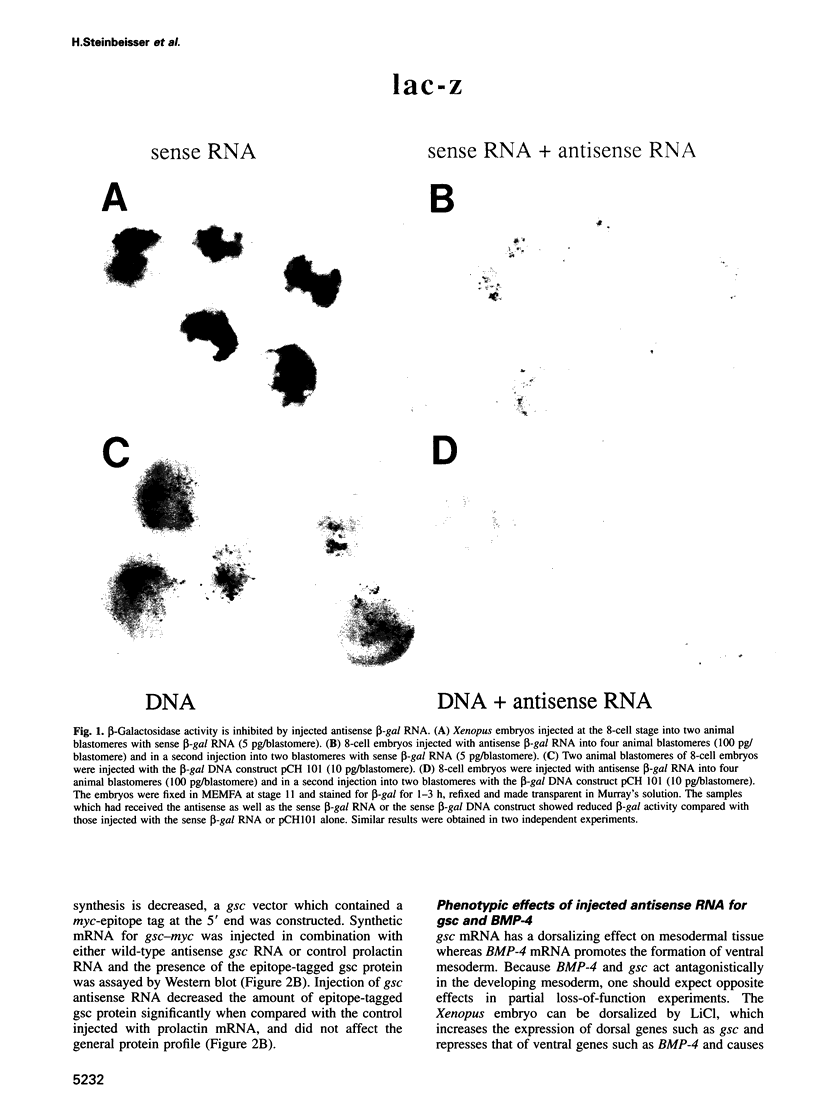
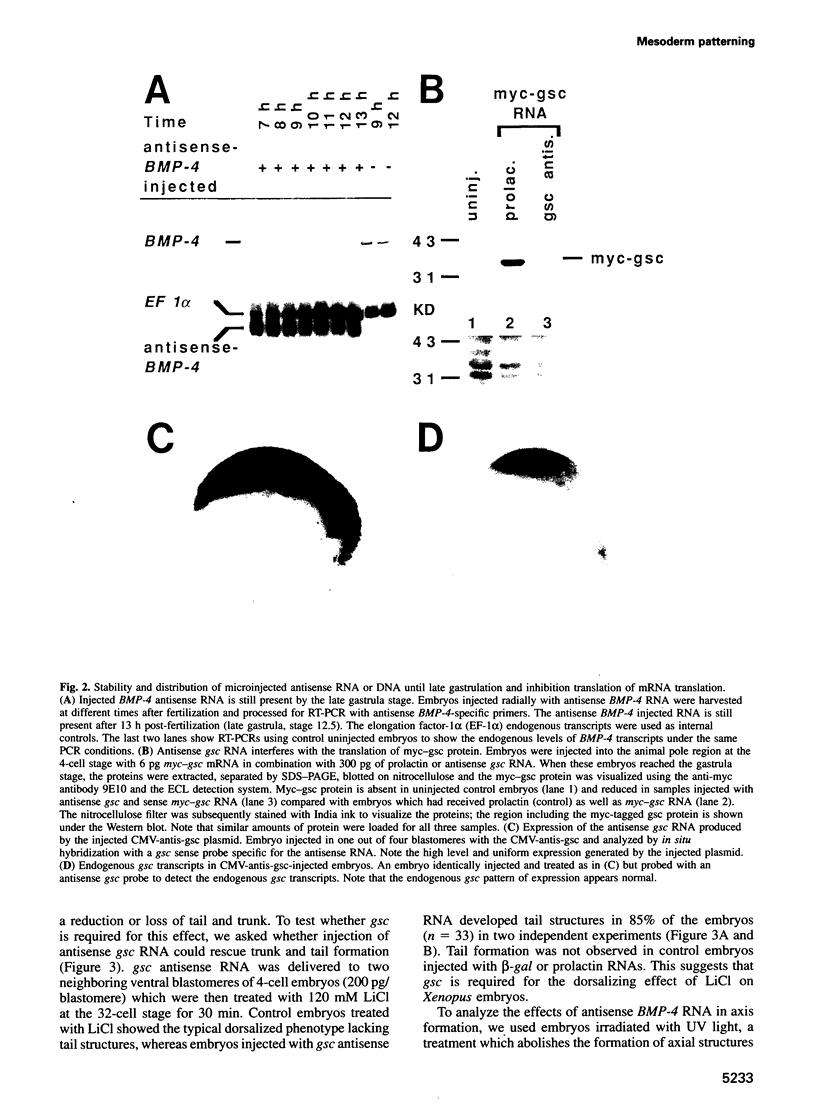
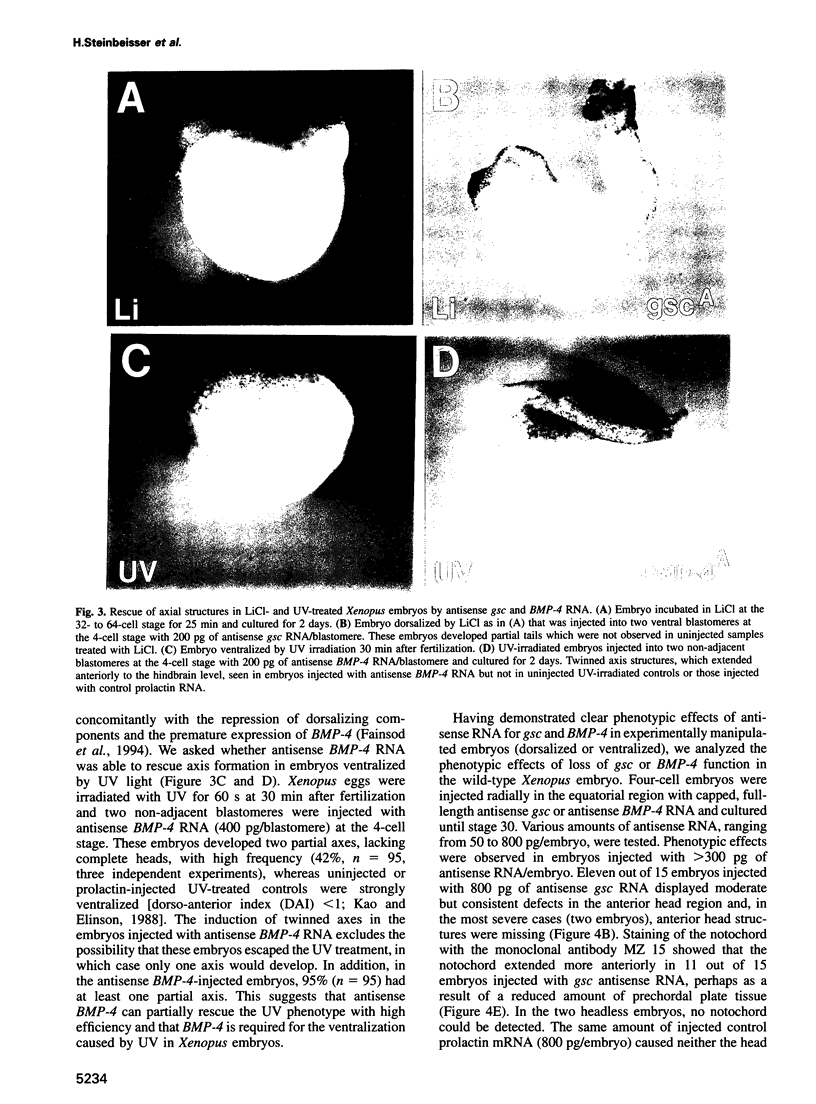
Abstract
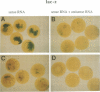
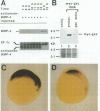
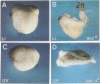
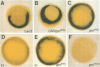
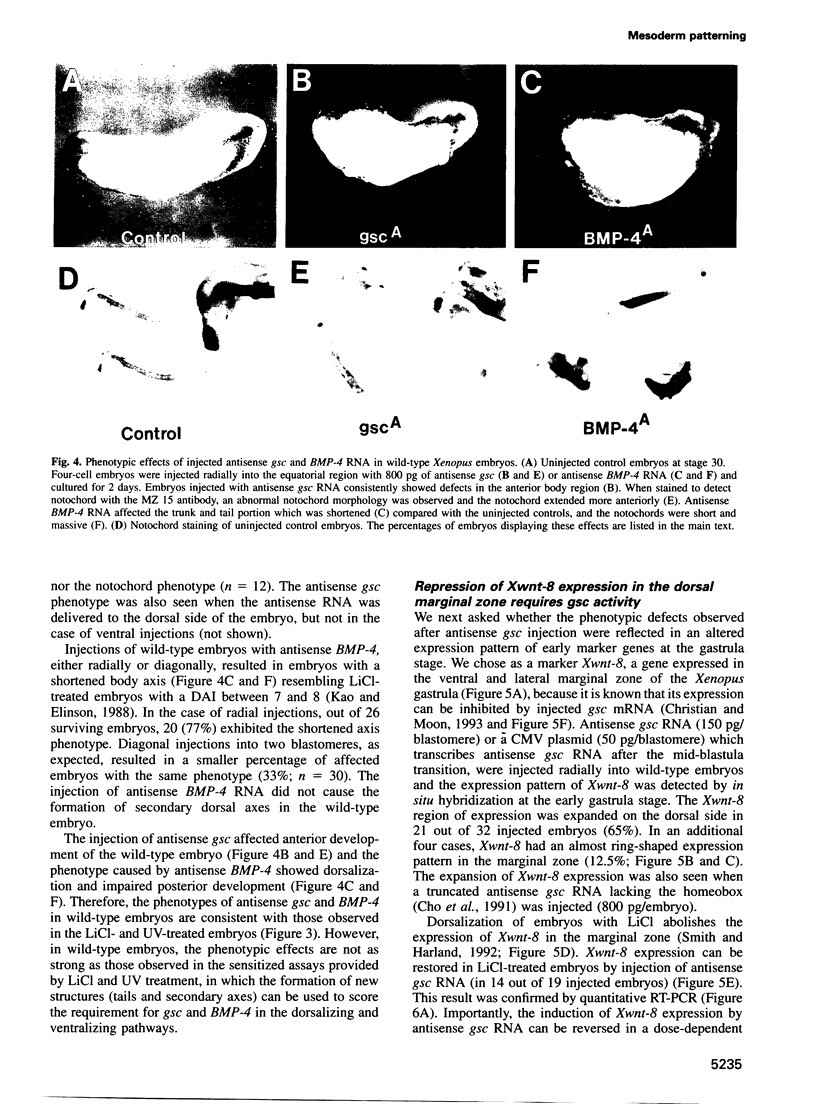
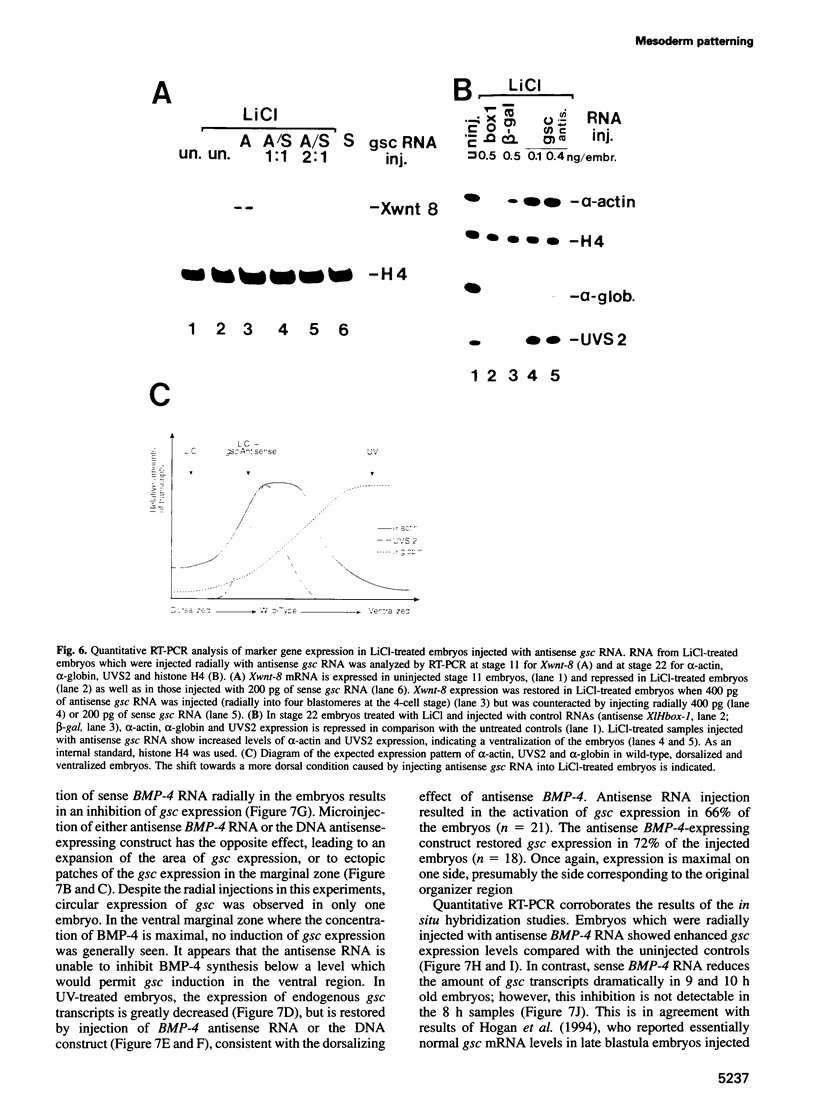
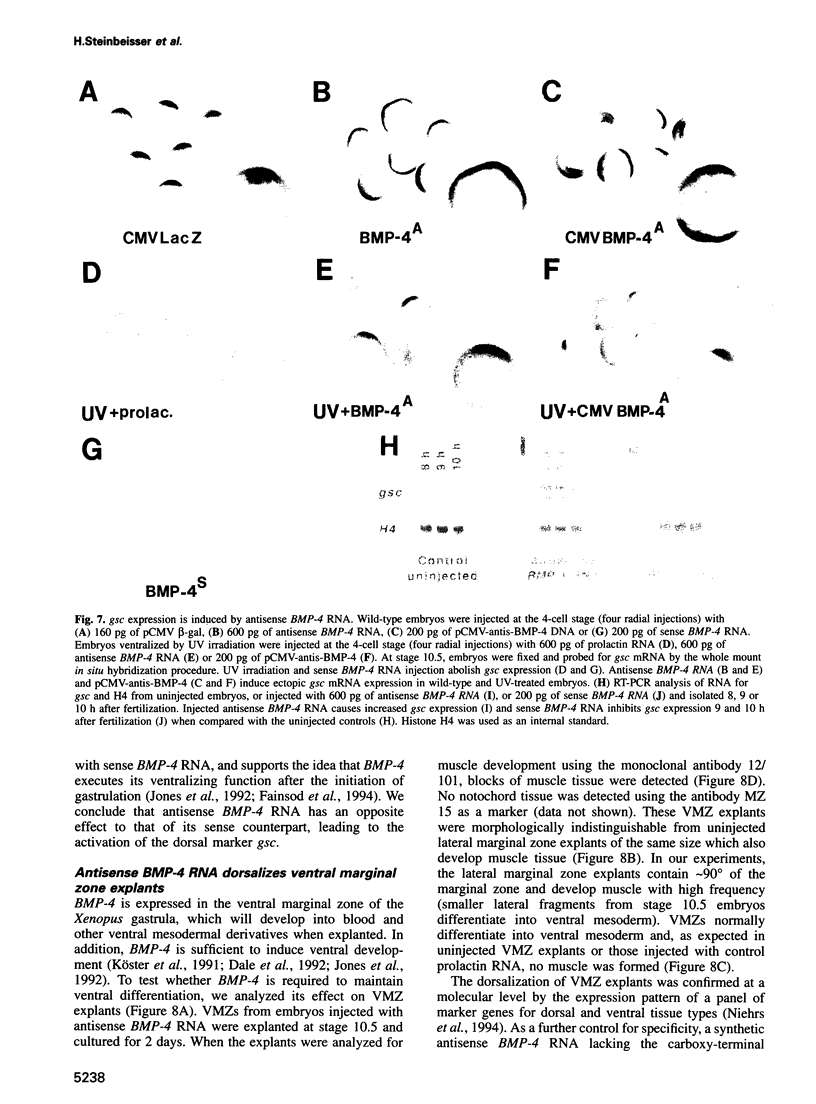
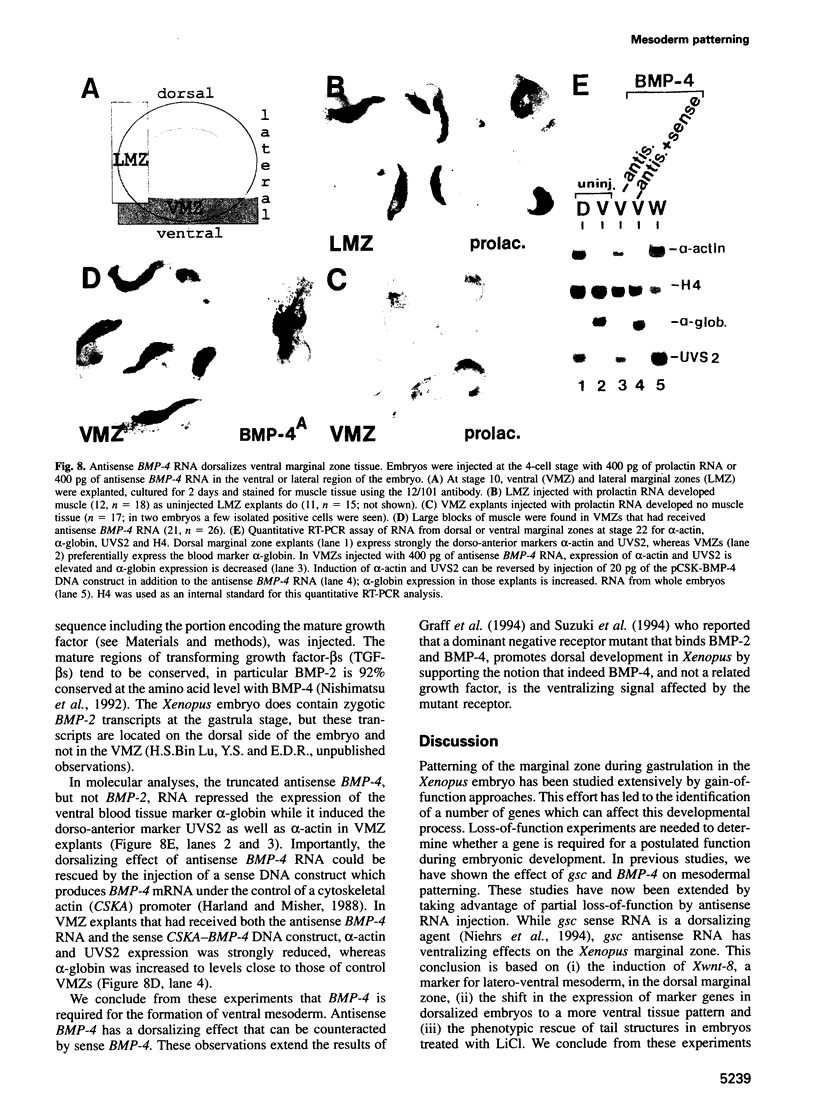
The dorsal-specific homeobox gene goosecoid (gsc) and the bone morphogenetic protein 4 gene (BMP-4) are expressed in complementary regions of the Xenopus gastrula. Injection of gsc mRNA dorsalizes ventral mesodermal tissue and can induce axis formation in normal and UV-ventralized embryos. On the other hand, BMP-4 mRNA injection, which has a strong ventralizing effect on whole embryos, has been implicated in ventralization by UV, and can rescue tail structures in embryos dorsalized by LiCl. The above-mentioned putative roles for BMP-4 and gsc are based on gain-of-function experiments. In order to determine the in vivo role of these two genes in the patterning of the Xenopus mesoderm during gastrulation, partial loss-of-function experiments were performed using antisense RNA injections. Using marker genes that are expressed early in gastrulation, we show that antisense gsc RNA has a ventralizing effect on embryos, whereas antisense BMP-4 RNA dorsalizes mesodermal tissue. These loss-of-function studies also show a requirement for gsc and BMP-4 in the dorsalization induced by LiCl and in the ventralization generated by UV irradiation, respectively. Thus, both gain- and loss-of-function results for gsc and BMP-4 support the view that these two genes are necessary components of the dorsal and ventral patterning pathways in Xenopus embryos.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass B. L., Weintraub H. A developmentally regulated activity that unwinds RNA duplexes. Cell. 1987 Feb 27;48(4):607–613. doi: 10.1016/0092-8674(87)90239-x. [DOI] [PubMed] [Google Scholar]
- Blumberg B., Wright C. V., De Robertis E. M., Cho K. W. Organizer-specific homeobox genes in Xenopus laevis embryos. Science. 1991 Jul 12;253(5016):194–196. doi: 10.1126/science.1677215. [DOI] [PubMed] [Google Scholar]
- Cho K. W., Blumberg B., Steinbeisser H., De Robertis E. M. Molecular nature of Spemann's organizer: the role of the Xenopus homeobox gene goosecoid. Cell. 1991 Dec 20;67(6):1111–1120. doi: 10.1016/0092-8674(91)90288-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. W., Goetz J., Wright C. V., Fritz A., Hardwicke J., De Robertis E. M. Differential utilization of the same reading frame in a Xenopus homeobox gene encodes two related proteins sharing the same DNA-binding specificity. EMBO J. 1988 Jul;7(7):2139–2149. doi: 10.1002/j.1460-2075.1988.tb03053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Christian J. L., McMahon J. A., McMahon A. P., Moon R. T. Xwnt-8, a Xenopus Wnt-1/int-1-related gene responsive to mesoderm-inducing growth factors, may play a role in ventral mesodermal patterning during embryogenesis. Development. 1991 Apr;111(4):1045–1055. doi: 10.1242/dev.111.4.1045. [DOI] [PubMed] [Google Scholar]
- Christian J. L., Moon R. T. Interactions between Xwnt-8 and Spemann organizer signaling pathways generate dorsoventral pattern in the embryonic mesoderm of Xenopus. Genes Dev. 1993 Jan;7(1):13–28. doi: 10.1101/gad.7.1.13. [DOI] [PubMed] [Google Scholar]
- Dale L., Howes G., Price B. M., Smith J. C. Bone morphogenetic protein 4: a ventralizing factor in early Xenopus development. Development. 1992 Jun;115(2):573–585. doi: 10.1242/dev.115.2.573. [DOI] [PubMed] [Google Scholar]
- Dent J. A., Polson A. G., Klymkowsky M. W. A whole-mount immunocytochemical analysis of the expression of the intermediate filament protein vimentin in Xenopus. Development. 1989 Jan;105(1):61–74. doi: 10.1242/dev.105.1.61. [DOI] [PubMed] [Google Scholar]
- Fainsod A., Steinbeisser H., De Robertis E. M. On the function of BMP-4 in patterning the marginal zone of the Xenopus embryo. EMBO J. 1994 Nov 1;13(21):5015–5025. doi: 10.1002/j.1460-2075.1994.tb06830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J., Danilchik M., Doniach T., Roberts S., Rowning B., Stewart R. Cortical rotation of the Xenopus egg: consequences for the anteroposterior pattern of embryonic dorsal development. Development. 1989;107 (Suppl):37–51. doi: 10.1242/dev.107.Supplement.37. [DOI] [PubMed] [Google Scholar]
- Giebelhaus D. H., Eib D. W., Moon R. T. Antisense RNA inhibits expression of membrane skeleton protein 4.1 during embryonic development of Xenopus. Cell. 1988 May 20;53(4):601–615. doi: 10.1016/0092-8674(88)90576-4. [DOI] [PubMed] [Google Scholar]
- Graff J. M., Thies R. S., Song J. J., Celeste A. J., Melton D. A. Studies with a Xenopus BMP receptor suggest that ventral mesoderm-inducing signals override dorsal signals in vivo. Cell. 1994 Oct 7;79(1):169–179. doi: 10.1016/0092-8674(94)90409-x. [DOI] [PubMed] [Google Scholar]
- Grunz H. The four animal blastomeres of the eight-cell stage of Xenopus laevis are intrinsically capable of differentiating into dorsal mesodermal derivatives. Int J Dev Biol. 1994 Mar;38(1):69–76. [PubMed] [Google Scholar]
- Harland R. M. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Harland R. M. The transforming growth factor beta family and induction of the vertebrate mesoderm: bone morphogenetic proteins are ventral inducers. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10243–10246. doi: 10.1073/pnas.91.22.10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland R., Misher L. Stability of RNA in developing Xenopus embryos and identification of a destabilizing sequence in TFIIIA messenger RNA. Development. 1988 Apr;102(4):837–852. doi: 10.1242/dev.102.4.837. [DOI] [PubMed] [Google Scholar]
- Heasman J., Crawford A., Goldstone K., Garner-Hamrick P., Gumbiner B., McCrea P., Kintner C., Noro C. Y., Wylie C. Overexpression of cadherins and underexpression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 1994 Dec 2;79(5):791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A., Kelly O. G., Melton D. A. Follistatin, an antagonist of activin, is expressed in the Spemann organizer and displays direct neuralizing activity. Cell. 1994 Apr 22;77(2):283–295. doi: 10.1016/0092-8674(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Hogan B. L., Blessing M., Winnier G. E., Suzuki N., Jones C. M. Growth factors in development: the role of TGF-beta related polypeptide signalling molecules in embryogenesis. Dev Suppl. 1994:53–60. [PubMed] [Google Scholar]
- Jones C. M., Lyons K. M., Lapan P. M., Wright C. V., Hogan B. L. DVR-4 (bone morphogenetic protein-4) as a posterior-ventralizing factor in Xenopus mesoderm induction. Development. 1992 Jun;115(2):639–647. doi: 10.1242/dev.115.2.639. [DOI] [PubMed] [Google Scholar]
- Kao K. R., Elinson R. P. The entire mesodermal mantle behaves as Spemann's organizer in dorsoanterior enhanced Xenopus laevis embryos. Dev Biol. 1988 May;127(1):64–77. doi: 10.1016/0012-1606(88)90189-3. [DOI] [PubMed] [Google Scholar]
- Kessler D. S., Melton D. A. Vertebrate embryonic induction: mesodermal and neural patterning. Science. 1994 Oct 28;266(5185):596–604. doi: 10.1126/science.7939714. [DOI] [PubMed] [Google Scholar]
- Kim U., Nishikura K. Double-stranded RNA adenosine deaminase as a potential mammalian RNA editing factor. Semin Cell Biol. 1993 Aug;4(4):285–293. doi: 10.1006/scel.1993.1034. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Kirschner M. W. An antisense mRNA directs the covalent modification of the transcript encoding fibroblast growth factor in Xenopus oocytes. Cell. 1989 Nov 17;59(4):687–696. doi: 10.1016/0092-8674(89)90015-9. [DOI] [PubMed] [Google Scholar]
- Kintner C. R., Brockes J. P. Monoclonal antibodies identify blastemal cells derived from dedifferentiating limb regeneration. Nature. 1984 Mar 1;308(5954):67–69. doi: 10.1038/308067a0. [DOI] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Köster M., Plessow S., Clement J. H., Lorenz A., Tiedemann H., Knöchel W. Bone morphogenetic protein 4 (BMP-4), a member of the TGF-beta family, in early embryos of Xenopus laevis: analysis of mesoderm inducing activity. Mech Dev. 1991 Mar;33(3):191–199. doi: 10.1016/0925-4773(91)90027-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemaire P., Garrett N., Gurdon J. B. Expression cloning of Siamois, a Xenopus homeobox gene expressed in dorsal-vegetal cells of blastulae and able to induce a complete secondary axis. Cell. 1995 Apr 7;81(1):85–94. doi: 10.1016/0092-8674(95)90373-9. [DOI] [PubMed] [Google Scholar]
- Maéno M., Ong R. C., Suzuki A., Ueno N., Kung H. F. A truncated bone morphogenetic protein 4 receptor alters the fate of ventral mesoderm to dorsal mesoderm: roles of animal pole tissue in the development of ventral mesoderm. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10260–10264. doi: 10.1073/pnas.91.22.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehrs C., Keller R., Cho K. W., De Robertis E. M. The homeobox gene goosecoid controls cell migration in Xenopus embryos. Cell. 1993 Feb 26;72(4):491–503. doi: 10.1016/0092-8674(93)90069-3. [DOI] [PubMed] [Google Scholar]
- Niehrs C., Steinbeisser H., De Robertis E. M. Mesodermal patterning by a gradient of the vertebrate homeobox gene goosecoid. Science. 1994 Feb 11;263(5148):817–820. doi: 10.1126/science.7905664. [DOI] [PubMed] [Google Scholar]
- Nishimatsu S., Suzuki A., Shoda A., Murakami K., Ueno N. Genes for bone morphogenetic proteins are differentially transcribed in early amphibian embryos. Biochem Biophys Res Commun. 1992 Aug 14;186(3):1487–1495. doi: 10.1016/s0006-291x(05)81574-8. [DOI] [PubMed] [Google Scholar]
- Rebagliati M. R., Melton D. A. Antisense RNA injections in fertilized frog eggs reveal an RNA duplex unwinding activity. Cell. 1987 Feb 27;48(4):599–605. doi: 10.1016/0092-8674(87)90238-8. [DOI] [PubMed] [Google Scholar]
- Rivera-Pérez J. A., Mallo M., Gendron-Maguire M., Gridley T., Behringer R. R. Goosecoid is not an essential component of the mouse gastrula organizer but is required for craniofacial and rib development. Development. 1995 Sep;121(9):3005–3012. doi: 10.1242/dev.121.9.3005. [DOI] [PubMed] [Google Scholar]
- Sanes J. R., Rubenstein J. L., Nicolas J. F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986 Dec 1;5(12):3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y., Kageyama R., Tagawa Y., Shigemoto R., Nakanishi S. Two mammalian helix-loop-helix factors structurally related to Drosophila hairy and Enhancer of split. Genes Dev. 1992 Dec;6(12B):2620–2634. doi: 10.1101/gad.6.12b.2620. [DOI] [PubMed] [Google Scholar]
- Sasai Y., Lu B., Steinbeisser H., De Robertis E. M. Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature. 1995 Jul 27;376(6538):333–336. doi: 10.1038/376333a0. [DOI] [PubMed] [Google Scholar]
- Sasai Y., Lu B., Steinbeisser H., Geissert D., Gont L. K., De Robertis E. M. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994 Dec 2;79(5):779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. E., Suzuki A., Ueno N., Kimelman D. Localized BMP-4 mediates dorsal/ventral patterning in the early Xenopus embryo. Dev Biol. 1995 May;169(1):37–50. doi: 10.1006/dbio.1995.1124. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S., Smith J. C., Dale L. Effects of truncated activin and FGF receptors and of follistatin on the inducing activities of BVg1 and activin: does activin play a role in mesoderm induction? EMBO J. 1994 Aug 1;13(15):3533–3541. doi: 10.1002/j.1460-2075.1994.tb06660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C., Watt F. M. Biochemical specificity of Xenopus notochord. Differentiation. 1985;29(2):109–115. doi: 10.1111/j.1432-0436.1985.tb00302.x. [DOI] [PubMed] [Google Scholar]
- Smith W. C., Harland R. M. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992 Sep 4;70(5):829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Smith W. C., Harland R. M. Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell. 1991 Nov 15;67(4):753–765. doi: 10.1016/0092-8674(91)90070-f. [DOI] [PubMed] [Google Scholar]
- Steinbeisser H., De Robertis E. M., Ku M., Kessler D. S., Melton D. A. Xenopus axis formation: induction of goosecoid by injected Xwnt-8 and activin mRNAs. Development. 1993 Jun;118(2):499–507. doi: 10.1242/dev.118.2.499. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Thies R. S., Yamaji N., Song J. J., Wozney J. M., Murakami K., Ueno N. A truncated bone morphogenetic protein receptor affects dorsal-ventral patterning in the early Xenopus embryo. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10255–10259. doi: 10.1073/pnas.91.22.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira M., Otani H., Saint-Jeannet J. P., Dawid I. B. Role of the LIM class homeodomain protein Xlim-1 in neural and muscle induction by the Spemann organizer in Xenopus. Nature. 1994 Dec 15;372(6507):677–679. doi: 10.1038/372677a0. [DOI] [PubMed] [Google Scholar]
- Yamada G., Mansouri A., Torres M., Stuart E. T., Blum M., Schultz M., De Robertis E. M., Gruss P. Targeted mutation of the murine goosecoid gene results in craniofacial defects and neonatal death. Development. 1995 Sep;121(9):2917–2922. doi: 10.1242/dev.121.9.2917. [DOI] [PubMed] [Google Scholar]
- von Dassow G., Schmidt J. E., Kimelman D. Induction of the Xenopus organizer: expression and regulation of Xnot, a novel FGF and activin-regulated homeo box gene. Genes Dev. 1993 Mar;7(3):355–366. doi: 10.1101/gad.7.3.355. [DOI] [PubMed] [Google Scholar]