Abstract
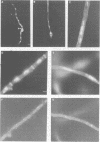
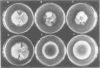
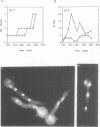
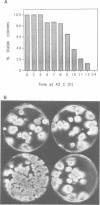
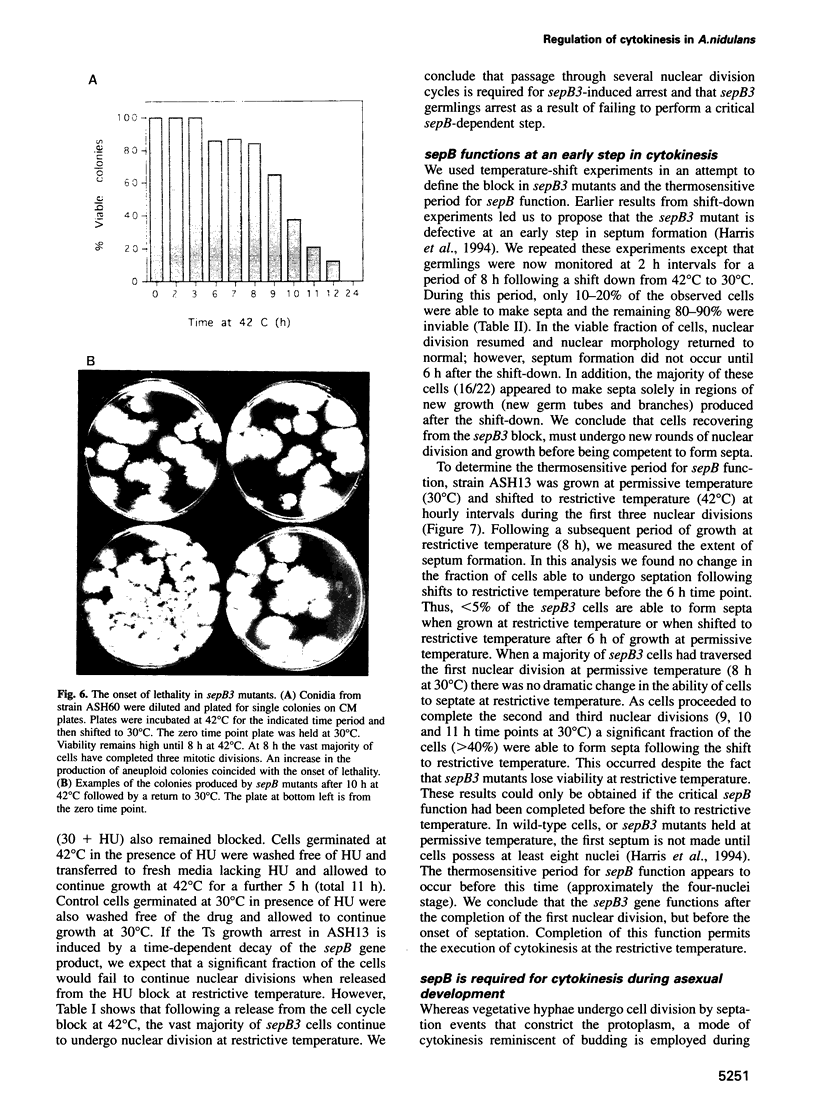
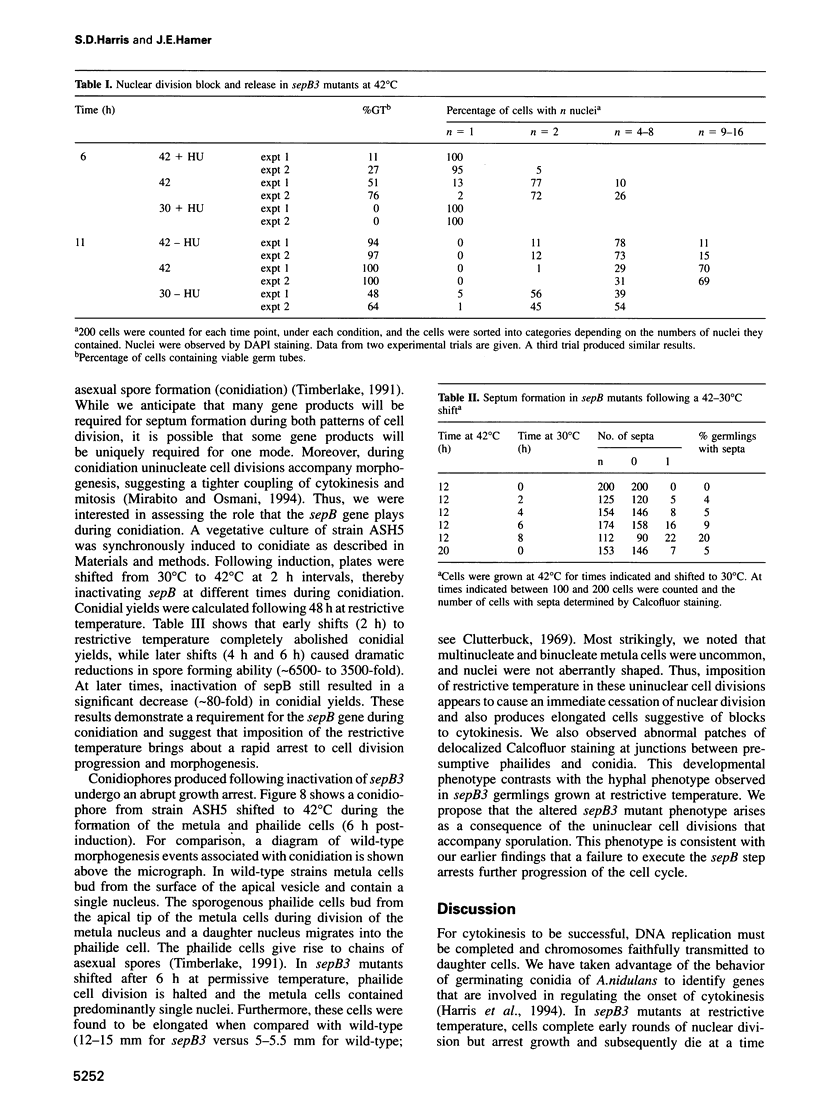
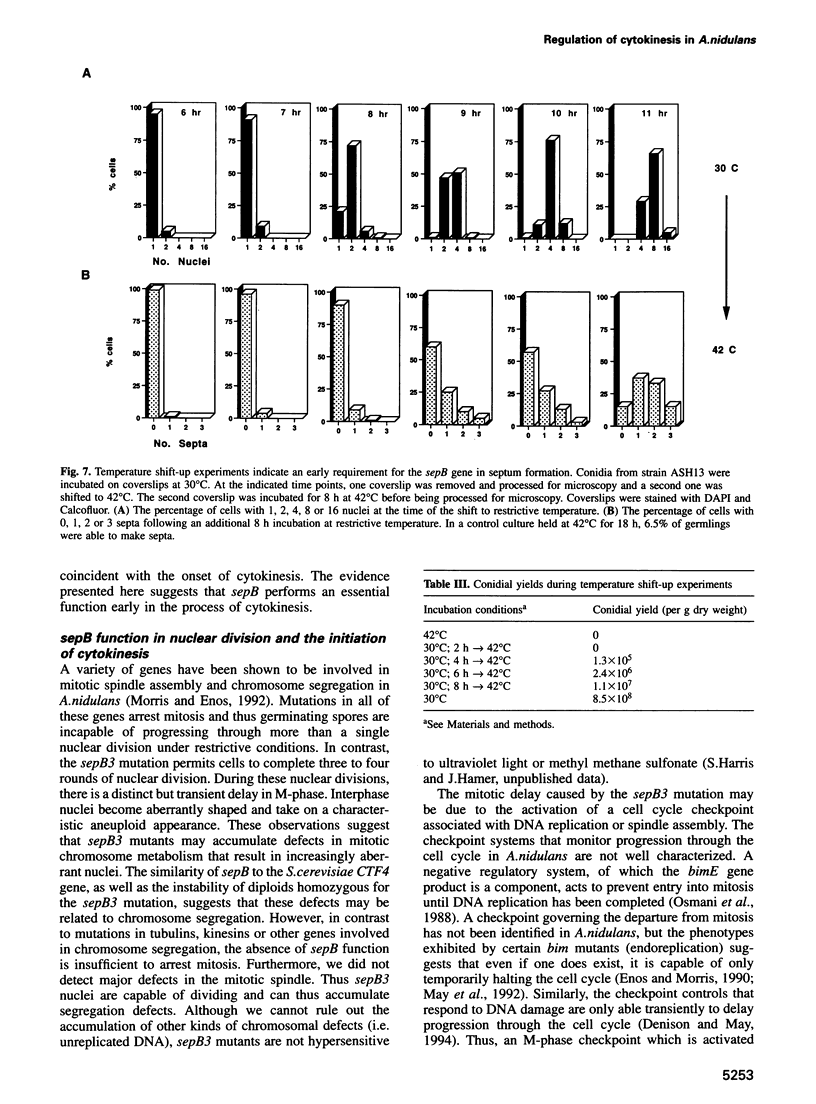
In Aspergillus nidulans conidia, cytokinesis (septation) is delayed until three rounds of nuclear division have been completed. This has permitted the identification of essential genes that are involved in the coordination of cytokinesis with nuclear division. Conditional mutations in the sepB gene block septation but allow germinating spores to complete the first three rounds of nuclear division at restrictive temperature. sepB3 mutants demonstrate transient delays in M-phase, accumulate aneuploid nuclei and show defects in chromosome segregation. Molecular analysis of the sepB gene reveals that it is essential and possesses limited similarity to the CTF4 gene of Saccharomyces cerevisiae. Using temperature-shift analysis we show that sepB is required after the first nuclear division but before the onset of cytokinesis. A failure to execute the sepB function results in a block to nuclear division and leads to cell death at a time when wild-type cells would be undergoing cytokinesis. Finally, we demonstrate that sepB is also required for the uninucleate cell divisions of developing conidiophores. Our results suggest that sepB3 mutants accumulate specific nuclear defects that do not arrest mitosis, but block the initiation of septum formation. Thus, proper chromosome segregation and a functional sepB gene are required to initiate cytokinesis.
Full text
PDF

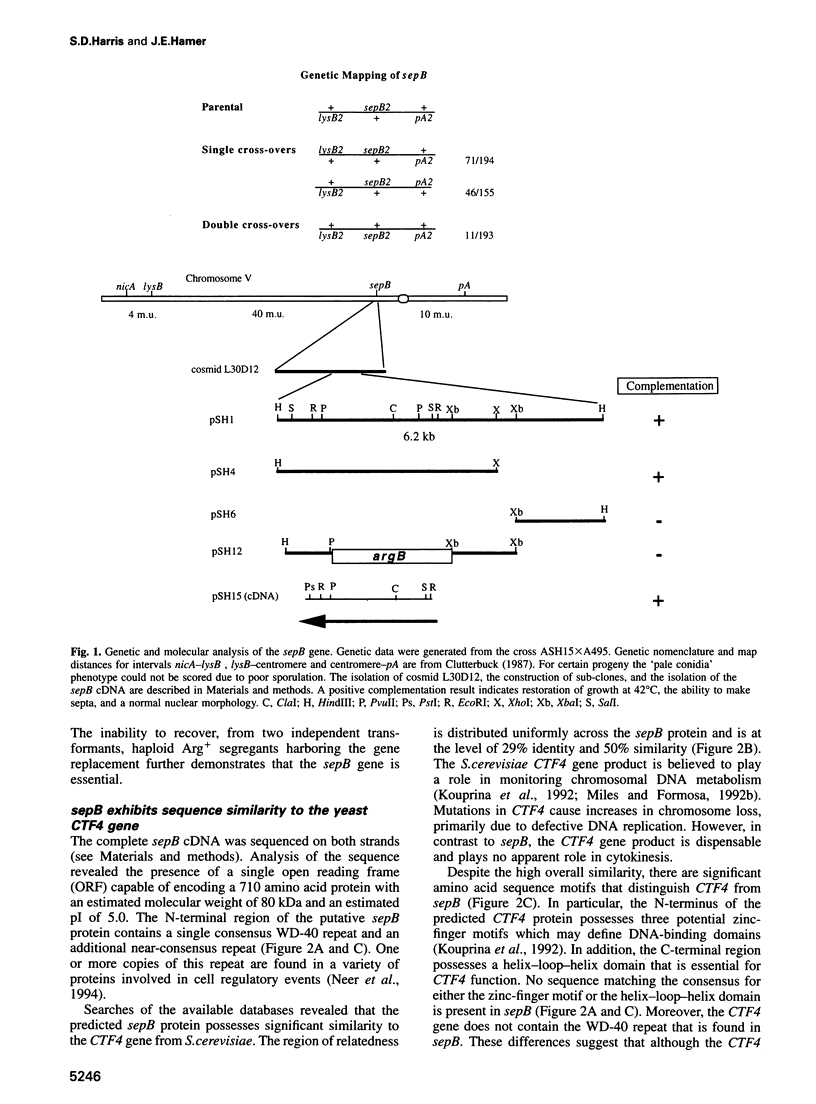


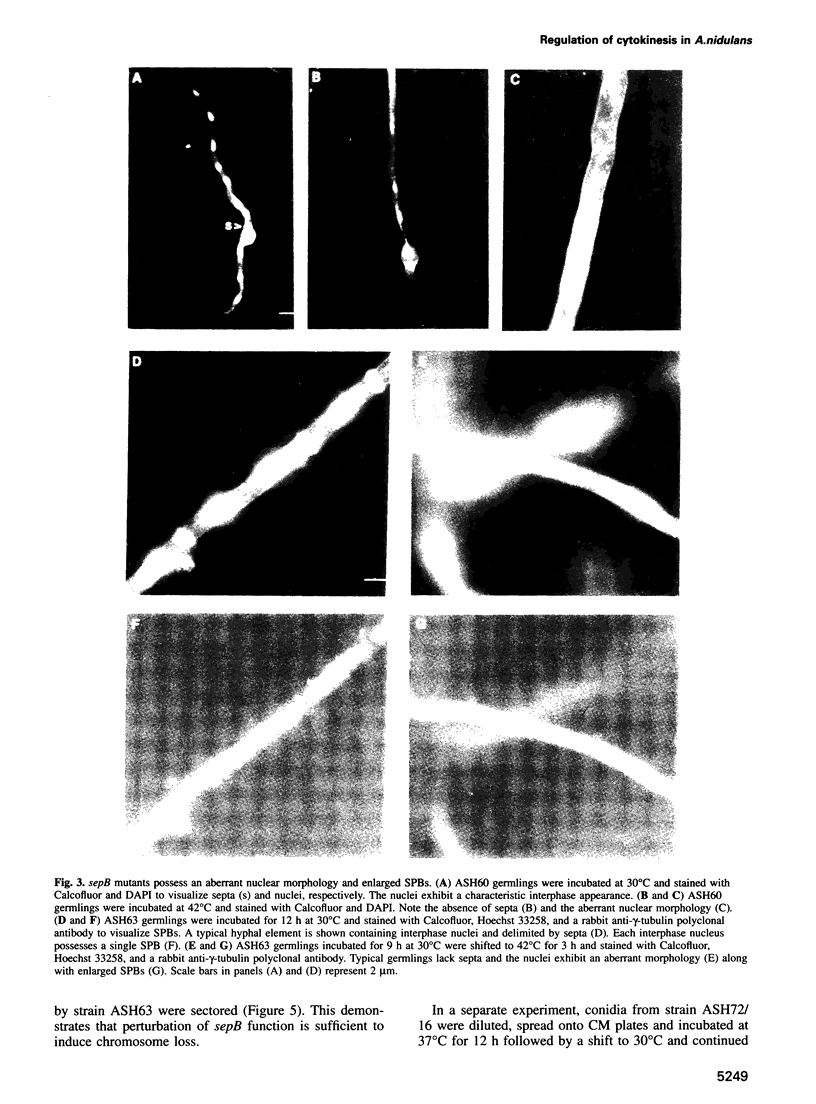
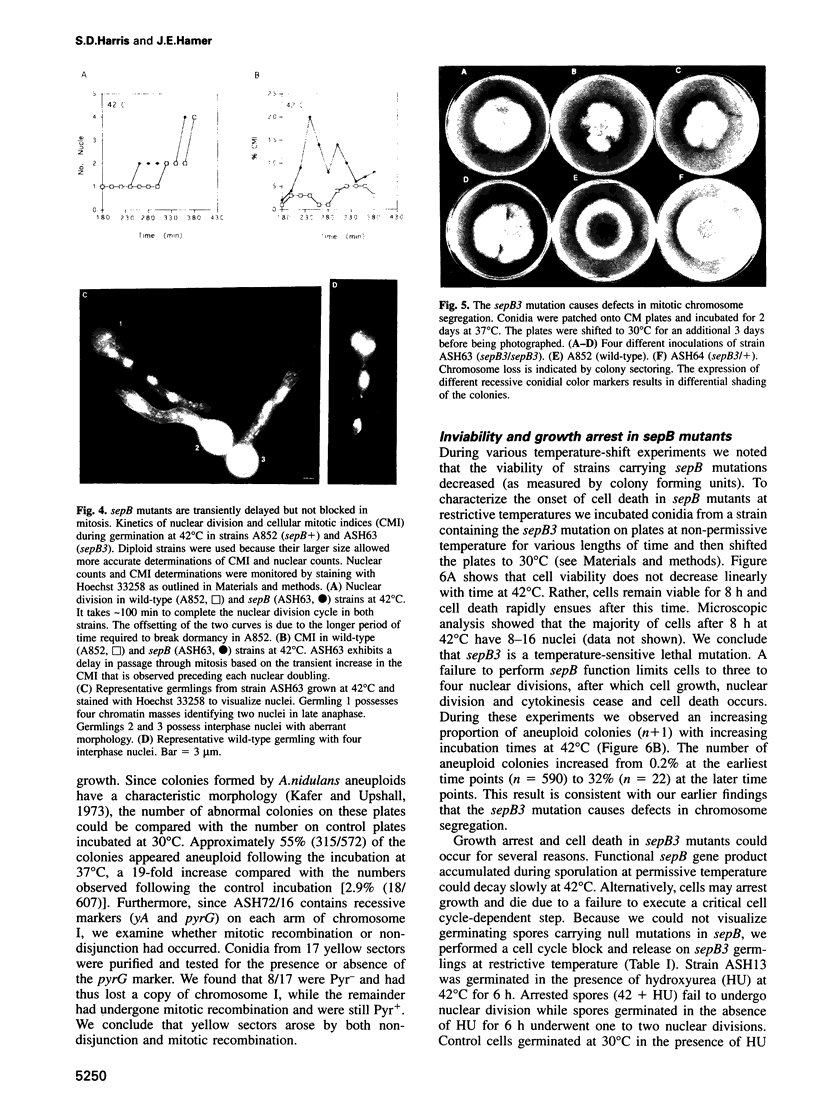




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Brody H., Griffith J., Cuticchia A. J., Arnold J., Timberlake W. E. Chromosome-specific recombinant DNA libraries from the fungus Aspergillus nidulans. Nucleic Acids Res. 1991 Jun 11;19(11):3105–3109. doi: 10.1093/nar/19.11.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutterbuck A. J. Cell volume per nucleus in haploid and diploid strains of Aspergillus nidulans. J Gen Microbiol. 1969 Feb;55(2):291–299. doi: 10.1099/00221287-55-2-291. [DOI] [PubMed] [Google Scholar]
- Cooke C. A., Heck M. M., Earnshaw W. C. The inner centromere protein (INCENP) antigens: movement from inner centromere to midbody during mitosis. J Cell Biol. 1987 Nov;105(5):2053–2067. doi: 10.1083/jcb.105.5.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lozanne A., Spudich J. A. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987 May 29;236(4805):1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- Denison S. H., May G. S. Mitotic catastrophe is the mechanism of lethality for mutations that confer mutagen sensitivity in Aspergillus nidulans. Mutat Res. 1994 Jan 16;304(2):193–202. doi: 10.1016/0027-5107(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobinson K. F., Harris R. E., Hamer J. E. Grasshopper, a long terminal repeat (LTR) retroelement in the phytopathogenic fungus Magnaporthe grisea. Mol Plant Microbe Interact. 1993 Jan-Feb;6(1):114–126. doi: 10.1094/mpmi-6-114. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Cooke C. A. Analysis of the distribution of the INCENPs throughout mitosis reveals the existence of a pathway of structural changes in the chromosomes during metaphase and early events in cleavage furrow formation. J Cell Sci. 1991 Apr;98(Pt 4):443–461. doi: 10.1242/jcs.98.4.443. [DOI] [PubMed] [Google Scholar]
- Enos A. P., Morris N. R. Mutation of a gene that encodes a kinesin-like protein blocks nuclear division in A. nidulans. Cell. 1990 Mar 23;60(6):1019–1027. doi: 10.1016/0092-8674(90)90350-n. [DOI] [PubMed] [Google Scholar]
- Fankhauser C., Marks J., Reymond A., Simanis V. The S. pombe cdc16 gene is required both for maintenance of p34cdc2 kinase activity and regulation of septum formation: a link between mitosis and cytokinesis? EMBO J. 1993 Jul;12(7):2697–2704. doi: 10.1002/j.1460-2075.1993.tb05931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C., Simanis V. Cold fission: splitting the pombe cell at room temperature. Trends Cell Biol. 1994 Mar;4(3):96–101. doi: 10.1016/0962-8924(94)90182-1. [DOI] [PubMed] [Google Scholar]
- Fankhauser C., Simanis V. The Schizosaccharomyces pombe cdc14 gene is required for septum formation and can also inhibit nuclear division. Mol Biol Cell. 1993 May;4(5):531–539. doi: 10.1091/mbc.4.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C., Simanis V. The cdc7 protein kinase is a dosage dependent regulator of septum formation in fission yeast. EMBO J. 1994 Jul 1;13(13):3011–3019. doi: 10.1002/j.1460-2075.1994.tb06600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluck R. A., Miller A. L., Jaffe L. F. Slow calcium waves accompany cytokinesis in medaka fish eggs. J Cell Biol. 1991 Dec;115(5):1259–1265. doi: 10.1083/jcb.115.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S. D., Morrell J. L., Hamer J. E. Identification and characterization of Aspergillus nidulans mutants defective in cytokinesis. Genetics. 1994 Feb;136(2):517–532. doi: 10.1093/genetics/136.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp Cell Res. 1971 Dec;69(2):265–276. doi: 10.1016/0014-4827(71)90223-0. [DOI] [PubMed] [Google Scholar]
- Hastie A. C. Benlate-induced instability of Aspergillus diploids. Nature. 1970 May 23;226(5247):771–771. doi: 10.1038/226771a0. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hirano T., Funahashi S., Uemura T., Yanagida M. Isolation and characterization of Schizosaccharomyces pombe cutmutants that block nuclear division but not cytokinesis. EMBO J. 1986 Nov;5(11):2973–2979. doi: 10.1002/j.1460-2075.1986.tb04594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karess R. E., Chang X. J., Edwards K. A., Kulkarni S., Aguilera I., Kiehart D. P. The regulatory light chain of nonmuscle myosin is encoded by spaghetti-squash, a gene required for cytokinesis in Drosophila. Cell. 1991 Jun 28;65(7):1177–1189. doi: 10.1016/0092-8674(91)90013-o. [DOI] [PubMed] [Google Scholar]
- Kouprina N., Kroll E., Bannikov V., Bliskovsky V., Gizatullin R., Kirillov A., Shestopalov B., Zakharyev V., Hieter P., Spencer F. CTF4 (CHL15) mutants exhibit defective DNA metabolism in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1992 Dec;12(12):5736–5747. doi: 10.1128/mcb.12.12.5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käfer E. Meiotic and mitotic recombination in Aspergillus and its chromosomal aberrations. Adv Genet. 1977;19:33–131. doi: 10.1016/s0065-2660(08)60245-x. [DOI] [PubMed] [Google Scholar]
- Käfer E., Upshall A. The phenotypes of the eight disomics and trisomics of aspergillus nidulans. J Hered. 1973 Jan-Feb;64(1):35–38. doi: 10.1093/oxfordjournals.jhered.a108334. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Andreassen P. R. The telophase disc: its possible role in mammalian cell cleavage. Bioessays. 1993 Mar;15(3):201–207. doi: 10.1002/bies.950150310. [DOI] [PubMed] [Google Scholar]
- May G. S., McGoldrick C. A., Holt C. L., Denison S. H. The bimB3 mutation of Aspergillus nidulans uncouples DNA replication from the completion of mitosis. J Biol Chem. 1992 Aug 5;267(22):15737–15743. [PubMed] [Google Scholar]
- Miles J., Formosa T. Evidence that POB1, a Saccharomyces cerevisiae protein that binds to DNA polymerase alpha, acts in DNA metabolism in vivo. Mol Cell Biol. 1992 Dec;12(12):5724–5735. doi: 10.1128/mcb.12.12.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles J., Formosa T. Protein affinity chromatography with purified yeast DNA polymerase alpha detects proteins that bind to DNA polymerase. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1276–1280. doi: 10.1073/pnas.89.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minet M., Nurse P., Thuriaux P., Mitchison J. M. Uncontrolled septation in a cell division cycle mutant of the fission yeast Schizosaccharomyces pombe. J Bacteriol. 1979 Jan;137(1):440–446. doi: 10.1128/jb.137.1.440-446.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris N. R., Enos A. P. Mitotic gold in a mold: Aspergillus genetics and the biology of mitosis. Trends Genet. 1992 Jan;8(1):32–37. doi: 10.1016/0168-9525(92)90022-v. [DOI] [PubMed] [Google Scholar]
- Morris N. R. Mitotic mutants of Aspergillus nidulans. Genet Res. 1975 Dec;26(3):237–254. doi: 10.1017/s0016672300016049. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Schmidt C. J., Nambudripad R., Smith T. F. The ancient regulatory-protein family of WD-repeat proteins. Nature. 1994 Sep 22;371(6495):297–300. doi: 10.1038/371297a0. [DOI] [PubMed] [Google Scholar]
- Neufeld T. P., Rubin G. M. The Drosophila peanut gene is required for cytokinesis and encodes a protein similar to yeast putative bud neck filament proteins. Cell. 1994 May 6;77(3):371–379. doi: 10.1016/0092-8674(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Nurse P., Thuriaux P., Nasmyth K. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1976 Jul 23;146(2):167–178. doi: 10.1007/BF00268085. [DOI] [PubMed] [Google Scholar]
- O'Connell M. J., Osmani A. H., Morris N. R., Osmani S. A. An extra copy of nimEcyclinB elevates pre-MPF levels and partially suppresses mutation of nimTcdc25 in Aspergillus nidulans. EMBO J. 1992 Jun;11(6):2139–2149. doi: 10.1002/j.1460-2075.1992.tb05273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmani S. A., Engle D. B., Doonan J. H., Morris N. R. Spindle formation and chromatin condensation in cells blocked at interphase by mutation of a negative cell cycle control gene. Cell. 1988 Jan 29;52(2):241–251. doi: 10.1016/0092-8674(88)90513-2. [DOI] [PubMed] [Google Scholar]
- Rappaport R. Establishment of the mechanism of cytokinesis in animal cells. Int Rev Cytol. 1986;105:245–281. doi: 10.1016/s0074-7696(08)61065-7. [DOI] [PubMed] [Google Scholar]
- Satterwhite L. L., Lohka M. J., Wilson K. L., Scherson T. Y., Cisek L. J., Corden J. L., Pollard T. D. Phosphorylation of myosin-II regulatory light chain by cyclin-p34cdc2: a mechanism for the timing of cytokinesis. J Cell Biol. 1992 Aug;118(3):595–605. doi: 10.1083/jcb.118.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timberlake W. E. Developmental gene regulation in Aspergillus nidulans. Dev Biol. 1980 Aug;78(2):497–510. doi: 10.1016/0012-1606(80)90349-8. [DOI] [PubMed] [Google Scholar]
- Timberlake W. E. Temporal and spatial controls of Aspergillus development. Curr Opin Genet Dev. 1991 Oct;1(3):351–357. doi: 10.1016/s0959-437x(05)80299-0. [DOI] [PubMed] [Google Scholar]
- Upshall A., Mortimore I. D. Isolation of aneuploid-generating mutants of Aspergillus nidulans, one of which is defective in interphase of the cell cycle. Genetics. 1984 Sep;108(1):107–121. doi: 10.1093/genetics/108.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakita Y., Yamashiro S., Matsumura F. In vivo phosphorylation of regulatory light chain of myosin II during mitosis of cultured cells. J Cell Biol. 1994 Jan;124(1-2):129–137. doi: 10.1083/jcb.124.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]