Abstract
Hypoxia inducible factor (HIF)-1α is the central transcriptional factor for the regulation of oxygen-associated genes in response to hypoxia. Erythropoietin (EPO), a hematopoietic growth factor, increases oxygen availability during hypoxia/ischemia and is associated with neuroprotection following hypoxia ischemia in laboratory models of stroke. However, EPO has failed to translate in a clinical setting. Thus it is critical to elucidate the key players in EPO-induced neuroprotection. Our preliminary studies have shown that EPO, as a downstream gene of hypoxia inducible factor (HIF), inhibits HIF-1α in a dose-dependent manner in an in-vitro model of hypoxia ischemia. This study is designed to elucidate the primary mediator of EPO-induced HIF-1α inhibition and subsequent cell survival/neuroprotection.
Oxygen and glucose deprivation (OGD) of nerve growth factor (NGF) differentiated rat pheochromocytoma (PC-12) cells were used to model hypoxia ischemia in an in vitro environment. The profile of HIF-1α, HIF-2α and PHD-2 expression, HIF-1α and prolyl hydroxylase (PHD-2) mRNA levels, MMP-9 and cell death was evaluated in the presence and absence of either EPO or PHD-2 inhibitor during OGD. Our findings showed that EPO treatment resulted in an increase in PHD-2 transcription and translation, inhibition of HIF-1α expression, reactive oxygen species (ROS) formation and matrix metalloproteinase (MMP)-9 activity, resulting in increased cell survival after OGD. We also observed that EPO-induced cell survival/neuroprotection was reversed by siRNA silencing of PHD-2. This led to the conclusion that PHD-2 is a key mediator of EPO-induced HIF-1α inhibition and subsequent neuroprotection in an in vitro model of hypoxia ischemia.
Keywords: HIF-1α, PHD-2, EPO, OGD
Introduction
Stroke continues to be among the leading causes of mortality and morbidity; ischemic stroke accounts for approximately 75% of all incidents (ASA, AHA). Despite extensive stroke research, there are still very limited therapeutic options available.
HIF-1α and HIF-2α are innate modulators of oxygen homeostasis in most aerobes (Zagorska & Dulak, 2004; Ratcliffe, 2007). However the specific HIF that is associated with EPO, neurons and neuroprotection is still a topic of frequent debate (Yeo et al., 2008). Cerebral ischemia promotes an accumulation of HIF-1α in neurons. During conditions of hypoxia, HIF-1α initiates the transcription of VEGF (Mu & Chang, 2003), EPO (Prass et al., 2003), glucose transporter (GLU-1), glycolytic enzymes (Jones & Bergeron, 2001a), and several prosurvival genes. However, HIF-1α is also associated in promoting the transcription of proapoptotic genes such as, MMP-9 (DeNiro et al., 2010), Bcl2/adenovirus EIB 19kD-interacting protein 3 (Bruick, 2003), Nix (Sowers et al., 2001), p53 and the caspases (Li et al., 2005a). Under normoxic condition, HIF-1α is continuously synthesized and degraded in less than five minutes through the ubiquitin–proteasome pathway (Huang et al., 1998; Kallio et al., 1999; Salceda & Caro, 1997). HIF-1α first undergoes hydroxylation by PHD-2, which allows it to interact with the von Hippel-Lindau (VHL) tumor suppressor gene (Ivan & Scaiano, 2003a; Jaakkola et al., 2001b; Lee et al., 2003). This interaction allows for the poly ubiquitination and proteasome-dependent degradation of HIF-1α.
PHDs are a family of three hydroxylase (PHD1-3) that are responsible for the hydroxylation of HIF in mammals. Recent studies have shown that PHD-2 activity is critical for HIF-1α degradation under normoxic conditions (Berra, 2003; Appelhoff, 2004). PHD-2 has a strikingly low O2 affinity, which is ideal for oxygen sensing (Epstein et al., 2001d; Hirsila et al., 2003a). The absence of oxygen or reactive oxygen species (ROS) promotes the destabilization of PHD-2 (Cash et al., 2007a; Epstein et al., 2001c; Evans et al., 2005c; Jones & Bergeron, 2001c). HIF degradation pathways require either hydroxylation or acetylation, which is PHD-2 and oxygen dependent (Cash et al., 2007a; Epstein et al., 2001c; Evans et al., 2005c; Jones & Bergeron, 2001c).
HIF-1α target gene, EPO, has been shown to modulate other target genes of HIF-1α such as matrix metalloproteinases (MMPs), vascular endothelial growth factor (VEGF), cyclooxygenase (COX), nitric oxide synthase (NOS) and p53 (Wang et al., 2008). Preclinical studies yielded promising results for the use of EPO in stroke therapy (Sun et al., 2005). However the effects of EPO on HIF-1α or its primary regulator PHD-2 have not been demonstrated. EPO was shown to stabilize mitochondrial membrane potential and decrease ROS in Abeta (25–35)-induced neuronal toxicity in PC12 cells, which are up-regulated anti-apoptotic and down-regulated pro-apoptotic proteins (Li et al., 2005b). Although clinical trials using EPO in stroke therapy failed (Ehrenreich et al., 2009), it is still imperative to identify the key mediators of EPO-induced neuroprotection. This information will allow for a better prediction of the side effects and ultimately improve clinical translation. We hypothesized that EPO-treatment will increase PHD-2 during hypoxia, attenuating ROS and HIF-1α accumulation. We have explored this hypothesis using the OGD model with NGF differentiated PC-12 cells. The protein expression of HIF-1α, HIF-2α, PHD-2 and VHL, as well as ROS formation in the presence and absence of EPO treatment during OGD were assessed. We then investigated if pharmacological inhibition of EPO receptor or siRNA silencing of PHD-2 increases ROS formation and negates EPO-induced neuroprotection during OGD. Neuroprotection was assessed by the percentage of cell death following OGD.
Material
The cell line (Rat pheochromocytoma cells, PC-12 cells) and serum supplements were obtained from ATCC (Manassas, VA.). Human recombinant EPO (Procrit ©) was from Loma Linda University, Hospital Pharmacy (Loma Linda, CA.). EPO receptor protein chimera (EPOR-Fc chimera) was purchased from Abcam (Cambridge, MA). NGF was obtained from Alomone Laboratories Ltd. (Jerusalem, Israel). Rat primary antibodies for HIF-1α and HIF-2α were from ABD Serortec (Raleigh, NC). HIF-PHD-2 antibody, β actin and all secondary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Poly L-lysine coated culture dishes were from BD Biosciences (San Diego, CA). Gelatin gel, TRIZOL reagent, siRNA and primers were from Invitrogen (Carlsbad, CA). All other reagents were obtained from Fisher Scientific (Tustin, CA).
Methods
PC-12 cells (ATCC Manassas, VA) passage 6 through 9 were used. Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM), Sigma Aldrich (St. Louis MO) with 5% Fetal bovine serum (FBS) and 10% horse serum (HS), ATCC (Manassas, VA), 1% Penicillin/streptomycin and 25mM glucose. Cells were incubated in 5% carbon dioxide incubators at 37°C. At 75–80% confluence cells were sub-cultured onto poly-L-lysine coated dishes (BD San Diego CA) and allowed to attach for 24 hours. NGF (Alamones lab Israel) 50ng/mL was then added and cells were allowed to differentiate. Approximately 75% of media was replaced every 2–3 days until 60–70% of the cells were differentiated, as determined by microscopy. Undifferentiated PC-12 cells exposed to NGF for 3 to 5 days exhibits neuronal phenotype such as responding to neurotransmitters (Dickson et al., 1986).
Treatment
Normal media was replaced with glucose-free, supplemented DMEM with either 0 or 10 U/ml EPO. Cells were then placed in a hypoxic chamber with less than 1% oxygen for 2 hours (Agani et al., 2002). The media was then replaced with normal media and cells were allowed to recover for 18 hours. Cells were then collected for cell assessment using cell death assay, western blotting, ROS assay, gelatin zymography or reverse transcriptase polymerase chain reaction (RT-PCR).
Assessment of Reactive Oxygen Species Formation
ROS formation was assessed immediately after OGD with OxiSelect™ ROS Assay Kit (Cell Biolabs Inc. San Diego CA). The assay used 2, 7-Dichlorodihydrofluorescin diacetate (DCFH-DA) and was performed according to manufacturer’s protocol. Briefly cells were washed with Dulbecco’s phosphate buffer saline (DPBS) and incubated 1mM of DCFH-DA in serum free media for 1 hour. Media was discarded and cells washed with DPBS to remove unabsorbed DCFH-DA. DCF was then evaluated by spectrophotometry at wavelength 480 nm excitation/530 nm emission wavelength in a 96 well plate reader (BioRad, Hercules CA). The levels of DCF in cells corresponded to the quantity of ROS formation.
Protein Extraction –and western blotting
Cells were collected by centrifugation and lysed using radio-immuno-precipitation assay (RIPA) lysis buffer [20 mM Tris, pH 7.5, 150 mMNaCl, 1%NP40, 0.5%Na deoxycholate, 1 mMEDTA, and 0.1% sodiumdodecyl sulfate (SDS)] supplemented with mammalian protease inhibitor cocktail, phenylmethanesulfonylfluoride (PMSF), (Sigma-Aldrich, St. Louis, MO) and sodium orthovanadate (Santa Cruz, Hercules CA). Prior to lysis, cells were rinsed with cold PBS. Cell lysis was enhanced by three rapid freeze thaw cycles using liquid nitrogen. Lysed cells were centrifugated at 4°C for 10 min and the supernatant collected. The protein concentration was assessed using the Bradford assay (BioRad, Hercules, CA). SDS-PAGE electrophoresis was performed as previously described (Dominguez et al. 2008).
Transfection
Prior to transfection, differentiated cells at a density of 2 ×105 cells in poly D-lysine coated 6 well plates (BD biosciences) were treated with Opti-MEM® reduced serum media. Plus reagent and siRNA were allowed to incubate for 15 minutes in a 1:1 ratio. Lipofectamine ™ LTX reagent was then added and mixture incubated for 25 minutes at room temperature. Reduced serum media was removed from cells and replaced with media containing 12.5pM concentration of siRNA complexed with Lipofectamine ™ Plates were incubated for 24 hours and transfection efficiency assessed. The transfected cells were then used for experiments.
Reverse Transcriptase Polymerase Chain Reaction
RNA was extracted using TRIZOL reagent (Invitrogen). DNA was synthesized using SuperScript® First-Strand Synthesis System for RT-PCR (Invitrogen). PCR for HIF-1α, PHD-2, VHL and internal standard GAPDH was performed using the following primers (forward 5′GGCTTTGTTATGTGCTAAC3′ and reverse 5′ACTTGATGTTCATCGTCCTC3′ for HIF-1α), (forward 5′TAAACGGCCGAACGAAAGC3′ and reverse 5′GGGTTATCAACGTGACGGACA3′ for PHD-2), (forward 5′ACAGGATGTGCAAGGACAAGTG3′ and reverse 5′TAGTCTGCAGCATTCCGTGAGT3′ for VHL) and (forward 5′ACCACAGTCCATGCCATCAC3′ and reverse 5′TCCACCACCCTGTTGCTGTA3′ for GAPDH). Samples were cycled using a 2720 thermal cycler (Applied Biosciences). The samples were denatured at 95°C for 5 minutes followed by 35 cycles of amplification for HIF-1α, PHD-2 and VHL and 25 cycles for GAPDH. The amplification cycle consisted of 1 minute denaturation at 94°C, 1 minute annealing at 55°C, 1.5 minute extension at 72°C and a final extension of 5 minutes at 72°C. Samples were electrophoresed on 2% agarose gel with 0.5ug/mL ethidium bromide. Gels were visualized under UV light and photographed, and the optical densities of the bands were analyzed with Quantity One software (BioRad) (Hull et al., 1999)
Cell Death Assessment
Cell death was also evaluated by trypan exclusion as previously described (Souvenir et al. 2011). The formula used to calculate the percentage of cell death was as follows: (Trypan blue positive cells)]/total cell counted) x 100. The average of all six counts was used.
Gelatin Zymography
Briefly, 60 μg samples of protein extract was prepared and separated by electrophoresis in 10% Tris-glycine gel with 0.1% gelatin as substrate (Bio-Rad, Hercules, CA). The gel was re-natured for 1 hour and then incubated with development buffer at 37°C for 48 hours. After development, the gel was stained with 0.5% coomassie brilliant blue R-250 for 1 hour and then distained for 18 to 24 hours. MMP activity was visualized and photographed with a Versa Doc imager (BioRad, Hercules, CA) and the optical densities were calculated with Image J (version 1.0) software (NIH).
Statistical Analysis
All data are presented as mean ± standard deviation. All data were analyzed using one way Analysis of Variance (ANOVA) followed by Tukey test. Data sets that failed the normality test were analyzed using Kruskal-Wallis ANOVA on Ranks. Sigma Stat software version 3.5 (DUNDAS software LTD. Germany) was used for all statistical evaluations.
Results
Determining if both HIF-1α and HIF-2α are inhibited by EPO treatment
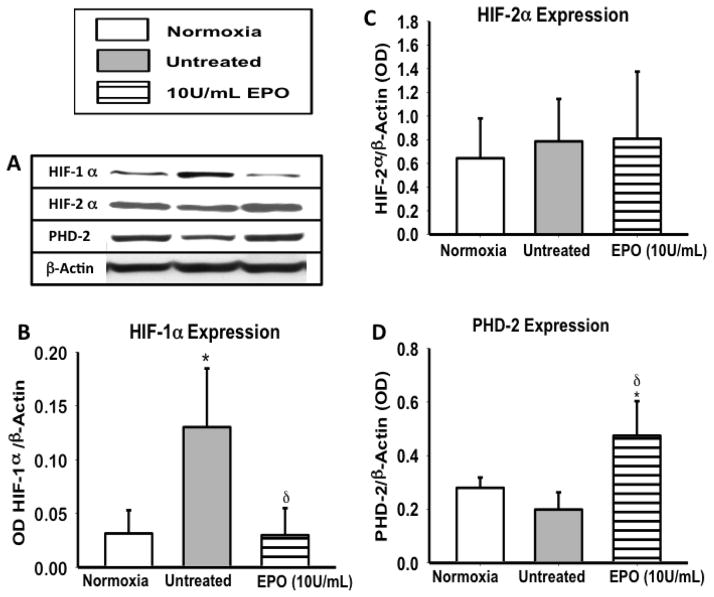
To determine if the reduction in HIF-1α expression, results in the upregulation of HIF-2α after EPO treatment (10U/mL), we evaluated the expression of both HIF-1α and HIF-2α following OGD. HIF-1α expression was significantly lower (p<0.5) in EPO treated groups, when compared to untreated (figure 1A&B). However HIF-2α expression remained unchanged in EPO treated groups (figure 1C), suggesting that HIF-1α not HIF-2α plays a key role in hypoxia during OGD. A significant increase (p< 0.01) in PHD-2 expression was also observed in the EPO treated group, when compared to normoxic and untreated groups. PHD-2 was the only PHD to play a critical role in HIF degradation therefore PHD-2 was the main focus of this study.
Figure 1. The profile of HIF-1α, HIF-2α, and PHD-2 following 2 hours of OGD.
(A) A representative image of a western blot of HIF-1α, HIF-2α, PHD-2 and the β-actin as the loading control (B) Densiometric quantification of HIF-1α expression. (C) HIF-2α expression following EPO treatment. (D) PHD-2 expression following OGD. Data represent mean (± SD) n=6 in all groups. *P<0.01 compared to normoxia. δP< 0.01 compared to untreated. #P< 0.01 compared to 10U/ml EPO treated group. One way ANOVA was used for all analysis. HIF-1α, P ≤ 0.001 using Kruskal-Wallis ANOVA on Ranks. PHD-2 P < 0.001. HIF-1α but not HIF-2α expression was altered in the presence of EPO treatment.
The effect of EPO and PHD-2 inhibition on ROS formation and HIF-1α and PHD-2 expression
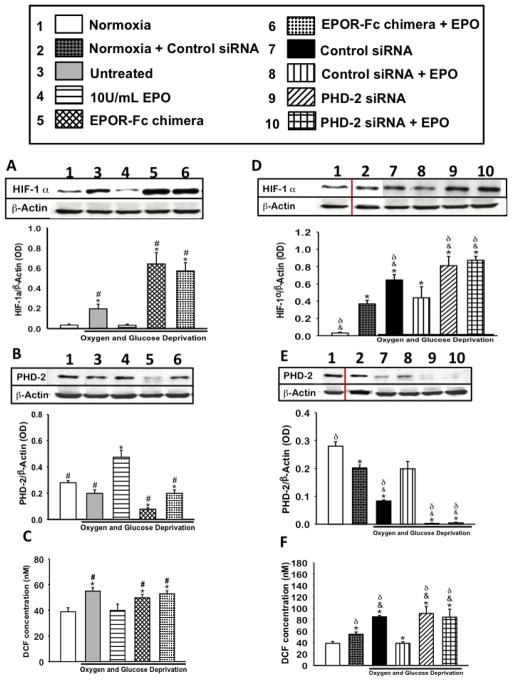
The primary mediators of HIF-α expression are ROS and hypoxia. Therefore, we evaluated the effects of EPO treatment on ROS formation by adding a treated group with PHD-2 siRNA or an EPOR-Fc chimera (1ng/mL). The siRNA or EPOR-Fc would competitively bind to the EPO receptor, diminishing the effects of the treatment. The optimal dose of EPOR-Fc chimera was determined experimentally; in its ability to inhibit JAK-2 phosphorylation in EPO treated differentiated PC-12 cells (data not shown). The formation of ROS was increased in all groups following OGD, except groups treated with 10U/mL EPO and control siRNA + EPO groups (figure 2C&F). In accordance to an increase in ROS formation, HIF-1α expression was also shown to be upregulted. HIF-1α expression was increased in all of groups exhibiting high ROS (p<0.01), yet decreased in EPO treated groups (figure 2A&D). Conversely, PHD-2 expression was inversely correlated with ROS formation and HIF-1α expression. PHD-2 expression was notably increased in the 10U/mL EPO treated groups (p<0.01) compared to normoxia, untreated, EPOR-Fc chimera and chimera with EPO (figure 2B). This data suggests that EPO directly or indirectly alters ROS formation, HIF-1α and PHD-2 expression in differentiated PC-12 cells. In the presence of control siRNA and OGD, EPO treatment restored PHD-2 protein levels to 2/3 of the basal levels observed in normoxic cells (figure 2E). All transfected groups, with or without EPO treatment, expressed high levels of ROS, when compared to normal groups. This data is not unusual, as transfected cells were exposed to low serum for approximately 24 hours. Both low serum and exposure to lipofectamine reagent negatively alters the mitochondrial dynamic, thereby promoting ROS formation. Cell death percentage was also higher in normoxia groups that had been transfected (figure 4E), yet had not gone through the OGD. ROS Assay has also seen an increase in ROS production in transfected normoxic groups (figure 2C). Although the control siRNA with EPO treatment group showed an increase in PHD-2 expression and decrease in HIF-1α, PHD-2 siRNA with EPO treatment group increased in HIF-1α and showed no increase in PHD-2 expression (Figure 2D,E, F). This suggests that EPO’s target protein is inhibited, decreasing the treatments neuroprotective effects.
Figure 2. The effect of EPO treatment on ROS formation, HIF-1α and PHD-2 expression in the presence of EPO and PHD-2 inhibition.
(A) HIF-1α protein expression in the presence and absence of EPOR chimera a competitive inhibitor of EPO. (B) Densiometric quantification of PHD-2 expression in the presence or absence of EPOR chimera (C) ROS formation during OGD in the presence or absence of EPO inhibitor. (D) HIF-1α expression after OGD in the presence of PHD-2 siRNA. (E) PHD-2 expression after OGD in the presence of siRNAs. (F) ROS formation during OGD in the presence or absence of PHD-2 siRNA. Data represent the mean and (± SD) n=6 in all groups. *P<0.01 compared to normoxia. #P< 0.01 compared to 10U/ml EPO treated group. &P<0.05 compared to Normoxia+Control siRNA. δP< 0.01 compared to PHD-2siRNA +EPO. One-way ANOVA was used for all analysis. pH P=0.015 using Kruskal-Wallis ANOVA on Ranks. HIF-1α P ≤ 0.001, using Kruskal-Wallis ANOVA on Ranks. PHD-2 P < 0.001. Inhibition of EPO or PHD-2 enhanced HIF-1α expression and ROS formation.
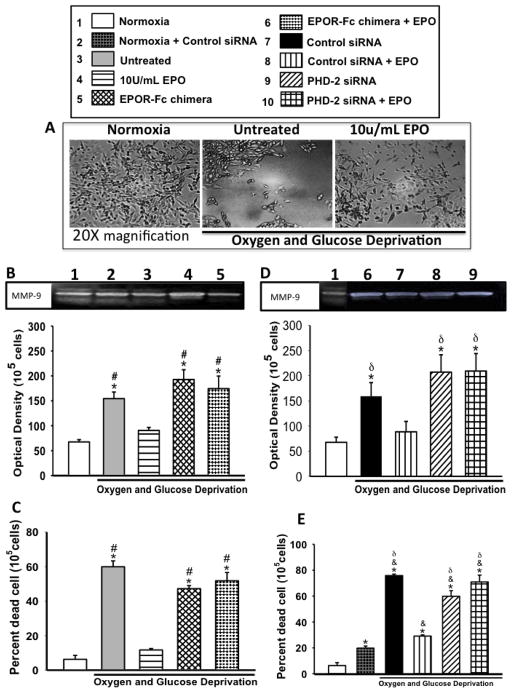
Figure 4. The effects of EPO treatment on MMP-9 activity and subsequent cell death after OGD.
(A) Shows representative image of NGF-differentiated cells with and without EPO and OGD. (B &D) Densiometric quantification of MMP-9 activity, (B) in the presence of EPOR chimera, (D) in the presence of PHD-2 siRNA. (C &E) Shows percent cell death as assess by trypan blue exclusion (C) in the presence of EPOR chimera, (E) in the presence of PHD-2 siRNA. Data represent the mean and (± SD) n=6 in all groups. *P<0.01 compared to 10U/ml EPO treated group. #P<0.01 compared to untreated. &P<0.05 compared to Normoxia+Control siRNA. δP<0.01 compared to untreated. MMP-9 P= 0.001, Cell death, P ≤ 0.001, using Kruskal-Wallis ANOVA on Ranks. Inhibition of PHD-2 significantly increased MMP-9 activity and cell death with and without EPO.
Determining the effects of EPO treatment on the mRNA levels of HIF-1α, PHD-2 and VHL
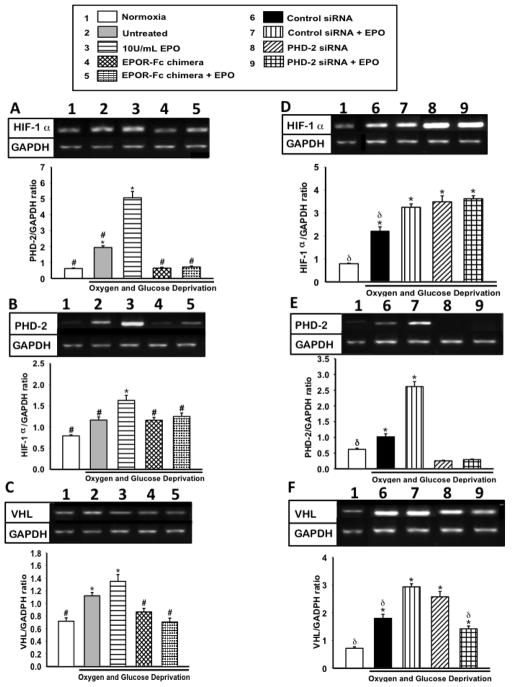
Changes in protein expression are not always indicative of transcriptional changes. Thus RT-PCR was performed to determine if the changes observed in HIF-1α and PHD-2 expression with EPO treatment and inhibitor were at the transcription or translational level. Silencing of PHD-2, via siRNA, triggered a significant increase (p<0.01) in HIF-1α transcription when compared to 10U/mL EPO treated group. On the contrary, competitive inhibition of EPO did not affect HIF-1α transcription levels (figure 3 A&D). This suggests that EPO inhibition of HIF-1α is only on the protein level alluding to possibly conformation change or indirect inhibition. Alternately, either inhibition of EPOR or silencing of PHD-2 significantly lowered mRNA levels of PHD-2 (figure 3B&E). Downstream effecter of HIF-1α degradation, VHL, was significantly increased in EPO treated group compared to normoxia and EPOR chimera with and without EPO. Additionally VHL mRNA was significantly higher in siRNA +EPO group compared to SiRNA control, normoxia and PHD-2 siRNA+ EPO (figure 3C&F). Although VHL is significantly changed, this finding is inconclusive in the absence of detectable protein levels of VHL in the cells. This observation alludes to PHD-2 as one of the primary effecter of HIF-1α inhibition and subsequent neuroprotection in PC-12 cells.
Figure 3. The effect of EPO treatment on mRNA levels of HIF-1α, PHD-2 and VHL in the presence of EPO and PHD-2 inhibition.
(A) HIF-1α mRNA expression after OGD in the presence of EPOR chimera. (B) PHD-2 mRNA expression after OGD in the presence of EPOR chimera. (C) Densiometric quantification of VHL in presence of EPO inhibitor following OGD. (D) HIF-1α mRNA expression after OGD during EPO inhibition. (E) PHD-2 mRNA expression in the presence of PHD-2 siRNA following OGD. (F) VHL mRNA expression in presence PHD-2 siRNA following OGD. Data represent the mean and (± SD) n=6 in all groups. *P<0.01 compared to normoxia. #P< 0.01 compared to 10U/ml EPO treated group. &P<0.05 compared to Normoxia+Control siRNA. δP< 0.01 compared to PHD-2 siRNA+EPO.. HIF-1α, P=< 0.001 P ≤ 0.001, using Kruskal-Wallis ANOVA on Ranks. PHD-2, P ≤ 0.001, using Kruskal-Wallis ANOVA on Ranks. VHL, P ≤ 0.001, using Kruskal-Wallis ANOVA on Ranks Inhibition of PHD-2 enhanced HIF-1α transcription in the presence and absence of EPO.
Inhibition of EPO or PHD-2 alters MMP-9 activity and reversed EPO’s neuroprotection
MMP-9 downstream gene of HIF-1α is associated with exacerbated injury and cell death in the acute stages of hypoxia ischemia. Thus, MMP-9 activity and Cell Death were assessed to determine if EPO-induced HIF-1α inhibition affects the downstream gene expression and subsequent cell death. As expected, EPO treatment was associated with lower MMP-9 activity. Inhibition of PHD-2 rapidly reversed EPO-induced MMP-9 inhibition; resulting in significantly higher level of MMP-9 activity in PHD-2 siRNA + EPO group compared to 10U/mL EPO and control siRNA + EPO (figure 4B-E). Yet, EPOR-Fc chimera did not appear to negatively affect MMP-9 activity in the presence of EPO. Cell death was also found to be correlated with an increase in MMP-9 activity. Inhibition of EPO or PHD-2 with or without EPO visibly reversed EPO-induced neuroprotection compared to 10U/mL (p<0.01) EPO (figure 4C&E).
Discussion
Our findings showed that EPO-induced inhibition of HIF-1α was associated with elevated transcription and translation of PHD-2. Inhibition of EPO had prompted an increase in HIF-1α protein expression, yet mRNA levels were not affected. EPO treatment enhanced PHD-2 mRNA production and protein expression, reducing ROS formation, MMP-9 activity and cell death.
HIF-1 and HIF-2α has 40% homology. HIF-2α has been shown to bind to the beta subunit of HIF1α during hypoxia (Krieg et al., 2000). An accumulation of HIF-1α or HIF-2α during hypoxia is tissue dependant. The specific HIF in neuronal tissue is uncertain and further investigation was needed (Yeo et al., 2008). However, this study evaluates both HIF-1α and HIF-2α revealing that HIF-2α protein expression was relatively unchanged during OGD with or without EPO treatment in differentiated PC-12 cells. This indicated that HIF-1α not HIF-2α is primarily associated with hypoxia in differentiated PC-12 cells. HIF-1α was increased in cells undergoing OGD,, but notably was decreased with EPO treatment. In the past, the attenuation of HIF-1α protein expression by the administration of exogenous EPO was not previously described, thus warranted further investigation. EPO is a downstream gene of HIF-1α (Semenza, 1998). It was speculated that the apparent EPO-induced inhibition of HIF-1α was the result of a negative feedback inhibition. However, the investigation of the primary regulators of HIF-1α degradation, PHD-2 and ROS, alluded to a much more complex mechanism.
The absence of oxygen and the presence of ROS promoted the destabilization of PHD-2 (Cash et al., 2007a; Epstein et al., 2001c; Evans et al., 2005c; Jones & Bergeron, 2001c). HIF degradation pathways require either hydroxylation or acetylation, which is PHD-2 and oxygen dependent. This interaction allows for the poly ubiquitination and proteasome-dependent degradation of HIF-1α.
There are three isoforms of HIF-PHDs (PHD1-3) hydroxylase involved in HIF hydroxylation in mammals. Previously it was thought that all three PHDs were necessary for HIF ubiquitination and subsequent degradation. However, knockdown studies showed that only PHD-2 is critical for HIF-1α degradation under normoxic conditions (Berra, 2003; Appelhoff, 2004) and so PHD-2 was manipulated and evaluated in this study. PHDs are extremely sensitive to low O2 concentration, making them ideal for oxygen sensing (Epstein et al., 2001b; Hirsila et al., 2003b). They are dioxygenases that catalyze oxygen-dependent hydroxylation of HIF-1α prolyl residues and promotes the degradation of both HIF-1α and HIF-2α (Chang et al., 2005). As expected, an inverse correlation between HIF-1α and PHD-2 protein expression after hypoxia (figure 1 and figure 2) and an increase in ROS formation that up regulated with HIF-1α expression. Our data comes into agreement with past publication, which demonstrated that reduced oxygen or increased ROS allows for the stabilization and accumulation of HIF-1α (Cash et al., 2007b; Evans et al., 2005b; Kamura et al., 1999b; Lisztwan et al., 1999b; Stebbins et al., 1999b). Also, others findings have shown that low levels of oxygen or high levels of ROS promote the destabilization of PHD-2 (Cash et al., 2007c; Epstein et al., 2001a; Evans et al., 2005a; Jones & Bergeron, 2001b).
Studies have shown that prolyl hydroxylation and acetylation control cellular HIF-1α levels by governing the physical interaction between HIF-1α and VHL. VHL binds with elongin B, elongin C, Rbx1 and Cul2 to form a E3 ubiquitin ligase. E3 ubiquitin ligase is unable to bind to HIF-1α during hypoxia because HIF-1α lacks hydroxylation or acetylation (Ivan & Scaiano, 2003b; Jaakkola et al., 2001a). Thus, it was expected that EPO-induced inhibition of HIF-1α would increase the expression of regulatory proteins, PHD and pVHL, and would not affect transcriptional levels. Yet, due to a low protein expression of pVHL, expression could not be quantified (data not shown). As expected, HIF-1α protein levels were significantly reduced by EPO treatment and did not affect mRNA levels. This suggests that EPO-induced inhibition of HIF-α has no direct effect on HIF-1α transcription, meaning that changes in HIF-1α protein expression were possibly mediated by either physical interaction of EPO with HIF-1α or through transcriptional regulation of a HIF-1α regulator (figure-2). The three-fold increase in HIF-1a observed in the presence of EPOR chimera was unexpected, yet not surprising. Since EPO is a downstream gene of HIF that is endogenously synthesized during hypoxia, it is plausible that the circulating EPOR were acting as a positive feedback loop for HIF-1α protein, possibly signaling that EPO levels are low. It was no surprise that EPO treatment propagated an increase in both transcriptional and translational levels of PHD-2. This alluded to the modulation of PHD-2 levels as one of the primary mechanism by which exogenous EPO inhibits HIF-1α, and confers neuroprotection in NGF differentiated PC-12 cells during oxygen and glucose deprivation. Conversely, Ratan and colleagues showed that inhibition of PHD and activation HIF-1α was associated with neuroprotection in vivo and in vitro (Ratan et al., 2008; Lee et al., 2009; Siddiq et al., 2009). The apparent contradiction in these reports attests to the biphasic nature of HIF-1α, which could be both beneficial as well as detrimental (Calvert et al., 2006; Halterman & Federoff, 1999; Leker et al., 2004). Baranova and colleagues who described an oscillation of HIF-1α levels following hypoxia ischemia best explain the dichotomy of HIF-1α neuroprotection. Additionally it was proposed that transient increases in HIF-1α within the first 24 hours after an injury was associated with elevation of proapoptotic genes however, downstream prosurvival genes of HIF-1α were later (>24hrs) unregulated with sustained elevation of HIF-1α (Baranova et al., 2007). Thus HIF-1α is detrimental in the acute stages of injury but beneficial in the delayed/recovery stage of injury.
Given the direct or indirect interaction of EPO with HIF-1α are biphasic and its primary regulator PHD-2, caution should be exercised when using EPO in stroke therapy. The time/duration of EPO exposure/treatment is the key to harness the beneficial neuroprotective effects of EPO, without triggering the detrimental effect of delayed/prolonged inhibition of HIF-1α. Silencing of PHD-2 prompted a notable increase in both protein and mRNA levels of HIF-1α, MMP-9 activity and the resultant cell death even in the presence of EPO treatment. These observations lends to the conclusion that PHD-2 is one of the key mediator of EPO-induced neuroprotection in NGF differentiated PC-12 cells.
Acknowledgments
This study was funded by NIH NS060936 to Jiping Tang
Abbreviations
- EPO
Erythropoietin
- OGD
Oxygen and glucose deprivation
- NGF
nerve growth factor
- HIF
hypoxia inducible factor
- PHD-2
prolyl hydroxylase domain 2
- MMP
matrix metalloproteinase
Footnotes
Compliance with Ethics Requirements:
Rhonda Souvenir declares that she has no conflict of interest.
Jerry J. Flores declares that he has no conflict of interest.
Robert P. Ostrowski declares that he has no conflict of interest.
Anatol Manaenko declares that he has no conflict of interest.
Kamil Duris declares that he has no conflict of interest.
Jiping Tang declares that she has no conflict of interest.
Reference List
- Agani FH, Puchowicz M, Chavez JC, Pichiule P, LaManna J. Role of nitric oxide in the regulation of HIF-1alpha expression during hypoxia. Am J Physiol Cell Physiol. 2002;283:C178–C186. doi: 10.1152/ajpcell.00381.2001. [DOI] [PubMed] [Google Scholar]
- Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, et al. Differential Function of the Prolyl Hydroxylases PHD1, PHD2, and PHD3 in the Regulation of Hypoxia-inducible Factor. J Biol Chem. 2004;279:38458–38465. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC. Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci. 2007;27:6320–6332. doi: 10.1523/JNEUROSCI.0449-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssergur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1 in normoxia. EMBO J. 2003;22:4082–4090. doi: 10.1093/emboj/cdg392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruick RK. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev. 2003;17:2614–2623. doi: 10.1101/gad.1145503. [DOI] [PubMed] [Google Scholar]
- Calvert JW, Cahill J, Yamaguchi-Okada M, Zhang JH. Oxygen treatment after experimental hypoxia-ischemia in neonatal rats alters the expression of HIF-1alpha and its downstream target genes. J Appl Physiol. 2006;101:853–865. doi: 10.1152/japplphysiol.00268.2006. [DOI] [PubMed] [Google Scholar]
- Cash TP, Pan Y, Simon MC. Reactive oxygen species and cellular oxygen sensing. Free Radic Biol Med. 2007;43:1219–1225. doi: 10.1016/j.freeradbiomed.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TC, Huang CJ, Tam K, Chen SF, Tan KT, Tsai MS, et al. Stabilization of hypoxia-inducible factor-1{alpha} by prostacyclin under prolonged hypoxia via reducing reactive oxygen species level in endothelial cells. J Biol Chem. 2005;280:36567–36574. doi: 10.1074/jbc.M504280200. [DOI] [PubMed] [Google Scholar]
- Chen W, Jadhav V, Tang J, Zhang JH. HIF-1alpha inhibition ameliorates neonatal brain injury in a rat pup hypoxic-ischemic model. Neurobiol Dis. 2008;31:433–441. doi: 10.1016/j.nbd.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioffi CL, Liu XQ, Kosinski PA, Garay M, Bowen BR. Differential regulation of HIF-1 alpha prolyl-4-hydroxylase genes by hypoxia in human cardiovascular cells. Biochem Biophys Res Commun. 2003;303:947–953. doi: 10.1016/s0006-291x(03)00453-4. [DOI] [PubMed] [Google Scholar]
- DeNiro M, Al-Halafi A, Al-Mohanna FH, Alsmadi O, Al-Mohanna FA. Pleiotropic effects of YC-1 selectively inhibit pathological retinal neovascularization and promote physiological revascularization in a mouse model of oxygen-induced retinopathy. Mol Pharmacol. 2010;77:348–367. doi: 10.1124/mol.109.061366. [DOI] [PubMed] [Google Scholar]
- Dickson G, Prentice H, Julien JP, Ferrari G, Leon A, Walsh FS. Nerve growth factor activates Thy-1 and neurofilament gene transcription in rat PC12 cells. EMBO J. 1986;5:3449–3453. doi: 10.1002/j.1460-2075.1986.tb04668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez E, Rivat C, Pommier B, Mauborgne A, Pohl M. JAK/STAT3 pathway is activated in spinal cord microglia after peripheral nerve injury and contributes to neuropathic pain development in rat. J Neurochem. 2008;107:50–60. doi: 10.1111/j.1471-4159.2008.05566.x. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40:e647–e656. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Evans AM, Mustard KJ, Wyatt CN, Peers C, Dipp M, Kumar P, et al. Does AMP-activated protein kinase couple inhibition of mitochondrial oxidative phosphorylation by hypoxia to calcium signaling in O2-sensing cells? J Biol Chem. 2005;280:41504–41511. doi: 10.1074/jbc.M510040200. [DOI] [PubMed] [Google Scholar]
- Greene T. Current therapy for acute leukemia in childhood. Nurs Clin North Am. 1976;11:3–19. [PubMed] [Google Scholar]
- Halterman MW, Federoff HJ. HIF-1alpha and p53 promote hypoxia-induced delayed neuronal death in models of CNS ischemia. Exp Neurol. 1999;159:65–72. doi: 10.1006/exnr.1999.7160. [DOI] [PubMed] [Google Scholar]
- Hirsila M, Koivunen P, Gunzler V, Kivirikko KI, Myllyharju J. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J Biol Chem. 2003;278:30772–30780. doi: 10.1074/jbc.M304982200. [DOI] [PubMed] [Google Scholar]
- Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull MA, Thomson JL, Hawkey Expression of cyclooxygenase 1 and 2 by human gastric endothelial cells. Gut. 1999;45:529–536. doi: 10.1136/gut.45.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivan MG, Scaiano JC. A new approach for the detection of carbon-centered radicals in enzymatic processes using prefluorescent probes. Photochem Photobiol. 2003a;78:416–419. doi: 10.1562/0031-8655(2003)078<0416:anaftd>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Jones NM, Bergeron M. Hypoxic preconditioning induces changes in HIF-1 target genes in neonatal rat brain. J Cereb Blood Flow Metab. 2001;21:1105–1114. doi: 10.1097/00004647-200109000-00008. [DOI] [PubMed] [Google Scholar]
- Jung SN, Yang WK, Kim HS, Kim EJ, Yun H, Park H, et al. Reactive oxygen species stabilize hypoxia-inducible factor-1 alpha protein and stimulate transcriptional activity via AMP-activated protein kinase in DU145 human prostate cancer cells. Carcinogenesis. 2008;29:713–721. doi: 10.1093/carcin/bgn032. [DOI] [PubMed] [Google Scholar]
- Kallio PJ, Wilson WJ, O’Brien S, Makino Y, Poellinger L. Regulation of the hypoxia-inducible transcription factor 1alpha by the ubiquitin-proteasome pathway. J Biol Chem. 1999;274:6519–6525. doi: 10.1074/jbc.274.10.6519. [DOI] [PubMed] [Google Scholar]
- Kamura T, Sakai K, Kaku T, Kobayashi H, Mitsumoto M, Tsuneyoshi M, et al. Comparison of p53 and CD44 variant 6 expression between paired primary and recurrent ovarian cancer: an immunohistochemical analysis. Oncol Rep. 1999;6:97–101. doi: 10.3892/or.6.1.97. [DOI] [PubMed] [Google Scholar]
- Krieg M, Haas R, Brauch H, Acker T, Flamme I, Plate KH. Up-regulation of hypoxia-inducible factors HIF-1alpha and HIF-2alpha under normoxic conditions in renal carcinoma cells by von Hippel-Lindau tumor suppressor gene loss of function. Oncogene. 2000;19:5435–5443. doi: 10.1038/sj.onc.1203938. [DOI] [PubMed] [Google Scholar]
- Lee C, Kim SJ, Jeong DG, Lee SM, Ryu SE. Structure of human FIH-1 reveals a unique active site pocket and interaction sites for HIF-1 and von Hippel-Lindau. J Biol Chem. 2003;278:7558–7563. doi: 10.1074/jbc.M210385200. [DOI] [PubMed] [Google Scholar]
- Lee DW, Rajagopalan S, Siddiq A, Gwiazda R, Yang L, Beal MF, et al. Inhibition of prolyl hydroxylase protects against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity: model for the potential involvement of the hypoxia-inducible factor pathway in Parkinson disease. J Biol Chem. 2009;284:29065–29076. doi: 10.1074/jbc.M109.000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Ahn HH, Kim KS, Lee JY, Kim MS, Lee B, et al. Polyethyleneimine-mediated gene delivery into rat pheochromocytoma PC-12 cells. J Tissue Eng Regen Med. 2008;2:288–295. doi: 10.1002/term.94. [DOI] [PubMed] [Google Scholar]
- Leker RR, Aharonowiz M, Greig NH, Ovadia H. The role of p53-induced apoptosis in cerebral ischemia: effects of the p53 inhibitor pifithrin alpha. Exp Neurol. 2004;187:478–486. doi: 10.1016/j.expneurol.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhou C, Calvert JW, Colohan AR, Zhang JH. Multiple effects of hyperbaric oxygen on the expression of HIF-1 alpha and apoptotic genes in a global ischemia-hypotension rat model. Exp Neurol. 2005;191:198–210. doi: 10.1016/j.expneurol.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Lieb ME, Menzies K, Moschella MC, Ni R, Taubman MB. Mammalian EGLN genes have distinct patterns of mRNA expression and regulation. Biochem Cell Biol. 2002;80:421–426. doi: 10.1139/o02-115. [DOI] [PubMed] [Google Scholar]
- Lisztwan J, Imbert G, Wirbelauer C, Gstaiger M, Krek W. The von Hippel-Lindau tumor suppressor protein is a component of an E3 ubiquitin-protein ligase activity. Genes Dev. 1999;13:1822–1833. doi: 10.1101/gad.13.14.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X, Chang C. TR3 orphan nuclear receptor mediates apoptosis through up-regulating E2F1 in human prostate cancer LNCaP cells. J Biol Chem. 2003;278:42840–42845. doi: 10.1074/jbc.M305594200. [DOI] [PubMed] [Google Scholar]
- Prass K, Scharff A, Ruscher K, Lowl D, Muselmann C, Victorov I, et al. Hypoxia-induced stroke tolerance in the mouse is mediated by erythropoietin. Stroke. 2003;34:1981–1986. doi: 10.1161/01.STR.0000080381.76409.B2. [DOI] [PubMed] [Google Scholar]
- Ratan RR, Siddiq A, Aminova L, Langley B, McConoughey S, Karpisheva K, et al. Small molecule activation of adaptive gene expression: tilorone or its analogs are novel potent activators of hypoxia inducible factor-1 that provide prophylaxis against stroke and spinal cord injury. Ann N Y Acad Sci. 2008;1147:383–394. doi: 10.1196/annals.1427.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998;8:588–594. doi: 10.1016/s0959-437x(98)80016-6. [DOI] [PubMed] [Google Scholar]
- Siddiq A, Aminova LR, Troy CM, Suh K, Messer Z, Semenza GL, et al. Selective inhibition of hypoxia-inducible factor (HIF) prolyl-hydroxylase 1 mediates neuroprotection against normoxic oxidative death via HIF- and CREB-independent pathways. J Neurosci. 2009;29:8828–8838. doi: 10.1523/JNEUROSCI.1779-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souvenir R, Fathali N, Ostrowski RP, Lekic T, Zhang JH, Tang J. Tissue Inhibitor of Matrix Metalloproteinase-1 Mediates Erythropoietin-Induced Neuroprotection in Hypoxia Ischemia. Neurobiol Dis. 2011;44:28–37. doi: 10.1016/j.nbd.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001;37:1053–1059. doi: 10.1161/01.hyp.37.4.1053. [DOI] [PubMed] [Google Scholar]
- Stebbins GT, Goetz CG, Lang AE, Cubo E. Factor analysis of the motor section of the unified Parkinson’s disease rating scale during the off-state. Mov Disord. 1999a;14:585–589. doi: 10.1002/1531-8257(199907)14:4<585::aid-mds1006>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Su EJ, Fredriksson L, Geyer M, Folestad E, Cale J, Andrae J, et al. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat Med. 2008;14:731–737. doi: 10.1038/nm1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Calvert JW, Zhang JH. Neonatal hypoxia/ischemia is associated with decreased inflammatory mediators after erythropoietin administration. Stroke. 2005;36:1672–1678. doi: 10.1161/01.STR.0000173406.04891.8c. [DOI] [PubMed] [Google Scholar]
- Wang L, Chopp M, Gregg SR, Zhang RL, Teng H, Jiang A, et al. Neural progenitor cells treated with EPO induce angiogenesis through the production of VEGF. J Cereb Blood Flow Metab. 2008;28:1361–1368. doi: 10.1038/jcbfm.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo EJ, Cho YS, Kim MS, Park JW. Contribution of HIF-1alpha or HIF-2alpha to erythropoietin expression: in vivo evidence based on chromatin immunoprecipitation. Ann Hematol. 2008;87:11–17. doi: 10.1007/s00277-007-0359-6. [DOI] [PubMed] [Google Scholar]
- Zagorska A, Dulak J. HIF-1: the knowns and unknowns of hypoxia sensing. Acta Biochim Pol. 2004;51:563–585. [PubMed] [Google Scholar]