Abstract
Individuals with atopic dermatitis (AD) are susceptible to a severe, potentially fatal, systemic infection and inflammatory response following exposure to vaccina virus (VV). IL-10 acts both as an inducer of Th2 responses and as a regulator of T cell activation. It has been shown to limit skin inflammation elicited by contact sensitizers. AD exacerbations have been associated with decreased IL-10 function. We used IL-10−/− mice to test the role of the cytokine in VV immunity. They exhibited larger primary lesions and increased cutaneous neutrophil infiltration compared to wild-type (WT) counterparts. This was associated with enhanced production of IL-17A, IL-17F and CXCL2. Paradoxically, despite intact adaptive immune responses, tissue viral burdens were increased in IL-10−/− mice. These findings suggest that IL-10 is important in limiting skin inflammation induced by VV and that abnormal IL-17-driven neutrophil recruitment at the primary infection site in the skin results in increased systemic viral dissemination.
Keywords: atopic dermatitis, eczema vaccinatum, vaccine
1. INTRODUCTION
Atopic dermatitis (AD) is a chronic skin disease affecting more than 10% of the population. It occurs in allergy-prone “atopic” individuals who are predisposed to the production of IgE antibodies directed against aeroallergens and foods. Acute AD lesions are infiltrated with CD4+ Th2 cells, which secrete predominately IL-4, IL-5 and IL-13 (1). In chronic AD, interferon (IFN)- γ producing Th1 and IL-22 producing cells arise as well and are probably kept in check by the Th2 cells which persist (2). Individuals with atopic dermatitis (AD) who are vaccinated against smallpox using vaccinia virus (VV), are at risk for developing a serious and potentially fatal disseminated infection called eczema vaccinatum (EV) (3). The basis of their abnormal susceptibility to VV is not well understood. However, due to the concern that smallpox might be used as a biological weapon, triggering a vaccination program, it is important to examine the immunological mechanisms underlying EV.
IL-10, a cytokine with pleiotropic functions, known both for its Th2-inducing and anti-inflammatory effects, is produced by a range of cell types including Th2 cells, monocytes, macrophages, dendritic cells, mast cells, B cells and keratinocytes (4,5). Published studies reveal complexity with respect to IL-10 regulation and function in AD (6). Several investigators have observed increased IL-10 levels in lesional skin (7). However, as might be anticipated for an immunoregulatory cytokine, there are settings in which decreased IL-10 is associated with flares of AD, a scenario that suggests that reduced IL-10 production is permissive for the emergence of inflammation (8,9). IL-10 regulates the production of IL-17, a cytokine implicated in a number of inflammatory diseases. IL-17 induces the local expression of chemokines that recruit neutrophils and monocytes. We hypothesized that IL-10 might function physiologically to dampen IL-17 production, thereby controlling both VV replication and the local neutrophil-driven inflammatory response to infection.
In order to test this hypothesis we took advantage of a well-characterized model of AD in which epicutaneous sensitization with the allergen ovalbumin (OVA) on skin subjected to periodic tape stripping (a surrogate for scratching) drives the appearance of AD-like lesions of thickened skin with a predominantly eosinophilic dermal infiltrate (10). We found that when compared with the response to VV infection in normal murine skin, the introduction of virus into these inflamed skin lesions gives rise to much more aggressive local lesions as well as increased systemic viral burdens, similar to the phenotype of patients with EV (11). To test the roles of IL-10 in EV we examined lesion size and histology, cutaneous cytokine responses, adaptive immune responses and viral burdens in wild type and IL-10−/− mice infected with VV at sites of allergen-induced skin inflammation. The contributions of IL-17 responses to VV pathology in IL-10−/− mice were investigated by treating infected animals with anti-IL-17 antibodies or by intradermal injection of IL-17 prior to VV infection.
2. MATERIAL AND METHODS
2.1 Mice
Animal procedures were performed under protocols approved by the Boston Children’s Hospital Institutional Animal Care and Use Committee. All mice were bred and maintained under barrier conditions. IL-10-deficient (B6.129P2-Il10tm1Cgn/J) and wild-type C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, Maine). The VV Western Reserve strain (VR-1354) was obtained from American Type Culture Collection (ATCC) (Manassas, VA). Mice were epicutaneously (EC) sensitized on the back with ovalbumin to induce local allergic skin inflammation, as described previously (10). Briefly, the backs of anesthetized mice were shaved and tape-stripped, and 100μg OVA was applied to the area on sterile gauze, held in place by Tegaderm™ transparent dressing. This procedure was repeated three days later, and the entire two-part process was completed three times total, each separated by two weeks. One day after the last sensitization, mice were anesthetized using avertin (2,2,2-tribromoethanol and tertiary amyl alcohol) and 10 μl VV (1 × 107 plaque-forming units (PFU)) were inoculated with 30 superficial scratches in the skin at the site of sensitization using a 27-gauge needle. Anti-IL-17 antibody (Bio X Cell, BE0173, West Lebanon, NH) or isotype control was administered retroorbitally, 3 doses of 100 μg per mouse, on days -1, 0, and 2 relative to infection. Recombinant mouse IL-17A (Biolegend, 576004, San Diego, CA) or vehicle was injected intradermally, one dose of 2.5 μg per mouse, at the site of sensitization and infection, immediately prior to infection with VV.
2.2 Viral load and cytokine transcript measurements
DNA was prepared from ovary, kidney and lung using the Qiagen DNeasy Kit (Qiagen, Valencia, CA) according to the manufacture’s guidelines. Viral genomes were quantified by real-time PCR using primers specific for vaccinia ribonucleotide reductase (Vvl4L) and an ABI 7300 Sequence Detection System (PE Applied Biosystems, Foster City, CA) (12). To quantify vaccinia virus genome copies, a standard curve was generated using DNA from purified VV stock with known PFU (determined by plaque assay). Viral copies determined using the standard curve were normalized to the amount of total input DNA.
Total RNA was isolated from homogenized skin tissue using TRIzol® reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. cDNA was generated with iScript cDNA synthesis kit (Bio-rad Laboratory). Quantitative real-time PCR was done using Taqman® Gene Expression Assay probes, Taqman® PCR master mix and the ABI Prism 7300 sequence detection system, all from Applied Biosystems, (Fostercity, CA). Expression of each cytokine transcript was determined relative to a reference gene transcript, GAPDH, calculated as 2^− (Ct,cytokine − Ct,GAPDH).
Flow cytometry
Single cell suspensions of splenocytes were prepared 7 days post infection. For enumeration of VV-specific CD8+ T cells, one million cells were incubated with 5 μl B8R20–27 (TSYKFESV) MHC class I Pro5TM pentamers conjugated to PE (ProImmune, Oxford, UK). Then CD8 staining was performed using APC-conjugated anti-mouse CD8a (Ly-2, clone 53-6.7) (eBioscience, San Diego, CA). Cells were also stained with FITC-conjugated anti-mouse CD45R/B220 (RA3-6B2) (BD Biosciences Pharmingen) in order to eliminate B cells from analysis. For intracellular IFN-γ staining, 1×106 splenocytes were incubated in complete medium with 1 nM B8R20–27 (TSYKFESV, H-2Kb restricted) peptide at 37°C in 5% CO2 for 5h to activate virus-specific T cells. To block the secretion of de novo synthesized IFN-γ, Brefeldin A was added after 1 h. Cells were fixed and permeabilized using BD Cytofix/Cytoperm™ Plus Kit (BD Biosciences Pharmingen) and stained with APC-conjugated anti-mouse IFN-γ Ab (BD Biosciences Pharmingen) and PE-conjugated CD8a and FITC-labeled anti-mouse CD4 (eBioscience). Samples were analyzed on a BD Biosciences FACSCanto flow cytometer (San Jose, CA). Analysis was performed using FloJo software.
2.3 Skin Histology
Seven days after infection with VV, sections of skin from the primary lesion site (or from the site of EC sensitization for uninfected mice) were fixed in 4% paraformaldehyde overnight. Cross-sections of the skin were prepared and a hematoxylin and eosin stain was used to visualize cellular infiltrate into the area. Neutrophilic infiltration was quantified by counting the number of neutrophils per high power field (HPF). Ten HPFs were evaluated per mouse, and the average of the ten values was used as the number of neutrophils per HPF.
3. RESULTS
3.1 IL-10 limits primary lesion size, IL-17 production and neutrophil recruitment but not adaptive immune responses
IL-10 is an important immunoregulatory cytokine capable of dampening neutrophil-dominant inflammation. Neutrophil recruitment to the skin occurs rapidly following immunization with VV. In humans undergoing small pox vaccination, VV is introduced by scarification (repeated pricking with a bifurcated needle). This triggers the formation of a neutrophil-filled blister that evolves to a small eschar. Similarly, mice infected with VV by repeated needle pricking of the skin exhibit eroded purulent lesions that eventually crust over. In order to test the role of IL-10 in limiting inflammatory responses to VV delivered in the setting of AD, we took advantage of a well-characterized murine model of the disease. In this model, EC sensitization of tape-stripped skin with OVA elicits many of the cardinal features of human AD, including dermal thickening, spongiosis and an eosinophil-dominant inflammatory infiltrate accompanied by strong anti-OVA Th2 and IgE responses (10,13,14).
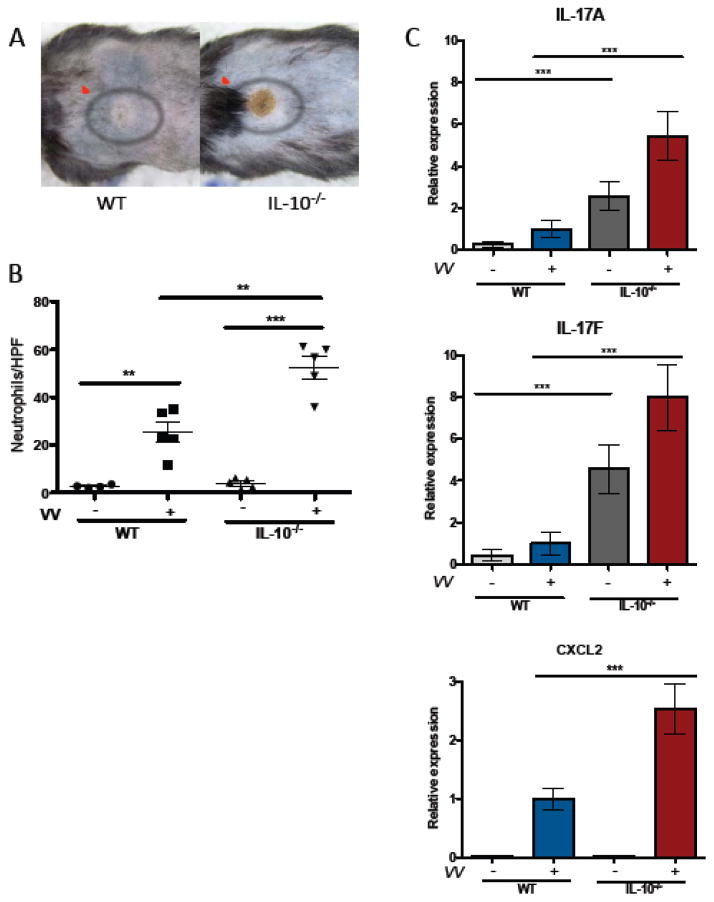
Wild-type (WT) and IL-10−/− mice subjected to the EC-OVA protocol were infected with VV by repeated needle pricking of sensitized skin. Primary lesions, evaluated 7 days post infection, were significantly larger (> 2-fold increased area) and were notably more purulent in appearance in IL-10−/− mice than in WT controls (Figure 1 A, Figure 3C). Neutrophil infiltration was evaluated by microscopic inspection of skin sections., IL-10−/− mice exhibited approximately 2-fold more neutrophils at the site of infection than their WT counterparts. (Figure 1 B). IL-17A and IL-17F mRNA levels were markedly increased in the skin of EC-OVA sensitized IL-10−/− vs. WT mice both before and after infection with VV (Figure 1 C). Transcripts for CXCL2, a potent neutrophil recruiting chemokine regulated by IL-17, were induced by VV infection in both WT and IL-10−/− mice with significantly higher levels observed in IL-10−/− animals (Figure 1 C). These findings suggest that IL-17 production, unchecked by IL-10, drives an exuberant neutrophil response and intense local inflammation following infection with VV.
Figure 1. IL-10 limits primary lesion size after VV inoculation and regulates neutrophil infiltration and transcription of IL-17 and CXCL2.
IL-10−/− and WT mice were VV-infected via skin scarification with 1×107 PFU. Primary lesions and cytokine production were analyzed 7 days later. A) Primary lesions (indicated by red ovals) on representative WT and IL-10−/− mice B) Neutrophils/HPF (mean ± SEM) enumerated by light microscopic examination of H&E-stained sections 4–5 mice/group C) IL-17A, IL-17F and CXCL2 mRNA levels (mean ± SEM) determined by quantitative PCR and normalized to the WT infected group, n=3 for IL-10−/−, uninfected mice; n=8–21 for other groups. ** p < 0.005, ***p < 0.0005 by unpaired t test, two-tailed.
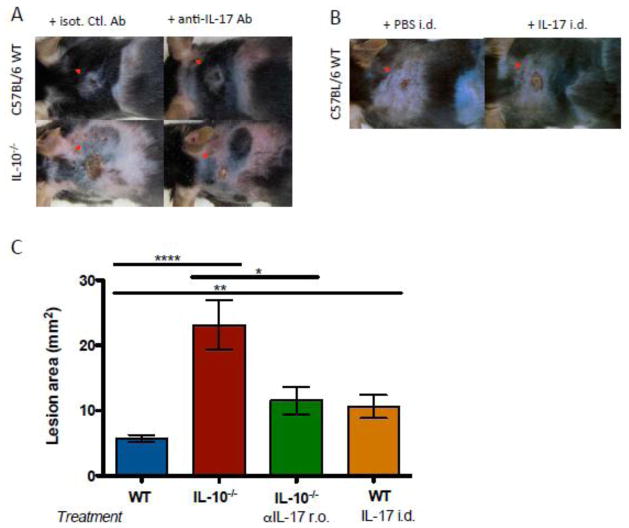
Figure 3. Lesion severity is increased in IL-10−/− mice in an IL-17-dependent manner.
IL-10−/− and WT mice were VV-infected via skin scarification with 107 PFU. Anti-IL-17 antibody or isotype control was administered retroorbitally, 3 doses of 100 μg per mouse, on days −1, 0, and 2 relative to infection. IL-17 was injected intradermally, one dose of 2.5 μg per mouse, immediately prior to infection. Primary lesions were evaluated 7 days after infection. A) Primary lesions of representative mice treated with anti-IL-17. B) Primary lesions of representative mice receiving intradermal IL-17. C) Lesion areas (5–22 mice per group, mean ± SEM). Administration of anti-IL-17 antibody decreased lesion sizes in IL-10−/− mice. * p < 0.05, ** p < 0.005, ****p < 0.0001, by unpaired t test, two-tailed.
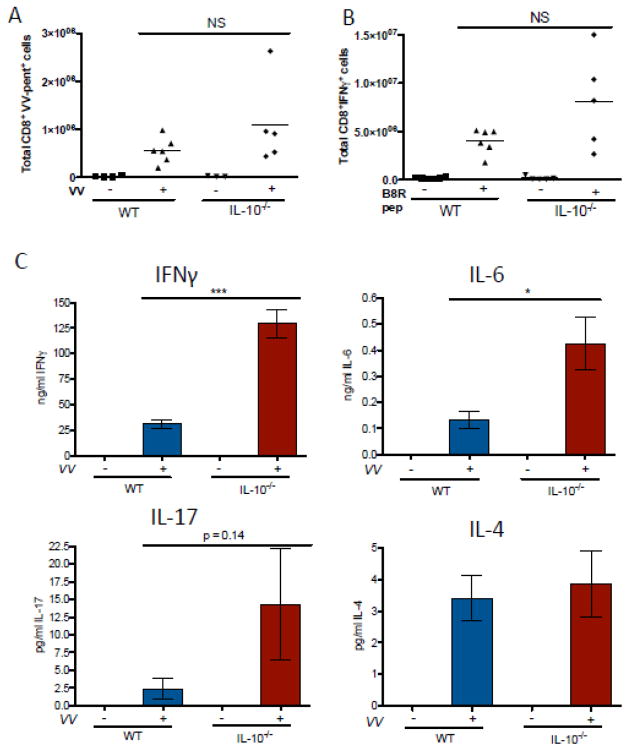
In order to determine whether the intense local inflammatory response to VV observed in IL-10−/− mice had any impact on adaptive immunity we tested the induction of VV-specific T cells as well as their ability to produce IFN-γ. The number of VV-specific CD8+ T cells, detected by their binding to B8R20–27 Class I MHC pentamers was similar in WT and IL-10−/− spleens. Effective viral clearance is dependent on the production of IFN-γ by these cells and we found that the frequency of cells producing this cytokine following stimulation with the B8R peptide was normal in IL-10−/− mice (Figure 2 A, B). Similarly, we observed robust IFN-γ production by VV-stimulated splenocytes, actually exceeding that observed in WT animals by more than 4-fold. IL-6 production was also enhanced (Figure 2 C). These findings indicate that IL-10 serves to control local inflammatory responses to VV but that effective adaptive immune responses to the virus can arise in the absence of IL-10.
Figure 2. Systemic adaptive immune responses to VV are intact in IL-10−/− mice.
IL-10−/− and WT mice were VV-infected via skin scarification with 1×107 PFU. Single-cell suspensions of splenocytes were prepared 7 days later and fluorescently stained to identify the number of cells A) CD8+ and VV-pentamer+ and B) CD8+ and IFNγ+ (3–6 mice per group). C) Splenocytes were cultured at 106 cells/ml, and release of IFNγ, IL-6, IL-17, and IL-4 into the cell medium was measured by ELISA after 72 hours in culture (3–9 mice per uninfected group, 5–19 mice per infected group, mean ± SEM). * p < 0.05, ***p < 0.0005, by unpaired t test, two-tailed.
3.2 IL-17 drives the local inflammatory response to VV in IL-10−/− mice
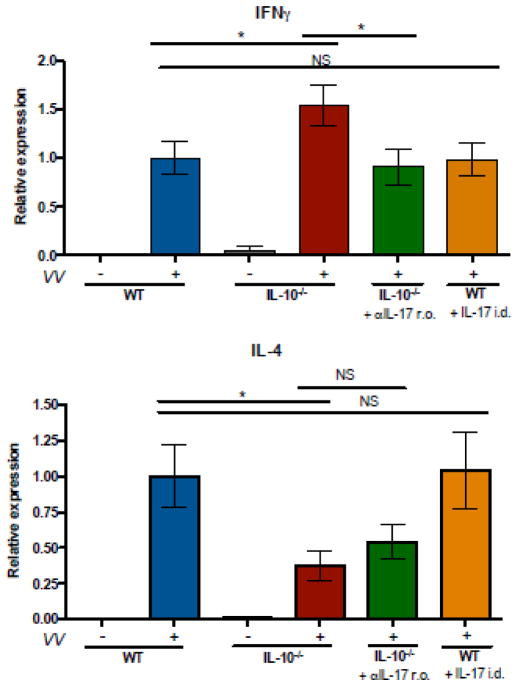
Blocking antibody and local reconstitution experiments were performed to test whether IL-17 was indeed responsible for increased lesion sizes observed in IL-10−/− mice. Consistent with our initial observation, IL-10−/− mice exhibited larger primary lesions after VV infection than did WT animals. Treatment with anti-IL-17 antibody significantly decreased lesion sizes in IL-10−/− mice (Figure 3 A, C). Conversely, intradermal administration of IL-17 to WT mice at the time of VV infections resulted in increased lesion sizes compared to mice receiving vehicle only (Figure 3 B, C). In addition, intradermal administration of IL-17 to WT mice significantly increased CXCL2 expression in the skin; mice receiving the treatment exhibited 2.46-fold greater CXCL2 expression than mice receiving vehicle alone (data not shown). IL-10−/− mice exhibited higher expression of IFNγ and lower expression of interleukin 4 (IL-4) in the skin 7 days after infection compared to their WT counterparts (Figure 4). The IFNγ response of IL-10−/− mice was attenuated in animals receiving anti-IL17. These results implicate IL-17 as the inducer of the larger purulent skin lesions arising in the absence of IL-10 and suggest that local IL-17 responses promote IFNγ production in the skin.
Figure 4. IL-10−/− mice exhibit increased IFN-γ and decreased IL-4 expression in the skin following infection.
IL-10−/− and WT mice were VV-infected via skin scarification with 107 PFU. Anti-IL-17 antibody or isotype control was administered retroorbitally, 3 doses of 100 μg per mouse, on days −1, 0, and 2 relative to infection. IL-17 was injected intradermally, one dose of 2.5 μg per mouse, immediately prior to infection. Transcripts encoding IFN-γ and IL-4 in skin RNA were evaluated by quanititative PCR and normalized to the WT infected group, mean ± SEM, (n=3 for IL-10−/−, uninfected mice; n=5–22 for all other groups). * p < 0.05, by unpaired t test, two-tailed.
3.3 Tissue viral burdens are increased in IL-10−/− mice
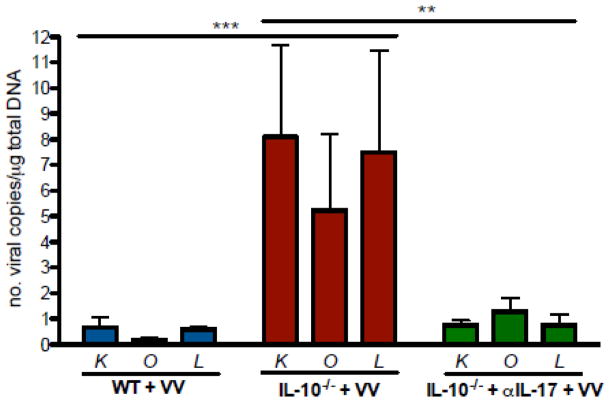
Although systemic adaptive immune responses to VV appeared to be intact in IL-10−/− mice (Figure 2 A–C), we hypothesized that the intense local inflammatory response observed in these animals might lead to abnormal viral proliferation and systemic dissemination. Applying a sensitive quantitative PCR assay for VV genomes to a number of the target organs of VV infection (kidney, ovary, and lung), we found that IL-10−/− mice harbored over 10-fold more virus than did WT controls (Figure 5). This appeared to be dependent on IL-17, since tissue viral burdens in IL-10−/− mice receiving anti-IL-17 were significantly less than viral burdens in IL-10−/− mice that did not receive the antibody, and were not significantly different from WT controls. Taken together, these findings support the hypothesis that dysregulated IL-17-driven inflammatory responses at the cutaneous site of VV infection result in enhanced systemic viral dissemination.
Figure 5. IL-10 deficiency results in and IL-17-dependent increase in viral burdens in VV-infected mice.
WT and IL-10−/− mice were VV-infected via skin scarification with 107 PFU. Anti-IL-17 antibody or isotype control was administered retroorbitally, 3 doses of 100 μg per mouse, on days −1, 0, and 2 relative to infection. Sections of kidney (K), ovary (O), and lung (L) were collected 7 days after the infection and frozen immediately. DNA was isolated from the organs and viral genomes were quantified by real-time PCR using primers specific for vaccinia ribonucleotide reductase (Vvl4L) (13–19 mice per group, mean ± SEM). Results were normalized to the total amount of DNA. ** p < 0.005, ***p < 0.0005, by two-way ANOVA.
4. DISCUSSION
Defining specific immune mechanisms underlying increased susceptibility to cutaneous viral infection in patients with AD is important both for the development of strategies to limit viral dissemination and pathology and for the implementation of safe and effective approaches to live viral vaccination. The findings presented here address the consequences of IL-10 dysregulation in AD with respect to local inflammatory responses, systemic adaptive immunity and viral dissemination and clearance. We report that IL-10−/− mice with allergen-induced allergic skin inflammation have increased primary lesion sizes and neutrophil recruitment with increased skin IL-17 and CXCL2 induction. The exaggerated local inflammatory response to VV in IL-10−/− animals is dependent on IL-17. Although IL-10−/− mice have intact systemic T cell immunity (pentamer+ CD8+ cells, IFN-γ+ CD8+ cells and IFN-γ production by VV-stimulated splenocytes), they exhibit increased systemic viral burdens. This observation suggests that dysregulated local inflammation at the cutaneous site of VV inoculation results in increased viral spread and proliferation even in the face of normal T cell responses.
These results add new insights into the immunobiology of IL-10 in AD. It appears that the cytokine both promotes the induction of Th2 responses and exerts a suppressive function in limiting pathology arising from allergic inflammation (6). Numerous investigators have assessed IL-10 production in the skin and circulating cells of patients with AD. Some of these reports have demonstrated increased IL-10, as might be expected given its function in inducing Th2 responses and its production by Th2 cells (7,15–18). Mouse models of AD similarly are characterized by increased IL-10. Fallon et al. reported enhanced T cell production of IL-10 in ft/ft mice. These animals, which spontaneously develop eczematous skin inflammation, harbor an early stop codon in the gene encoding filaggrin, which is also mutated in a significant proportion of patients with severe AD (19). We have previously reported that IL-10 is rapidly induced in the skin of mice following disruption of the epidermal barrier by tape stripping (a surrogate for scratching) and is required for inflammatory cell recruitment and Th2 cytokine responses following epicutaneous allergen exposure in a murine model of AD (13). In this setting IL-10, together with TSLP, acts directly on dendritic cells (DC) to promote a Th2-skewing phenotype (14).
Despite its role in skewing Th2 responses in the skin it appears that deficiency of IL-10 is associated with severe disease and acute flares, probably because of its anti-inflammatory actions. An inverse relationship between plasma IL-10 levels and AD severity was reported both by Niwa and colleagues and by Seneviratne et al. (8,9). The latter group demonstrated decreased IL-10+ CD4+ T cells in severely symptomatic vs. mildly affected individuals. IL-10 receptor levels are similarly down-regulated in acute vs. chronic AD (20). Children with AD, who tend to have a strong inflammatory component to their disease, also exhibit decreased IL-10 responses (21). Taken together these findings suggest that while patients with AD may have a tendency at baseline towards increased IL-10 production, the occurrence of severe disease and acute exacerbations are associated with a failure to produce this regulatory cytokine. This concept is supported by a recent study showing that AD patients’ acute exacerbations are associated with transient suppression of IL-10 transcription in Foxp3-negative regulatory T cells (22).
Our findings suggest that unconstrained IL-17 production mediates the intense neutrophil-dominant inflammatory response elicited by VV infection of IL-10−/− mice and that this local process ultimately affects systemic viral clearance. We have previously reported that VV inoculation into sites of tape stripping and EC-OVA sensitization induces strong cutaneous IL-17 responses (23). The same study further revealed that addition of IL-17 to skin explants led to increased VV proliferation implicating local effects of IL-17 in the increased viral burden occurring when VV is introduced via sites of allergic skin inflammation. Dysregulation of the IL-17 axis cytokines (including IL-23, IL-6 and IL-17) has been implicated in other inflammatory processes, including colitis, observed in IL-10−/− mice (24). It is possible that IL-17 produced in this setting suppresses the natural killer cell response, as has been reported by Kawakami and colleagues (25).
Consistent with our observations in cutaneous VV infection, IL-10-mediated regulation of IL-17 has recently been shown to have significant effects on immune responses to other skin tropic infectious agents. In one study, increased IL-17-mediated cutaneous immunopathology was found in IL-10−/− mice infected with Leishmania major (26). The same report revealed that IL-10 down-regulated IL-17 production in leishmaniasis patients. Another skin pathogen, Francisella tularensis, has also been shown to exhibit IL-10-dependent suppression of IL-17 responses (27) and Th17 responses to Bacillus Calmette-Guérin (BCG) are enhanced in IL-10−/− mice (28). In contrast to the situation with VV and L. major however, the enhanced IL-17 response elicited in the absence of IL-10 appeared to be favorable to host responses to F. tularensis and BCG.
Our own finding of increased VV replication in IL-10−/− mice is specific to the setting of percutaneous infection at sites of allergic skin inflammation. It is important to note that a different result has been reported for i.p. VV infection in IL-10−/− mice. Van den Broek and colleagues showed increased inflammatory cytokine production and enhanced viral elimination in IL-10−/− mice infected i.p. (29). In contrast, our results indicate that when virus is introduced in the setting of allergic skin inflammation that IL-10 plays an important role in controlling inflammatory cytokine production (IL-17) and that unfettered IL-17 production arising in the absence of IL-10 is permissive for increased viral replication.
The tendency of AD patients to acquire serious cutaneous viral infections remains a major challenge in their management. Defining the immunological mechanisms underlying this susceptibility is an important first step in developing novel strategies to identifying risk factors for viral skin infection and in designing novel interventions to enhance viral immunity. In the case of EV such approaches may be important in preventing small pox vaccine reactions. Our results suggest that IL-10, a cytokine which given its role in Th2 induction might at first glance have seemed a candidate for blockade in AD, is actually an important regulator of skin inflammation, keeping detrimental IL-17 responses in check. Additional studies focused on approaches to boosting IL-10 responses or to blocking Th17 induction and activation might prove useful in identifying effective strategies.
Highlights.
AD patients are prone to eczema vaccinatum, a severe vaccinia virus infection.
AD exacerbations are associated with decreased IL-10 function.
IL-10−/− mice were used to test the role of IL-10 in immunity to vaccinia virus.
IL-10 was found to limit the size of primary lesions induced by vaccinia virus.
IL-10 suppressed local IL-17 and CXCL2 production and neutrophil infiltration.
Acknowledgments
FUNDING
This work was supported by the Atopic Dermatitis Research Network of the National Institutes of Health [Grant number HHSN272201000020C] and by a National Institutes of Health training grant [T32AI007512] (OTB).
ABBREVIATIONS
- AD
atopic dermatitis
- EV
eczema vaccinatum
- VV
vaccinia virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leung DY, Bhan AK, Schneeberger EE, Geha RS. Characterization of the mononuclear cell infiltrate in atopic dermatitis using monoclonal antibodies. The Journal of allergy and clinical immunology. 1983;71:47. doi: 10.1016/0091-6749(83)90546-8. [DOI] [PubMed] [Google Scholar]
- 2.Gittler JK, Shemer A, Suarez-Farinas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, Mitsui H, Cardinale I, de Guzman Strong C, Krueger JG, Guttman-Yassky E. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. The Journal of allergy and clinical immunology. 2012;130:1344. doi: 10.1016/j.jaci.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engler RJ, Kenner J, Leung DY. Smallpox vaccination: Risk considerations for patients with atopic dermatitis. J Allergy Clin Immunol. 2002;110:357. doi: 10.1067/mai.2002.128052. [DOI] [PubMed] [Google Scholar]
- 4.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annual review of immunology. 2011;29:71. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 5.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nature reviews Immunology. 2010;10:170. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 6.Brandt EB, Sivaprasad U. Th2 Cytokines and Atopic Dermatitis. Journal of clinical & cellular immunology. 2011:2. doi: 10.4172/2155-9899.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohmen JD, Hanifin JM, Nickoloff BJ, Rea TH, Wyzykowski R, Kim J, Jullien D, McHugh T, Nassif AS, Chan SC, et al. Overexpression of IL-10 in atopic dermatitis. Contrasting cytokine patterns with delayed-type hypersensitivity reactions. Journal of immunology. 1995;154:1956. [PubMed] [Google Scholar]
- 8.Niwa Y. Elevated RANTES levels in plasma or skin and decreased plasma IL-10 levels in subsets of patients with severe atopic dermatitis. Archives of dermatology. 2000;136:125. doi: 10.1001/archderm.136.1.125. [DOI] [PubMed] [Google Scholar]
- 9.Seneviratne SL, Jones L, Bailey AS, Black AP, Ogg GS. Severe atopic dermatitis is associated with a reduced frequency of IL-10 producing allergen-specific CD4+ T cells. Clinical and experimental dermatology. 2006;31:689. doi: 10.1111/j.1365-2230.2006.02172.x. [DOI] [PubMed] [Google Scholar]
- 10.Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott JE, ElKhal A, Freyschmidt EJ, MacArthur DH, McDonald D, Howell MD, Leung DY, Laouar A, Manjunath N, Bianchi T, Boes M, Oettgen HC, Geha RS. Impaired immune response to vaccinia virus inoculated at the site of cutaneous allergic inflammation. J Allergy Clin Immunol. 2007;120:1382. doi: 10.1016/j.jaci.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Freyschmidt EJ, Mathias CB, MacArthur DH, Laouar A, Narasimhaswamy M, Weih F, Oettgen HC. Skin inflammation in RelB(−/−) mice leads to defective immunity and impaired clearance of vaccinia virus. J Allergy Clin Immunol. 2007;119:671. doi: 10.1016/j.jaci.2006.12.645. [DOI] [PubMed] [Google Scholar]
- 13.Laouini D, Alenius H, Bryce P, Oettgen H, Tsitsikov E, Geha RS. IL-10 is critical for Th2 responses in a murine model of allergic dermatitis. J Clin Invest. 2003;112:1058. doi: 10.1172/JCI18246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oyoshi MK, Larson RP, Ziegler SF, Geha RS. Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. The Journal of allergy and clinical immunology. 2010;126:976. doi: 10.1016/j.jaci.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aleksza M, Lukacs A, Antal-Szalmas P, Hunyadi J, Szegedi A. Increased frequency of intracellular interleukin (IL)-13 and IL-10, but not IL-4, expressing CD4+ and CD8+ peripheral T cells of patients with atopic dermatitis. The British journal of dermatology. 2002;147:1135. doi: 10.1046/j.1365-2133.2002.05013.x. [DOI] [PubMed] [Google Scholar]
- 16.Aiba S, Manome H, Yoshino Y, Tagami H. Alteration in the production of IL-10 and IL-12 and aberrant expression of CD23, CD83 and CD86 by monocytes or monocyte-derived dendritic cells from atopic dermatitis patients. Experimental dermatology. 2003;12:86. doi: 10.1034/j.1600-0625.2003.120111.x. [DOI] [PubMed] [Google Scholar]
- 17.Simon D, Braathen LR, Simon HU. Increased lipopolysaccharide-induced tumour necrosis factor-alpha, interferon-gamma and interleukin-10 production in atopic dermatitis. The British journal of dermatology. 2007;157:583. doi: 10.1111/j.1365-2133.2007.08050.x. [DOI] [PubMed] [Google Scholar]
- 18.Howell MD, Novak N, Bieber T, Pastore S, Girolomoni G, Boguniewicz M, Streib J, Wong C, Gallo RL, Leung DY. Interleukin-10 downregulates anti-microbial peptide expression in atopic dermatitis. The Journal of investigative dermatology. 2005;125:738. doi: 10.1111/j.0022-202X.2005.23776.x. [DOI] [PubMed] [Google Scholar]
- 19.Fallon PG, Sasaki T, Sandilands A, Campbell LE, Saunders SP, Mangan NE, Callanan JJ, Kawasaki H, Shiohama A, Kubo A, Sundberg JP, Presland RB, Fleckman P, Shimizu N, Kudoh J, Irvine AD, Amagai M, McLean WH. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat Genet. 2009;41:602. doi: 10.1038/ng.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muschen A, Mirmohammadsadegh A, Jarzebska-Deussen B, Abts HF, Ruzicka T, Michel G. Differential IL-10 receptor gene expression in acute versus chronic atopic eczema. Modulation by immunosuppressive drugs and cytokines in normal cultured keratinocytes. Inflammation research: official journal of the European Histamine Research Society … [et al.] 1999;48:539. doi: 10.1007/s000110050500. [DOI] [PubMed] [Google Scholar]
- 21.Dunstan JA, Hale J, Breckler L, Lehmann H, Weston S, Richmond P, Prescott SL. Atopic dermatitis in young children is associated with impaired interleukin-10 and interferon-gamma responses to allergens, vaccines and colonizing skin and gut bacteria. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2005;35:1309. doi: 10.1111/j.1365-2222.2005.02348.x. [DOI] [PubMed] [Google Scholar]
- 22.Oh SH, Park CO, Wu WH, Kim JY, Jin S, Byamba D, Bae BG, Noh S, Lim BJ, Noh JY, Lee KH. Corticotropin-releasing hormone downregulates IL-10 production by adaptive forkhead box protein 3-negative regulatory T cells in patients with atopic dermatitis. The Journal of allergy and clinical immunology. 2012;129:151. doi: 10.1016/j.jaci.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Oyoshi MK, Elkhal A, Kumar L, Scott JE, Koduru S, He R, Leung DY, Howell MD, Oettgen HC, Murphy GF, Geha RS. Vaccinia virus inoculation in sites of allergic skin inflammation elicits a vigorous cutaneous IL-17 response. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14954. doi: 10.1073/pnas.0904021106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. The Journal of clinical investigation. 2006;116:1310. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawakami Y, Tomimori Y, Yumoto K, Hasegawa S, Ando T, Tagaya Y, Tagaya Y, Crotty S, Kawakami T. Inhibition of NK cell activity by IL-17 allows vaccinia virus to induce severe skin lesions in a mouse model of eczema vaccinatum. The Journal of Experimental Medicine. 2009;206:1219. doi: 10.1084/jem.20082835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez-Lombana C, Gimblet C, Bacellar O, Oliveira WW, Passos S, Carvalho LP, Goldschmidt M, Carvalho EM, Scott P. IL-17 Mediates Immunopathology in the Absence of IL-10 Following Leishmania major Infection. PLoS pathogens. 2013;9:e1003243. doi: 10.1371/journal.ppat.1003243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metzger DW, Salmon SL, Kirimanjeswara G. Differing Effects of IL-10 in Cutaneous and Pulmonary Francisella tularensis LVS Infection. Infection and immunity. 2013;81:2022. doi: 10.1128/IAI.00024-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pitt JM, Stavropoulos E, Redford PS, Beebe AM, Bancroft GJ, Young DB, O’Garra A. Blockade of IL-10 signaling during bacillus Calmette-Guerin vaccination enhances and sustains Th1, Th17, and innate lymphoid IFN-gamma and IL-17 responses and increases protection to Mycobacterium tuberculosis infection. Journal of immunology. 2012;189:4079. doi: 10.4049/jimmunol.1201061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Broek M, Bachmann MF, Köhler G, Barner M, Escher R, Zinkernagel R, Kopf M. IL-4 and IL-10 antagonized IL-12-mediated protection against acute vaccinia virus infection with a limited role of IFN-g and nitric oxide synthase. Journal of Immunology. 2013;164:371. doi: 10.4049/jimmunol.164.1.371. [DOI] [PubMed] [Google Scholar]