Abstract
Initial therapy of multiple myeloma with lenalidomide-based regimens can compromise stem cell collection, which can be overcome with the addition of plerixafor. Plerixafor is typically given subcutaneously (SQ), with collection approximately 11 hours later for maximum yield. Intravenous (IV) administration may allow more rapid and predictable mobilization. This trial was designed to assess the efficacy and feasibility of IV plerixafor in patients receiving initial therapy with a lenalidomide-based regimen. Patients received G-CSF at 10 μg/kg/day for 4 days followed by IV plerixafor at 0.24 mg/kg/dose starting on day 5; plerixafor administered early in the morning with apheresis 4–5 hours later. Thirty-eight (97%) patients collected at least 3×106 CD34+ cells/kg within 2 days of apheresis. The median CD34+ cells/kg after 1 day of collection was 3.9×106 (range; 0.7–9.2) and after two days of collection was 6.99×106 (range: 1.1–16.5). There were no grade 3 or 4 non-hematological adverse events and one patient experienced grade 4 thrombocytopenia. The most common adverse events were nausea, diarrhea and abdominal bloating. IV plerixafor is an effective strategy for mobilization with low failure rate and is well tolerated. It offers flexibility with a schedule of early morning infusion followed by apheresis later in the day.
Keywords: Plerixafor, Multiple Myeloma, apheresis
INTRODUCTION
Autologous stem cell transplantation (ASCT) remains an integral part of current management of multiple myeloma in transplant eligible patients.1–3 Traditionally, patients undergo 4–6 months of initial therapy with one of several commonly used regimens followed by peripheral blood stem cell mobilization. Following a successful stem cell harvest, patients either proceed to an immediate ASCT or continue with the initial therapy and use ASCT at the time of relapse.4 One of the critical steps in this process remains the ability to collect adequate number of stem cells for a successful ASCT. Nearly 10% of patients may fail to collect the minimum number of stem cells required for the ASCT, depending on the mobilization process utilized.5–7 In addition, the initial therapy employed for myeloma management also has significant impact on the success of stem cell mobilization.8–12 While alkylating agents that can impair stem cell mobilization are rarely used currently as part of initial therapy in transplant eligible patients, newer drugs such as lenalidomide can also impair the collection process.11–14 The most common approaches to stem cell mobilization until recently have been the use of G-CSF alone or G-CSF following pulse dose chemotherapy.5 The chemotherapy approach has lower failure rates, but is associated with increased risk of neutropenic fever and consequent complications. More recently, the introduction of plerixafor, a CXCR4 antagonist, has radically changed stem cell mobilization, considerably reducing the rate of mobilization failures when used in conjunction with G-CSF.15–19 Risk adapted strategies for the use of plerixafor based on circulating CD34+ cell numbers or apheresis yields have allowed us to successfully mobilize and collect stem cells in nearly all patients and provide the opportunity to proceed with a stem cell transplant when recommended.18, 20–25 However, the current schedule for plerixafor administration late in the evening prior to collection and the relatively narrow window for collecting the stem cells introduce logistical difficulties.26 While the majority of the studies have used plerixafor by the subcutaneous route, and the current label indicates SQ route, intravenous administration has been studied in a limited fashion. Following SQ administration, plerixafor is absorbed rapidly with 70–80% bioavailability in healthy volunteer studies. Estimates of Cmax and AUC were higher following IV administration compared with SC dosing, while terminal half-lives were comparable between the two routes. In the healthy volunteer studies, the peak peripheral blood CD34+ cell counts were seen 10–14 hours after administration of plerixafor, leading to the current recommendations of injection and apheresis schedules. We designed this trial with two objectives: (1) to determine the risk of failure of stem cell mobilization with plerixafor and G-CSF among patients receiving a lenalidomide based induction therapy for myeloma and, (2) to determine the safety and efficacy of intravenously administered plerixafor in the setting of patients with myeloma undergoing peripheral blood stem cell mobilization.
SUBJECTS AND METHODS
Patients
Patients with a diagnosis of symptomatic multiple myeloma receiving initial treatment with a lenalidomide based treatment regimen started ≤12 months prior to registration were enrolled. Patients should have received at least 2 cycles of treatment with the lenalidomide regimen with the last dose of lenalidomide > 2 weeks prior to registration and patients should be eligible for and be considered for stem cell transplant. The trial was approved by the Mayo Foundation Institutional Review Board and was carried out in accordance with the Helsinki Principle. The clinical trial was registered at www.clinicaltrials.gov as NCT00998049.
The objectives of the trial were to determine the proportion of patients reaching a stem cell yield of 3 × 106 CD34+ cells/kg by the second day of apheresis with intravenously administered plerixafor, the safety and tolerability of intravenously administered plerixafor, and the overall rate of failure to mobilize minimum required number of stem cells for an ASCT (< 2.5 × 106 CD34+ cells/kg). Toxicities were graded using CTCAE v 4.0. Adverse event assessment was performed daily during the study.
Treatment
Patients received G-CSF (10 ug/kg), daily subcutaneous injection beginning Day 1, once they completed the required pre-transplant evaluation. On the morning of day 5 of G-CSF administration, plerixafor was administered at a dose of 240 mcg/kg (160 mcg/kg if CrCl < 30 ml/min) intravenously. The same formulation as that used for subcutaneous administration was used, but diluted in a larger volume. The dose to be administered was added to 50 ml of normal saline. The drug was administered using standard infusion tubing via slow infusion over 30 minutes. At the end of the infusion, the line was flushed with 10 ml of normal saline. Patients then proceeded to large volume leukapheresis on the Fenwal Amicus (Fenwal Inc., Lake Zurich IL, USA) utilizing version 2.5 software. The collection method has previously been described.27 In brief, patients underwent leukapheresis for five hours with patients with white blood cell counts of less than 35×109/L processed at a blood flow rate of 90 ml/min utilizing a cycle volume of 1,400 ml and those with white blood cell counts greater than 35×109/L processed at a blood flow rate of 65 ml/min utilizing a cycle volume of 1,000 ml.28 Anticoagulant consisted of a mixture of ACD-A (Baxter Healthcare Corp., Deerfield, IL, USA), normal saline, and heparin. The citrate infusion rate was 2.50 mg/kg/min and anticoagulant ratio was 13:1. MNC offset was 1.5 ml and RBC offset was 5 ml and adjusted during the procedure as necessary. 27 Patients began collection approximately 4 hours after the completion of the plerixafor infusion. Patients continued to receive daily G-CSF and IV plerixafor each morning of apheresis for a maximum of four doses or until collection goal met. Patients were allowed to undergo additional apheresis collections beyond the fourth collection at the discretion of the treating physician, but only four doses of plerixafor could be administered.
Patients were typically conditioned with melphalan 200 mg/m2, with dose reduction to 140 mg/m2 for patients with reduced renal function or patients over 70 years. Post transplant GCSF was not routinely used for any of the patients. Engraftment kinetics was examined in the subgroup of patients who proceeded to a stem cell transplant. Neutrophil engraftment was defined as neutrophil count more than or equal to 0.5 × 109/L for 3 days or more than or equal to 1.0 × 109/L for 1 day. Platelet engraftment was defined as platelet count more than or equal to 20 × 109/L without a transfusion for the preceding 7 days.
Statistical analysis
For primary endpoint, success was defined as collection of 3 × 106 CD34+ cells/kg after two days of apheresis. The largest success proportion where the proposed treatment regimen would be considered ineffective in this population was 60%, and the smallest success proportion supporting future studies in this patient population was 80%. This design required 36 evaluable patients, where at least 26 successes were required to conclude that further studies be recommended. This design has 91% power and a 9% Type I error rate.
RESULTS
Forty patients were accrued between December 2009 – October 2011, and 39 were eligible for analysis. The baseline characteristics of the patients as well as other myeloma related details are provided in Table 1. The patients had received a median of 4 cycles with a lenalidomide-based regimen, mostly lenalidomide and dexamethasone. Nearly a fourth of the patients received a bortezomib and lenalidomide combination. The majority of patients had remained on full dose lenalidomide prior to proceeding with study registration and stem cell collection.
Table 1.
Baseline Characteristics
| Total (N=39) | |
|---|---|
| Age | |
| Median | 60.0 |
| Range | (28.0–73.0) |
| Gender: Male | 25 (64.1%) |
| ECOG Performance Score | |
| 0 | 27 (69.2%) |
| 1 | 12 (30.8%) |
| Months from initial myeloma therapy to registration | |
| Median | 4.9 |
| Range | (2.6–11.1) |
| Days from first dose of lenalidomide to registration | |
| Median | 141.0 |
| Range | (78.0–311.0) |
| Days from last dose of lenalidomide to registration | |
| Median | 24.0 |
| Range | (14–110.0) |
| Ending lenalidomide Dose | |
| 10 mg | 1 (2.6%) |
| 15 mg | 7 (18.4%) |
| 25 mg | 30 (78.9%) |
| Total number of cycles of lenalidomide | |
| Median | 4.0 |
| Range | (3.0–11.0) |
| Other drugs used in combination with lenalidomide | 39 (100.0%) |
| Dexamethasone | 39 |
| Cyclophosphamide | 1 |
| Velcade | 10 |
| Other | 2 |
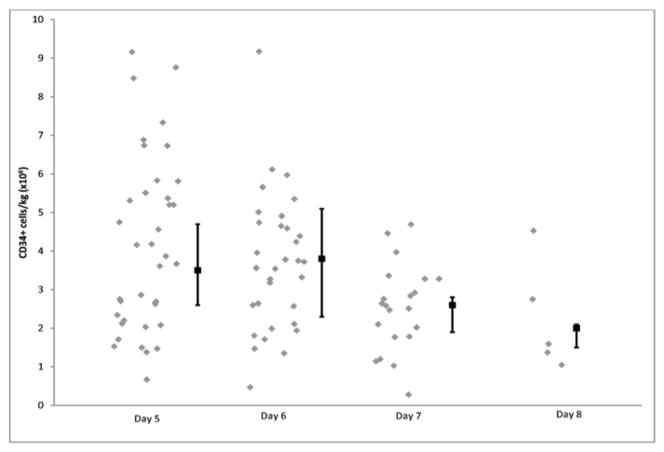
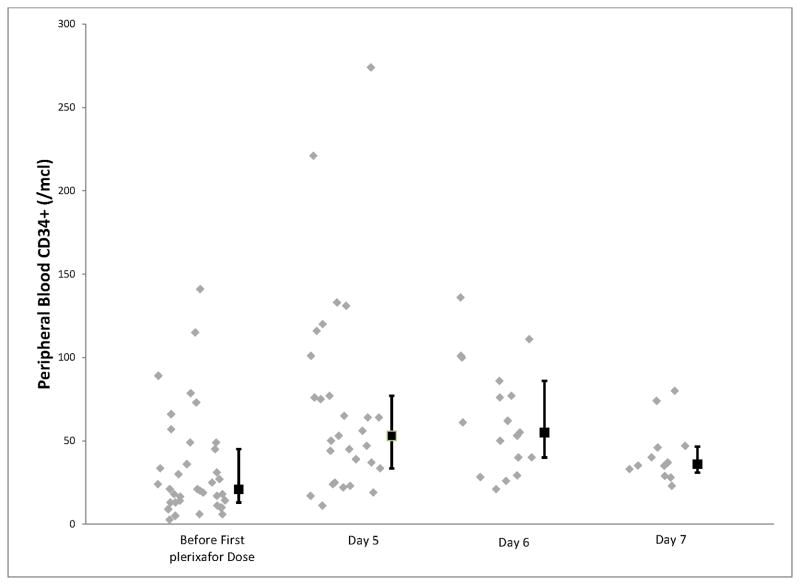
In terms of the primary endpoint, thirty-eight (97%) of the patients achieved at least 3 × 106 CD34+ cells/kg, adequate to proceed to one stem cell transplant, within 2 days of apheresis (Table 2). The median CD34+ cells/kg after 1 day of collection was 3.9 × 106 (range; 0.7 to 9.2) and after two days of collection was 6.99 × 106 (range: 1.1–16.5). The median number of cells collected on each apheresis day is shown in figure 1. We then examined the time taken to reach 4 × 106 and 8 × 106 CD34+ cells/kg, given that these are typically considered the ideal numbers required for 1 and 2 transplants respectively. As shown in figure 2, 38 patients were able to reach the 4 × 106 threshold after 4 apheresis sessions and 25 of the patients in whom the target was to collect for more than one transplant, achieved 8 × 106 target after 4 sessions. The sole patient who failed to reach the primary goal of 3 × 106 was a 61-year-old male who had received 4 cycles of previous lenalidomide at 25 mg (with dexamethasone). The total CD34+ cell yield for this patient, over the course of 3 days, was 1.42 × 106 cells/kg. The kinetics of the peripheral blood CD34+ cell counts are shown in figure 3.
Table 2.
Stem cell mobilization and harvest outcomes
| Rate of achieving 3 × 106 CD34+ cells/kg after 2 days of apheresis1 | 97% (95%CI: 86–99) |
| Number of patients | 38 |
| Median CD34+cell yield Day 1 | 3.87 × 106 cells/kg (range: 0.67–9.16) |
| Median CD34+cell yield Day 2 | 3.55 × 106 cells/kg (range: 0.47–9.17) |
| Median number of days of apheresis | 4 (range: 2–5) |
| Median time (from first GCSF dose) to reach 6 × 106 CD34+ cells/kg 2 | 5 days (95%CI: 5–6) |
| Rate of failure to mobilize (never achieve 2.5 × 106 cells/kg) 1 | 3% (95%CI: 0.006–13) |
| Number of patients | 1 |
CI: confidence interval
Binomial distribution
Kaplan Meier
Figure 1.
Figure shows the median number of CD34+cells collected (/kg body weight) on each day of apheresis. X-axis shows the day of apheresis and the Y-axis show the median CD34+ cells (× 106)/kg. The error bars denote the interquartile range.
Figure 2.
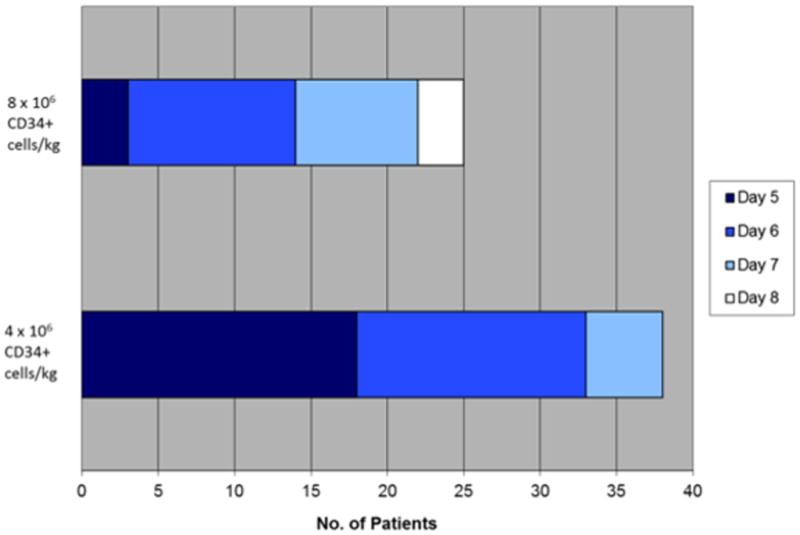
Figure shows the number of days to reach specific targets (4 × 106/kg and 8 × 106/kg from start of GCSF administration. X-axis shows the number of patients. The number of days from start of GCSF administration (Day 5 is the first day of plerixafor) is denoted by the color of the shaded portion.
Figure 3.
Figure shows the kinetics of peripheral blood CD34+ cell counts. Data is presented from before the administration of plerixafor and from one, two and three days after the initiation of plerixafor. The error bars show interquartile range. Day 5 is the first day of plerixafor.
The IV administration was well tolerated with no grade 3 or higher adverse events (Table 3). The most common grade 1 or 2 adverse events seen were gastrointestinal, namely nausea, diarrhea and abdominal pain or bloating. Grade 1 dizziness was reported in 8 patients. Infusion site reactions were observed in one patient.
Table 3.
Maximum Severity of Toxicities1 (N=39)
| Toxicity2 | Grade 1 | Grade 2 | Grade 3 or higher | Total |
|---|---|---|---|---|
| Anemia | 0 | 1 | 0 | 1 |
| Abdominal Pain | 5 | 2 | 0 | 7 |
| Bloating | 0 | 1 | 0 | 1 |
| Diarrhea | 9 | 1 | 0 | 10 |
| Nausea | 12 | 1 | 0 | 13 |
| Injection Site Reaction | 1 | 0 | 0 | 1 |
| Thrombocytopenia | 0 | 1 | 0 | 1 |
| Dizziness | 4 | 0 | 0 | 4 |
| Headache | 4 | 1 | 0 | 5 |
Possibly, probably or definitely related
Common Terminology Criteria for Adverse Events version 3.0.
We then performed additional analysis to identify factors potentially contributing to slower collection. Given that all but one patient achieved the primary goal of 3 × 106 CD34+ cells/kg in two days, we compared the baseline clinical and laboratory characteristics between patients achieving 6 × 106 in 2 days (N=25) vs. those who did not. Specifically, we examined if age, time from diagnosis to registration, lenalidomide dose at start of therapy and at end of therapy, days between stopping lenalidomide and start of mobilization, duration of lenalidomide therapy, and blood counts, serum creatinine, serum albumin, bone marrow plasma cell percentage, plasma cell labeling index (PCLI) and beta2 microglobulin from study registration influenced the ability to collect stem cells. Presence of active myeloma, as reflected in a higher percentage of (bone marrow) plasma cells, higher beta 2 microglobulin, and higher plasma cell labeling index, was the only factor affecting the ability to mobilize and the rate of collection.
At the time of data analysis, 34 (87%) patients had received autologous stem cell transplantation. The median time to ANC engraftment was 14 days (range; 11–21) and to platelet engraftment was 15.5 days (range; 12–38).
DISCUSSION
The results of the current trial highlights two aspects of plerixafor and GCSF based stem cell mobilization; the ability to administer the drug intravenously in a safe and effective manner and the ability of plerixafor based mobilization to overcome the adverse impact of lenalidomide based initial therapy. The current study represents the first trial specifically designed to evaluate the feasibility of intravenous administration of plerixafor for stem cell mobilization. The results of the current trial should be interpreted in the context of the previous trials evaluating the subcutaneous administration.16, 29 In the randomized trial comparing plerixafor and G-CSF to G-CSF alone in patients with myeloma undergoing peripheral blood stem cell mobilization, a similar treatment schedule and dosing was utilized.16 The proportion of patients collecting 6×106 CD34+ cells/kg in 2 days in this study was 67% (26 of 39), similar to the 71.6% seen with use of SQ plerixafor in the randomized trial. With respect to the minimal collection, 97% of the patients collected at least 3 × 106 CD34+ cells/kg, which is comparable to the 95.3% who collected at least 2 × 106 CD34+ cells/kg in 4 apheresis sessions in the randomized trial. However, the peripheral blood CD34+ cell counts were lower with IV plerixafor than those seen in the plerixafor arm of the randomized trial. Overall, the results are comparable with what has been observed previously in this patient population with the use of SQ plerixafor. In terms of toxicity, the types of toxicity and the severity were comparable with the SQ administration with gastrointestinal symptoms being the most common.
It is difficult to directly compare the results of the current study with those from the randomized trials given that less than 5% of the patients in the plerixafor arm had received lenalidomide. We have previously shown that lenalidomide therapy can adversely affect the ability to mobilize stem cells in the context of G-CSF based mobilization.11 In that study, patients who had received lenalidomide therapy for induction had a significantly lower total CD34+ cell yield, lower average daily CD34+ cell and lower CD34+ cell collection on first day, first 2 days and first 3 days and a greater number of collections compared with those receiving VAD (Vincristine, adriamycin, dexamethasone) or dexamethasone alone for induction therapy. Overall, 7% of patients failed mobilization and stem cell collection in the context of prior therapy with lenalidomide. The median CD34+ cell collection in the lenalidomide treated patients on days 1 and 2 of apheresis were 1.7 × 106 CD34+ cells/kg each, compared with 3.55 and 3.7×106 CD34+ cells/kg in the current trial, respectively. Other studies have also reported higher failure rates with G-CSF based mobilization among lenalidomide treated patients. Popat et al reported a mobilization failure (<2 × 106 CD34+ cells/kg) rate of 25% with G-CSF alone in patients who had previously received lenalidomide.14 In a case series, mobilization with G-CSF (10 mg/kg/day) alone or G-CSF (7.5 mg/kg/day) plus GM-CSF (7.5 mg/kg/day) resulted in a failure rate of 43%.13 In the series by Paripati et al, 45% of patients failed to reach the target of at least 2 × 106 CD34+ cells/kg with G-CSF (10 mg/kg/day).12 Given the difficulty seen across multiple reports, several studies have looked at the utility of plerixafor in patients receiving initial therapy with lenalidomide. In a study examining the efficacy of plerixafor among lenalidomide treated patients from across multiple studies, the overall median number of CD34+ cells collected was 5.6 × 106/kg (range, 0.45×106–37.2×106/kg).30 Of 60 patients, 52 (86.7%) had the minimum number of 2×106 CD34+ cells/kg collected, and 38 (63.3%) had 5×106/kg CD34+ cells collected. In the European compassionate use program, thirty-five patients previously treated with lenalidomide were given plerixafor plus G-CSF for remobilization.31 The overall median number of CD34+ cells collected was 3.4 × 106/kg (range: 1.1–14.8). The minimum required number of CD34+ cells (>/=2.0 × 106/kg) was collected from 69% of patients in a median of 2 days. More recently, risk adapted strategies for use of plerixafor have substantially reduced the failure rate in these patients.
Introduction of plerixafor clearly has increased the options for stem cell mobilization, enabling a substantial number of patients who otherwise would not have been able to proceed to stem cell transplantation due to failure to collect adequate stem cells, to receive the benefit of this therapy. However, this has to be viewed in the context of the cost associated with the agent, which is substantial. The high cost of the drug has led to evaluation and development of several risk-adapted therapy models, all aimed at selectively using the drug in the patients who are most likely to fail with GCSF alone. In addition, the duration of therapy with plerixafor is an important determinant of the cost and randomized controlled trials have shown the maximum benefit during the initial 2–4 days of therapy with diminishing returns beyond that time point.
In conclusion, intravenous administration is a safe and effective approach to plerixafor administration. The intravenous administration offers flexibility in patient scheduling with a schedule of early morning infusion followed by apheresis later in the day. However, prospective randomized controlled trials will have to be performed for a more accurate comparison of the pros and cons of the two routes of administration. Use of plerixafor clearly allows for effective stem cell mobilization in patients previously treated with lenalidomide, an important finding given the common use of lenalidomide for initial therapy of myeloma.
Acknowledgments
This work is supported in part by: Mayo Clinic Hematological Malignancies Program, Paul Calabresi K12 Award (CA96028), Mayo Clinic Cancer Center and the Mayo Foundation.
Footnotes
Author Contributions: SKK, BL and KL designed the study, collected and analyzed the data, and wrote the manuscript, JM, MQL, FKB, DD, MAG, TM, MM, LB, SRHG, CR, AKS, and AD contributed patients and was involved in writing the manuscript, DAG and JLW managed the apheresis unit and were involved in writing the manuscript.
Disclosures: SKK has research support for clinical trials from Celgene, Millennium, Novartis, and Genzyme and is a consultant for Merck. AD and MQL received clinical trial support from Celgene.
References
- 1.Kumar SK, Mikhael JR, Buadi FK, Dingli D, Dispenzieri A, Fonseca R, et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines. Mayo Clin Proc. 2009;84(12):1095–110. doi: 10.4065/mcp.2009.0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335(2):91–7. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 3.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. The New England journal of medicine. 2003;348(19):1875–83. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 4.Kumar S. Multiple myeloma - current issues and controversies. Cancer Treat Rev. 2010;36 (Suppl 2):S3–11. doi: 10.1016/S0305-7372(10)70006-2. [DOI] [PubMed] [Google Scholar]
- 5.Giralt S, Stadtmauer EA, Harousseau JL, Palumbo A, Bensinger W, Comenzo RL, et al. International myeloma working group (IMWG) consensus statement and guidelines regarding the current status of stem cell collection and high-dose therapy for multiple myeloma and the role of plerixafor (AMD 3100) Leukemia. 2009;23(10):1904–12. doi: 10.1038/leu.2009.127. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S, Giralt S, Stadtmauer EA, Harousseau JL, Palumbo A, Bensinger W, et al. Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell collection following initial therapy with thalidomide-, lenalidomide-, or bortezomib-containing regimens. Blood. 2009;114(9):1729–35. doi: 10.1182/blood-2009-04-205013. [DOI] [PubMed] [Google Scholar]
- 7.Sinha S, Gastineau D, Micallef I, Hogan W, Ansell S, Buadi F, et al. Predicting PBSC harvest failure using circulating CD34 levels: developing target-based cutoff points for early intervention. Bone marrow transplantation. 2011;46 (7):943–9. doi: 10.1038/bmt.2010.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knudsen LM, Rasmussen T, Jensen L, Johnsen HE. Reduced bone marrow stem cell pool and progenitor mobilisation in multiple myeloma after melphalan treatment. Med Oncol. 1999;16(4):245–54. doi: 10.1007/BF02785870. [DOI] [PubMed] [Google Scholar]
- 9.Boccadoro M, Palumbo A, Bringhen S, Merletti F, Ciccone G, Richiardi L, et al. Oral melphalan at diagnosis hampers adequate collection of peripheral blood progenitor cells in multiple myeloma. Haematologica. 2002;87(8):846–50. [PubMed] [Google Scholar]
- 10.de la Rubia J, Blade J, Lahuerta JJ, Ribera JM, Martinez R, Alegre A, et al. Effect of chemotherapy with alkylating agents on the yield of CD34+ cells in patients with multiple myeloma. Results of the Spanish Myeloma Group (GEM) Study. Haematologica. 2006;91(5):621–7. [PubMed] [Google Scholar]
- 11.Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Gastineau DA, et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21(9):2035–42. doi: 10.1038/sj.leu.2404801. [DOI] [PubMed] [Google Scholar]
- 12.Paripati H, Stewart AK, Cabou S, Dueck A, Zepeda VJ, Pirooz N, et al. Compromised stem cell mobilization following induction therapy with lenalidomide in myeloma. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2008;22(6):1282–4. doi: 10.1038/sj.leu.2405100. [DOI] [PubMed] [Google Scholar]
- 13.Mazumder A, Kaufman J, Niesvizky R, Lonial S, Vesole D, Jagannath S. Effect of lenalidomide therapy on mobilization of peripheral blood stem cells in previously untreated multiple myeloma patients. Leukemia. 2008;22(6):1280–1. doi: 10.1038/sj.leu.2405035. author reply 1281–2. [DOI] [PubMed] [Google Scholar]
- 14.Popat U, Saliba R, Thandi R, Hosing C, Qazilbash M, Anderlini P, et al. Impairment of filgrastim-induced stem cell mobilization after prior lenalidomide in patients with multiple myeloma. Biol Blood Marrow Transplant. 2009;15(6):718–23. doi: 10.1016/j.bbmt.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stiff P, Micallef I, McCarthy P, Magalhaes-Silverman M, Weisdorf D, Territo M, et al. Treatment with plerixafor in non-Hodgkin’s lymphoma and multiple myeloma patients to increase the number of peripheral blood stem cells when given a mobilizing regimen of G-CSF: implications for the heavily pretreated patient. Biol Blood Marrow Transplant. 2009;15(2):249–56. doi: 10.1016/j.bbmt.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 16.DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113(23):5720–6. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
- 17.Mohty M, Duarte RF, Croockewit S, Hubel K, Kvalheim G, Russell N. The role of plerixafor in optimizing peripheral blood stem cell mobilization for autologous stem cell transplantation. Leukemia. 2011;25(1):1–6. doi: 10.1038/leu.2010.224. [DOI] [PubMed] [Google Scholar]
- 18.Micallef IN, Sinha S, Gastineau DA, Wolf R, Inwards DJ, Gertz MA, et al. Cost-Effectiveness Analysis of a Risk-Adapted Algorithm of Plerixafor Use for Autologous Peripheral Blood Stem Cell Mobilization. Biol Blood Marrow Transplant. 2012 doi: 10.1016/j.bbmt.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Nademanee AP, DiPersio JF, Maziarz RT, Stadtmauer EA, Micallef IN, Stiff PJ, et al. Plerixafor plus granulocyte colony-stimulating factor versus placebo plus granulocyte colony-stimulating factor for mobilization of CD34(+) hematopoietic stem cells in patients with multiple myeloma and low peripheral blood CD34(+) cell count: results of a subset analysis of a randomized trial. Biol Blood Marrow Transplant. 2012;18(10):1564–72. doi: 10.1016/j.bbmt.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Hamilton E, Vaughn L, Graiser M, Renfroe H, Lechowicz MJ, et al. Effectiveness and cost analysis of “just-in-time” salvage plerixafor administration in autologous transplant patients with poor stem cell mobilization kinetics. Transfusion. 2011;51(10):2175–82. doi: 10.1111/j.1537-2995.2011.03136.x. [DOI] [PubMed] [Google Scholar]
- 21.Abhyankar S, DeJarnette S, Aljitawi O, Ganguly S, Merkel D, McGuirk J. A risk-based approach to optimize autologous hematopoietic stem cell (HSC) collection with the use of plerixafor. Bone Marrow Transplant. 2012;47(4):483–7. doi: 10.1038/bmt.2011.133. [DOI] [PubMed] [Google Scholar]
- 22.Chen AI, Bains T, Murray S, Knight R, Shoop K, Bubalo J, et al. Clinical experience with a simple algorithm for plerixafor utilization in autologous stem cell mobilization. Bone marrow transplantation. 2012;47(12):1526–9. doi: 10.1038/bmt.2012.74. [DOI] [PubMed] [Google Scholar]
- 23.Costa LJ, Abbas J, Hogan KR, Kramer C, McDonald K, Butcher CD, et al. Growth factor plus preemptive (‘just-in-time’) plerixafor successfully mobilizes hematopoietic stem cells in multiple myeloma patients despite prior lenalidomide exposure. Bone marrow transplantation. 2012;47(11):1403–8. doi: 10.1038/bmt.2012.60. [DOI] [PubMed] [Google Scholar]
- 24.Gopal AK, Karami M, Mayor J, Macebeo M, Linenberger M, Bensinger WI, et al. The effective use of plerixafor as a real-time rescue strategy for patients poorly mobilizing autologous CD34(+) cells. J Clin Apher. 2012;27(2):81–7. doi: 10.1002/jca.21206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horwitz ME, Chute JP, Gasparetto C, Long GD, McDonald C, Morris A, et al. Preemptive dosing of plerixafor given to poor stem cell mobilizers on day 5 of G-CSF administration. Bone Marrow Transplant. 2012;47(8):1051–5. doi: 10.1038/bmt.2011.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper DL, Pratt K, Baker J, Medoff E, Conkling-Walsh A, Foss F, et al. Late afternoon dosing of plerixafor for stem cell mobilization: a practical solution. Clin Lymphoma Myeloma Leuk. 2011;11(3):267–72. doi: 10.1016/j.clml.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Burgstaler EA, Winters JL. Manual color monitoring to optimize hematopoietic progenitor cell collection on the Fenwal Amicus. J Clin Apher. 2011;26(3):123–30. doi: 10.1002/jca.20280. [DOI] [PubMed] [Google Scholar]
- 28.Burgstaler EA, Pineda AA, Winters JL. Hematopoietic progenitor cell large volume leukapheresis (LVL) on the Fenwal Amicus blood separator. J Clin Apher. 2004;19(2):103–11. doi: 10.1002/jca.20011. [DOI] [PubMed] [Google Scholar]
- 29.DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27(28):4767–73. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- 30.Micallef IN, Ho AD, Klein LM, Marulkar S, Gandhi PJ, McSweeney PA. Plerixafor (Mozobil) for stem cell mobilization in patients with multiple myeloma previously treated with lenalidomide. Bone marrow transplantation. 2011;46(3):350–5. doi: 10.1038/bmt.2010.118. [DOI] [PubMed] [Google Scholar]
- 31.Malard F, Kroger N, Gabriel IH, Hubel K, Apperley JF, Basak GW, et al. Plerixafor for autologous peripheral blood stem cell mobilization in patients previously treated with fludarabine or lenalidomide. Biol Blood Marrow Transplant. 2012;18(2):314–7. doi: 10.1016/j.bbmt.2011.10.003. [DOI] [PubMed] [Google Scholar]