Abstract
Ceramide is a precursor of complex sphingolipids and also plays important roles in cell signaling. With the advances in lipid analytical technologies, the structural diversity of ceramide species have become evident, and the complexity of cellular metabolism and function associated with distinct ceramide species is beginning to be revealed. One of the common structural variations of ceramide is 2′-hydroxylation of the N-acyl chain. Fatty acid 2-hydroxylase (FA2H) is one of the enzymes that introduce the hydroxyl group during de novo synthesis of ceramide. FA2H is essential for the normal functioning of the nervous system, as evidenced by demyelinating disorder associated with FA2H mutations in humans and mice. Studies of Fa2h mutant mice indicate that lack of 2′-hydroxy galactosylceramide in the myelin membrane results in loss of long-term stability of myelin and eventual demyelination. FA2H also regulates differentiation of various cell types (epidermal keratinocytes, schwannoma cells, adipocytes). When provided exogenously, ceramide induces apoptosis in many cell types. Interestingly, the effective concentration of 2′-hydroxy ceramide that induces apoptosis is significantly lower compared to non-hydroxy ceramide, and cells die much more rapidly, suggesting that 2′-hydroxy ceramide can mediate proapoptotic signaling distinct from non-hydroxy ceramide. Collectively, current evidence clearly shows that 2′-hydroxy ceramide and 2′-hydroxy complex sphingolipids have unique functions in membrane homeostasis and cell signaling that could not be substituted by non-hydroxy counterparts.
Keywords: Ceramide, 2-hydroxy-ceramide, sphingolipids, 2-hydroxy-sphingolipids
Introduction
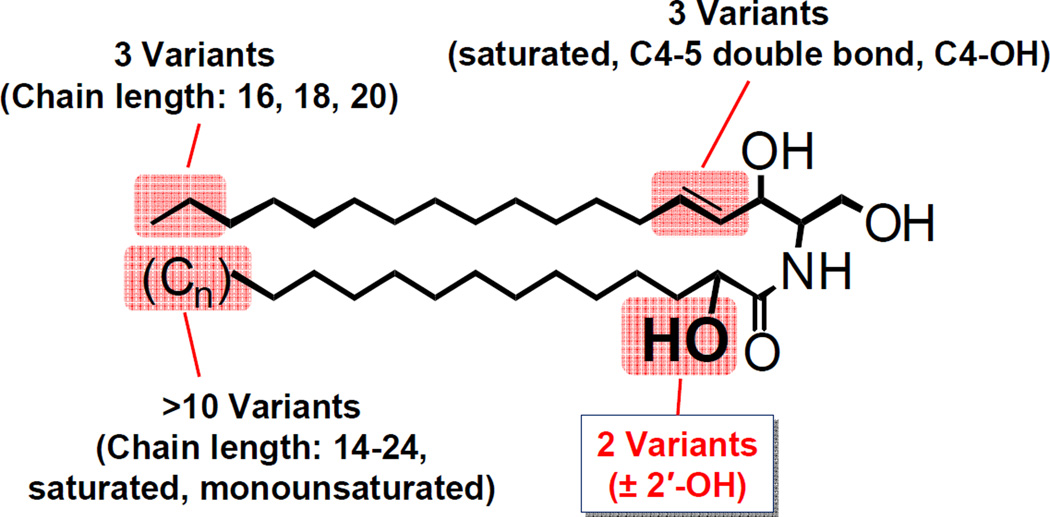
The sphingolipid ceramide is a precursor of all complex sphingolipids as well as an essential component of sphingolipid-mediated cell signaling. Ceramide is composed of a long-chain base and an amide-linked fatty acid (the N-acyl chain). Both the long-chain base and the N-acyl chain are structurally highly diverse (Fig. 1). Commonly found ceramide species in mammalian cells contain a long-chain base of 16–20 carbons in length that can be saturated, C4–5 unsaturated, or C4-hydroxylated. The N-acyl chain is mostly 14–24 carbons in length and saturated or monounsaturated. Some of the ceramide species also contain 2′-hydroxy N-acyl chain, which are highly abundant in the brain and in the epidermis of the skin. Further complexity exists in ceramide species in the epidermis, testis and epididymal spermatozoa. The long-chain base in epidermal ceramide can be 12–28 carbons in length, some of which contain C6 hydroxyl group (Robson et al., 1994; Stewart and Downing, 1999). The epidermal ceramides also contain very unique ultra-long-chain fatty acids (28–36 carbons) with omega-hydroxyl group, which is either esterified with linoleic acid or linked to proteins (Breiden and Sandhoff, 2013; Madison, 2003; Rabionet et al., 2013). The unique ceramides in testis and epididymal spermatozoa contain polyunsaturated N-acyl chain of 27–32 carbons with or without 2′-hydroxyl group (Zanetti et al., 2010). Thus, there can be hundreds of different ceramide molecular species present in a single cell. Although the reason for the extreme diversity in ceramide molecular species is not fully understood, current evidence indicates that many of the structural modifications are associated with distinct biological activities. This review will focus on 2′-hydroxylation of the N-acyl chain and describe recent advances in our understanding of the biosynthesis and function of 2′-hydroxy ceramide.
Fig. 1.
Common ceramide species in mammalian cells. The structural variations of ceramide species commonly found in most mammalian cells are shown. Additional diversity exists in unique ceramide species in the epidermis, testis, and epididymal spermatozoa (see text).
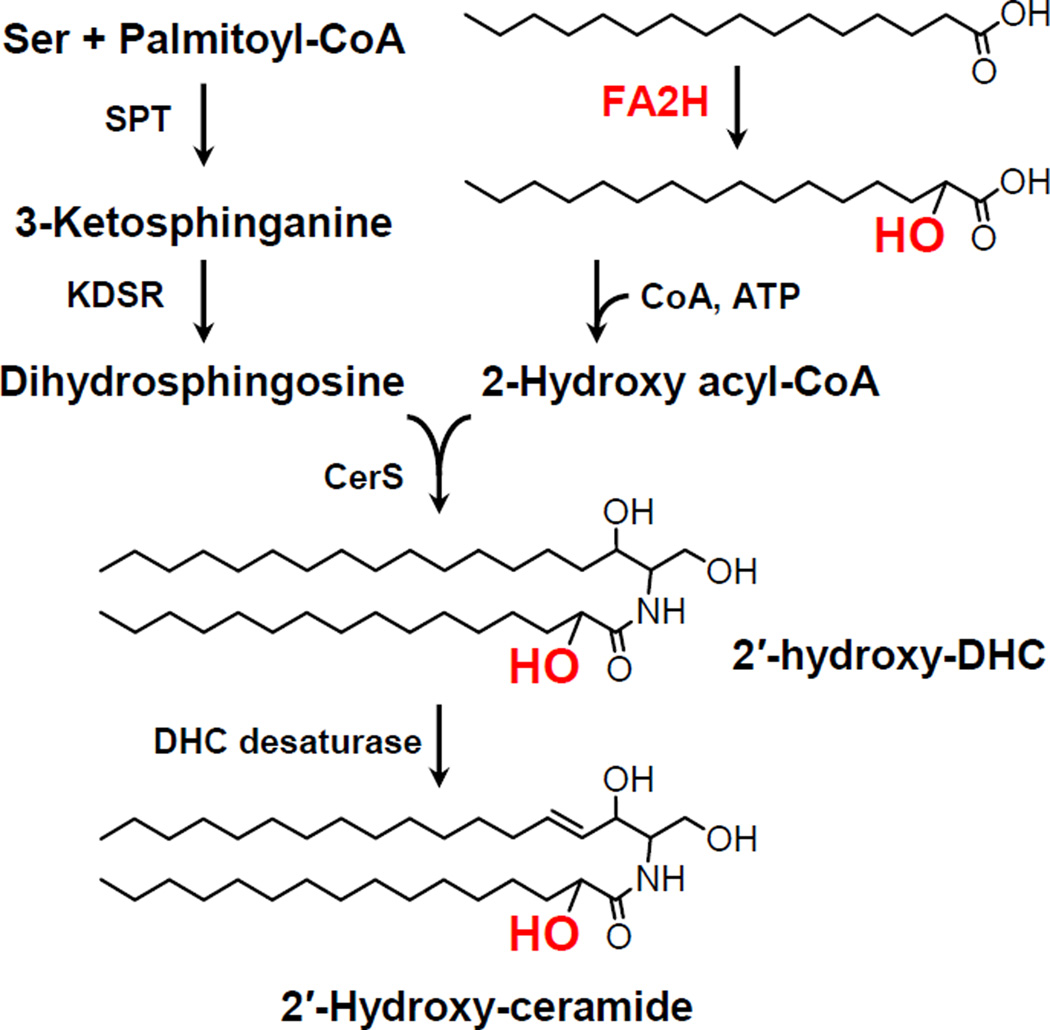
Synthesis of 2′-hydroxy ceramide
In the first step of de novo synthesis of ceramide, serine and a fatty acid (primarily palmitate) are condensed to 3-keto-dihydrosphingosine by serine palmitoyltrasnferase (SPT), and then reduced to dihydrosphingosine by 3-keto-dihydrosphingosine reductase (KDSR). Dihydroceramide synthase (CerS) catalyzes the N-acylation of dihydrosphingosine to form dihydroceramide using acyl-CoA. The 2′-hydroxyl group can be introduced in this step to form 2′-dihydroceramide. All six isoforms of CerS can utilize 2-hydroxy acyl-CoA to synthesize 2′-hydroxy dihydroceramide (Mizutani et al., 2008). Fatty acid 2-hydroxylase (FA2H) is one of the enzymes that supply 2-hydroxy fatty acids (Alderson et al., 2004) (Fig. 2).
Fig. 2.
De novo synthesis of 2′-hydroxy ceramide. SPT, serine palmitoyltransferase; KDSR, 3-keto-dihydrosphingosine reductase; CerS, dihydroceramide synthase; DHC, dihydroceramide.
Primary cells from patients with FA2H deficiency provided an opportunity to study FA2H-independent synthesis of 2′-hydroxy sphingolipids. FA2H-deficient fibroblasts, lymphocytes, and erythrocytes all contained 2′-hydroxy sphingomyelin, indicating there is at least one other 2-hydroxylase (Dan et al., 2011). Identity of the second enzyme is currently unknown, and the substrate for the 2-hydroxylation has not been determined.
Upon 2-hydroxylation of a fatty acid, the C2 carbon becomes chiral, creating two possible stereoisomers, 2R- and 2S-hydroxy fatty acid. Interestingly, the N-acyl chain of mammalian 2′-hydroxy sphingolipids were reported to be exclusively in the 2′R configuration (Karlsson et al., 1969; Mislow and Bleicher, 1954). This stereo-specificity is consistent with the sterospecific 2-hydroxylation by FA2H (Guo et al., 2012). There is some evidence of 2′S-hydroxy fatty acid in mammalian sphingolipids in foodstuff (Jenske and Vetter, 2008). The mechanism of enzymatic synthesis of the 2′S isomer is currently unknown.
2′-Hydroxy-ceramides/sphingolipids in the nervous system
In the legendary 1884 publication “A Treatise on the Chemical Constitution of the Brain” Thudicum noted high concentrations of a group of lipids containing nitrogen and sugar, but no phosphorus, which he named cerebrosides (Thudicum, 1884). Although the precise structures of cerebrosides were not known at the time, Thudicum did determine that the most abundant cerebroside contained a 2-hydroxy fatty acid. Later it was determined to be galactosylceramide (GalCer) with 2-hydroxy tetracosanoic acid (Klenk, 1928). In the following decades, additional 2′-hydroxy GalCer species with other fatty acids differing in chain lengths and desaturation were identified [see (Deuel, 1951) for review of early studies]. These sphingolipids are the main components of mammalian myelin in both the central nervous system (CNS) and the peripheral nervous system (PNS).
As mentioned above, there is redundancy in 2-hydroxylase enzymes in most tissues. An exception is myelin-forming cells (oligodendrocytes in the CNS and Schwann cells in the PNS), which exclusively depend on FA2H for the production of 2′-hydroxy GalCer as demonstrated by the discovery of FA2H deficiency in 2008 (Edvardson et al., 2008). Children with mutations in the FA2H gene developed leukodystrophy and spastic paraparesis. Since then, several groups have reported various FA2H mutations/deletions in a total of 35 cases as of this writing (Cao et al., 2013; Dick et al., 2010; Donkervoort et al., 2013; Garone et al., 2011; Kruer et al., 2010; Pierson et al., 2012; Rupps et al., 2012; Tonelli et al., 2012). In most cases, affected children develop normally until 2–6 years of age, and then start to exhibit frequent falls and walking difficulties. Progressive spasticity follows, and they eventually lose ability to move and communicate, eventually leading to death. Interestingly, initial developmental myelination appears unaffected, and PNS myelin is less affected in the early stage of the disease. These observations indicate that 2′-hydroxy GalCer is dispensable for the myelination process but critical for the long-term stability of myelin.
A timely report on Fa2h knockout mice corroborated the findings in FA2H deficiency (Zoller et al., 2008). Myelin in Fa2h knockout mice were devoid of 2′-hydroxy GalCer. While such myelin appears morphologically and functionally indistinguishable from normal myelin of wild type mice, its long-term stability is compromised, leading to eventual demyelination. Using cell type-specific Fa2h mutant (Fa2hflox/flox Cnp1-Cre) mice, Potter et al. demonstrated that the CNS demyelination and neuronal cell loss was caused by loss of Fa2h in oligodendrocytes, and therefore resulted from abnormalities in myelin lipids (Potter et al., 2011). Further, Fa2h null mice had additional deficits in spatial learning and memory that were not present in the cell type-specific mutants (summarized in Table 1). These findings indicate that a major role of FA2H in the nervous system is to produce 2′-hydroxy GalCer in oligodendrocytes to confer structural stability to myelin sheath. The mouse phenotypes also indicate that FA2H plays a role in non-oligodendrocytes involved in the learning and memory function of the brain. An intriguing possibility is that 2′-hydroxy ceramide/sphingolipids regulate neurogenesis or neuronal connectivity, which could be tested using neuron-specific Fa2h mutant mice.
Table 1.
CNS phenotypes of Fa2h null mice and conditional mutant lacking Fa2h in oligodendrocytes*
| CNS Phenotype | Fa2h−/− | Fa2hflox/flox Cnp1-Cre |
|---|---|---|
| Loss hFA-GalCer | Yes | Yes |
| Demyelination | Yes | Yes |
| Cerebellar Purkinje cell loss | Yes | Yes |
| Cerebellar dysfunction | Yes | Yes |
| Learning and memory deficits | Yes | No |
Data from (Potter et al., 2011)
The idea that 2′-hydroxy GalCer confers structural stability to myelin membrane is supported by the current knowledge about 2′-hydroxy ceramide/GalCer in lipid packing. Several biophysical studies used synthetic GalCer and model membranes to show how 2′-hydroxyl group affects lipid-lipid interactions. The hydroxyl group is located near the polar region of the membrane where it can form hydrogen bonds with neighboring lipids and the polar head group (Pascher and Sundell, 1977). The 2′-hydroxyl group facilitates tight lipid packing via a network of hydrogen bonds, thereby stabilizes the gel phase of the lipids (Boggs et al., 1988; Lofgren and Pascher, 1977; Pascher, 1976). The 2′-hydroxyl group also enhances carbohydrate-carbohydrate interactions between the head groups of galactolipids on apposing membranes (Stewart and Boggs, 1993). In the absence of 2′-hydroxy GalCer, lipid packing within myelin would be less tight, possibly making myelin more susceptible to chemical and physical insults such as inflammation and mechanical damage. It is of note that one of the patients with FA2H deficiency “developed normally until age three, when he suffered a febrile illness and afterwards ‘started tripping often’” (Pierson et al., 2012). It is conceivable that neuroinflammation triggered demyelination in this patient.
Hydroxy-ceramide in cell signaling
The concept of ceramide as a signaling molecule was established during the 1990s. While a large number of studies have shown various roles of ceramide in numerous cellular processes, 2′-hydroxy ceramide had been largely dismissed as nonexistent in most cells, and, when examined, it was inactive in inducing apoptosis (Ji et al., 1995). This landscape is gradually changing. A number of tissues and cell types have been shown to contain 2′-hydroxy ceramide and 2′-hydroxy complex sphingolipids (Hama, 2010). In most cells, however, it is unknown whether 2′-hydroxy ceramide and its metabolites play a role in cell signaling. Below are recent developments that indicate involvement of FA2H in cell differentiation (summarized in Table 2).
Table 2.
Effects of FA2H knockdown on cellular phenotypes
| Cell type | Phenotype | Reference |
|---|---|---|
| Cultured keratinocytes | Partial loss of 2′-hydroxy ceramide | |
| Aberrant lamellar body formation | (Uchida et al., 2007) | |
| Loss of secreted lamellar materials | ||
| D6P2T schwannoma | Partial loss of 2′-hydroxy ceramide | (Maldonado et al., 2008) |
| Enhanced migratory property | ||
| Impaired cAMP-induced cell cycle exit | (Alderson and Hama, 2009) | |
| 3T3-L1 adipocyte | Diminished adipocyte marker expression | |
| Impaired glucose uptake and lipogenesis | ||
| Enhanced diffusional mobility of raft-associated lipids | (Guo et al., 2010) | |
| Increased GLUT4 endocytosis | ||
First evidence for the involvement of FA2H in cell differentiation was reported in a study of epidermal keratinocytes (Uchida et al., 2007). As mentioned above, 2′-hydroxy ceramide is a major component of the cornified layer of the epidermis. Uchida et al. showed that FA2H was highly upregulated during differentiation of cultured human keratinocytes, and that FA2H knockdown resulted in a reduced proportion of 2′-hydroxy ceramide. Unexpectedly, FA2H knockdown also resulted in formation of abnormal epidermal lamellar bodies, and the keratinocytes failed to form the extracellular lamellar membranes. These findings indicate that FA2H not only supply the precursor for skin 2′-hydroxy ceramide but also regulates the differentiation process in keratinocytes. The mechanism for this regulation remains unknown.
Schwann cells are the myelin-forming cells of the peripheral nervous system. Cultured Schwann cells upregulate the genes involved in producing myelin components when treated with cAMP. We reported that Fa2h expression was upregulated by cAMP treatment in primary rat Schwann cells (Maldonado et al., 2008). Interestingly, the level of upregulation was an order of magnitude greater compared to other myelin-associated genes Cgt and P0, suggesting that FA2H may have an additional function other than producing myelin lipids. A subsequent study of D6P2T schwannoma cells showed evidence for a role of FA2H in cell differentiation (Alderson and Hama, 2009). As Schwann cells, D6P2T cells respond to cAMP and show differentiated phenotypes, including withdrawal from the cell cycle and upregulation of myelin basic protein (Clark et al., 1998; Friessen et al., 1997). In this process, the cyclin-dependent kinase inhibitors p21 and p27 are upregulated in response to cAMP (Atanasoski et al., 2006; Friessen et al., 1997). In our study, the upregulation of p21 and p27 by cAMP treatment was greatly diminished by Fa2h knockdown. This result indicates that cAMP-induced upregulation of these genes is facilitated by elevated 2′-hydroxy-ceramide (or its metabolite) resulting from concomitant upregulation of FA2H.
Guo et al. reported strong evidence that FA2H mediates adipocyte differentiation (Guo et al., 2010). Fa2h expression increases during hormone-induced differentiation in 3T3-L1 adipocytes along with other genes involved in lipogenesis. Fa2h knockdown had striking effects in this process; expression of adipocyte markers was diminished, and accumulation of triacylglycerol was blocked. In mature adipocytes, Fa2h knockdown inhibited basal and insulin-stimulated glucose uptake and lipogenesis. These effects were attributed, at least in part, to enhanced diffusional mobility of raft-associated lipids in the plasma membrane, which was associated with facilitated endocytosis of GLUT4 glucose transporter. As the authors suggested, it is also possible that FA2H regulates signaling pathways and/or transcription factors necessary for adipocyte differentiation.
The aforementioned studies on cell differentiation did not identify specific 2′-hydroxy lipids that mediate the biological activities of FA2H. In a recent study, we focused on the growth inhibitory activity of exogenously provided 2′-hydroxy ceramide. In an earlier study Kyogashima et al. demonstrated higher pro-apoptotic activity of mixed 2′-hydroxy ceramide fraction isolated from equine kidneys compared to non-hydroxy ceramide fractions (Kyogashima et al., 2008). Szulc et al. showed that synthetic 2R′-hydroxy-C6-ceramide showed stronger growth inhibitory activity against MCF7 cells compared to the 2′S isomer or non-hydroxy-C6-ceramide (Szulc et al., 2010), though the mechanism of the growth inhibition was not determined. Using synthetic 2′R- and 2′S-hydroxy-C16-ceramide, we demonstrated that proapoptotic activity of 2′R isomer was much more potent compared to the 2′S isomer or non-hydroxy C16-ceramide (Kota et al., 2013). Treatment with 2′R-hydroxy-C16-ceramide induced rapid dephosphorylation of Akt and MAP kinases, while the 2′S isomer and non-hydroxy C16-ceramide did not induce dephosphorylation at the same concentrations. These findings strongly suggest that 2′R-hydroxy ceramide induces apoptosis via specific cellular targets, possibly protein phosphatases.
In the recent years, a tantalizing research area on 2-hydroxyoleic acid (2-OHOA) has emerged. When exogenously provided, 2-OHOA shows remarkable biological activities. It suppresses glioma cell growth and induces differentiation in vitro and in vivo (Barcelo-Coblijn et al., 2011; Teres et al., 2012). It also induces ER stress and autophagy in tumor cells (Marcilla-Etxenike et al., 2012). Many of its biological activities are explained by altered plasma membrane lipid composition and aberrant sphingolipid metabolism (Martin et al., 2013a). At least one sphingolipid enzyme (sphingomyelin synthase) is directly activated by 2-OHOA (Barcelo-Coblijn et al., 2011; Martin et al., 2013b). It is plausible to speculate that some of the actions of 2-OHOA is due to 2′-hydroxy-C18:1-ceramide produced upon 2-OHOA administration.
Concluding remark
In the past several years, evidence has accumulated that 2′-hydroxy ceramide/sphingolipids have distinct biological functions to regulate various cellular processes. The works summarized above point to two modes of action. First, physical properties of cell membranes are modulated by 2′-hydroxy ceramide/sphingolipids. This is evident from the findings in myelin of Fa2h null mice and the plasma membrane properties of Fa2h-knockdown adipocytes. Second, 2′-hydroxy ceramide likely regulate cell differentiation and apoptosis by binding to specific target proteins. Identifying such target proteins will be a key to understanding the mechanism of cell regulation by 2′-hydroxy ceramide.
Acknowledgement
The work from the author’s laboratory was supported by National Institutes of Health Grants R01NS060807, P30CA138313, and P20RR017677.
Abbreviations
- FA2H
fatty acid 2-hydroxylase
- SPT
serine palmitoyltransferase
- CerS
dihydroceramide synthase
- GalCer
galactosylceramide
- CNS
central nervous system
- PNS
peripheral nervous system
- 2-OHOA
2-hydroxyoleic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alderson NL, Hama H. Fatty acid 2-hydroxylase regulates cAMP-induced cell cycle exit in D6P2T Schwannoma cells. J Lipid Res. 2009;50:1203–1208. doi: 10.1194/jlr.M800666-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson NL, Rembiesa BM, Walla MD, Bielawska A, Bielawski J, Hama H. The human FA2H gene encodes a fatty acid 2-hydroxylase. J Biol Chem. 2004;279:48562–48568. doi: 10.1074/jbc.M406649200. [DOI] [PubMed] [Google Scholar]
- Atanasoski S, Boller D, De Ventura L, Koegel H, Boentert M, Young P, Werner S, Suter U. Cell cycle inhibitors p21 and p16 are required for the regulation of Schwann cell proliferation. Glia. 2006;53:147–157. doi: 10.1002/glia.20263. [DOI] [PubMed] [Google Scholar]
- Barcelo-Coblijn G, Martin ML, De Almeida RF, Noguera-Salva MA, Marcilla-Etxenike A, Guardiola-Serrano F, Luth A, Kleuser B, Halver JE, Escriba PV. Sphingomyelin and sphingomyelin synthase (SMS) in the malignant transformation of glioma cells and in 2-hydroxyoleic acid therapy. Proc Natl Acad Sci U S A. 2011;108:19569–19574. doi: 10.1073/pnas.1115484108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs JM, Koshy KM, Rangaraj G. Influence of structural modifications on the phase behavior of semi- synthetic cerebroside sulfate. Biochim. Biophys. Acta. 1988;938:361–372. doi: 10.1016/0005-2736(88)90134-4. [DOI] [PubMed] [Google Scholar]
- Breiden B, Sandhoff K. The role of sphingolipid metabolism in cutaneous permeabilitybarrier formation. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbalip.2013.08.010. in press. [DOI] [PubMed] [Google Scholar]
- Cao L, Huang XJ, Chen CJ, Chen SD. A rare family with Hereditary Spastic Paraplegia Type 35 due to novel FA2H mutations: a case report with literature review. J Neurol Sci. 2013;329:1–5. doi: 10.1016/j.jns.2013.02.026. [DOI] [PubMed] [Google Scholar]
- Clark R, Stewart M, Miskimins WK, Miskimins R. Involvement of MAP kinase in the cyclic AMP induction of myelin basic protein gene expression. Int J Dev Neurosci. 1998;16:323–331. doi: 10.1016/s0736-5748(98)00045-8. [DOI] [PubMed] [Google Scholar]
- Dan P, Edvardson S, Bielawski J, Hama H, Saada A. 2-Hydroxylated sphingomyelin profiles in cells from patients with mutated fatty acid 2-hydroxylase. Lipids Health Dis. 2011;10:84. doi: 10.1186/1476-511X-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel HJ. Chemistry of the Phosphatides and Cerebrosides. In: Deuel HJ, editor. The Lipids, Their Chemistry and Biochemistry. New York: Interscience Publishers; 1951. pp. 405–496. [Google Scholar]
- Dick KJ, Eckhardt M, Paisan-Ruiz C, Alshehhi AA, Proukakis C, Sibtain NA, Maier H, Sharifi R, Patton MA, Bashir W, Koul R, Raeburn S, Gieselmann V, Houlden H, Crosby AH. Mutation of FA2H underlies a complicated form of hereditary spastic paraplegia (SPG35) Hum Mutat. 2010;31:E1251–E1260. doi: 10.1002/humu.21205. [DOI] [PubMed] [Google Scholar]
- Donkervoort S, Dastgir J, Hu Y, Zein W, Marks H, Blackstone C, Bonnemann C. Phenotypic variability of a likely FA2H founder mutation in a family with complicated hereditary spastic paraplegia. Clin Genet. 2013 doi: 10.1111/cge.12185. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvardson S, Hama H, Shaag A, Gomori JM, Berger I, Soffer D, Korman SH, Taustein I, Saada A, Elpeleg O. Mutations in the fatty acid 2-hydroxylase gene are associated with leukodystrophy with spastic paraparesis and dystonia. Am J Hum Genet. 2008;83:643–648. doi: 10.1016/j.ajhg.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friessen AJ, Miskimins WK, Miskimins R. Cyclin-dependent kinase inhibitor p27kip1 is expressed at high levels in cells that express a myelinating phenotype. J Neurosci Res. 1997;50:373–382. doi: 10.1002/(SICI)1097-4547(19971101)50:3<373::AID-JNR3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Garone C, Pippucci T, Cordelli DM, Zuntini R, Castegnaro G, Marconi C, Graziano C, Marchiani V, Verrotti A, Seri M, Franzoni E. FA2H-related disorders: a novel c.270+3A>T splice-site mutation leads to a complex neurodegenerative phenotype. Dev Med Child Neurol. 2011;53:958–961. doi: 10.1111/j.1469-8749.2011.03993.x. [DOI] [PubMed] [Google Scholar]
- Guo L, Zhang X, Zhou D, Okunade AL, Su X. Stereospecificity of fatty acid 2-hydroxylase and differential functions of 2-hydroxy fatty acid enantiomers. J Lipid Res. 2012;53:1327–1335. doi: 10.1194/jlr.M025742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Zhou D, Pryse KM, Okunade AL, Su X. Fatty acid 2-hydroxylase mediates diffusional mobility of Raft-associated lipids, GLUT4 level, and lipogenesis in 3T3-L1 adipocytes. J Biol Chem. 2010;285:25438–25447. doi: 10.1074/jbc.M110.119933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H. Fatty acid 2-Hydroxylation in mammalian sphingolipid biology. Biochim Biophys Acta. 2010;1801:405–414. doi: 10.1016/j.bbalip.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenske R, Vetter W. Enantioselective analysis of 2- and 3-hydroxy fatty acids in food samples. J Agric Food Chem. 2008;56:11578–11583. doi: 10.1021/jf802772a. [DOI] [PubMed] [Google Scholar]
- Ji L, Zhang G, Uematsu S, Akahori Y, Hirabayashi Y. Induction of apoptotic DNA fragmentation and cell death by natural ceramide. FEBS Lett. 1995;358:211–214. doi: 10.1016/0014-5793(94)01428-4. [DOI] [PubMed] [Google Scholar]
- Karlsson K, Nilsson K, Samuelsson BE, Steen GO. The presence of hydroxy fatty acids in sphingomyelins of bovine rennet stomach. Biochim Biophys Acta. 1969;176:660–663. doi: 10.1016/0005-2760(69)90239-2. [DOI] [PubMed] [Google Scholar]
- Klenk E, et al. Über die Cerebronsäure. (9. Mitteilung über Cerebroside.). Hoppe Seylers Z. Physiol. Chem. 1928;179:312–319. [Google Scholar]
- Kota V, Dhople VM, Fullbright G, Smythe NM, Szulc ZM, Bielawska A, Hama H. 2 -Hydroxy C16-ceramide induces apoptosis-associated proteomic changes in C6 glioma cells. J Proteome Res. 2013 doi: 10.1021/pr4003432. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruer MC, Paisan-Ruiz C, Boddaert N, Yoon MY, Hama H, Gregory A, Malandrini A, Woltjer RL, Munnich A, Gobin S, Polster BJ, Palmeri S, Edvardson S, Hardy J, Houlden H, Hayflick SJ. Defective FA2H leads to a novel form of neurodegeneration with brain iron accumulation (NBIA) Ann Neurol. 2010;68:611–618. doi: 10.1002/ana.22122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyogashima M, Tadano-Aritomi K, Aoyama T, Yusa A, Goto Y, Tamiya-Koizumi K, Ito H, Murate T, Kannagi R, Hara A. Chemical and apoptotic properties of hydroxy-ceramides containing long-chain bases with unusual alkyl chain lengths. J Biochem. 2008;144:95–106. doi: 10.1093/jb/mvn050. [DOI] [PubMed] [Google Scholar]
- Lofgren H, Pascher I. Molecular arrangements of sphingolipids. The monolayer behavior of ceramides. Chem. Phys. Lipids. 1977;20:273–284. doi: 10.1016/0009-3084(77)90068-8. [DOI] [PubMed] [Google Scholar]
- Madison KC. Barrier function of the skin: "la raison d'etre" of the epidermis. J Invest Dermatol. 2003;121:231–241. doi: 10.1046/j.1523-1747.2003.12359.x. [DOI] [PubMed] [Google Scholar]
- Maldonado EN, Alderson NL, Monje PV, Wood PM, Hama H. FA2H is responsible for the formation of 2-hydroxy galactolipids in peripheral nervous system myelin. J Lipid Res. 2008;49:153–161. doi: 10.1194/jlr.M700400-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcilla-Etxenike A, Martin ML, Noguera-Salva MA, Garcia-Verdugo JM, Soriano-Navarro M, Dey I, Escriba PV, Busquets X. 2-Hydroxyoleic acid induces ER stress and autophagy in various human glioma cell lines. PLoS One. 2012;7:e48235. doi: 10.1371/journal.pone.0048235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin ML, Barcelo-Coblijn G, De Almeida RF, Noguera-Salva MA, Teres S, Higuera M, Liebisch G, Schmitz G, Busquets X, Escriba PV. The role of membrane fatty acid remodeling in the antitumor mechanism of action of 2-hydroxyoleic acid. Biochim Biophys Acta. 2013a;1828:1405–1413. doi: 10.1016/j.bbamem.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Martin ML, Liebisch G, Lehneis S, Schmitz G, Alonso-Sande M, Bestard-Escalas J, Lopez DH, Garcia-Verdugo JM, Soriano-Navarro M, Busquets X, Escriba PV, Barcelo-Coblijn G. Sustained activation of sphingomyelin synthase by 2-hydroxyoleic acid induces sphingolipidosis in tumor cells. J Lipid Res. 2013b;54:1457–1465. doi: 10.1194/jlr.M036749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mislow K, Bleicher S. The configuration of cerebronic acid. J. Am. Chem. Soc. 1954;76:2825–2826. [Google Scholar]
- Mizutani Y, Kihara A, Chiba H, Tojo H, Igarashi Y. 2-Hydroxy-ceramide synthesis by ceramide synthase family: enzymatic basis for the preference of FA chain length. J Lipid Res. 2008;49:2356–2364. doi: 10.1194/jlr.M800158-JLR200. [DOI] [PubMed] [Google Scholar]
- Pascher I. Molecular arrangements in sphingolipids. Conformation and hydrogen bonding of ceramide and their implication on membrane stability and permeability. Biochim. Biophys. Acta. 1976;455:433–451. doi: 10.1016/0005-2736(76)90316-3. [DOI] [PubMed] [Google Scholar]
- Pascher I, Sundell S. Molecular arrangements in sphingolipids. The crystal structure of cerebroside. Chem. Phys. Lipids. 1977;20:175–191. [Google Scholar]
- Pierson TM, Simeonov DR, Sincan M, Adams DA, Markello T, Golas G, Fuentes-Fajardo K, Hansen NF, Cherukuri PF, Cruz P, Mullikin JC, Blackstone C, Tifft C, Boerkoel CF, Gahl WA. Exome sequencing and SNP analysis detect novel compound heterozygosity in fatty acid hydroxylase-associated neurodegeneration. Eur J Hum Genet. 2012;20:476–479. doi: 10.1038/ejhg.2011.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter KA, Kern MJ, Fullbright G, Bielawski J, Scherer SS, Yum SW, Li JJ, Cheng H, Han X, Venkata JK, Khan PA, Rohrer B, Hama H. Central nervous system dysfunction in a mouse model of FA2H deficiency. Glia. 2011;59:1009–1021. doi: 10.1002/glia.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabionet M, Gorgas K, Sandhoff R. Ceramide synthesis in the epidermis. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbalip.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Robson KJ, Stewart ME, Michelsen S, Lazo ND, Downing DT. 6-Hydroxy-4-sphingenine in human epidermal ceramides. J Lipid Res. 1994;35:2060–2068. [PubMed] [Google Scholar]
- Rupps R, Hukin J, Balicki M, Mercimek-Mahmutoglu S, Rolfs A, Dias C. Novel Mutations in FA2H-Associated Neurodegeneration: An Underrecognized Condition? J Child Neurol. 2012 doi: 10.1177/0883073812458538. [DOI] [PubMed] [Google Scholar]
- Stewart ME, Downing DT. A new 6-hydroxy-4-sphingenine-containing ceramide in human skin. J Lipid Res. 1999;40:1434–1439. [PubMed] [Google Scholar]
- Stewart RJ, Boggs JM. A carbohydrate-carbohydrate interaction between galactosylceramide-containing liposomes and cerebroside sulfate-containing liposomes: dependence on the glycolipid ceramide composition. Biochemistry. 1993;32:10666–10674. doi: 10.1021/bi00091a017. [DOI] [PubMed] [Google Scholar]
- Szulc ZM, Bai A, Bielawski J, Mayroo N, Miller DE, Gracz H, Hannun YA, Bielawska A. Synthesis, NMR characterization and divergent biological actions of 2'-hydroxyceramide/dihydroceramide stereoisomers in MCF7 cells. Bioorg. Med. Chem. 2010;18:7565–7579. doi: 10.1016/j.bmc.2010.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teres S, Llado V, Higuera M, Barcelo-Coblijn G, Martin ML, Noguera-Salva MA, Marcilla-Etxenike A, Garcia-Verdugo JM, Soriano-Navarro M, Saus C, Gomez-Pinedo U, Busquets X, Escriba PV. 2-Hydroxyoleate, a nontoxic membrane binding anticancer drug, induces glioma cell differentiation and autophagy. Proc Natl Acad Sci U S A. 2012;109:8489–8494. doi: 10.1073/pnas.1118349109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thudicum JLW. A treatise on the chemical composition of the brain. London: Bailliere, Tindall, and Cox; 1884. [Google Scholar]
- Tonelli A, D'angelo MG, Arrigoni F, Brighina E, Arnoldi A, Citterio A, Bresolin N, Bassi MT. Atypical adult onset complicated spastic paraparesis with thin corpus callosum in two patients carrying a novel FA2H mutation. Eur J Neurol. 2012;19:e127–e129. doi: 10.1111/j.1468-1331.2012.03838.x. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Hama H, Alderson NL, Douangpanya S, Wang Y, Crumrine DA, Elias PM, Holleran WM. Fatty acid 2-hydroxylase, encoded by FA2H, accounts for differentiation-associated increase in 2-OH ceramides during keratinocyte differentiation. J Biol Chem. 2007;282:13211–13219. doi: 10.1074/jbc.M611562200. [DOI] [PubMed] [Google Scholar]
- Zanetti SR, De Los Angeles Monclus M, Rensetti DE, Fornes MW, Aveldano MI. Ceramides with 2-hydroxylated, very long-chain polyenoic fatty acids in rodents: From testis to fertilization-competent spermatozoa. Biochimie. 2010;92:1778–1786. doi: 10.1016/j.biochi.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Zoller I, Meixner M, Hartmann D, Bussow H, Meyer R, Gieselmann V, Eckhardt M. Absence of 2-hydroxylated sphingolipids is compatible with normal neural development but causes late-onset axon and myelin sheath degeneration. J Neurosci. 2008;28:9741–9754. doi: 10.1523/JNEUROSCI.0458-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]