Abstract
Purpose
Ewing sarcoma is a tumor of the bone and soft tissue characterized by diffuse cell membrane expression of CD99 (MIC2). Single-site, surgically resectable disease is associated with an excellent 5-year event-free survival; conversely, patients with distant metastases have a poor prognosis. Non-invasive imaging is the standard approach to identifying sites of metastatic disease. We sought to develop a CD99-targeted imaging agent for staging Ewing sarcoma and other CD99-expressing tumors.
Experimental Design
We identified a CD99 antibody with highly specific binding in vitro and labeled this antibody with 64Cu. Mice with either subcutaneous Ewing sarcoma xenograft tumors or micrometastases were imaged with the 64Cu-labeled anti-CD99 antibody and these results were compared to conventional MRI and FDG-PET imaging.
Results
64Cu-labeled anti-CD99 antibody demonstrated high avidity for the CD99 positive subcutaneous tumors, with a high tumor-to-background ratio, greater than that demonstrated with FDG-PET. Micrometastases, measuring 1-2 mm on MRI, were not detected with FDG-PET but readily visualized with the 64Cu-labeled anti-CD99 antibody. Probe biodistribution studies demonstrated high specificity of the probe for CD99 positive tumors.
Conclusions
64Cu-labeled anti-CD99 antibody can detect subcutaneous Ewing sarcoma tumors and metastatic sites with high sensitivity, outperforming FDG-PET in preclinical studies. This targeted radiotracer may have important implications for the diagnosis, surveillance, and treatment of Ewing sarcoma. Similarly, it may impact the management of other CD99 positive tumors.
Introduction
Ewing sarcoma is a tumor of the bone or soft tissue affecting approximately 250 children, adolescents, and young adults each year.(1, 2) Patients with localized disease are treated with chemotherapy, surgery for local control, and radiotherapy in patients for whom surgical margins remain positive.(3) For patients with metastatic disease, chemotherapy and radiotherapy are the mainstays of treatment.(4) While patients with localized disease have a good prognosis with 70% 5-year event-free survival (EFS), those with metastatic or relapsed disease fare poorly with only 15-20% 5-year EFS.(1, 2, 5) Accordingly, accurate assessment of the extent of disease at the time of diagnosis plays a critical role in directing appropriate therapy and assessing prognosis.
Non-invasive studies including magnetic resonance imaging (MRI), computed X-ray tomography (CT), or positron emission tomography (PET) are used to determine disease stage. While these techniques are optimally utilized in different clinical scenarios, their limits of detection, constrained by system resolution, vary by modality: approximately millimeter range for CT and MRI, and 1 cm for PET.(6-10) Apart from resolution limitation, the detection of micrometastases for molecular imaging modalities such as PET, is highly dependent upon the signal-to-background ratio provided by the imaging probe. Micrometastatic disease that cannot be detected with existing imaging modalities is a known risk for distant recurrence, hence the need exists for new imaging approaches that can more accurately identify remote sites of disease with high specificity and sensitivity.(11)
Imaging also plays a primary role in assessing the response of patients to therapy. After completion of therapy, patients typically undergo surveillance with MRI of the primary disease site as well as chest CT, or serial chest X-rays, for evaluation of pulmonary nodules, a common site of disease recurrence.(12) Although CT imaging is excellent at detecting abnormal lung lesions of small size, it cannot accurately distinguish between scar tissue, vascular structures, inflammation, infection and malignant disease. A targeted imaging approach for Ewing sarcoma would help alleviate the diagnostic quandary posed by the finding of small lung nodules detected using existing imaging approaches, both at the time of diagnosis and during post-treatment surveillance.
By routine histological staining, Ewing sarcoma cells are similar to other so-called “small round blue cell tumors.” The most specific diagnostic marker used for the histopathological diagnosis of Ewing sarcoma is CD99 (MIC2), a 32-kDa integral membrane glycoprotein that is highly expressed in Ewing sarcoma.(13, 14) CD99 plays a key role in cell adhesion, migration, and apoptosis in lymphocytes, and appears to affect differentiation and malignancy of Ewing cells. (15, 16) In clinical Ewing sarcoma cancer specimens, immunohistochemical staining of CD99 demonstrates high expression in a diffuse membrane distribution with nearly 100% of specimens staining positive, speaking to its use as a diagnostic tool.(17) CD99 is also expressed on circulating leukocytes; however, the degree of CD99 expression in white blood cells as compared with Ewing sarcoma cells is not well described.(18, 19)
Given a known high level of expression of CD99 at the cell surface in Ewing sarcoma, we sought to create an imaging probe targeting CD99 to allow detection of Ewing sarcoma micrometastases in a more sensitive and specific manner than is possible with existing imaging modalities. While therapeutic studies have targeted CD221 (insulin-like growth factor receptor), a well-researched Ewing sarcoma cell surface protein, we opted to focus on CD99 given that expression of CD221 has been shown to be variable.(20) Given the routine use of PET imaging at diagnosis and during surveillance, we chose to develop a 64Cu-labeled anti-CD99 antibody, which may have utility not only in Ewing sarcoma, but other cancers in which CD99 is over-expressed, including lymphomas, synovial cell sarcoma, rhabdomyosarcoma, spindle cell tumors, and other soft tissue sarcomas.(21, 22)
Methods and Materials
Cell Culture and Flow Cytometry
TC32 Ewing sarcoma cells (CD99+) were cultured in RPMI (Gibco); A673 Ewing sarcoma cells (CD99+) and Kelly neuroblastoma cells (CD99-) were cultured in DMEM, all supplemented with 10% FBS and 1% penicillin-streptomycin (Gibco) in 5% CO2 at 37°C. A673 and TC32 cell lines were provided by Dr. Todd Golub (Broad Institute, Cambridge, MA). The Kelly cell line was provided by Dr. Rani George (Dana-Farber Cancer Institute, Boston, MA). All Ewing sarcoma cell lines were authenticated via confirmation of a translocation of EWS and FLI1 genes by FISH for disruption of the EWS locus, and RT-PCR cloning of the fusion junction (data not shown). TC32 cells were transduced with a lentivirus containing the FUW-Luc-mCherry-puro construct to enable bioluminescence imaging (BLI).(23) In preparation for flow cytometry, cells were washed with phosphate buffered saline (Gibco, pH 7.0-7.2), dissociated from flasks using Cellstripper (Cellgro), a non-enzymatic dissociation solution, centrifuged, and re-suspended in PBS. Approximately 1 million cells were aliquoted, filtered and incubated on ice with 2 μg/mL anti-CD99 antibody (Abcam #23617, Abcam #48530) or anti-CD221 antibody (BD-556000, Abcam #16890) for one hour. Cells were washed three times, and incubated on ice with 2 μg/mL FITC-conjugated secondary anti-mouse IgG (BD-554001) for one hour. At the end of the incubation, samples were again washed with PBS and maintained at 4°C until analyzed on a Becton Dickinson FACScan. A non-specific IgG isotype control was obtained from Southern Biotech (#0107-01). Human blood leukocytes for flow cytometry studies were isolated using Ficoll-Paque™ density gradient separation after centrifugation for 30 minutes. Cells were stained with 1 μg/μL PE-conjugated anti-CD3 (Biolegend #317308), per manufacturer recommendations, and subsequently labeled with anti-CD99 and a FITC-conjugated secondary anti-mouse IgG as above.
Innoculation of subcutaneous and micro-metastatic lesions
TC32, A673, or Kelly cells in mid-logarithmic growth phase were trypsinized, centrifuged, and re-suspended in PBS. NCr Nude mice were obtained from the Charles River Animal Facility in Wilmington, MA. NOD/SCID- IL2Rγnull (NSG) mice were obtained from the Jackson Laboratory in Bar Harbor, Maine. For subcutaneous xenografts, 2-3 million TC32 or A673 cells, and 4-6 million Kelly cells were suspended in 100 μL of PBS and injected subcutaneously in NCr Nude mice. For the experimental metastasis model, 5,000-15,000 TC32 cells were suspended in 200 μL of PBS and injected via tail vein into NSG mice. Based on pilot studies, 1-2 mm isolated liver metastases appeared approximately 4 weeks post-injection, whereas larger secondary lung lesions grew at approximately 6 weeks post-injection.
Antibody radiolabeling with 64Cu
Chemicals and reagents were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise specified. EDC (1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide hydrochloride) was purchased from Pierce (Rockford, IL). Metal-naïve pipette tips were purchased from Rainin Instrument (Oakland, CA) and were used to prepare all samples. Glass and plasticware were acid washed with 10% HNO3 and rinsed thoroughly with ultra-pure water (>15 MΩ resistivity; US Filter/Siemens Water Technologies, Warrendale, PA). Ultra-pure water was also used in all buffer preparations. Metal contaminants in buffers were decreased by passing through a Chelex-100 resin column (Bio-Rad Laboratories, Hercules, CA). 64Cu was purchased from Washington University (St. Louis, MO).
The anti-CD99 antibody (Abcam #23617, DN16) and its non-specific IgG control were conjugated with the bifunctional chelator p-NH2-Bn-NOTA and radiolabeled with 64Cu according to previously reported methods.(24) Briefly, the antibodies were purified by high-performance liquid chromatography (HPLC) using a BioSep SEC-S3000 column (Phenomonex, Torrance, CA) with an isocratic aqueous phase consisting of 0.1 M sodium acetate, pH 5.0, at a flow rate of 1 mL/min. The antibodies were then concentrated to 8 mg/ml in sodium acetate buffer using centrifugal filter units (3,000 MWCO; Millipore, Billerica, MA). They were then mixed with p-NH2-Bn-NOTA (Macrocyclics, Dallas, TX) and then with EDC at reagent/antibody molar ratios of 250:1 and 500:1, respectively. The reaction was diluted to a protein concentration of 5 mg/mL with sodium acetate buffer, mixed by gentle pipetting, centrifuged to remove air bubbles, and placed in a water bath at 37°C for 30 minutes. The antibody was separated from unreacted p-NH2-Bn-NOTA using the same HPLC conditions as before, concentrated in sodium acetate buffer, aliquoted, and stored at -80°C. Flow cytometry was performed on TC32 cells to confirm antibody binding after conjugation.
For radiolabeling, conjugated antibody aliquots were thawed, mixed with 64Cu at a ratio of 4:1 (μCi/μg) and incubated for 30 min at 25°C. Thin layer chromatography (TLC) was used to assess radiolabeling. An aliquot (1 μL) of the radiolabeling reaction was added to 9 μL of phosphate buffer (0.1 M, pH 8, 100 mM EDTA). After 5 min, a 1 μL aliquot of the mixture was spotted on an instant TLC glass microfiber strip (Biodex Medical Systems, Inc., NY), allowed to dry, and developed using phosphate buffer/EDTA as the mobile phase. Using these conditions, the radiolabeled antibody remains at the baseline and free 64Cu moves with the solvent front. The TLC strip was cut in half and the radioactivity in each half was assayed. If the TLC assay showed that the radiochemical purity was <95%, the product was purified using centrifugal filter units (as described above). The radioimmunoconjugate was diluted with saline and sterile filtered (0.2 μM filter) before injection.
Tumor volume measurements and bioluminescence imaging
The volumes of subcutaneous tumors were determined by caliper measurements using the formula Vol=0.5*L*W2. For PET studies, mice with 50-150 mm3 tumors were utilized to minimize necrosis. For the experimental metastasis model, the mice that underwent tail vein injection of tumor cells were serially imaged using bioluminescence imaging (IVIS Spectrum, Caliper Life Sciences, Hopkinton, MA) as previously described to identify mice with lung and liver lesions.(25)
MRI imaging
Mice were imaged with a Bruker 7T BioSpec system. Mice were anesthetized with 1-1.5% isoflurane and rapid acquisition with relaxation enhancement (RARE) methods were used to obtain the images with TR=1200ms, effective TE=20ms, in plane geometry: FOV=25.6×19.2mm, matrix size=256×192, and slice thickness=1mm.
PET imaging
For 2-deoxy-2-(18F)fluoro-D-glucose ([18F]-FDG) imaging, mice were fasted overnight (∼12 to 16 h) with free access to water. Mice were warmed for at least an hour, anesthetized with inhaled sevoflurane, and intravenously injected with ∼14 MBq (∼400 μCi) of 18F-FDG in a volume of 250 μL through the lateral tail vein. Mice were maintained under anesthesia for a one-hour uptake period and then scanned (350-650 keV energy window, 10 min listmode acquisition, 3D rebinning followed by OSEM-MAP reconstruction) on a multi-modality preclinical imaging system (Inveon™, Siemens Healthcare). For selected mice, CT acquisitions (80 kVp, 0.5 mA, 220 degree rotation, 600 ms/degree exposure time, 80 μm reconstruction pixel size) were also performed immediately before the PET imaging. The reconstructed PET/CT images were analyzed using Inveon Research Workplace (Siemens Healthcare). Where applicable, tumor uptake was quantified by the software and reported as PET standardized uptake values (SUV).
For 64Cu PET studies, similar procedures were followed except that no fasting was performed and imaging occurred at multiple time points after tracer injection. Each mouse was intravenously injected with ∼4-7MBq (∼100-200 μCi, 50 μg) of radiotracer (anti-CD99- or IgG-conjugates) in a volume of 250 μL via the lateral tail vein. At later imaging time points (e.g., 48 h), an acquisition time of 20 minutes was used to compensate for diminished counting statistics due to radioisotope decay.
Bio-distribution and washout
One mouse with bilateral subcutaneous tumors was imaged at 4, 14, 24, and 44 h post-injection and time-activity curves (TAC) were generated from regions-of-interest (ROIs). Terminal tracer biodistribution studies were also carried out at 24 and 48 h after tracer injection (n=3 at each time point). Tumor, blood, and other selected tissues were dissected and weighed. Radioactivity in the tissue samples was assayed using a Packard Cobra II automated gamma counter (Meriden, CT).
Immunohistochemistry of paraffin-embedded tissue
Expression of CD99 in human Ewing sarcoma in murine tissues was evaluated by immunohistochemical staining (MIC2 Convance antibody, dilution: 1/150) using a manual method. Formalin-fixed paraffin-embedded tissue sections were mounted on Superfrost plus charged microscope slides. Tissues were incubated with primary antibody for 60 min followed by secondary anti-mouse antibody linked to horseradish peroxidase (Envision, Dako) for 30 min, both at room temperature. Expression of CD99 in Ewing sarcoma and human lymph node specimens was evaluated by immunohistochemical staining (DN16 Abcam antibody, dilution: 1/100) using an automated method on a Ventana Discovery XT platform according to the manufacturer's instructions. Following the Closed Loop Assay Development (CLAD) protocol (Ventana Medical Systems, Tucson, AZ), antibody staining was developed using the OmniMap DAB anti-Mouse (HRP) detection kit (Ventana Medical Systems, Tucson, AZ). In both murine and human tissue staining studies, standard quality control procedures were undertaken to optimize antigen retrieval, primary antibody dilution, secondary antibody detection, and other factors for both “signal and noise”. All human tumor specimens were studied in accordance with an IRB approved protocol from Boston Children's Hospital for the study of discarded human sarcoma tissue (protocol #S10-12-0617).
Statistical analysis
Statistical analysis was conducted using the Student's two-tailed t-test. Data are expressed as means of two or more experiments ± standard error of the mean (SEM).
Results
Immunostaining of Ewing sarcoma cells
To develop a Ewing sarcoma targeted imaging probe, we first sought to identify an antibody with high affinity for Ewing sarcoma cells. Based on prior studies, we assessed antibodies targeting CD221 (insulin-like growth factor receptor), and CD99 (MIC2) using flow cytometric analysis after in vitro immunofluorescent staining.(26, 27) While the Ewing sarcoma cell line TC32 showed CD221 positive staining (Figure 1A), the degree of immunofluorescence was less than that for CD99, which consistently demonstrated a 2.5-log shift in fluorescence (Figure 1B). CD99 staining was consistently positive in all Ewing sarcoma cell lines tested (Supplemental Figure 1), while no immunoreactivity was found in cells without CD99 expression, such as the neuroblastoma cell line Kelly (Figure 1C). The mouse anti-CD99 monoclonal antibody DN16 was derivitized for all subsequent studies.
Figure 1. Flow cytometric analysis of CD99 expression in Ewing sarcoma cells.
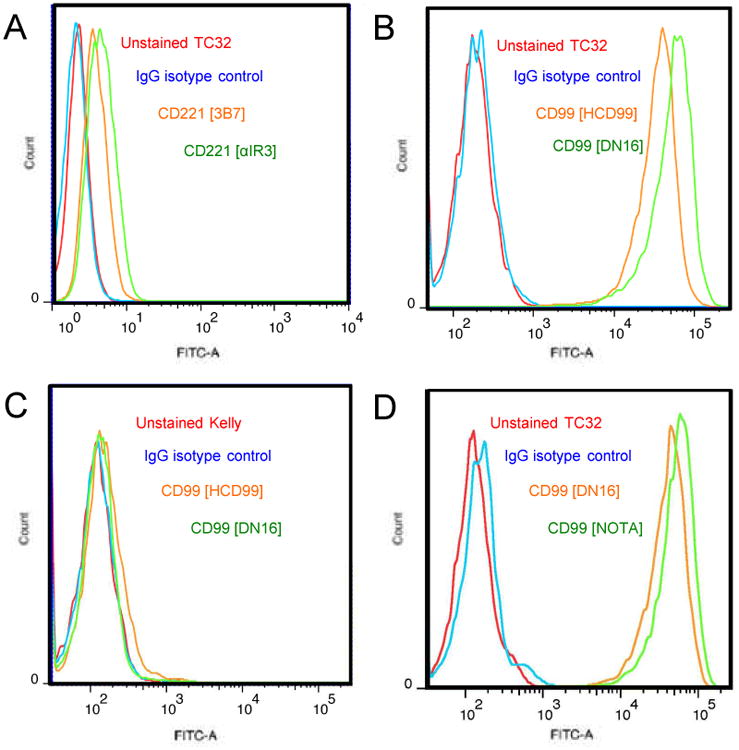
A. TC32 Ewing sarcoma cells stained with anti-CD221 antibodies (3B7, αIR3). B. TC32 cells stained with anti-CD99 antibodies (HCD99, DN16). C. Kelly neuroblastoma cells stained with anti-CD99 antibodies (HCD99, DN16). D. TC32 cells stained with anti-CD99 antibody (DN16) compared to DN16 conjugated to NOTA. In all panels, unstained and isotype matched IgG are controls.
Creation of a 64Cu anti-CD99 probe
With the goal of enhancing tumor contrast and, in turn, maximizing image resolution and sensitivity, we chose to develop a PET probe for in vivo imaging. Based on our prior studies, we first conjugated the bifunctional chelator p-NH2-Bn-NOTA to the DN16 antibody.(24) After purification, retention of antibody affinity for CD99 was verified by flow cytometry (Figure 1D). Aliquots of NOTA-DN16 were radiolabeled with 64Cu to form a 64Cu-DN16 probe, and the product with >95% radiochemical purity was used for in vivo studies.
PET imaging of Ewing sarcoma xenografts
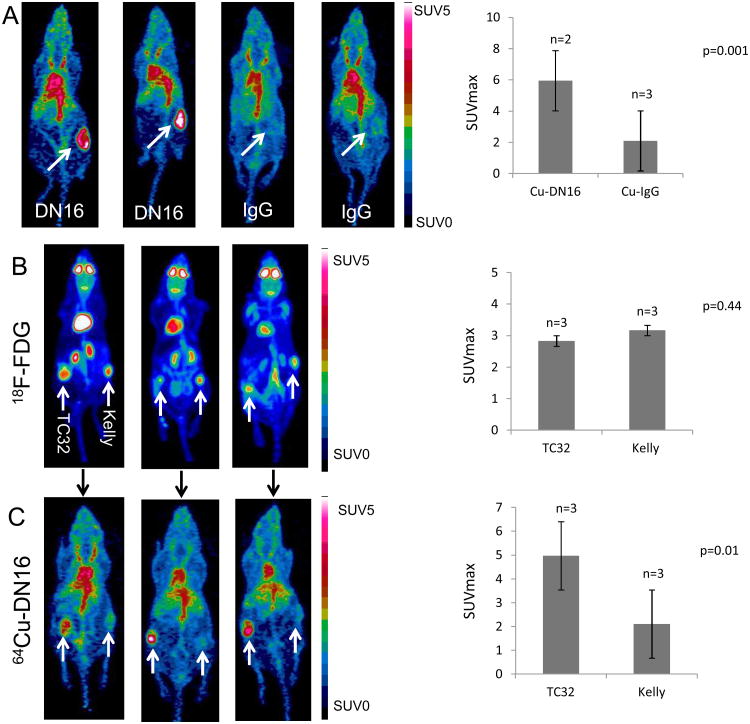
The 64Cu-DN16 probe was first evaluated in mice bearing subcutaneous xenograft tumors. Mice with A673 (CD99+) xenograft tumors between 50-150 mm3 were injected with either 64Cu-DN16 or an isotype-matched 64Cu-IgG probe. Both probes showed distribution through the blood pool (Figure 2A), but only the 64Cu-DN16 probe demonstrated strong avidity for A673 xenografts with a statistically significant difference (p=0.001) in maximum SUV values comparing the 64Cu-DN16 probe to 64Cu-IgG (Figure 2A).
Figure 2. PET imaging with a 64Cu-labeled anti-CD99 antibody compared to 18F-FDG.
A. Mice with A673 (CD99+) xenografts (white arrows) were injected with either 64Cu-DN16 or 64Cu-IgG and PET imaged 24 h post-injection. Quantitated data plotted as mean +/- SEM for n=5 animals. B. Mice with TC32 (CD99+) xenografts on the left and Kelly (CD99-) xenografts on the right were PET imaged one hour after injection of 18F-FDG PET. Data plotted as mean +/- SEM for n=3 animals. C. The same mice were injected with 64Cu-DN16 and PET imaged 24 h after injection. Data plotted as mean +/- SEM for n=3 animals. Significance determined by the Student's t-test.
To further assess specificity, we established mice with bilateral xenograft tumors in which TC32 (CD99+ Ewing sarcoma) xenografts were established on the left flank, and Kelly (CD99− neuroblastoma) xenografts were established on the right flank. Mice with similarly sized tumors (50-150 mm3) were injected with 18F-FDG, followed by PET imaging at 1 h post-injection, to demonstrate the location of tumors and show equivalency for 18F-FDG uptake in the two tumor types (Figures 2B). The same mice were then injected with 64Cu-DN16 and PET imaged 24 h later. In contrast to the 18F-FDG results (Figure 2B), uptake of the 64Cu-DN16 radiotracer was restricted to the CD99+ TC32 tumors (Figure 2C). Whereas tracer activity in the brain, kidneys and heart was apparent in animals injected with 18F-FDG (Figure 2B), in mice injected with the 64Cu-DN16 probe, tracer background was restricted to the blood pool (Figure 2C). Together, these results demonstrate that the 64Cu-DN16 is highly specific for CD99 expressing tumors.
PET imaging of micrometastatic disease
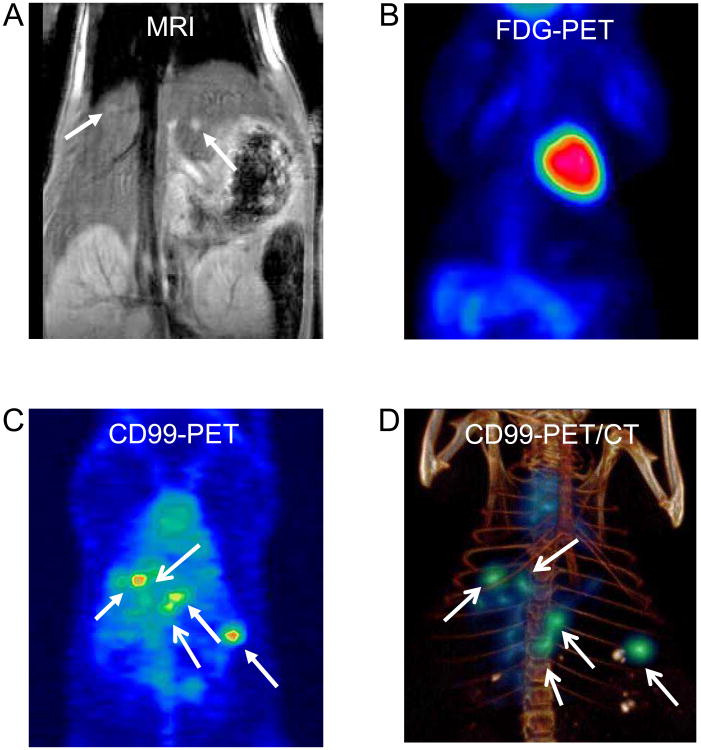
Having established the specificity of 64Cu-DN16, we next assessed the sensitivity of the probe. To establish a model of micrometastatic disease, mice were injected intravenously with TC32 cells (transduced with Luc-mCherry-puro), and the distribution and size of lesions in the liver and lungs was serially assessed using bioluminescence and MRI. Animals with disease in the liver were first identified using bioluminescence imaging (data not shown). MRI was then used to identify animals with 1-2 mm nodules in the liver (Figure 3A, Supplemental Figure 2).
Figure 3. 64Cu-DN16 PET imaging of TC32 metastases.
A mouse with 1-2 mm experimental liver metastases (same mouse as Supplemental Figure 2) was imaged with A. MRI, B. 18F-FDG, and C. 64Cu-labeled anti-CD99 antibody. D. Three-dimensional volume rendering of co-registered PET and microCT images.
To determine whether 18F-FDG PET imaging can detect lesions in this size range, mice with 1-2 mm liver nodules were imaged 1 h after injection of 18F-FDG. We were consistently unable to visualize any liver lesions using 18F-FDG PET imaging (Figure 3B). Following FDG imaging, mice were immediately injected with 64Cu-DN16, and repeat PET imaging was performed 24 h later. In contrast to the 18F-FDG PET results, 64Cu-DN16 uptake within the micrometastatic lesions was readily visualized (Figure 3C-D).
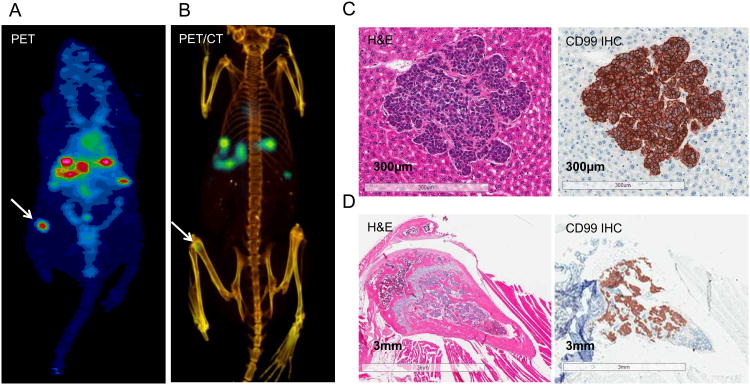
We performed histopathological validation in a mouse with 64Cu-DN16 PET positive liver lesions, in which we also noted a focus of uptake in the femur (Figure 4A-B). Routine hematoxylin-eosin staining of the liver confirmed the presence of a deposit of small round blue cells consistent with Ewing sarcoma that stained positive for CD99 (Figure 4C). Of interest, a similar focus of cells was found infiltrating the marrow space in the distal femur (Figure 4D), corresponding to the location of the lesion identified by 64Cu-DN16 PET imaging (Figure 4A-B). Together, these results demonstrate that preclinical PET imaging with the 64Cu-DN16 probe improves signal-to-background ratio and enables detection of 1-2 mm CD99+ micrometastatic lesions that are below the threshold of detection using 18F-FDG.
Figure 4. 64Cu-DN16 PET imaging of micrometastatic lesions.
A-B. An independent mouse with 1-2 mm liver metastases was imaged 24 hrs after injection of 64Cu-DN16. An ectopic focus of activity in the femur was noted (white arrow). C. Hematoxylin-eosin (H&E) staining of a metastatic liver lesion, as well as anti-CD99 immunohistochemistry (CD99 IHC). D. H&E and CD99 IHC right femur, corresponding to the PET-focus in A-B.
Probe performance
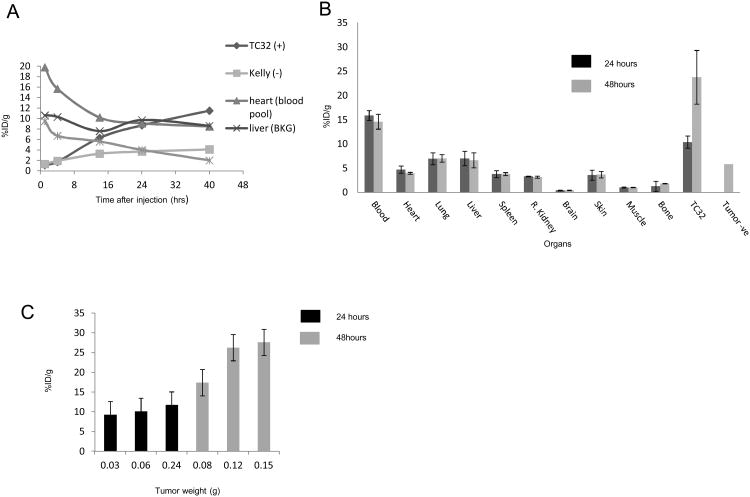
To more thoroughly define the biodistribution and pharmacokinetics of the 64Cu-DN16 probe, we performed longitudinal PET imaging and ex vivo validation in a subset of animals with subcutaneous and metastatic xenograft tumors. Time-activity curves for major organs and xenograft tumors in one mouse demonstrated a progressive decrease in activity in the liver, heart and spleen over time (Figure 5A). The TC32 tumor had a progressive increase in activity over the 40 h of imaging (Figure 5A). There was likewise a small increase in activity within the CD99- Kelly tumor over time, perhaps due to EPR (enhanced permeability and retention) effects commonly associated with tumors.(28) However, the absolute activity within the Kelly tumor was substantially less than that previously shown in the TC32 tumor at all points later than 4 h (Figure 5A).
Figure 5. 64Cu-DN16 biodistribution.
A. Time-activity-curves (TAC) generated from serial imaging of a mouse with a subcutaneous TC32 (CD99+) and Kelly (CD99-) xenograft tumor following injection of 64Cu-DN16. B-C. Terminal radiotracer biodistribution in mice with subcutaneous xenograft tumors, performed at 24 and 48 h post-injection.
Terminal tracer biodistribution studies were performed at 24 and 48 h after probe injection, with three mice at each time point (Figures 5B). Each mouse had a unilateral TC32 tumor, while one mouse sacrificed at 48 h had bilateral tumors with a TC32 tumor on one flank, and a Kelly tumor on the contralateral flank. Figures 5B and 5C validate our imaging results (Figures 2-4), demonstrating specificity of probe for CD99+ tumors and accumulation of probe over time.
Comparative staining of lymphocytes and Ewing sarcoma
Within normal tissues, CD99 expression is highly restricted to lymphocytes and endothelial cells. To compare the expression of CD99 in Ewing sarcoma cells to that in lymphocytes, we isolated normal human mononuclear cells, and stained a normalized number of mononuclear and TC32 Ewing sarcoma cells with the DN16 antibody. Cells were also stained with a CD3 antibody to identify T-cells. CD99 expression was found on both CD3-positive and negative lymphocytes. The mean fluorescence in Ewing sarcoma cells (Figure 6A) was nearly 100-fold higher than the staining of normal human lymphocytes (Figure 6B).
Figure 6. Comparative CD99 staining of normal lymphocytes compared to Ewing sarcoma cells.
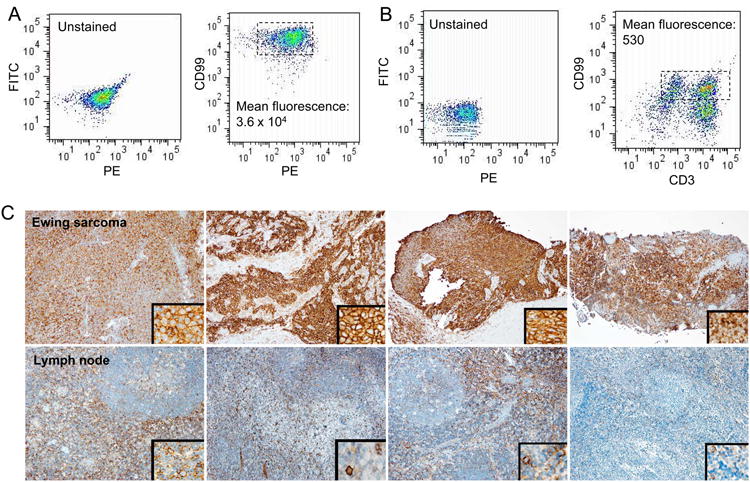
A. TC32 Ewing sarcoma cells were stained with DN16-FITC. B. Normal human lymphocytes were stained with DN16-FITC and anti-CD3-PE. C. Formalin-fixed paraffin-embedded tissue sections of human Ewing sarcoma (top row) and reactive human lymph node (bottom row) specimens were stained with anti-CD99 antibody (DN16). Each panel is from an independent patient.
To further compare the expression of CD99 in clinical specimens, we performed immunohistochemistry on four independent human Ewing sarcoma and human lymph node specimens. Staining in lymph nodes was limited to T-cell zones and occasional cells morphologically consistent with dendritic cells (Figure 6C, bottom row) whereas staining in Ewing sarcoma cells was diffuse, membranous, and qualitatively more intense (Figure 6C, top row).
Discussion
In the clinic, imaging plays a key role in the management of patients with solid tumors, helping to determine the primary site of disease, sites of dissemination, and response to therapy. While some forms of cancer utilize surgical sampling to determine the extent of local spread (e.g., regional lymph node sampling), in most cases imaging is the only modality used to determine whether distant metastases are present. Despite tremendous advances in the last few decades, the clinical limit of detection of current imaging modalities is on the order of 5-10 mm.(6-10) Moreover, lesions in this size range are often indistinguishable from non-malignant processes, resulting in uncertainty in interpretation. There is thus an unmet need for more sensitive and specific cancer imaging approaches.
Molecular imaging sensitivity is directly dependent on system resolution as well as other characteristics of the scanner and, equally important, the tumor (signal)-to-background ratio provided by the imaging probe. In these experimental models, our data show that small lesions detectable with preclinical MRI cannot be detected using preclinical FDG-PET but can be detected using the same PET scanner with the 64Cu-labeled anti-CD99 antibody probe. Preclinical imaging devices offer superior resolution to clinical PET scanners, therefore it may be difficult to extrapolate these results to the clinical realm. However, we anticipate that this targeted imaging approach will translate to a similarly improved signal-to-background ratio when used with human scanners, leading to enhanced detection of metastatic lesions, and ultimately tailored therapy for patients with Ewing sarcoma.
There are multiple requirements for the development of an antibody-targeted imaging probe. First, the epitope of interest must be extracellular and must be expressed at high levels. Second, the radionuclide should have a sufficiently long half-life to allow delayed imaging to match the pharmacokinetic properties of intact antibodies. Finally, for clinical use, the antibody must be humanized to allow repeated use. The radionuclide dose in humans, extrapolated from standard 18F-FDG dosing, is approximately 10-15 times that dosed to mice. Assuming a similar antibody-to-radionuclide ratio, the antibody dose in humans would be less than 5mg which is clinically acceptable. For targeted imaging of Ewing sarcoma, we have demonstrated that a 64Cu-labeled anti-CD99 radioconjugate is both sensitive and specific for PET imaging of Ewing sarcoma in experimental models. These encouraging first results establish the rationale for proceeding with development of a human probe for future clinical translation.
Assessment of specificity in xenograft models is confounded by lack of cross-species reactivity for most antibodies. Moreover, CD99 is normally expressed on leukocytes and is highly expressed on thymocytes, where it is believed to augment T-cell adhesion, apoptosis of double positive T-cells, and migration and activation.(18) The immunodeficient animals used in this study lack lymphocytes, limiting the ability to assess whether the antibody is cross-reactive. To this end, we stained normal human lymphocytes and found that only a small fraction of total lymphocytes stain positive for CD99, and that this staining is quantitatively lower than that for Ewing sarcoma cells. We also performed immunohistochemistry on paraffin-embedded human Ewing sarcoma versus human lymph node specimens to determine the relative expression of CD99 in clinical specimens. We again found that a much higher proportion of Ewing sarcoma cells stain positive for CD99 and that the staining is far more intense than for lymphoid tissues. These results suggest that the level of expression of CD99 in Ewing sarcoma cells significantly exceeds that in normal human lymphocytes, and predicts that a CD99 targeted imaging agent would have potential utility for imaging of CD99-overexpressing tumors.
The scope of a CD99-targeted imaging probe extends beyond Ewing sarcoma. CD99 is expressed in tumors arising from immature mesenchymal tissue, including rhabdomyosarcoma, synovial sarcoma, clear cell sarcoma, and other soft tissue sarcomas.(21, 22) T-cell leukemia and lymphomas likewise express CD99 as a lineage-specific marker. The opportunity to expand use to these additional cancer types helps bolster the rationale for development of a CD99-targeted imaging probe.
Finally, the current results suggest that an anti-CD99 antibody may have utility not only for delivering imaging moieties to CD99+ tumors, but also for delivering therapeutic payloads. Several therapeutic antibodies have been engineered into imaging probes, and the characteristics desired for an imaging probe are generally congruent with those of a therapeutic. Future studies will explore the use of a radionuclide with a longer half-life (e.g. 89Zr), allowing an extended period of tracer imaging and longer period of tracer washout to further reduce background. We will likewise explore DN16 as a therapeutic radioconjugate and as an antibody-drug conjugate. Ultimate antibody modification, including the creation of antibody fragments and/or antibody humanization may aid in translation to the clinic.
Supplementary Material
Statement of Translational Relevance.
In this manuscript, we describe the creation of a CD99-targeted imaging probe that is highly sensitive for the visualization of CD99-expressing tumors. The 64Cu-labeled anti-CD99 probe is able to visualize 1-2 mm lesions, in xenograft models of Ewing sarcoma micrometastases, below the threshold of detection using FDG-PET. The performance of the current probe establishes a rationale for the development of a CD99 targeted imaging probe for clinical translation. This radiotracer may significantly impact the determination of disease extent at diagnosis, interpretation of conventional imaging findings, and ultimate approach to therapy for patients with Ewing sarcoma. It may similarly impact the management of other CD99 positive tumors.
Acknowledgments
The authors of this paper would like to thank the Hyundai Hope on Wheels Foundation for its much appreciated funding and support. Copper-64 was produced at Washington University School of Medicine (St. Louis, MO) under the support of National Cancer Institute Grant R24CA86307. Murine tissue histologic and immunohistologic analyses were carried out in the Dana Farber-Harvard Cancer Center Specialized Histopathology Services Core Laboratory.
Footnotes
The authors have no conflicts of interest to report.
References
- 1.Gurney JG, Swensen AR, Bulterys M. Malignant Bone Tumors SEER Data Pediatrics. National Cancer Institute; 1975-1995. pp. 99–110. [Google Scholar]
- 2.Gurney JG, Young JL, Jr, Roffers SD, Smith MA, Bunin GR. Soft Tissue Sarcomas SEER Data Pediatrics. National Cancer Institute; 1975-1995. pp. 111–124. [Google Scholar]
- 3.Donaldson SS. Ewing sarcoma: radiation dose and target volume. Pediatr Blood Cancer. 2004;42:471–76. doi: 10.1002/pbc.10472. [DOI] [PubMed] [Google Scholar]
- 4.Liu AK, Stinauer M, Albano E, Greffe B, Tello T, Maloney K. Local control of metastatic sites with radiation therapy in metastatic Ewing sarcoma and rhabdomyosarcoma. Pediatr Blood Cancer. 2011;57:169–71. doi: 10.1002/pbc.23063. [DOI] [PubMed] [Google Scholar]
- 5.Cotterill SJ, Ahrens S, Paulussen M, Jurgens HF, Voute PA, Gadner H, et al. Prognostic factors in Ewing's tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing's Sarcoma Study Group. J Clin Oncol. 2000;18:3108–14. doi: 10.1200/JCO.2000.18.17.3108. [DOI] [PubMed] [Google Scholar]
- 6.Franzius C, Daldrup-Link HE, Sciuk J, Rummeny EJ, Bielack S, Jurgens H, et al. FDG-PET for detection of pulmonary metastases from malignant primary bone tumors: comparison with spiral CT. Ann Oncol. 2001;12:479–86. doi: 10.1023/a:1011111322376. [DOI] [PubMed] [Google Scholar]
- 7.Heye T, Ley S, Heussel CP, Dienemann H, Kauczor HU, Hosch W, et al. Detection and size of pulmonary lesions: how accurate is MRI? A prospective comparison of CT and MRI. Acta Radiol. 2012;53:153–60. doi: 10.1258/ar.2011.110445. [DOI] [PubMed] [Google Scholar]
- 8.Blyth S, Blakeborough A, Peterson M, Cameron IC, Majeed AW. Sensitivity of magnetic resonance imaging in the detection of colorectal liver metastases. Ann R Coll Surg Engl. 2008;90:25–28. doi: 10.1308/003588408X242303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sica GT, Ji H, Ros PR. CT and MR imaging of hepatic metastases. AJR Am J Roentgenol. 2000;174:691–98. doi: 10.2214/ajr.174.3.1740691. [DOI] [PubMed] [Google Scholar]
- 10.Biederer J, Hintze C, Fabel M. MRI of pulmonary nodules: technique and diagnostic value. Cancer Imaging. 2008;8:125–30. doi: 10.1102/1470-7330.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saeter G, Elomaa I, Wahlqvist Y, Alvegard TA, Wiebe T, Monge O, et al. Prognostic factors in bone sarcomas. Acta Orthop Scand Suppl. 1997;273:156–60. doi: 10.1080/17453674.1997.11744723. [DOI] [PubMed] [Google Scholar]
- 12.Meyer JS, Nadel HR, Marina N, Womer RB, Brown KL, Eary JF, et al. Imaging guidelines for children with Ewing sarcoma and osteosarcoma: a report from the Children's Oncology Group Bone Tumor Committee. Pediatr Blood Cancer. 2008;51:163–70. doi: 10.1002/pbc.21596. [DOI] [PubMed] [Google Scholar]
- 13.Bernstein M, Kovar H, Paulussen M, Randall RL, Schuck A, Teot LA, et al. Ewing's sarcoma family of tumors: current management. Oncologist. 2006;11:503–19. doi: 10.1634/theoncologist.11-5-503. [DOI] [PubMed] [Google Scholar]
- 14.Kovar H, Dworzak M, Strehl S, Schnell E, Ambros IM, Ambros PF, et al. Overexpression of the pseudoautosomal gene MIC2 in Ewing's sarcoma and peripheral primitive neuroectodermal tumor. Oncogene. 1990;5:1067–70. [PubMed] [Google Scholar]
- 15.Cerisano V, Aalto Y, Perdichizzi S, Bernard G, Manara MC, Benini S, et al. Molecular mechanisms of CD99-induced caspase-independent cell death and cell-cell adhesion in Ewing's sarcoma cells: actin and zyxin as key intracellular mediators. Oncogene. 2004;23:5664–74. doi: 10.1038/sj.onc.1207741. [DOI] [PubMed] [Google Scholar]
- 16.Rocchi A, Manara MC, Sciandra M, Zambelli D, Nardi F, Nicoletti G, et al. CD99 inhibits neural differentiation of human Ewing sarcoma cells and thereby contributes to oncogenesis. J Clin Invest. 2010;120:668–80. doi: 10.1172/JCI36667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weidner N, Tjoe J. Immunohistochemical profile of monoclonal antibody O13: antibody that recognizes glycoprotein p30/32MIC2 and is useful in diagnosing Ewing's sarcoma and peripheral neuroepithelioma. Am J Surg Pathol. 1994;18:486–94. [PubMed] [Google Scholar]
- 18.Choi EY, Park WS, Jung KC, Kim SH, Kim YY, Lee WJ, et al. Engagement of CD99 induces up-regulation of TCR and MHC class I and II molecules on the surface of human thymocytes. J Immunol. 1998;161:749–54. [PubMed] [Google Scholar]
- 19.Dubois SG, Epling CL, Teague J, Matthay KK, Sinclair E. Flow cytometric detection of Ewing sarcoma cells in peripheral blood and bone marrow. Pediatr Blood Cancer. 2010;54:13–18. doi: 10.1002/pbc.22245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asmane I, Watkin E, Alberti L, Duc A, Marec-Berard P, Ray-Coquard I, et al. Insulin-like growth factor type 1 receptor (IGF-1R) exclusive nuclear staining: a predictive biomarker for IGF-1R monoclonal antibody (Ab) therapy in sarcomas. Eur J Cancer. 2012;48:3027–35. doi: 10.1016/j.ejca.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Llombart-Bosch A, Navarro S. Immunohistochemical detection of EWS and FLI-1 proteins in Ewing sarcoma and primitive neuroectodermal tumors: comparative analysis with CD99 (MIC-2) expression. Applied immunohistochemistry & molecular morphology: AIMM/official publication of the Society for Applied Immunohistochemistry. 2001;9:255–60. doi: 10.1097/00129039-200109000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Perlman EJ, Dickman PS, Askin FB, Grier HE, Miser JS, Link MP. Ewing's sarcoma--routine diagnostic utilization of MIC2 analysis: a Pediatric Oncology Group/Children's Cancer Group Intergroup Study. Hum Pathol. 1994;25:304–07. doi: 10.1016/0046-8177(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 23.Kimbrel EA, Davis TN, Bradner JE, Kung AL. In vivo pharmacodynamic imaging of proteasome inhibition. Mol Imaging. 2009;8:140–47. [PubMed] [Google Scholar]
- 24.Dearling JL, Voss SD, Dunning P, Snay E, Fahey F, Smith SV, et al. Imaging cancer using PET--the effect of the bifunctional chelator on the biodistribution of a (64)Cu-labeled antibody. Nucl Med Biol. 2011;38:29–38. doi: 10.1016/j.nucmedbio.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giubellino A, Woldemichael GM, Sourbier C, Lizak MJ, Powers JF, Tischler AS, et al. Characterization of two mouse models of metastatic pheochromocytoma using bioluminescence imaging. Cancer Lett. 2012;316:46–52. doi: 10.1016/j.canlet.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scotlandi K, Benini S, Sarti M, Serra M, Lollini PL, Maurici D, et al. Insulin-like growth factor I receptor-mediated circuit in Ewing's sarcoma/peripheral neuroectodermal tumor: a possible therapeutic target. Cancer Res. 1996;56:4570–74. [PubMed] [Google Scholar]
- 27.Scotlandi K, Manara MC, Serra M, Marino MT, Ventura S, Garofalo C, et al. Expression of insulin-like growth factor system components in Ewing's sarcoma and their association with survival. Eur J Cancer. 2011;47:1258–66. doi: 10.1016/j.ejca.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–92. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.