Abstract
Background
Obesity rates in the United States have escalated in recent decades and present a major challenge in public health prevention efforts. Currently, testing to identify genetic risk for obesity is readily available through several direct-to-consumer companies. Despite the availability of this type of testing, there is a paucity of evidence as to whether providing people with personal genetic information on obesity risk will facilitate or impede desired behavioral responses.
Purpose
We describe the key issues in the design and implementation of a randomized controlled trial examining the clinical utility of providing genetic risk information for obesity.
Methods
Participants are being recruited from the Coriell Personalized Medicine Collaborative, an ongoing, longitudinal research cohort study designed to determine the utility of personal genome information in health management and clinical decision-making. The primary focus of the ancillary Obesity Risk Communication Study is to determine whether genetic risk information added value to traditional communication efforts for obesity, which are based on lifestyle risk factors. The trial employs a 2x2 factorial design in order to examine the effects of providing genetic risk information for obesity, alone or in combination with lifestyle risk information, on participants’ psychological responses, behavioral intentions, health behaviors, and weight.
Results
The factorial design generated four experimental arms based on communication of estimated risk to participants: 1) no risk feedback (control), 2) genetic risk only, 3) lifestyle risk only, 4) both genetic and lifestyle risk (combined). Key issues in study design pertained to the selection of algorithms to estimate lifestyle risk and determination of information to be provided to participants assigned to each experimental arm to achieve a balance between clinical standards and methodological rigor. Following the launch of the trial in September 2011, implementation challenges pertaining to low enrollment and differential attrition became apparent and required immediate attention and modifications to the study protocol. Although monitoring of these efforts is ongoing, initial observations show a doubling of enrollment and reduced attrition.
Limitations
The trial is evaluating the short-term impact of providing obesity risk information as participants are followed for only 3 months. This study is built upon the structure of an existing personalized medicine study wherein participants have been provided with genetic information for other diseases. This nesting in a larger study may attenuate the effects of obesity risk information and has implications for the generalizability of study findings.
Conclusions
This randomized trial examines value of obesity genetic information, both when provided independently and when combined with lifestyle risk assessment, to motivate individuals to engage in healthy lifestyle behaviors. Study findings will guide future intervention efforts to effectively communicate genetic risk information.
Keywords: obesity, personalized medicine, clinical utility, genetic testing, risk assessment, enrollment strategies, differential attrition, randomized controlled trial
Introduction
Obesity rates in the United States have escalated in recent decades and present a major challenge in public health prevention efforts. Currently, approximately two-thirds of adults are either overweight (body mass index [BMI]: 25-29.9 kg/m2) or obese (BMI ≥ 30 kg/m2) and a third of children and adolescents are at risk of becoming overweight [1-2]. Obesity is a multifactorial condition [1]; advances in genomics have begun to shed light on its genetic underpinnings. Heritability is the percent of a disease or trait that is due to genetics. With heritability estimates ranging from 81 to 92%, there is strong evidence that genetics has a significant impact on the variation in the occurrence of obesity [3-4].
Currently, testing to identify genetic risk for obesity is readily available through several companies including 23andMe and Pathway Genomics. The potential utility of these tests rests, in part, on the premise that the information they provide will be useful to individuals and will motivate those at elevated risk to engage in health behaviors to avoid obesity or to reduce body weight [5]. Yet, despite the availability of this type of testing, there is a paucity of evidence as to whether providing people with personal genetic information on obesity risk will facilitate or impede desired behavioral responses [5-6]. Moreover, it is also unknown whether information about one's personal genetic predisposition to obesity will provide any “added value” to communication efforts that attempt to convey traditional, non-genetic, risk information [5].
To date, evidence for the potential benefit of genetic information for common complex diseases has been limited, often based on opinion surveys or hypothetical, vignette-based studies wherein a participant is asked to imagine he/she has undergone genetic testing for obesity or other chronic conditions and to respond to the genetic results. For example, clinical surveys on patient populations have found that a high percentage (71%) of individuals endorsed the notion that getting a ‘high risk” genetic result for diabetes would increase their motivation to adopt healthy behavior changes [7]. Similarly, vignette-based studies have demonstrated that genetic risk feedback for obesity increased perceptions of risk and intentions to eat a healthy diet [8-9]. Notably, some of these studies also have found that genetic information decreased perceptions of confidence in ability to eat a healthy diet, raising the possibility of fatalistic responses to this type of information [8]. Yet, other research examining the actual provision of personal genetic information has not confirmed that finding. For example, investigators of a study of 30 post-menopausal obese women reported that providing personalized obesity genetic information increased participants’ confidence in their ability to control eating and lose weight, regardless of genetic test result [10]. Results from the available studies must be interpreted with caution given the various study limitations (e.g., small sample size) as well as differences in study populations (undergraduates versus obese women) and study design (vignette versus real feedback).
Is genetic risk perceived differently from non-genetic risk?
Findings from some studies suggest that genetic risk information may have a greater influence on risk perceptions and decision making compared to non-genetic risk information [11-14], lending support to the potential added value of genetic information. For example, one study in the context of Alzheimer's disease (AD) found that risk estimates derived from genetic testing results (apoE) had a greater impact on risk perceptions compared to risk estimates derived from family history information [11]. Similar results were reported in a vignette-based study, which showed greater chemotherapy preferences among women previously treated for early stage breast cancer when estimates of breast cancer recurrence were based on a genetic test versus standard prognostic factors (e.g., age, tumor site, tumor grade) [12]. In part, the potentially greater influence of risk communications based on genetic information may be due to a perception of greater accuracy or certainty from this type of information [11-14]. Nonetheless, findings in the literature are inconsistent, with some vignette-based studies reporting either no difference based on “source” of the risk estimate or the reverse effect [15-16]. Moreover, only one study [12] has examined responses to risk when information from different sources was discrepant, i.e., estimate from genetic test is low, but estimate from other clinical indicators is high. Research examining the impact of actual DNA based risk information on people's attitudes and behavior compared to risk information derived from other indicators would help to clarify reported findings to date.
A randomized controlled trial to examine obesity risk communication
We describe the key issues in the design and implementation of a randomized controlled trial to evaluate the clinical utility of providing genetic risk information for obesity. Because prior study designs lacked the ability to test the added value of personalized genetic information, our trial employed a 2x2 factorial design to address the following specific aims:
Aim 1: To examine the effects of providing genetic risk information (factor 1), alone or in combination with lifestyle risk information (factor 2), on participants’ behavioral intentions, health behaviors, and weight. Hypothesis 1: Providing both genetic and lifestyle risk feedback combined will result in the greatest levels of intentions to change diet and activity behaviors, actual diet and activity behavior change, and weight loss, compared to the other conditions.
Aim 2: To determine the extent to which the effects of any factor vary according to the risk level conferred (elevated versus non-elevated). Hypothesis 2. Receiving elevated risk feedback for either genetics or lifestyle will result in greater levels of intentions to change diet and activity behaviors, actual diet and activity behavior change, and weight loss, compared to non-elevated risk feedback. The impact will be greatest among participants who receive elevated risk feedback for both genetics and lifestyle.
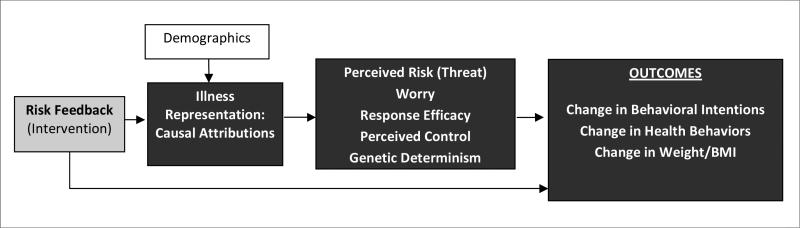
The conceptual framework guiding this study draws from theories of self-regulation and illness representations [17-18]. These theories describe how information about an illness threat is processed within an individual's pre-existing cognitive schema and how the representations within these schemas activate coping responses (e.g., behavioral action) to deal with the perceived threat. Figure 1 outlines these possible mechanisms, guided by research evidence regarding the role of illness representations in health behaviors and in relation to other psychosocial mediators such as perceptions of risk, control, and response efficacy [18-23]. We will use data generated from this study to test and refine our conceptual model and to clarify the relationship between the receipt of genetic risk information, illness representations, and health behaviors.
Figure 1.
Conceptual Model
Our team faced several issues in the design and implementation of the Obesity Risk Communication Study, the subject of this report. Challenges in designing the study included 1) the selection of algorithms to estimate lifestyle risk and 2) the need to achieve a balance between clinical standards and methodological rigor when presenting information to participants assigned to each experimental arm. Moreover, designing a trial nested within an existing longitudinal cohort presented its own set of challenges. Finally, following the launch of the trial in September 2011, implementation challenges pertaining to low enrollment and differential attrition across experimental arms became apparent and required modifications to the study protocol.
Thus, we describe critical methodological decisions made by our study team related to patient eligibility, recruitment and randomization; details about the formulation of risk algorithms for both genetic and lifestyle risk estimates and development of risk feedback reports specific to each experimental arm; and the modifications made to our study protocol to address enrollment and retention concerns and the impact of these changes.
Methods
Overview of Coriell Personalized Medicine Collaborative (CPMC) Parent Study
This randomized clinical trial was built upon the structure of an existing personalized medicine study – the Coriell Personalized Medicine Collaborative (CPMC). Described in detail elsewhere [24], the CPMC is an ongoing evidence-based longitudinal research study designed to determine the utility of using personal genome information in health management and clinical decision-making. The CPMC collects saliva samples, performs genetic analysis, and provides online genetic risk feedback for several potentially medically actionable conditions including coronary artery disease, type 2 diabetes, melanoma and aspects of drug metabolism. Participants in the CPMC were required to be at least 18 years of age, have a valid email address, provide written informed consent, and submit a saliva sample for genomic analysis.
The CPMC enrolled four distinct cohorts: the Community cohort, the Cancer cohort, the Chronic Disease cohort and the Air Force cohort. Community cohort participants were recruited from the general population, not selected for disease or health history, and agreed to participate in the study by providing the required medical and family history information and a saliva sample. Cancer cohort participants had either prostate or breast cancer confirmed by diagnosis by personal physicians, agreed to participate in the study, and provided specified medical records to the CPMC study. Chronic disease participants had either hypertension or congestive heart failure confirmed by diagnosis by their physicians, agreed to participate in the study, and provided the necessary medical records to the CPMC. Participants in the Air Force cohort were active-duty United States Air Force Medical Service personnel who had an interest in genetic testing and agreed to provide saliva samples, share their medical records, and participate in the study.
Following informed consent and saliva sample submission, participants were prompted to activate an online web portal account, which serves as the communication channel through which all CPMC-related activities are conducted. Following account activation, participants completed detailed medical history, family history and lifestyle questionnaires. Upon completion of required questionnaires, participants were genotyped and subsequently eligible to view their personalized risk results online for health conditions and drug responses included within the parent CPMC study. Unlike most direct-to-consumer genetic testing services, the CPMC provided information on non-genetic risk factors, such as family history and lifestyle, in addition to genetic results.
As of January 7, 2013, when enrollment closed, the source population eligible for the Obesity Risk Communication Study consisted of 3238 individuals with an average age of 52.8 (range 20-97). The source population was predominantly Caucasian (91.4%) with more women (60.3%) than men (39.7%). The average BMI was 26.8; 29.4% and 27.2% of the source population were considered overweight and obese, respectively.
Obesity Risk Communication Study
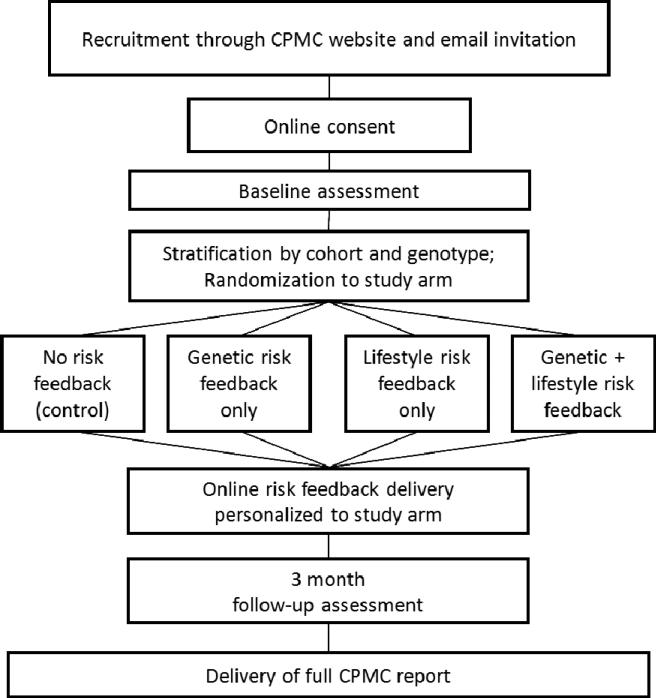
The Obesity Risk Communication Study was an ancillary study of the CPMC designed to examine participant responses to risk information derived from both genetic test results and a lifestyle risk algorithm. This randomized trial employed a 2×2 factorial design, with the provision of genetic risk feedback and/or and lifestyle risk feedback as the factors, resulting in four experimental arms: 1) no risk feedback (control arm), 2) genetic risk feedback only, 3) lifestyle risk feedback only, 4) both genetic and lifestyle risk (combined) feedback. The study schema is presented in Figure 2.
Figure 2.
Study Schema
All “active enrollees” in the CPMC were eligible to participate in the obesity study 90 days after they had received their initial CPMC risk results. The 90 day waiting period was implemented to reduce the carry-over effects of genetic risk information for the other conditions provided by the parent study. Active enrollees were defined as having provided a saliva sample for DNA testing and completed all baseline CPMC parent study questionnaires. Because genotyping had been performed once questionnaires had been completed, genetic results for obesity (FTO variant rs9939609) already were available for CPMC active enrollees eligible for the obesity study.
Eligible CPMC participants were informed of the availability of the optional obesity study through tailored email communications and an “optional study page” within the secure CPMC web portal used to communicate with CPMC participants. Following completion of the online informed consent process, study participants were stratified by CPMC cohort and FTO genotype and randomized to one of four types of risk feedback they would receive. The randomization sequence was created using nQuery Advisor 7.0 (Statistical Solutions, Saugus, MA) software with stratification factors of cohort and FTO genotype, permuted block size of 4, and a 1:1:1:1 allocation ratio. Since the randomization and delivery of risk feedback was completely automated, allocation concealment was maintained and all study personnel remain blinded.
Results
Selection of Genetic and Lifestyle Risk Factors and Development of Risk Algorithms
This study followed the same approach to risk reporting used in the CPMC as described by Stack and colleagues [25]. Briefly, genetic variants were selected for inclusion based on a rigorous process of literature review followed by vetting by an independent advisory board called the Informed Cohort Oversight Board (ICOB) for a determination of actionability and inclusion in the study. While the ICOB selected specific genetic variants, the study staff determined which published risk value to report in association with that variant as well as other risk factors, and associated risk values, to report to participants. Other risk factors are limited to established risk factors, i.e., those consistently shown to be associated with disease. Reported risk values were derived from published studies, selected based upon the strength of their design and ability to provide representative and valid estimates of association.
A variant in intro 1 of the FTO (fat mass and obesity) gene, rs9939609, was selected based on its association with obesity in both adults and children [26-27]. It was the first gene to be replicated across multiple studies as a risk factor for obesity [28-31] and has been shown to increase body weight by 1.2 kg per allele [26]. Approximately 16% of adults are homozygous for the high risk allele (AA), which increases the odds of being obese (OR=1.47 in males and 1.46 in females) [32]. Although the exact mechanism of FTO in obesity is unknown, murine models and human studies suggest a possible role for FTO in appetite regulation, energy expenditure, and energy (food) intake [33-35].
Genetic variant risk values for reporting were drawn from a large study by Qi et al. [32] that evaluated the impact of the rs9939609 variant on obesity in two prospective cohorts, the Nurses’ Health Study and the Health Professionals Follow Up Study. The study by Qi et al. was selected based on its size, prospective design and biennially collected BMI values. Obesity study participants were presented with genotype-specific relative risk information, with risk estimates ranging from 1.0 (homozygous wildtype) to 1.3 (homozygous variant) based on presence of 0, 1 or 2 copies of the rs9939609.
The number of potential studies that provided evidence regarding lifestyle risk factors was limited by the requirements that associations be reported in terms of obesity rather than continuous BMI or weight. Publications that met this requirement reported lifestyle risk factors of fruit and vegetable intake, leisure time physical activity, fast food consumption and sitting while watching television. Hu et al [36], reported associations between obesity and sitting while watching television, using data from the Nurses’ Health Study, and was the only study that had employed a prospective design. Associations with fruit and vegetable consumption and leisure-time physical activity were based upon data from a large national study, the National Health Interview Study [37], but used a cross-sectional design, Studies of fast food intake used cross-sectional designs and were based on non-U.S. populations [38-39]. Because the prospective study by Hu et al. was determined to have the strongest design with the most robust and representative risk estimates, time spent sitting while watching television was selected as the only risk factor for the personalized lifestyle risk algorithm.
Thus, participants were provided with lifestyle risk information based on their self-reported hours spent sitting while watching television. Risk estimates ranged from 1.0 (no more than one hour per week spent watching television) to 1.9 (greater than 40 hours per week spent watching television). The decision to use a single lifestyle risk estimate in the trial rather than the simultaneous presentation of several lifestyle risk estimates allowed for a simpler, more rigorous study of how study participants process risk estimates from different sources, particularly when risk estimates are discrepant (average vs. elevated risk). Other obesity risk estimates based on lifestyle factors, such as physical activity and diet, will be presented to participants in the full CPMC report for obesity following completion of the follow-up survey for the obesity trial. This full report also will be released to CPMC members who did not participate in the obesity trial, following the close of the study.
Considerations for the Study Intervention – Online Risk Feedback Reports
Risk feedback reports for the obesity study were modeled on the CPMC reports for other disease conditions (see http://cpmc.coriell.org/Demo/DemoPeople.aspx). Risk reports, regardless of study arm, contained background educational information on obesity, including definitions of obesity and BMI and the prevalence of obesity in the U.S. population. Facts about genetic and/or lifestyle risk factors also were presented to participants as appropriate based on the random assignment.
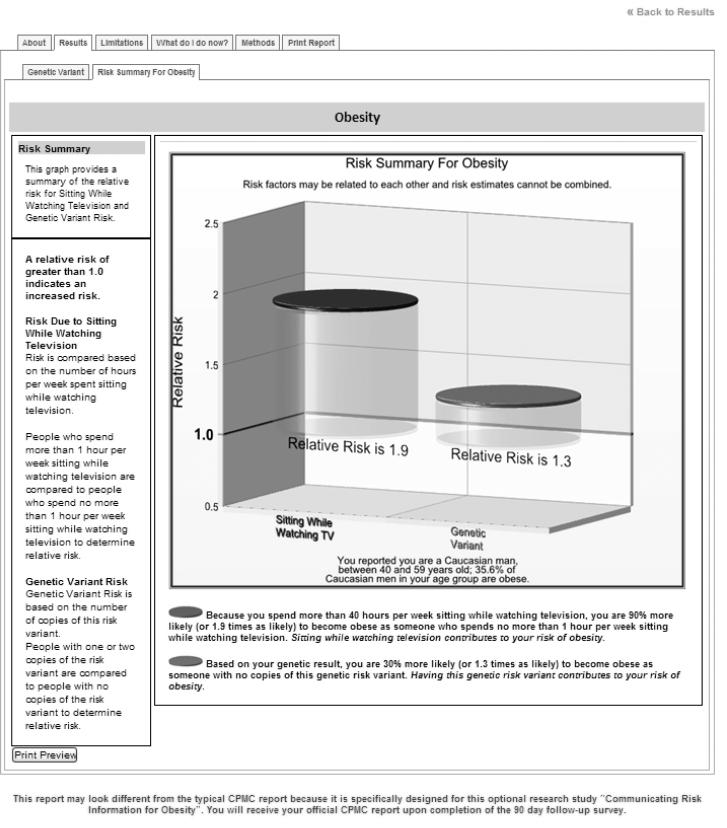
Personalized risk feedback also varied by experimental arm. Risk feedback was presented using a graphic illustration that highlighted the range of relative risks conferred by the risk factor(s); Figure 3 provides an example. A colored disk at the top of each graphic cylinder depicted the participant's personal risk along the risk continuum. Accompanying text was provided with the illustration to aid interpretation of risk. For example, participants in the genetic risk feedback only arm received relative risk estimates based on their FTO variant, presented as a single cylinder graphic. In contrast, those in the lifestyle risk feedback only arm received relative risk estimates based on self-reported sedentary television watching behavior. Participants in the genetic + lifestyle combined arm were presented with both their genetic and lifestyle risk estimates (two side-by-side cylinders). No personal risk feedback was presented to control arm participants.
Figure 3.
Sample Screen from Risk Report for a Participant Assigned to the Combined Genetic and Lifestyle Risk Feedback Arm.
A key challenge for this study was finding a balance between providing clinically standard education (a core component of the parent CPMC study) versus the need to maintain methodological rigor for the obesity trial. For example, the communication of heritability information is standard in the provision of genetic counseling services and is included in CMPC reports to provide a context in which to interpret genetic results for multi-factorial diseases. As noted above, heritability is estimated to be as high as 92% for obesity [3-4]. However, due to the nature of the study aims and the hypothesized importance of people's pre-existing beliefs about disease causation in shaping responses to risk messages, we concluded that inclusion of the heritability estimate would undermine the hypotheses to be tested in the obesity trial. After extensive discussions among study team members and the CPMC management team, it was decided that heritability information would be omitted from the risk reports in the obesity study, but would be presented in the full CPMC obesity report, which is provided to all patients following completion of the 3-month follow-up survey.
Implementation Issues and Considerations
Two issues became apparent once the study was launched in September, 2011: 1) low study enrollment, and 2) inadequate study retention with differential attrition among treatment groups.
1) Low Study Enrollment
The initial targeted enrollment for the obesity study was 1200 participants. During the initial recruitment period (mid-Sept 2011 – mid-Aug 2012), a total of 301 participants enrolled in the study, 292 of whom completed the 20-minute baseline survey. During this period, eligible CPMC participants were notified via email of the opportunity to participate in the obesity study and encouraged to read more information through the CPMC web portal. Those interested could contact the Coriell site pbesity study coordinator, who provided them with instructions on how to access the online consent document. Eligible individuals who did not enroll in the obesity study were sent 4 to 6 email messages during a 3-month period to remind them about the study and their eligibility to participate. The number of email messages depended on personal genetic risk for obesity, with more messages sent to those at elevated risk based on genotype. Participants were offered a $10 incentive for completion of the study, including both the baseline and 3-month follow-up surveys.
Because of low enrollment observed in the first year, a second recruitment phase was implemented in in mid-August 2012. After consulting a survey research expert and a communications expert, we made the following modifications to our recruitment procedures.
a) Personalized messages
Individual names were added to the majority of contact messages from the initial enrollment period, including the initial emailed letter of invitation, reminders to enroll, and reminders to complete the follow-up survey. The appearance of email messages was modified to add logos and signatures from team members in order to make the email messages look like personal letters.
b) Emphasized key message components
All correspondence focused on i) ease of participation (e.g., no travel, only two online surveys), ii) appreciation for participation (e.g., incentive framed as a token of appreciation for their time rather than as a payment for a task), and iii) study timeline and expectations (e.g., graphic representation of the steps involved in study participation to communicate what we were asking participants to do).
c) Modified study enrollment
A direct link to access the study was provided on the CPMC online web portal to bypass contacting the study coordinator.
d) Modified email message schedule and source
After the initial emailed letter of invitation, up to 5 emails were sent over a 3-month period to remind individuals about the opportunity to participate. The sender or source of the message was varied to change the appearance of messages when they appeared in CPMC participants’ inboxes and to increase the chance that emails would be read rather than treated as spam. (CPMC participants could opt out of receiving future email messages about the obesity study.)
e) Increased financial incentives
The total incentive for study participation was increased from $10 to $40. Participants received a $20 gift card after completing each of the baseline and 3-month follow-up questionnaires. A “bonus” incentive ($5 gift card) was offered to the first 100 individuals to enroll during the second enrollment period
During Phase 2, from mid-August 2012 to the close of study enrollment on January 7, 2013, a total of 798 participants enrolled in the trial, an increase of 497 participants over a 5-month period. Notably, 167 participants enrolled in the study within the first week after receiving the initial invitation email message, compared to 76 when the study was first launched a year earlier.
2) Study Retention and Differential Attrition
Three months after completing the baseline survey and viewing obesity risk results, participants were notified to complete a 20-minute follow-up survey. Phase 1 participants became eligible for the 3-month surveys in mid-December 2011. By February 2012, the overall response rate for the 3-month follow-up survey was 69% and response rates varied across treatment groups: 1) no risk feedback − 82%, 2) genetic risk feedback only – 72%, 3) lifestyle risk feedback only– 63%, 4) both genetic and lifestyle risk feedback– 58%. In addition, it was discovered that planned reminder emails for the follow-up surveys had inadvertently not been sent out. Subsequently, reminder emails were implemented and sent out at intervals of 2 weeks, 1 month, and 2 months to all individuals who had not completed the follow-up survey.
Phase 2 modifications included a series of four reminder emails to be deployed for non-respondents (10 days, 1 month, 2 month, 3 months from the initial follow-up survey study invitation), and contingent use of follow-up telephone calls from the study coordinator. Follow-up reminder email messages included more personalized language to emphasize how valuable participants’ responses are to the research and to thank them again for their contribution. A timeline also was included to show that they were “almost done” with the study. As of June 2013, the overall follow-up response rate had increased to 92%, with more uniformity by study arm: 1) no risk feedback – 89%, 2) genetic risk feedback only – 90% 3) lifestyle risk feedback only – 93% 4) both genetic and lifestyle risk feedback− 91%.
Discussion
We have designed and conducted a randomized trial to test the effect of providing obesity genotype information on people's attitudes and beliefs about obesity, their health behaviors, and their actual weight. The obesity study is one of first studies to examine responses to personal obesity genotype information in a real life setting among both overweight and non-overweight individuals [40]. In designing the study, several key decisions were made by the study team in order to overcome current limitations in the published literature, reduce biases, and provide empirical evidence to address currently unresolved issues.
Although we were targeting obesity, we included both participants with healthy weights (BMI <25) and those overweight (BMI≥25). Thus we are able to examine the role of BMI in responses to genetic risk information, building upon prior vignette-based research in this area [8],and to examine how existing phenotypes may influence behavioral responses to genetic risk information differentially. For healthy weight individuals, the study will reveal whether genetic information motivates behavioral efforts to prevent weight gain or results in false reassurance and encourages weight gain. In contrast, individuals who are overweight may feel that learning about the genetic underpinnings of obesity reduces the stigma associated with being overweight [41], which may increase (or undermine) efforts to lose weight [42-43].
The obesity study recruited participants from an existing longitudinal cohort, presenting several challenges. Our team struggled with the format for presenting risk information, which had to conform to the existing structure and standards of the parent CPMC study. In addition, due to the inclusion of other risk reports in the CPMC parent study, selecting the best time to present obesity risk information was a significant challenge. Finally, while selection of the non-genetic risk factor for the obesity trial, i.e., sedentary television watching, was based on the CPMC standard approaches for choosing the most statistically robust risk factor(s) as described in Stack et al. [25], this risk behavior is not commonly targeted in adult obesity prevention efforts in contrast to others such as fruit and vegetable intake or physical activity.
Lessons Learned
Table 1 presents a summary of the implementation issues and potential solutions that were employed within this trial. As with other online research, the use of email invitations to participate in our study had diminishing returns over time. Our team decided to use multiple strategies simultaneously to increase enrollment rate during the second recruitment phase since it was our last chance to motivate participation prior to the onset of email fatigue. Thus, we did not test the relative effectiveness of different approaches deployed and are unable to determine which of the strategies was most influential at motivating enrollment.
Table 1.
Summary of Implementation Issues and Potential Solutions
| Implementation Issue | Potential Solutions |
|---|---|
| Low study enrollment | 1. Modify emailed recruitment letters and reminders |
| a. Increase personalization and visual appeal (e.g., logos, graphics) | |
| b. Emphasize ease of participation, appreciation for time and effort | |
| c. Provide individual study timeline and expectations | |
| d. Revise email schedule regarding number of messages and intervals | |
| e. Use different email sending addresses for reminder emails to limit effect of spam filters | |
| 2. Streamline study entry to remove potential barriers (real or perceived) | |
| 3. Provide incentive for enrollment | |
| a. Mail pre-paid monetary incentive | |
| b. Provide bonus incentive for early enrollment | |
| Low study retention and differential attrition | 1. Modify emailed reminders |
| a. Emphasize value of survey completion to the trial and appreciation for participants’ contributions | |
| b. Display timeline to depict individual participant milestones and remaining tasks | |
| 2. Telephone participants who have not completed follow-up | |
| 3. Modify financial incentives | |
| a. Link incentives to each follow-up survey completed | |
| b. Increase amount of incentives | |
A strategy that we wanted to employ was to mail a small pre-paid monetary incentive, which has been demonstrated in prior online research to boost enrollment [44]. Although the parent CPMC study collects mailing addresses, consent documents for that study indicated that all communication would be electronic unless a problem arose (e.g., undeliverable email messages). Given consent limitations, we were unable to mail letters directly to eligible candidates to recruit them for the obesity study. Future online studies should consider consenting participants to allow for all forms of communication for all research purposes to allow for greater flexibility in added ancillary studies and retention efforts.
The $5 bonus offered to early enrollees in Phase 2 raised questions among IRB board members about whether this crossed the line as payment for participation instead of compensation for participants’ time. It is unknown whether the monetary bonus for early enrollment had an effect on the number of individuals who enrolled or the speed at which they enrolled or whether the concurrent increase in total compensation ($10 in phase 1 vs. $40 in phase 2) had a greater impact on enrollment rates. Additional research examining alternative approaches to up-front incentives are needed.
Study retention improved dramatically over the course of implementing different approaches and the issue of differential attrition that we observed during the first year disappeared as later recruitment and retention strategies were deployed. We speculate that the reason for the initial differences observed in retention may have been due to personal motivation to receive the risk information. Because participants in the combined risk feedback arm already had received both genetic and lifestyle risk feedback about obesity, they may have concluded that they had little to gain by completing the follow-up survey. Designers of future trials may wish to employ some or all of the strategies that were employed in the present study to achieve high retention.
There are several limitations to this study. Specifically, we are examining the short-term impact of providing obesity genotype information as participants are followed for only 3 months. Moreover, this randomized trial has been conducted within the framework of an existing personalized medicine study wherein all participants will have been provided with genetic information for other diseases, thus possibly attenuating the effects of obesity risk information and limiting the generalizability of study findings. However, this trial also had several noteworthy strengths. First, the CPMC cohort provided a sizable source population from which to recruit for the obesity study. In addition, the infrastructure of the CPMC allowed for easy communication with eligible participants through the CPMC's secure web portal. Finally, because all active participants in the CPMC already had been genotyped, genetic risk information was readily available, thus avoiding otherwise prohibitive logistical challenges and costs associated with genotyping. These strengths, along with the unique opportunity to conduct an experimental study about reactions to obesity genetic risk information in a real life setting (as opposed to a hypothetical scenario-based one), greatly outweighed the challenges and limitations we faced.
In sum, we will learn from the randomized trial the value of genetic information, both when provided independently and when combined with lifestyle risk assessment, to motivate individuals to engage in healthy lifestyle behaviors. If genetic information is demonstrated to add value to risk communication efforts by increasing motivation to change behaviors, not only will it be a useful tool to encourage people who participate in traditional weight management programs, it also will provide the much needed behavioral science evidence to inform public health practice and policy and to contribute significantly to the understanding of the effective translation of promising genomic applications into evidence-based guidelines [45-46]. Study findings may serve as a model for future intervention efforts to communicate genetic risk information with the goal of improving overall population health.
Acknowledgements
The Obesity Risk Communication Study is funded by a grant from the National Human Genome Research Institute (R21 HG006073). Dr. Wang also is supported by the National Cancer Institute (K07 CA131103) and a Peter T. Paul career development professorship from Boston University. Dr. Bowen also is supported by a Cooperative Agreement from the Centers for Disease Control and Prevention (U48DP001922). Dr. Green also receives support from the Partners Center for Personalized Genetic Medicine and by NIH grants HG02213, HG005092, HG006500, AG27841 and HG003170. Ms. Gordon, Ms. Wawak, and Dr. Christman and the underlying structure of the Coriell Personalized Medicine Collaborative are supported by the Rohr Foundation, the RNR Foundation, and supported by the Air Force Medical Support Agency (via Johns Hopkins University Applied Physics Laboratory Contract 103438).
We acknowledge Mick Couper and Amanda Dalia for their advice on the strategies deployed to enhance study enrollment and retention and Rachel Kasper for her efforts in data management and extraction of pertinent data for this manuscript.
Footnotes
Clinicaltrials.gov identifier: NCT01355224 Communicating Risk Information for Obesity.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Beydoun MA. The obesity epidemic in the United States--gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 3.Bulik CM, Sullivan PF, Kendler KS. Genetic and environmental contributions to obesity and binge eating. Int J Eat Disord. 2003;33:293–8. doi: 10.1002/eat.10140. [DOI] [PubMed] [Google Scholar]
- 4.Thomsen SF, Ulrik CS, Kyvik KO, et al. Association between obesity and asthma in a twin cohort. Allergy. 2007;62:1199–204. doi: 10.1111/j.1398-9995.2007.01480.x. [DOI] [PubMed] [Google Scholar]
- 5.McBride CM, Bowen D, Brody LC, et al. Future health applications of genomics: priorities for communication, behavioral, and social sciences research. Am J Prev Med. 2010;38:556–65. doi: 10.1016/j.amepre.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng PC, Murray SS, Levy S, Venter JC. An agenda for personalized medicine. Nature. 2009;461:724–6. doi: 10.1038/461724a. [DOI] [PubMed] [Google Scholar]
- 7.Grant RW, Hivert M, Pandiscio JC, Florez JC, Nathan DM, Meigs JB. The clinical application of genetic testing in type 2 diabetes: a patient and physician survey. Diabetologia. 2009 doi: 10.1007/s00125-009-1512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frosch DL, Mello P, Lerman C. Behavioral consequences of testing for obesity risk. Cancer Epidemiol Biomarkers Prev. 2005;14:1485–9. doi: 10.1158/1055-9965.EPI-04-0913. [DOI] [PubMed] [Google Scholar]
- 9.Sanderson SC, Persky S, Michie S. Psychological and Behavioral Responses to Genetic Test Results Indicating Increased Risk of Obesity: Does the Causal Pathway from Gene to Obesity Matter? Public Health Genomics. 2009;13:34–42. doi: 10.1159/000217794. [DOI] [PubMed] [Google Scholar]
- 10.Harvey-Berino J, Gold EC, West DS, et al. Does genetic testing for obesity influence confidence in the ability to lose weight? A pilot investigation. J Am Diet Assoc. 2001;101:1351–3. doi: 10.1016/S0002-8223(01)00323-6. [DOI] [PubMed] [Google Scholar]
- 11.LaRusse S, Roberts JS, Marteau TM, et al. Genetic susceptibility testing versus family history-based assessment: Impact on perceived risk of Alzheimer disease. Genet Med. 2005;7:48–53. doi: 10.1097/01.gim.0000151157.13716.6c. [DOI] [PubMed] [Google Scholar]
- 12.Brewer NT, Edwards AS, O'Neill SC, Tzeng JP, Carey LA, Rimer BK. When genomic and standard test results diverge: implications for breast cancer patients’ preference for chemotherapy. Breast Cancer Res Treat. 2009;117:25–9. doi: 10.1007/s10549-008-0175-2. [DOI] [PubMed] [Google Scholar]
- 13.Markowitz SM, Park ER, Delahanty LM, O'Brien KE, Grant RW. Perceived impact of diabetes genetic risk testing among patients at high phenotypic risk for type 2 diabetes. Diabetes Care. 2011;34:568–73. doi: 10.2337/dc10-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wijdenes-Pijl M, Dondorp WJ, Timmermans DR, Cornel MC, Henneman L. Lay perceptions of predictive testing for diabetes based on DNA test results versus family history assessment: a focus group study. BMC Public Health. 2011;11:535. doi: 10.1186/1471-2458-11-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarini BA, Singer D, Clark SJ, Davis MM. Parents’ concern about their own and their children's genetic disease risk: potential effects of family history vs genetic test results. Arch Pediatr Adolesc Med. 2008;162:1079–83. doi: 10.1001/archpedi.162.11.1079. [DOI] [PubMed] [Google Scholar]
- 16.Hicken B, Tucker D. Impact of genetic risk feedback: Perceived risk and motivation for health protective behaviours. Psychol Health Med. 2002;7:25–36. [Google Scholar]
- 17.Petrie KJ, Weinman JA. Perceptions of Health & Illness: Current Research and Applications. Harwood Academic Publishers; Singapore: 1997. [Google Scholar]
- 18.Marteau TM, Weinman J. Self-regulation and the behavioural response to DNA risk information: A theoretical analysis and framework for future research. Soc Sci Med. 2006;62:1360–8. doi: 10.1016/j.socscimed.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Cameron LD. Illness risk representations and motivations to engage in protective behavior: The case of skin cancer risk. Psychol Health. 2007;23:91–112. doi: 10.1080/14768320701342383. [DOI] [PubMed] [Google Scholar]
- 20.Shiloh S, Rashuk-Rosenthal D, Benyamini Y. Illness causal attributions: an exploratory study of their structure and associations with other illness cognitions and perceptions of control. J Behav Med. 2002;25:373–94. doi: 10.1023/a:1015818532390. [DOI] [PubMed] [Google Scholar]
- 21.Marteau TM, Senior V. Illness representations after the Human Genome Project: The perceived role of genes in causing illness. In: Petrie KJ, Weinman JA, editors. Perceptions of Health & Illness. Harwood Academic Publishers; Amsterdam: 1997. pp. 241–66. [Google Scholar]
- 22.Pijl M, Timmermans DR, Claassen L, et al. Impact of communicating familial risk of diabetes on illness perceptions and self-reported behavioral outcomes: a randomized controlled trial. Diabetes Care. 2009;32:597–9. doi: 10.2337/dc08-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marteau T, Senior V, Humphries SE, et al. Psychological impact of genetic testing for familial hypercholesterolemia within a previously aware population: A randomized controlled trial. Am J Med Genet. 2004;128A:285–93. doi: 10.1002/ajmg.a.30102. [DOI] [PubMed] [Google Scholar]
- 24.Keller MA, Gordon ES, Stack CB, et al. Coriell Personalized Medicine Collaborative (R): a prospective study of the utility of personalized medicine. Per Med. 2010;7:301–17. doi: 10.2217/pme.10.13. [DOI] [PubMed] [Google Scholar]
- 25.Stack CB, Gharani N, Gordon ES, Schmidlen T, Christman MF, Keller MA. Genetic risk estimation in the Coriell Personalized Medicine Collaborative. Genet Med. 2011;13:131–9. doi: 10.1097/GIM.0b013e318201164c. [DOI] [PubMed] [Google Scholar]
- 26.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–94. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scuteri A, Sanna S, Chen WM, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3:e115. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andreasen CH, Stender-Petersen KL, Mogensen MS, et al. Low physical activity accentuates the effect of the FTO rs9939609 polymorphism on body fat accumulation. Diabetes. 2008;57:95–101. doi: 10.2337/db07-0910. [DOI] [PubMed] [Google Scholar]
- 29.Haupt A, Thamer C, Machann J, et al. Impact of variation in the FTO gene on whole body fat distribution, ectopic fat, and weight loss. Obesity (Silver Spring) 2008;16:1969–72. doi: 10.1038/oby.2008.283. [DOI] [PubMed] [Google Scholar]
- 30.Hunt SC, Stone S, Xin Y, et al. Association of the FTO gene with BMI. Obesity (Silver Spring) 2008;16:902–4. doi: 10.1038/oby.2007.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peeters A, Beckers S, Verrijken A, et al. Variants in the FTO gene are associated with common obesity in the Belgian population. Mol Genet Metab. 2008;93:481–4. doi: 10.1016/j.ymgme.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Qi L, Kang K, Zhang C, et al. Fat mass-and obesity-associated (FTO) gene variant is associated with obesity: longitudinal analyses in two cohort studies and functional test. Diabetes. 2008;57:3145–51. doi: 10.2337/db08-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fischer J, Koch L, Emmerling C, et al. Inactivation of the Fto gene protects from obesity. Nature. 2009;458:894–8. doi: 10.1038/nature07848. [DOI] [PubMed] [Google Scholar]
- 34.Fredriksson R, Hagglund M, Olszewski PK, et al. The obesity gene, FTO, is of ancient origin, up-regulated during food deprivation and expressed in neurons of feeding-related nuclei of the brain. Endocrinology. 2008;149:2062–71. doi: 10.1210/en.2007-1457. [DOI] [PubMed] [Google Scholar]
- 35.Wardle J, Carnell S, Haworth CM, Farooqi IS, O'Rahilly S, Plomin R. Obesity associated genetic variation in FTO is associated with diminished satiety. J Clin Endocrinol Metab. 2008;93:3640–3. doi: 10.1210/jc.2008-0472. [DOI] [PubMed] [Google Scholar]
- 36.Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289:1785–91. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 37.Kruger J, Ham SA, Prohaska TR. Behavioral risk factors associated with overweight and obesity among older adults: the 2005 National Health Interview Survey. Prev Chronic Dis. 2009. 6:A14. [PMC free article] [PubMed] [Google Scholar]
- 38.Schroder H, Fito M, Covas MI. Association of fast food consumption with energy intake, diet quality, body mass index and the risk of obesity in a representative Mediterranean population. Br J Nutr. 2007;98:1274–80. doi: 10.1017/S0007114507781436. [DOI] [PubMed] [Google Scholar]
- 39.Smith KJ, McNaughton SA, Gall SL, Blizzard L, Dwyer T, Venn AJ. Takeaway food consumption and its associations with diet quality and abdominal obesity: a cross-sectional study of young adults. Int J Behav Nutr Phys Act. 2009;6:29. doi: 10.1186/1479-5868-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meisel SF, Beeken RJ, van Jaarsveld CH, Wardle J. Genetic test feedback with weight control advice: study protocol for a randomized controlled trial. Trials. 2012;13:235. doi: 10.1186/1745-6215-13-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puhl RM, Heuer CA. Obesity stigma: important considerations for public health. Am J Public Health. 2010;100:1019–28. doi: 10.2105/AJPH.2009.159491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rief W, Conradt M, Dierk JM, et al. Is information on genetic determinants of obesity helpful or harmful for obese people?--A randomized clinical trial. J Gen Intern Med. 2007;22:1553–9. doi: 10.1007/s11606-007-0353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conradt M, Dierk JM, Schlumberger P, et al. A consultation with genetic information about obesity decreases self-blame about eating and leads to realistic weight loss goals in obese individuals. J Psychosom Res. 2009;66:287–95. doi: 10.1016/j.jpsychores.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 44.Alexander GL, Divine GW, Couper MP, et al. Effect of Incentives and Mailing Features on Recruitment for an Online Health Program. American Journal of Preventive Medicine. 2008;34:382–8. doi: 10.1016/j.amepre.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agurs-Collins T, Khoury MJ, Simon-Morton D, Olster DH, Harris JR, Milner JA. Public health genomics: translating obesity genomics research into population health benefits. Obesity (Silver Spring) 2008;16(Suppl 3):S85–94. doi: 10.1038/oby.2008.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henrikson NB, Bowen D, Burke W. Does genomic risk information motivate people to change their behavior? Genome Med. 2009;1:37. doi: 10.1186/gm37. [DOI] [PMC free article] [PubMed] [Google Scholar]