Abstract
Background
Therapy for Staphylococcal infections may be complicated by the possibility of inducible macrolide-lincosamide-streptogramin B resistance (MLSBi). We studied the prevalence of MLSBi in community associated (CA) and hospital associated (HA) Staphylococcus aureus isolates from clinical samples.
Methods
A total of 305 strains of S. aureus comprising 140 (45.9%) [95% CI 40.36–51.52] methicillin resistant S. aureus (MRSA) and 165 (54%) [95% CI 48.48–59.64] methicillin-sensitive S. aureus (MSSA) were identified by conventional methods. The double disc test (D test) was applied by placing erythromycin and clindamycin discs to investigate inducible and constitutive MLSBi resistant phenotypes.
Results
16.6% of MRSA showed constitutive resistance and 37.5% inducible MLSBi resistance. Community associated MRSA (CA-MRSA) represented 10% of all isolates and had lower prevalence of MLSBi than hospital associated MRSA (HA-MRSA).
Conclusion
Routine screening for inducible MLSBi resistance by double disc test can screen for potential treatment failures such that clindamycin can be used effectively and judiciously when indicated for staphylococcal infections especially for treating skin and soft tissue infections (SSTIs) in CA-MRSA due to low prevalence of MLSBi among CA-MRSA.
Keywords: D test, Inducible clindamycin resistance, Staphylococcus aureus
Introduction
Staphylococcus aureus (S. aureus) is increasingly recognized as a cause of hospital associated (HA) and community associated (CA) infections. Macrolide, lincosamide and streptogramin B (MLSB) antibiotics are commonly used in treatment of staphylococcal infections. Widespread use of MLSB antibiotics has led to an increase in resistance to these antibiotics especially clindamycin, amongst staphylococcal strains.1–3 Macrolides such as erythromycin, roxithromycin, clarithromycin and lincosamides such as clindamycin and lincomycin belong to different classes of antimicrobials but act through the same mechanism that is by inhibition of protein synthesis.4 Clindamycin has long been an option for treating both methicillin-susceptible S. aureus (MSSA) and methicillin resistant S. aureus (MRSA) infections. Expression of inducible resistance to clindamycin could limit the effectiveness of this drug.5 Macrolide resistance may be constitutive or inducible in the presence of a macrolide inducer.6
This mechanism can be constitutive, where methylase is always produced, or can be inducible, where methylase is produced only in presence of a macrolide inducer. Among MLSB drugs only macrolides are good inducers of the enzyme erythromycin ribosome methylase (erm). Once induced, the gene product confers cross-resistance to other members of the group including lincosamides and streptogramin B.7 S. aureus isolates with constitutive resistance show resistance to erythromycin and clindamycin on in vitro testing, whereas isolates with inducible resistance show resistance to erythromycin but appear sensitive to clindamycin on disc diffusion testing. A double disc diffusion test (D test) for detecting inducible resistance to clindamycin in erythromycin-resistant isolates can be performed by placing a 15 μg erythromycin disc in proximity to a 2 μg clindamycin disc in adjacent positions.8 This test helps to distinguish staphylococci that have inducible resistance from those with constitutive resistance. For erythromycin-resistant isolates, D test can help to determine whether clindamycin could be used as a therapeutic option (reported as susceptible when the D test is negative or reported as resistant when the D test is positive). Data describing MLSBi prevalence or clinical predictors of the presence of macrolide-lincosamide-streptogramin B resistance (MLSBi) among community acquired methicillin resistant S. aureus (CA-MRSA) and hospital acquired methicillin resistant S. aureus (HA-MRSA) isolates are limited. In the present study, we aimed to determine the prevalence of MLSBi resistance in both hospital and community-associated S. aureus isolates, including MRSA and MSSA.
Materials and methods
This study included 305 nonduplicate isolates of S. aureus from various clinical samples. Isolated microorganisms were identified by using conventional methods (colony morphology, Gram stain, catalase test, slide and tube coagulase test and DNase test). Methicillin resistance was detected using 1 μg oxacillin disc (HiMedia) on a swab inoculated Mueller–Hinton agar plate supplemented with 2% NaCl and incubating at 35 °C for 24 h.
Antimicrobial susceptibilities were studied by Kirby–Bauer disc diffusion method as per guidelines from Clinical and Laboratory Standards Institute (CLSI). Interpretation of the diameters of zones of inhibition was as depicted in Table 1.
Table 1.
Interpretation of erythromycin and clindamycin zone sizes in S. aureus.a
| Sensitive | Intermediate | Resistant | |
|---|---|---|---|
| Erythromycin | ≥23 mm | 14–22 mm | ≤13 mm |
| Clindamycin | ≥21 mm | 15–20 mm | ≤14 mm |
CLSI Guidelines 2010: Performance std for Antimicrobial Disk Susceptibility Tests.
To detect inducible clindamycin resistance, 15 μg erythromycin and 2 μg clindamycin discs (HiMedia) were placed on Mueller–Hinton plate that had been inoculated with a staphylococcal isolate. The discs were placed at a distance of 15–20 mm edge to edge from each other. Plates were incubated overnight at 37 °C. S. aureus ATCC 25923 was used as control for these tests.
A positive D test was taken as flattening of the zone of inhibition around clindamycin disc proximal to erythromycin disc (D shaped zone of inhibition) and was defined as inducible MLSBi resistance (Fig. 1). Strains that were resistant to both erythromycin and clindamycin were defined as exhibiting constitutive MLSB resistance, and those that were resistant to erythromycin and sensitive to clindamycin were the MS phenotype.9 The D test phenotype categories were recorded as noted in Table 2.
Fig. 1.
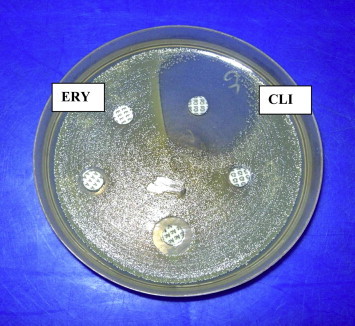
Double disc test (D test) showing flattening of the zone of inhibition around clindamycin disc proximal to erythromycin disc (D shaped zone of inhibition).
Table 2.
D test phenotype categories and their characteristics.
| D test phenotype | Resistance phenotype | CLI result | ERY result | Double disc test description |
|---|---|---|---|---|
| D+ | Inducible MLSB | S | R | Blunted, D shaped clear zone around CLI disc proximal to ERY disc |
| D− | MS | S | R | Clear zone around CLI |
| R | Constitutive MLSB | R | R | Growth upto CLI and ERY discs |
| S | No resistance | S | S | Clear zone around discs |
S – Sensitive, R – Resistant, CLI – Clindamycin, ERY – Erythromycin.
Medical records for the source patients were reviewed for presence of major comorbid conditions such as diabetes mellitus, post surgical status, malignancy, solid-organ transplant, trauma, and burn injury. Based on available records, determination was made as to whether a clinical infection due to the S. aureus cultured was present, as opposed to asymptomatic colonization. Infection was assumed to be present in all cases in which bacterial isolates were derived from blood or cerebrospinal fluid. MRSA isolates were designated HA-MRSA if the source patient had any of the following risk factors or comorbidities: a history of hospitalization, dialysis or surgery in recent past; growth of MRSA 48 h or more upon admission to a hospital; presence of a permanent indwelling catheter or percutaneous device at the time of culture; or prior positive MRSA culture. If none of the above risk factors was present, isolate was considered CA-MRSA.
Statistical analysis
Univariate analysis was carried out. Chi-square test was used for categorical variables and student's 't' test was carried out for quantitative variables.
Results
During the study period, 305 S. aureus isolates were collected prospectively. Among these 140 (45.9%) [95% CI 40.36–51.52] were MRSA and 165 (54%) [95% CI 48.48–59.64] were MSSA The difference in proportion was found to be statistically significant between the MRSA and MSSA (p = 0.04). The presence of MLSBi was confirmed by the D test. A blunted edge with otherwise clear zone of inhibition around clindamycin disc was seen in D test positive strains. The overall prevalence of MLSBi among all Staphylococcus isolates was 20.3% (Table 3).
Table 3.
MLSB resistance phenotype of S. aureus.
| Total isolates | Constitutive MLSB resistance (ERY-R, CLI-R) | Inducible MLSB resistance (ERY-R, CLI-S, D+) | MS phenotype (ERY-R, CLI-S, D−) | |
|---|---|---|---|---|
| S. aureus | 305 | – | – | – |
| MSSA | 165 (54%) | 8 (4.8%) | 10 (6%) | 52 (31.5%) |
| MRSA | 140 (45.9%) | 23 (16.6%) | 52 (37.1%) | 32 (22.8%) |
ERY – Erythromycin, CLI – Clindamycin.
In MRSA 37.5% exhibited the MLSBi, 16.6% exhibited the constitutive phenotype while 22.8% strains exhibited the MS phenotype. Among the 140 MRSA isolates, 30 (21.4%) were designated community associated MRSA (CA-MRSA) and 110 (78.5%) hospital associated MRSA (HA-MRSA). CA-MRSA represented 10% of all isolates.
The presence of MLSBi was detected in 23.3% CA-MRSA and 40.9% HA-MRSA. The proportion of MLSBi amongst HA-MRSA and CA-MRSA was compared and found to be statistically significant in HA-MRSA (86.5%).
Amongst MSSA, 6% exhibited the MLSBi, 8% the constitutive resistance phenotype while 31.5% exhibited MS phenotype. 93.94% exhibited sensitivity to clindamycin.
When the results were compared statistically for presence of inducible resistance in MRSA and MSSA, there was a highly significant difference amongst MRSA showing much higher proportion of MLSBi than MSSA (37.5%, 6% respectively) (p = 0.000). In our isolates the susceptibilities to erythromycin and clindamycin were higher in MSSA than among MRSA (93.94% versus 23.5% respectively). Univariate analysis revealed that methicillin resistance, classification into health care or community associated and presence of comorbidity were statistically significant factors (p < 0.05) as shown in Table 4.
Table 4.
Comparison of D test positive and D test negative S. aureus isolates by the chi square analysis.
| Variable | Isolates n = 305 |
MRSA isolate n = 140 |
Sex n = 305 |
Comorbidity n = 305 |
||||
|---|---|---|---|---|---|---|---|---|
| MRSA | MSSA | Healthcare associated | Community associated | Male | Female | Present | Absent | |
| D+ | 52 (84%) | 10 (16%) | 45 (87%) | 7 (13%) | 44 (71%) | 18 (29%) | 47 (76%) | 15 (24%) |
| D− | 88 (36%) | 155 (64%) | 65 (74%) | 23 (26%) | 182 (75%) | 61 (25%) | 37 (15%) | 206 (85%) |
| p Value | <0.001 | 0.077 | 0.528 | <0.001 | ||||
| Result | Highly significant | Not significant | Not significant | Highly significant | ||||
Discussion
Empirical outpatient treatment options for staphylococcal infections have become more limited as concerns about the prevalence of MRSA have increased. Changing patterns in antimicrobial resistance have led to renewed interest in the use of clindamycin. Clindamycin is frequently used to treat skin and soft tissue infections (SSTIs) because of good oral bioavailability, low cost, excellent tissue penetration and the fact that it accumulates in abscesses. However therapeutic failures caused by MLSBi resistant strains are now being reported commonly.
Resistance to macrolide, lincosamide and streptogramin B (MLSB) antibiotics results from acquisition of erm gene. Expression of MLSB resistance can be constitutive or inducible.
Staphylococcal strains with MLSBi are resistant to inducer macrolides (eg. erythromycin) but susceptible to noninducer macrolides such as spiramycin and lincosamides (clindamycin) and streptogramin B (quinupristin). Inducible clindamycin resistance may not be detected if erythromycin and clindamycin discs are placed in nonadjacent positions.10 We found a high prevalence of 20.3% of MLSBi amongst all staphylococcal isolates. A study conducted in Turkey observed a prevalence of MLSBi as 21.9%.11 Gadepalli et al found 21 per cent inducible MLSBi resistance phenotype.12
Our study suggests a higher prevalence of MLSBi in health care-associated than community associated S. aureus (86.5% versus 13.4% respectively). Implication of this finding is that patients who have infections due to CA-MRSA may reasonably be offered clindamycin as a treatment option because of a lower likelihood of the strains exhibiting MLSBi.
Different studies have found varying prevalence rates of inducible CLI resistance. In one study conducted at the university of Iowa, 65 (62%) of 105 Staphylococcus isolates showed MLSBi resistance phenotype.13 In another study at University of Texas Health Science Centre, 29% of 114 S. aureus isolates showed inducible resistance while 34% showed constitutive resistance to clindamycin.
In a study by Goyal et al, all MLSBi and MS phenotypes displayed resistance to macrolides and exhibited either a low level resistance or susceptibility to clindamycin before the induction by erythromycin. After induction with erythromycin, MICs of clindamycin were noticed to rise from 16 to 256 g/mL in MLSBi phenotypes indicating inducible resistance.14 Almost 50% of our isolates belonged to the MS Phenotype. These isolates showed resistance to erythromycin but were susceptible to clindamycin even in the presence of erythromycin, also 93.94% of MSSA isolates were susceptible to clindamycin suggesting a possible role of treatment with clindamycin in such strains.
Staphylococci exhibiting inducible resistance to MLS antibiotics are now common in clinical practice. Only a few reports describing patients who received clindamycin for S. aureus infections with MLSBi are available, and some of these patients developed constitutive resistance during therapy.3,15,16 One should be cautious about using clindamycin in patients with major infections, especially where treatment is likely to be prolonged or infection difficult to eradicate as constitutive mutants can be selected during the course of clindamycin therapy in patients with MLSBi.17
Currently, some authors recommend avoidance of clindamycin for treatment of complicated infections which may have a high bacterial burden, such as abscesses or osteomyelitis.18 Conversely labeling all erythromycin-resistant staphylococci as clindamycin resistant would prevent the use of clindamycin in infections caused by truly clindamycin susceptible isolates. Clindamycin may be useful for non-MLSBi infections esp. less severe S. aureus infections.
This study reflects the prevalence of MLSBi at a tertiary care centre; however prevalence may differ from institute to institute. Microbiological laboratories should adopt testing for MLSBi among S. aureus isolates and report isolates exhibiting MLSBi as clindamycin resistant.
Conflicts of interest
All authors have none to declare.
References
- 1.Lim J.A., Kwon A.E., Kim S.K., Chong Lee K., Choi E.C. Prevalence of resistance to macrolide, lincosamide and streptogramin antibiotics in Gram-positive cocci isolated in Korean hospital. J Antimicrob Chemother. 2002;49:489–495. doi: 10.1093/jac/49.3.489. [DOI] [PubMed] [Google Scholar]
- 2.Lina G., Quaglia A., Reverdy, Leclercq R., Vandenesch, Etienne Distributon of genes encoding resistance to macrolides, lincosamides, and streptogramins among staphylococci. Antimicrob Agents Chemother. 1999;43:1062–1066. doi: 10.1128/aac.43.5.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drinkovic D., Fuller E.R., Shore K.P., Holland D.J., Ellis Pegler Clindamycin treatment of Staphylococcus aureus expressing inducible clindamycin resistance. J Antimicrob Chemother. 2001;48:315–316. doi: 10.1093/jac/48.2.315. [DOI] [PubMed] [Google Scholar]
- 4.Steward Christine D., Raney Patti M., Morell Allison. Testing for induction of clindamycin resistance in erythromycin resistant isolates of Staphylococcus aureus. J Clin Microbiol. 2005;43:1716–1721. doi: 10.1128/JCM.43.4.1716-1721.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel Mukesh, Waites Ken B., Moser Stephen A., Cloud Gretchen, Hoesley Craig. Prevalence of inducible clindamycin resistance among community and hospital associated Staphylococcus aureus isolates. J Clin Microbiol. 2006;44:2481–2484. doi: 10.1128/JCM.02582-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delialioglu Nuran, Aslan Gonul, Ozturk Candan, Baki Vildan, Sen Sebahat. Inducible clindamycin resistance in staphylococci isolated from clinical samples. J Infect Dis. 2005;58:104–106. [PubMed] [Google Scholar]
- 7.Leclercq Roland. Mechanisms of resistance to macrolides and lincosamides: nature of the resistance elements and their clinical implications. Clin Infect Dis. 2002;34:482–492. doi: 10.1086/324626. [DOI] [PubMed] [Google Scholar]
- 8.Fiebelkorn K.R., Crawford S.A., McElmeel M.L., Jorgensen J.H. Practical disc diffusion method for detection of inducible clindamycin resistance in Staphylococcus aureus and coagulase-negative staphylococci. J Clin Microbiol. 2003;41:4740–4744. doi: 10.1128/JCM.41.10.4740-4744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamilton-Miller J.M.T., Shah Patterns of phenotypic resistance to the macrolide-lincosamide-ketolide-streptogramin group of antibiotics in staphylococci. J Antimicrob Chemother. 2000;46:941–949. doi: 10.1093/jac/46.6.941. [DOI] [PubMed] [Google Scholar]
- 10.Schreckenberger Paul, Ilendo Elizabeth, Ristow Kathryn. Incidence of constitutive and inducible clindamycin resistance in Staphylococcus aureus and coagulase – negative staphylococci in a community and a tertiary care hospital. J.Clin Microbiol. 2004;42:2777–2779. doi: 10.1128/JCM.42.6.2777-2779.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yilmaz Gurdal, Aydin Kemalettin. Detection and prevalence of inducible clindamycin resistance in staphylococci. J Med Microbiol. 2007;56:342–345. doi: 10.1099/jmm.0.46761-0. [DOI] [PubMed] [Google Scholar]
- 12.Gadepalli Ravisekhar, Dhawan Benu, Mohanty Srujana, Kapil Arti. Inducible clindamycin resistance in clinical isolates of Staphylococcus aureus. Indian J Med Res. 2006;123:571–573. [PubMed] [Google Scholar]
- 13.Sanchez, Flint, Jones Occurrence of macrolide-lincosamide-streptogramin resistance among staphylococcal clinical isolates at a university medical center. Is false susceptibility to new macrolides and clindamycin a contemporary clinical and in vitro testing problem. Diagn Microbiol Infect Dis. 1993;16:205–213. doi: 10.1016/0732-8893(93)90111-j. [DOI] [PubMed] [Google Scholar]
- 14.Goyal R., Singh N.P., Manchanda V., Mathur M. Detection of clindamycin susceptibility in macrolide resistant phenotypes of Staphylococcus aureus. Indian J Med Microbiol. 2004;22:251–254. [PubMed] [Google Scholar]
- 15.Lewis J.S., Jorgensen J.H. Inducible clindamycin resistance in staphylococci: should clinicians and microbiologists be concerned? Clin Infect Dis. 2005;40:280–285. doi: 10.1086/426894. [DOI] [PubMed] [Google Scholar]
- 16.Rao G.G. Should clindamycin be used in treatment of patients with infections caused by erythromycin resistant staphylococci? J Antimicrob Chemother. 2000;45:715. doi: 10.1093/jac/45.5.715. [DOI] [PubMed] [Google Scholar]
- 17.Levin T.P. Potential clindamycin resistance in clindamycin susceptible, erythromycin resistant Staphylococcus aureus: report a clinical failure. Antimicrob Agents Chemother. 2005;49:1222–1224. doi: 10.1128/AAC.49.3.1222-1224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siberry G.K., Tekle T., Carroll K., Dick J. Failure of clindamycin treatment of methicillin resistant Staphylococcus aureus expressing inducible clindamycin resistance in vitro. Clin Infect Dis. 2003;37:1257–1260. doi: 10.1086/377501. [DOI] [PubMed] [Google Scholar]


