Abstract
Objective
To prospectively study the techniques and outcomes of transcatheter closure of complex Atrial septal defects (ASD).
Study design and settings
Prospective single center study with experience in catheter closure of ASD. All patients with complex ASD suitable for device closure.
Objective
Analysis of outcomes of transcatheter closure of complex ASD in JIPMER Hospital over the past 5-year period.
Methods
Complex ASD was predefined and patients satisfying inclusion and exclusion criteria are included. All the patients had meticulous Transesophageal echocardiography (TEE) imaging beforehand. Modifications of the conventional techniques were allowed on a case per case basis according to operator preference. Successfully intervened patients were followed up clinically.
Results
Out of the 75 patients enrolled, 69 patients had successful device closure (success rate 92%) despite challenging anatomy. Fifty-six (74%) patients had ASD ≥25 mm. Fifteen patients (20%) had defect size ≥35 mm and 20 patients (26.6%) had devices implanted with ≥35 mm waist size. Fifty percent of patients had complete absence of aortic rim and 25% had deficient posterior rim. Twenty percent of patients had malaligned septum. Mean follow up period was 3.2 years.
Conclusions
Trans catheter closure is feasible in anatomically complex substrates of Secundum ASD. Careful case selection, scrupulous imaging protocol, and expertise in modified techniques are mandatory for successful outcomes.
Keywords: Complex atrial septal defects, Device closure of ASD, Balloon assisted technique
1. Introduction
Trans catheter closure for Atrial Septal Defects (ASD) has been in vogue for more than two decades.1 As the experience and the techniques evolved, the operators are daring to take up more challenging defects for the catheter based treatment. No doubt, surgery remains the gold standard as it carries negligible mortality and 100% operative success rates, not to say that it can deal with any number of complex anatomies. But the technical ease, the comfort of the procedure, lack of a scar and incredibly less morbidity has taken transcatheter closure miles ahead in patient and operator preference as the choice of modality.
2. Objective
We analyzed prospectively the pattern, techniques and outcomes of transcatheter closure of complex atrial septal defects over the past 5 years in our institute.
3. Design and settings
Prospective single center registry in a tertiary care hospital was done. All patients referred for ASD closure were initially evaluated with transthoracic echocardiography (TTE) and then with Transesophageal echocardiogram (TEE). In very small children and infants, transesophageal echo was avoided if the transthoracic imaging is adequate with details.
3.1. Inclusion criteria
3.1.1. Patients with complex ostium secundum ASD
Definition of complex ASD includes satisfying one of the following criteria:
-
1.
Large ASD – long axis in any view measuring ≥25 mm.
-
2.
Malaligned septum.
-
3.
Multiple ASD.
-
4.
ASD with septal aneurysm.
-
5.
ASD with deficiency/floppy posterior or inferior rims with or without complete absence of aortic rim.
-
6.
ASD with associated lesions like mitral/pulmonic stenosis, post-tricuspid shunts, coronary anomalies.
3.2. Exclusion criteria
-
1.
Atrial septal defects involving primum and sinus venosus locations.
-
2.
Pulmonary artery hypertension with hemodynamics suggestive of inoperability.
-
3.
Complete absence of Inferior/Superior vena caval rim.
-
4.
Size more than 44 mm.
4. Methods
4.1. Imaging
All patients referred for device closure were scrutinized for satisfaction of inclusion and exclusion criteria. All adult patients had a pre procedure TTE as well as TEE. TEE imaging of all complex defects were done with 120° sweeping in addition to the standard imaging angles at 0, 45 and 90°. The echo was done in presence of the operators themselves in view of the complexity of the anatomy. The size of the defect, shape of the defect, number of defects, and location with regard to mitral valve, and aortic valve were noted. Each of the important rims were measured and documented. Malalignment and aneurysm of the septum were also noted.
4.2. Intervention
All procedures in adult patients were done under local anesthesia and mild sedation under TEE guidance. Pediatric patients were subjected to general anesthesia with or without TEE. All patients had an arterial line with a 5F sheath in view of predictable long duration of procedure and anticipated impingement on vital structures as the defects were either large or with challenging rims. All the procedures were done under full heparinization with 100 U/kg of unfractionated heparin and maintenance of ACT between 250 and 300 s. All patients received a bolus dose of Aspirin 300 mg (4 mg/kg in pediatric patients) followed by 150 mg daily (2 mg/kg in pediatric patients) for 6 months after successful device closure.
The ASD is crossed with a multipurpose (5F/6F) or Cournard catheter and a 0.035″ Guidewire (Terumo Inc.) and catheter was parked in the left upper/right upper pulmonary veins. Exchanged for the 0.035″/0.038 super stiff wire depending on the support required for large delivery sheaths.
4.3. Modified techniques for complex ASD closures
The ASD closure was attempted in the standard ways in many patients, and if fails modified techniques was used. In some patients, where very large devices/floppy rims were present, we straightway went ahead with modified techniques.
4.4. Balloon sizing of defects
ASD sizing balloon with stop flow technique was used upon operator's discretion. In cases where there is a malalignment defect, defects with septal aneurysm and where there is suboptimal sizing with TEE, balloon sizing was done with stop flow echo technique and fluoroscopic measurement.
4.5. Pulmonary vein deployment technique
In this method, the left atrial disc is completely deployed in the pulmonary vein and keeping the disc in pulmonary vein, the whole device is stretched out to open the right atrial disc. Momentary release of the left atrial disc was done when the right atrial disc fans out to catch out the two sides of the septum. We have used this technique in left upper, right upper and some times in left lower pulmonary vein.
4.6. Left atrial roof deployment method
The same principle as in pulmonary veins, but the left disc is opened against the left atrial roof.
4.7. Modified/Cut sheath approach
Here the operator cuts the sheath tip in an oblique fashion to allow for asymmetric expansion of the left atrial disc to catch the rims of the defect followed by the asymmetric deployment of the right atrial disc. This method was used in some cases of malaligned septum.
4.8. Dilator/Catheter assisted method
With the help of a contralateral venous access, a long dilator/Diagnostic catheter–wire assembly was kept across the defect to support the device delivery. This method was used in some cases of deficient posterior/aortic rims, where the device tends to slip back in to right atrium in the conventional approach.
4.9. Balloon assisted technique (BAT)
Here again with the help of a contralateral venous sheath, a highly compliant sizing balloon was used to occlude the defect fully or partially for supporting the device delivery. The device is then delivered from either the left upper / right upper pulmonary vein and the balloon over the wire placed on the contra lateral side. With the inflated balloon in situ across the defect, we deploy the LA disc and then pull back the assembly to the septum. The balloon supports and prevents prolapse of the LA disc in to RA and then allows the delivery of the RA disc outside of the sheath. Then after checking the discs on their respective sides of the septum by TEE, the operator deflates the balloon slowly allowing the discs to expand and stent the septum followed by careful slow and steady withdrawal of the balloon. This method was useful in deployment of large devices above 35 mm and with deficient/floppy margins.
We have used the Amplatzer (St. Jude) and Cocoon (Vascular Innovations) sizing balloons measuring 24 and 34 mm for the balloon assisted technique.
5. Results
Of the total number of 169 patients referred for trans catheter closure of ASD during the period of last 5 years in JIPMER Hospital from Jan 2008 to Dec 2012, 75 patients had complex atrial septal defects satisfying the inclusion and exclusion criteria (44.38%). The baseline characteristics of the patients are given in Table 1. 17 patients were under the age of 12. The mean device size was 33.1 mm. The device size chosen was larger by 2.4–4.9 mm than the measured maximum ASD size measured.
Table 1.
Baseline characteristics.
| Baseline characteristics | n (%) Patientsa |
|---|---|
| Age (range) | 15 months–64 years |
| Mean age (years) | 21.4 |
| Gender male/female | 59/16 (78.6/21.4) |
| Sinus rhythm | 70 (93.3) |
| Atrial fibrillation | 5 (6.6) |
| Left to right shunt & RV volume overload | 75 (100) |
| RV systolic pressure>40 mmHg | 32 (42.6) |
| Previous stroke | 3 (4) |
| Congestive heart failure | 6 (8) |
| Hypertension | 7 (9.3) |
| Coronary artery disease | 3 (4) |
| RV systolic dysfunction | 4 (5.3) |
| Large ASD (≥25 mm) | 56 (74.6) |
| Very large ASD (≥35 mm) | 15 (20) |
| Malaligned septum | 17 (22.6) |
| Multiple/Fenestrated ASD | 4 (5.3) |
| Septal aneurysm | 5 (6.6) |
| Deficient/floppy posterior rim | 23 (30.6) |
| Complete absence of aortic rim | 37 (49.3) |
| ASD with mitral stenosis (Lutembacher's syndrome) | 1 (1.3) |
| ASD with valvular PS | 2 (2.6) |
| ASD with PDA | 3 (4) |
| ASD with VSD | 1 (1.3) |
| ASD with partial ALCAPA | 1 (1.3) |
n/% = Number/Percentage of patients.
One patient had Dextrocardia who had a large ASD as part of Kartagener's syndrome underwent successful intervention. 5 patients had severe pulmonary artery hypertension (PAH) with systolic pressures above 70 mmHg. Systolic pulmonary artery pressures came below 40 mmHg in three patients immediately post intervention and in one patient during follow up. One patient continues to have moderate pulmonary hypertension.
The technique of intervention varied in different patients (Table 2). Balloon sizing of the ASD was done in 35 patients in this group according to the operator's discretion. However it was done in all cases of septal aneurysm and fenestrated ASD. In cases where balloon sizing was done, we found a good correlation between balloon sizing by stop flow echo and balloon waist measurement by fluoroscopy with a mean difference of 1.6 mm. We did not observe any complications secondary to balloon sizing.
Table 2.
Techniques used for device placement.
| Technique of intervention | n (%) |
|---|---|
| Balloon assisted technique | 21 (29.5) |
| Left upper/lower pulmonary vein method | 16 (22.5) |
| Right upper pulmonary vein method | 11 (15.4) |
| Modified/cut sheath method | 10 (14) |
| Left atrial roof method | 9 (12.6) |
| Conventional technique | 6 (8.4) |
| Catheter/dilator support | 2 (2.8) |
| Total number of Interventions | 71 |
One patient underwent balloon mitral valvuloplasty before device closure. Mitral valve area improved to 1.7 cm2 and then ASD was closed. Two patients had successful balloon opening pulmonic valve before closing ASD. Patients who had post tricuspid shunts were closed transcatheter wise before undertaking ASD closure. The patient with ALCAPA had uneventful closure of the ASD without any device impingement on the anomalous artery.
5.1. Immediate outcomes
The device closure was successful in 69 patients (success rate of 92%) The devices used in this study include the double umbrella type discs {Amplatzer Septal Occluder (St. Jude), Cocoon Septal Occluder (Vascular Innovations) and Heart R Septal Occluders (Lifetech)}. In four patients intervention was unsuccessful. In 3 patients having large defects with deficient posterior margin the device closure was a failure. The fourth patient developed hemodynamic compromise while device positioning warranting immediate recapture of the device. Later it was confirmed that the patient had an anomalous left circumflex artery from the right sinus coursing transversely near the septum getting compressed by the device. All these patients were referred for elective surgical closure. Four patients had multiple ASD; Use of a larger device resulted in closure of all defects in 2 patients, where as two patients had small additional defect located far away and could not be closed with single device. None of the patients in this study had more than one device implanted. There was no procedure related cardiac tamponade or any vascular complications. All pediatric patients had an uneventful anesthesia recovery. There was no sustained arrhythmia or device related thromboembolic complications.
5.2. Procedure time and fluoroscopy
The complex defects definitely required longer procedure and fluoroscopic time. The mean fluoroscopic time was 28 min and procedure time was 52 min.
5.3. Follow-up outcomes
The mean follow-up period for the study is 3.2 years (range 6 months–5 years). All patients who underwent closure had symptomatic improvement. In large defects with deficient posterior margin, we observed residual shunting in 5 patients, but all of them disappeared at follow up TEE done one month after the procedure. 2 patients had small additional ASD, which remained patent after intervention. All other patients had 100% occlusion rates. There was no procedure related late mortality. None of the patients in Atrial fibrillation reverted to sinus rhythm.
5.4. Closure of very large ASD (≥35 mm)
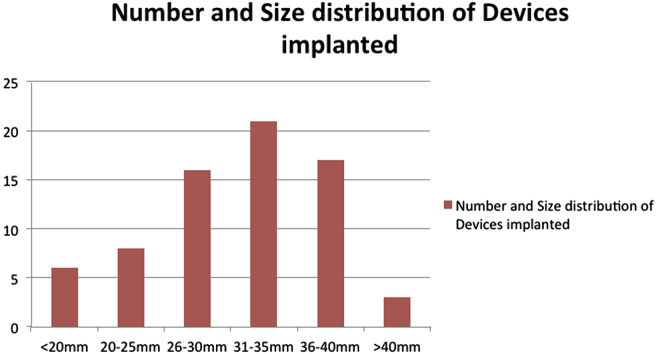
Overall 20 patients (26.6%) had devices implanted with more than 35 mm waist size (Fig. 1). Four patients had 40 mm implants while 2 had 42 mm devices and one patient the largest in the study i.e. 44 mm. Fig. 2, Fig. 3 show the sequential echocardiographic and fluoroscopic images of balloon assisted intervention of an extremely large ASD of 38.5 mm with a 42 mm device. Video 1 shows balloon assisted device closure in a patient with large 36 mm defect and posterior malaligned atrial septum being successfully closed with a 40 mm ASD occluder.
Fig. 1.
Number and sizes of devices used.
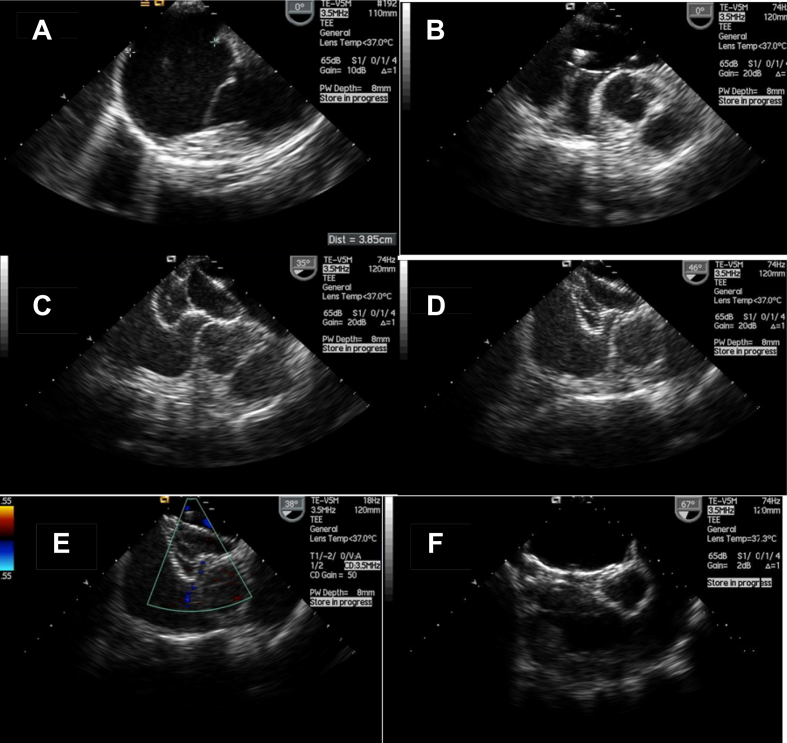
Fig. 2.
Serial transesophageal echocardiography images of balloon assisted device closure of a large 38.5 mm atrial septal defect with a 42 mm ASD device (A–F). A) Very large ASD measuring 38.5 mm with deficient posterior rim, B) Sizing balloon occluding the defect, C) Device in hour glass shape with either discs in respective atria and waist compressed by inflated balloon, D) Balloon is deflated and waist expands, E) Device in good position snugly holding on after removal of the balloon, F) Device after deployment.
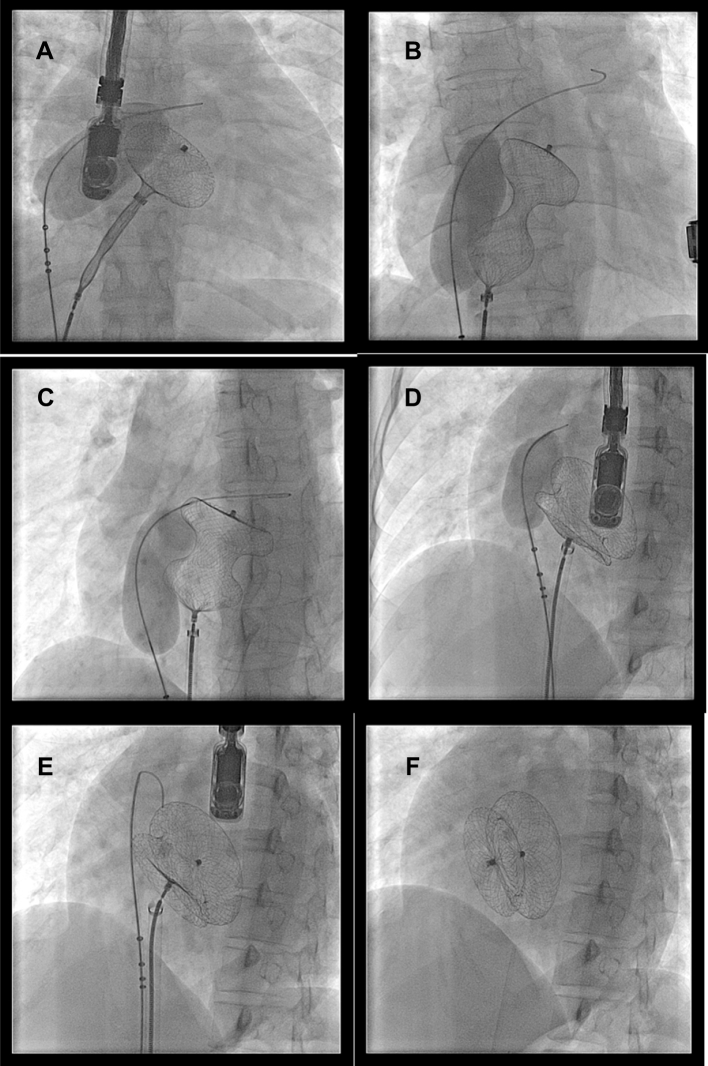
Fig. 3.
Serial fluoroscopic images of balloon assisted device closure of a large 38.5 mm atrial septal defect with a 42 mm ASD device (A–F). A) Left disc being delivered with balloon inflated across the defect, B) Whole device delivered with balloon inflated, device assumes a hour glass shape with waist being compressed by the balloon, C) The waist expands as balloon starts deflating, D) The waist expands further as balloon gets smaller, E) Device in good position after complete deflation, F) Device deployed after withdrawal of the balloon.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ihj.2013.12.016.
The following is the supplementary data related to this article:
5.5. Complications
Coronary air embolism and transient ST elevation occurred in 5 patients during procedure. But none of them resulted in any form of hemodynamic compromise. All of them settled spontaneously with high flow oxygen inhalation.
There were three patients who had device embolization, two of them on table and the third 5 h after procedure, two to the pulmonary artery and one device in to the left atrium. All of them were successfully retrieved percutaneously. Two of these patients where in the deficient posterior rim and large size of the defect (38 mm, 35 mm) were the reasons for instability, were referred for elective surgery. In the third patient, the complete absence of aortic rim with malaligned septum made the procedure difficult. Here we downsized the device size from 24 mm to 20 mm and we were successful. We feel that oversizing the device made high tension on the device framework which made it prolapse and embolise in to pulmonary artery.
Two patients developed new onset pericardial effusion albeit mild, at one month follow up. It was a cause of concern; both of them were admitted and evaluated for early onset erosion. No findings of erosion were seen in TEE and computed tomography and the effusion subsided spontaneously. We presume the cause to be metal allergy.
Three patients had mild mitral regurgitation post-procedure and were accepted in view of the large device touching the anterior mitral valve leaflet. On follow up, none of the leaks progressed. There was no occurrence of aortic regurgitation.
Three patients developed aspirin intolerance (gastric side effects) and needed switching over to clopidogrel.
6. Conclusions
Transcatheter closure has become the default treatment option for ASD closure. Even complex cases can be successfully treated with judicious case selection and modified techniques for device implantation. Our success rate in this study was 92% with no immediate or late mortality. We had three cases of device embolization (4%) where the substrate was large defects with deficient/floppy posterior margin. All the embolised devices retrieved successfully in the cath lab. There was no referral for emergency surgery. Balloon sizing was very useful in predicting device size in cases were TEE imaging is sub-optimal as in with septal aneurysm. We did not experience any complications related to balloon sizing. Our patients did extremely well on clinical follow up. Catheter closure of complex ASD can be done in experienced centers with very good success rates, however we recommend extreme caution in case selection with TEE playing a pivotal role in understanding defect characteristics and planning strategy.
7. Discussion
Our study is a single center experience of closing complex ASD. More than 50% of our study population had defects more than 30 mm, a subset in which catheter closure is often challenging. In addition to the size of the defect, understanding the overall morphology of the septum is crucial.2 Posterior location, complete absence of aortic rim and floppy posterior rims are difficult situations for device positioning.3 Our success rate is 92% with an embolization rate of 4%, which we think is because of extremely complex anatomy.
We should understand that the success of complex ASD closure mainly lies on the proper imaging techniques.4 A thorough interpretation of the septal anatomy is paramount to success. Complex anatomical substrates like sinusoidal septum, aneurysm, and fenestrated defects require careful delineation before planning intervention.5 We did study all patients with a detailed transesophageal examination beforehand. The pediatric subjects did have good trans thoracic views and because of logistic reasons, we had to plan interventions without TEE in some of the cases. TEE also is suboptimal in septal aneurysms and fenestrations/tissue tags straddling ASD. Balloon sizing was useful in all the cases where TEE imaging was suboptimal.
Lack of proper aortic rim is often granted for success, but we found that complete absence of retro aortic tissue is often challenging because almost always the LA disc flips tangentially across the defect. Proper alignment almost always requires one of the modified techniques already described, especially in large defects with floppy posterior rims. TEE imaging can add clues in such scenario, if diametrically opposite points in TEE are inadequate, then device closure is difficult. Short axis (45°) TEE would be of great value for this. We have never attempted a case of absent inferior vena caval (IVC) rim. Even in cases of deficient posterior rim, we made sure that a proper IVC rim is there before deciding for catheter closure. We did not use three dimensional TEE (3 DTEE), but believe that as we move along this would be a very useful tool in the future in understanding complex septal morphology and thus aid in catheter closures.6 Intracardiac echocardiography (ICE) is another emerging tool for imaging trans catheter closure of ASD. But given the learning curve and cost involved in ICE and the expertise we have over TEE, it surely would take precedence over ICE for complex defects.7
The crux of the success is the device alignment with respect to the defect and surrounding structures. In complex cases, modified techniques would help alignment in more predictable fashion by allowing the LA disc to remain expanded over the left side of the septum while the RA disc fans out over the right side of the septum. Conventional technique was successful in less than 10% cases in our study. This essentially helps the device “stent” the defect. We would like to make a special mention the usefulness of balloon assisted technique in closing very large defects like 40 mm. The technique proposed by Dalvi et al8 deserves special mention as we used this technique more often although we used the sizing balloon supplied along with the device.
ASD is never a round structure and often it's oval in shape. Measurement in long and short axis views is thus helpful in deciding the device size. In pediatric subsets, we gave importance to total septal length so as to see whether the left atrial side which of often smaller would accommodate the larger (12–14 mm) LA disc. Mitral valve impingement is a source of another concern, and we did see 2 cases of MR albeit mild. We did not encounter a case where in two devices would be required for complete closure of fenestrated defects. We were able to close the moderately sized adjacent defects with a single large occluder. But surely large multiple ASD's situated farther away in the septum would require multiple devices. Definitely surgery continues to be a gold standard for complex ASD closure, but we understand that with growing experience, a significant proportion of the complex defects could be treated successfully by the percutaneous method with modified techniques and precise understanding of the anatomy.
Conflicts of interest
All authors have none to declare.
References
- 1.Rao P.S., Sideris E.B., Hausdorf G. International experience with secundum atrial septal defect occlusion by the buttoned device. Am Heart J. 1994;128:1022–1035. doi: 10.1016/0002-8703(94)90602-5. [DOI] [PubMed] [Google Scholar]
- 2.Masura J., Gavora P., Formanek A., Hijazi Z. Transcatheter closure of secundum atrial septal defects using the new self-centering Amplatzer Septal Occluder: initial human experience. Cathet Cardiovasc Diag. 1997;42:388–393. doi: 10.1002/(sici)1097-0304(199712)42:4<388::aid-ccd7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Chan K.C., Godman M.J. Morphological variations of fossa ovalis atrial septal defects (secundum): feasibility for transcutaneous closure with the clam-shell device. Br Heart J. 1993;69:52–55. doi: 10.1136/hrt.69.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwinger M.E., Gindea A.J., Freedberg R.S., Kronzon I. The anatomy of the interatrial septum: a transesophageal echocardiographic study. Am Heart J. 1990;119:1401–1405. doi: 10.1016/s0002-8703(05)80191-7. [DOI] [PubMed] [Google Scholar]
- 5.Santoro G., Bigazzi M.C., Lacono C. Transcatheter closure of complex atrial septal defects: feasibility and mid-term results. J Cardiovasc Med (Hagerstown) 2006 Mar;7:176–181. doi: 10.2459/01.JCM.0000203852.82643.f2. [DOI] [PubMed] [Google Scholar]
- 6.Vasilyev N.V., Martinez J.F., Freudenthal F.P., Suematsu Y., Marx G.R., del Nido P.J. Three-dimensional echo and videocardioscopy-guided atrial septal defect closure. Ann Thorac Surg. 2006 Oct;82:1322–1326. doi: 10.1016/j.athoracsur.2006.05.002. discussion 1326. [DOI] [PubMed] [Google Scholar]
- 7.Budts W., Troost E., Voigt J.U., Gewillig M. Intra cardiac echocardiography in atrial septal interventions: Impact on hospitalization costs. Acta Cardiol. 2010 Apr;65:147–152. doi: 10.2143/AC.65.2.2047047. [DOI] [PubMed] [Google Scholar]
- 8.Dalvi B.V., Pinto R.J., Gupta A. New technique for device closure of large atrial septal defects. Catheter Cardiovasc Interv. 2005 Jan;64:102–107. doi: 10.1002/ccd.20248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.